- 1Department of Surgery, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain
- 2College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain
- 3Department of Pathology, King Hamad University Hospital, Busaiteen, Bahrain
- 4Department of Physiology, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain
- 5Department of Clinical Physiology, Faculty of Medicine, Menoufia University, Menoufia, Egypt
- 6Department of Surgery, Jordan University of Science and Technology, Irbid, Jordan
Introduction: A mesenteric inflammatory myofibroblastic tumor (IMT) is a rare solid tumor of intermediate malignant potential that affects children, adolescents, and young adults predominantly. IMT is mostly encountered in the lung. We report a case of malignant jejunal mesenteric IMT in a 61-year-old male patient who presented with vague abdominal pain and generalized weakness. CT scan revealed a mesenteric mass displacing the attached jejunum. Surgical resection was curative.
Discussion: An extensive literature review was performed to update and further analyze the already available data. A total of 35 cases with mesenteric IMT were reported previously. Only five cases of jejunal mesenteric IMT were reported. Mesenteric IMT demands vast effort to reveal the diagnosis due to its vagueness in the clinical presentation. Mesenteric IMT resembles each other in plenty of pathological and immunohistochemical characteristics.
Conclusion: To the best of our knowledge, this is the first case of malignant jejunal mesenteric IMT in the elderly. Surgical resection was curative.
Introduction
An inflammatory myofibroblastic tumor (IMT) of the lung was first reported in 1939 by Brunn (1). Classically, IMT was known for its benign propensity and local involvement. Locally malignant variants (with metastases seldomly) were later described. Currently, the World Health Organization states its nature as an intermediate malignant neoplasm with mesenchymal origin (2). IMT has been reported in almost all body and soft tissue visceral organs (3, 4). Most recorded cases were in the lung, followed by the mesentery and the omentum (5, 6). IMT has also been known as inflammatory pseudotumor, plasma cell granuloma, omental mesenteric myxoid hamartoma, and inflammatory fibrosarcoma. This reflects the diversity in the clinicopathological pictures of this tumor (7). The etiology of IMT remains unclear. However, it has been significantly related to viral infection (HHV-8), trauma, and surgery (8). The anaplastic lymphoma kinase (ALK) gene exists in 33–67% of IMT cases, with a higher prevalence in children and young adults (9). Microscopically, IMT is composed of spindle-shaped cells of myofibroblasts accompanied by inflammatory cells (10). There are no distinctive signs or symptoms for IMT, due to its ability to affect various anatomical sites (11). Herein, we report a rare case of malignant IMT in the jejunal mesentery of a 61-year-old male patient who presented with abdominal pain and generalized weakness.
Case report
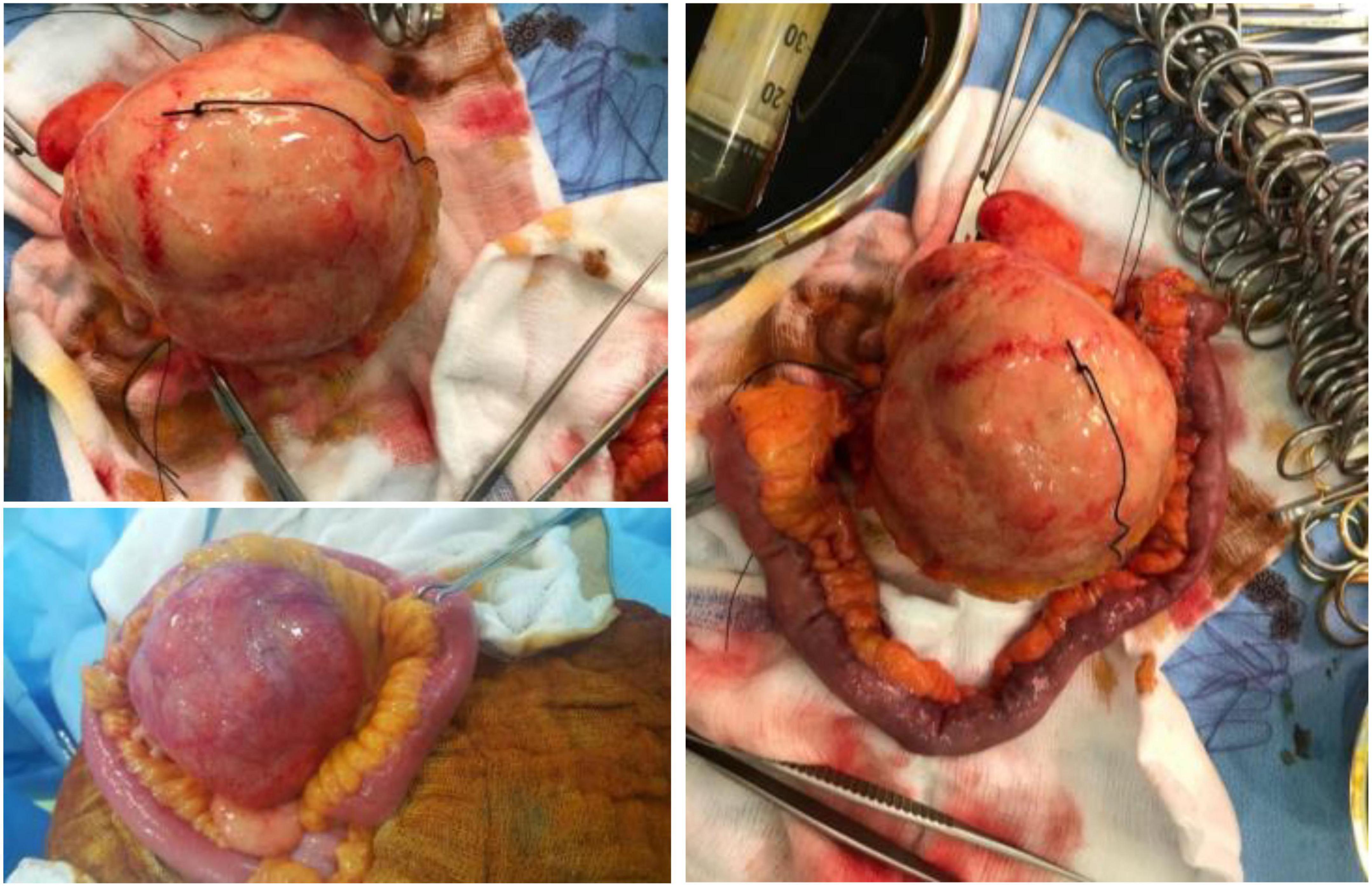
A 61-year-old male patient presented with vague progressive abdominal pain and generalized weakness for a 6-month duration. He is married with four children. He is a non-smoker and a mechanical engineer. General clinical examination revealed paleness. Abdominal examination revealed a central intra-abdominal mass. Complete blood count (CBC), liver, and renal function tests were normal apart from a low hemoglobin (Hb; 12.15 g/dl) and a high erythrocyte sedimentation rate (ESR; 22 mm/h). Abdominal ultrasound (US) revealed a well-defined solid hypoechoic mesenteric mass measuring about 7.8 × 8 cm that was situated in the upper abdomen above the umbilicus. CT scan showed a mesenteric mass with no evidence of intestinal obstruction (Figure 1). These findings gave an ambiguous diagnosis of a solid mass in the mesentery (Figure 1). Fine-needle aspiration cytology (FNAC) or true-cut biopsy was not recommended by the radiologist due to the absence of a safe window. Exploratory laparotomy was the decisive treatment for this patient. Intraoperatively, a spheroid-like mass was occupying the mesentery of the jejunum with an intact jejunal wall (Figure 2). The whole mass was resected with partial jejunectomy followed by end-to-end anastomosis. There was no gross lymphadenopathy. Cytological evaluation of the peritoneal fluid was negative for malignant cells.
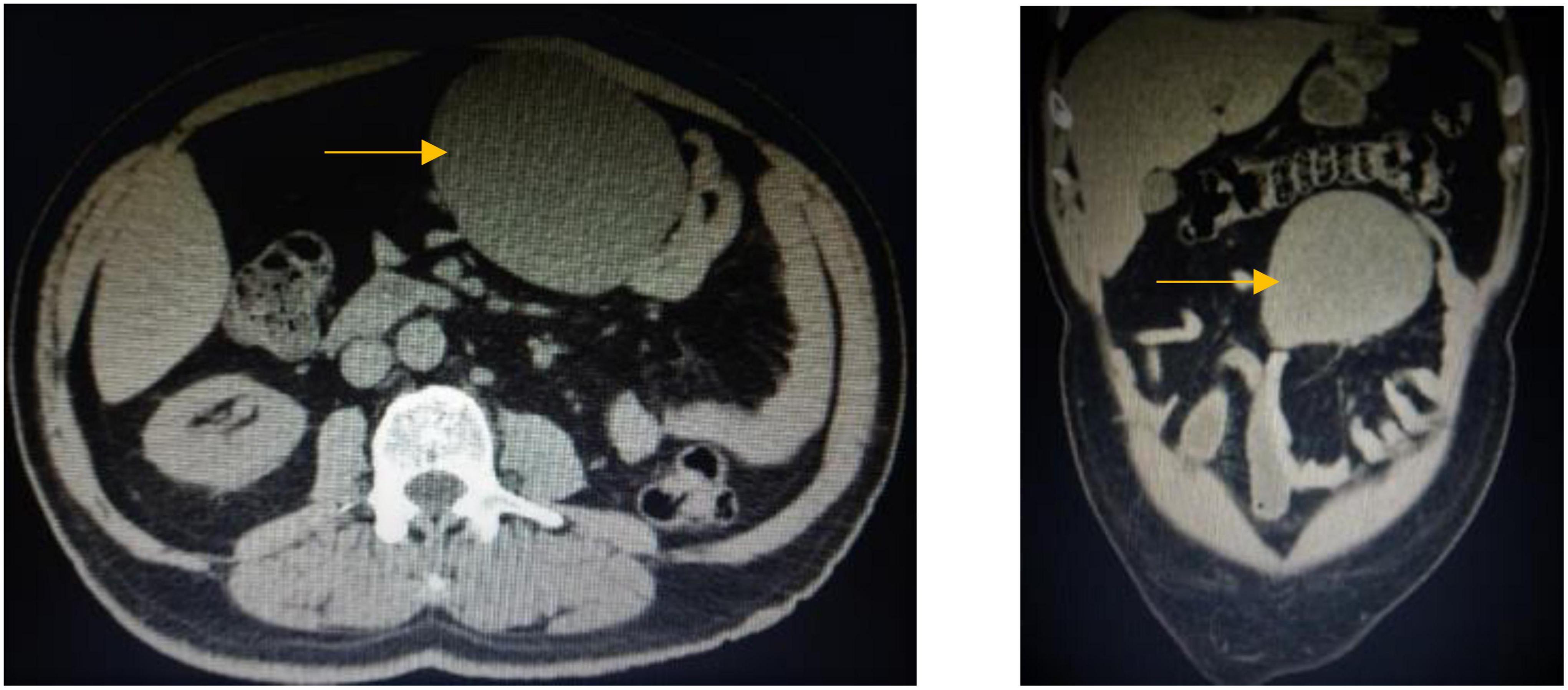
Figure 1. CT scan of the abdomen, coronal, and axial images shows large well-defined opacity at mesentery on the left side of the upper abdomen surrounded by small and large bowel loops with no enlarged mesenteric or retroperitoneal lymph nodes.
Provisional diagnosis included gastrointestinal stromal tumor (GIST) and IMT. The literature reported other differential diagnoses of mesenteric mass (12). The most common one was desmoid tumor with other possibilities such as teratoma, liposarcoma, hemangioma, neuroblastoma, carcinoid tumor, and adenocarcinoma (12).
A gross examination of the specimen revealed a large tannish glistening tumor mass in the center of the bowel loop. The rest of the bowel and the attached pericolic fat were normal (Figure 2).
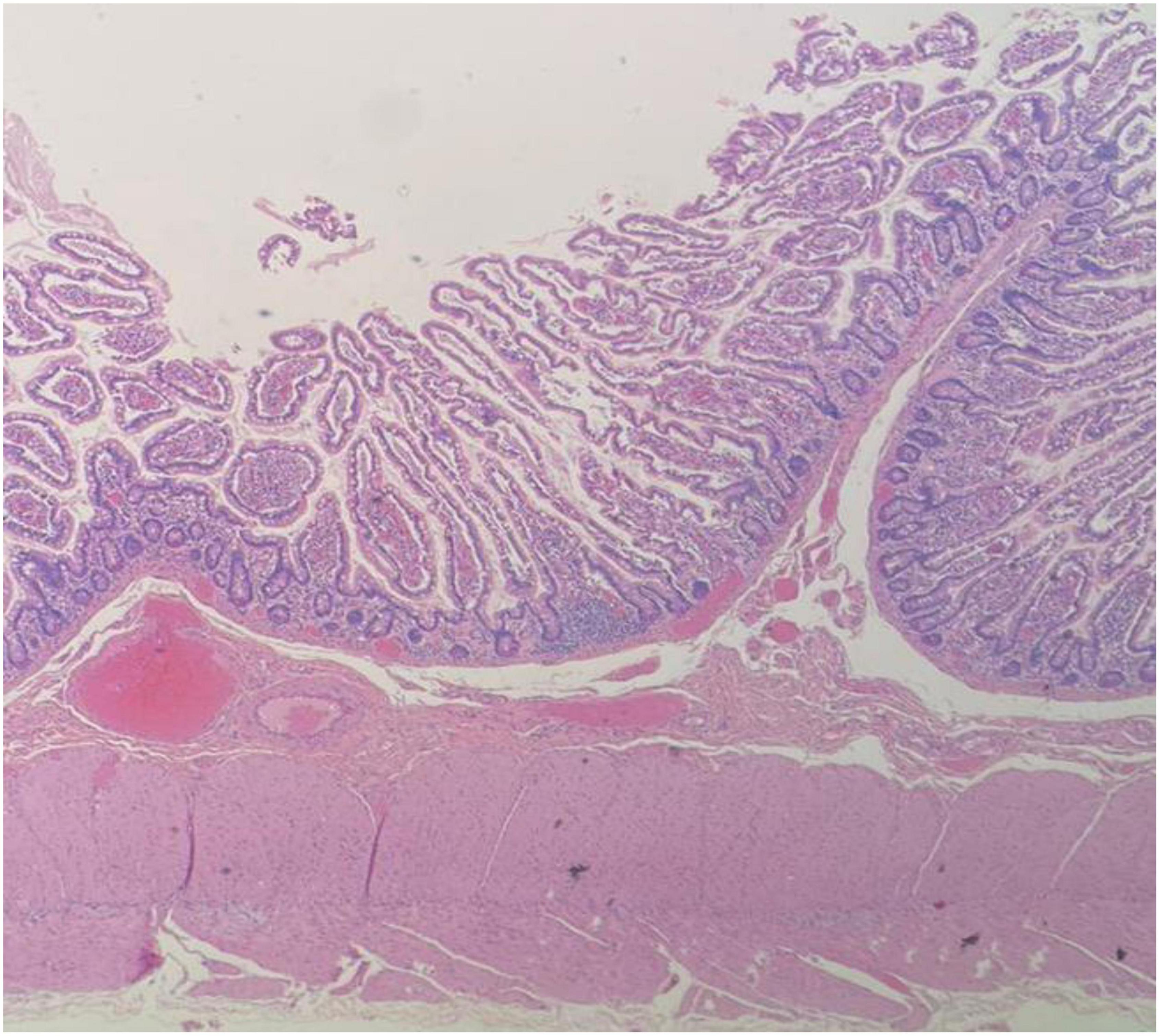
The pathologist described a mesenteric-origin tumor that occupied the visceral peritoneum with an intact jejunal loop (Figure 3). Microscopically, the tumor was composed of myofibroblastic spindle-shaped cells with absent mitosis and minimal atypia (Figure 4). The tumor contained abundant chronic mononuclear and inflammatory cells, mostly lymphocytes, plasma cells, and histiocytes accompanied by eosinophils. Lymphoid aggregations were scant (Figure 5). Further immunohistochemistry studies were performed by another independent pathologist: the tumor had negative immunoreaction to CD117, DOG1, desmin, and anaplastic lymphoma kinase (ALK-1). There was an infrequent immunoreaction to smooth muscle actin (SMA).
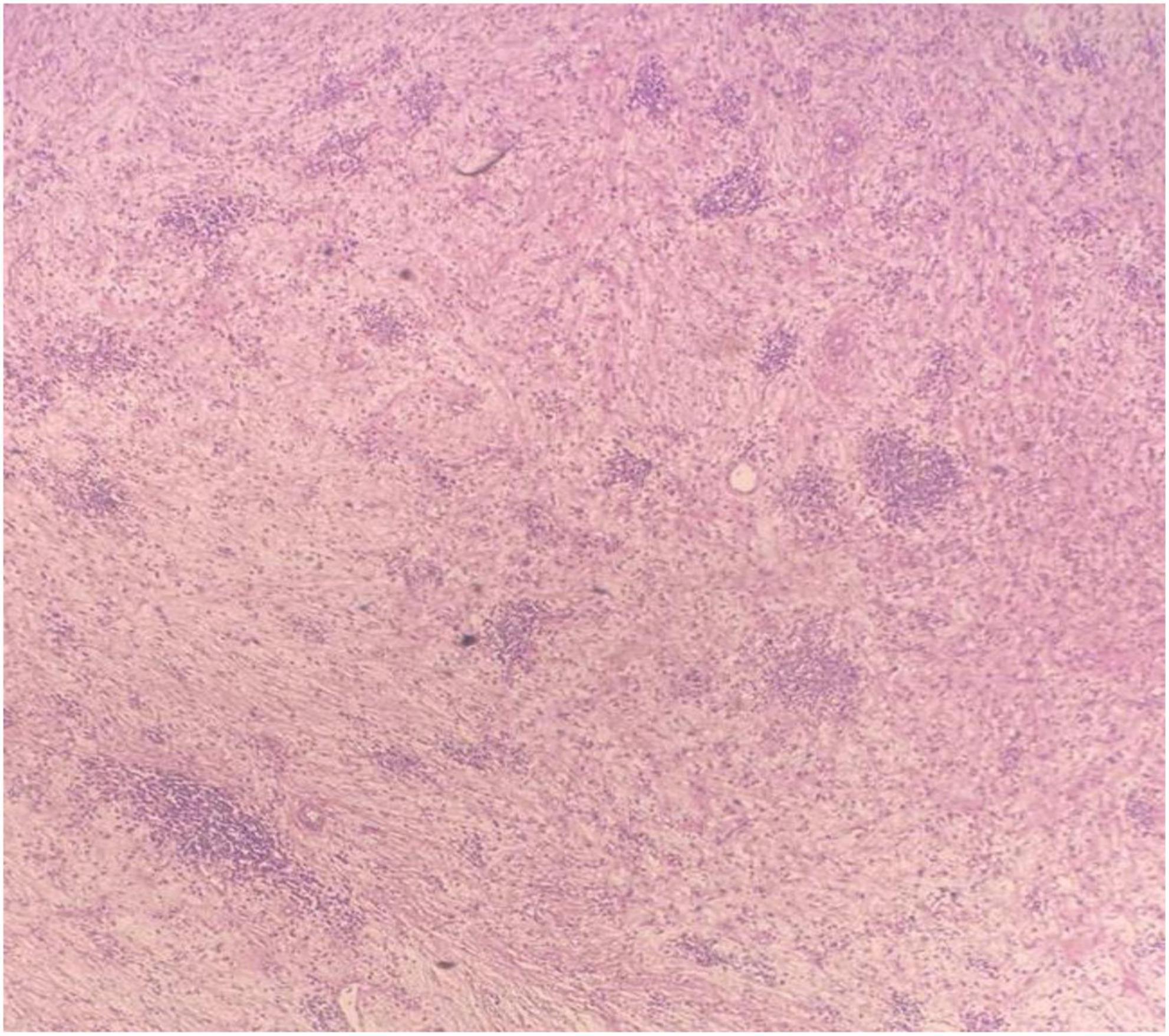
Figure 4. Microscopic image of loosely arranged spindle cells growing in haphazardly arranged fascicles, interspersed by abundant chronic inflammatory cells, LPF.
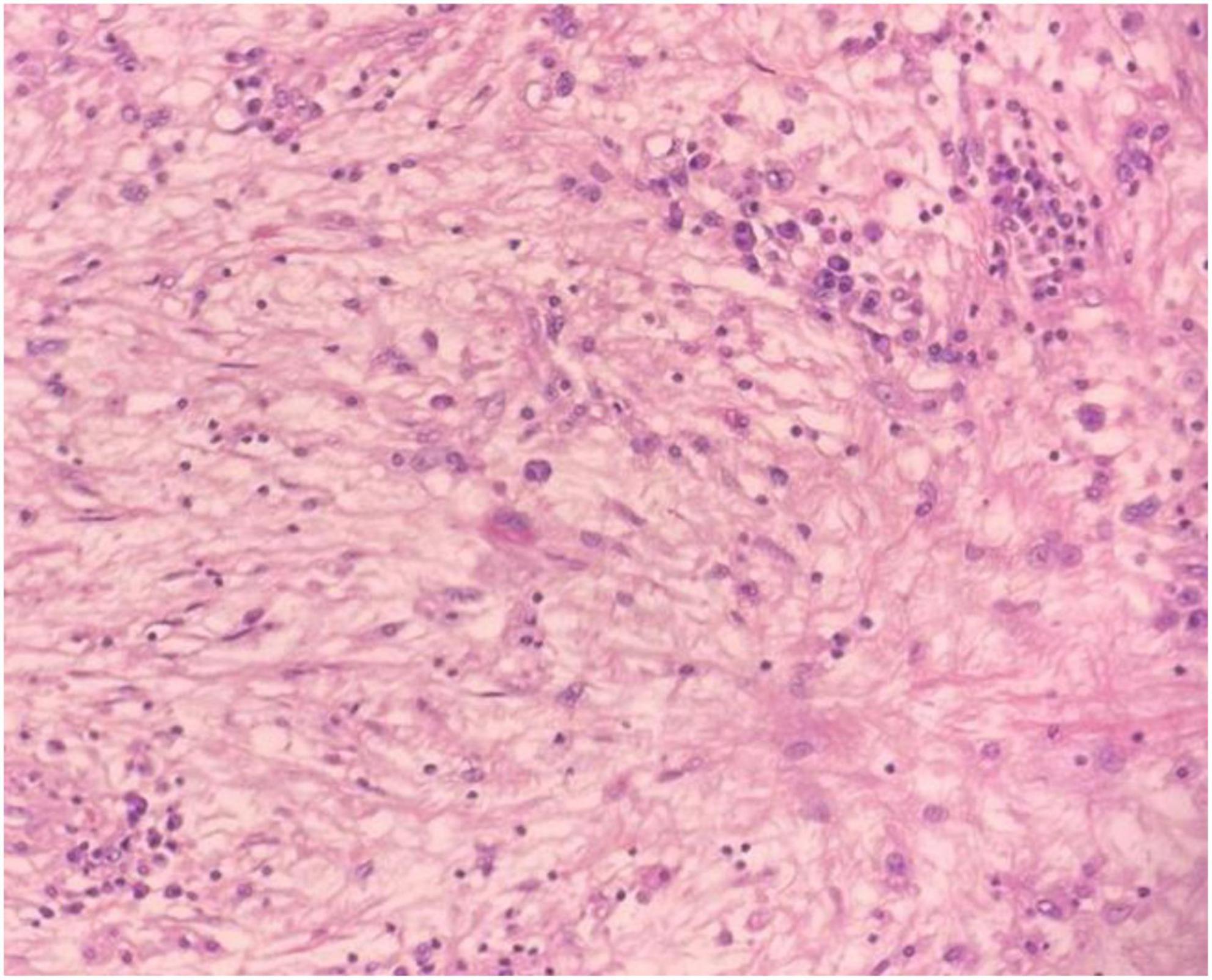
Figure 5. Microscopic image of abundant chronic mononuclear and inflammatory cells, mostly lymphocytes, plasma cells, and histiocytes accompanied by eosinophils and scanty lymphoid aggregations, HPF.
The post-operative course was uneventful, and he returned to his job within 2 weeks of the surgery. He was asymptomatic for the last four and a half years. Follow-up US of the abdomen yearly and CT scan of the abdomen every 2 years were done with no clue of tumor recurrence or mass lesion.
Discussion
Inflammatory myofibroblastic tumor is a mesenchymal, rare solid tumor of intermediate malignant potential (2). IMT imitates malignant neoplasm, with a local recurrence rate of up to 25% (8, 13). Based on demographic data, IMT often affects children and young adults (14). While some reported female predominance in IMT cases (female:male = 1.67:1) (15), others reported the opposite sex ratio (16). A high recurrence rate is much attributed to the tumor site, pathological features (multifocal or ill-defined), and incomplete resection of the tumor. A significant correlation between IMT that arises in the abdomen, mesentery, omentum, or retroperitoneum and the aggressive local recurrence has been documented. In addition, IMT with gross multinodular features and a diameter of more than 8 cm has a more aggressive course (6, 17).
Tables 1–3 summarize 36 case reports (e.g., the present case) of mesenteric IMT, emphasizing clinical, radiological, and pathological features, respectively. The literature review was performed by searching electronic databases (PubMed and ScienceDirect) and using the search terms “Inflammatory, myofibroblastic, tumor, Jejunum, mesentery, mass.”
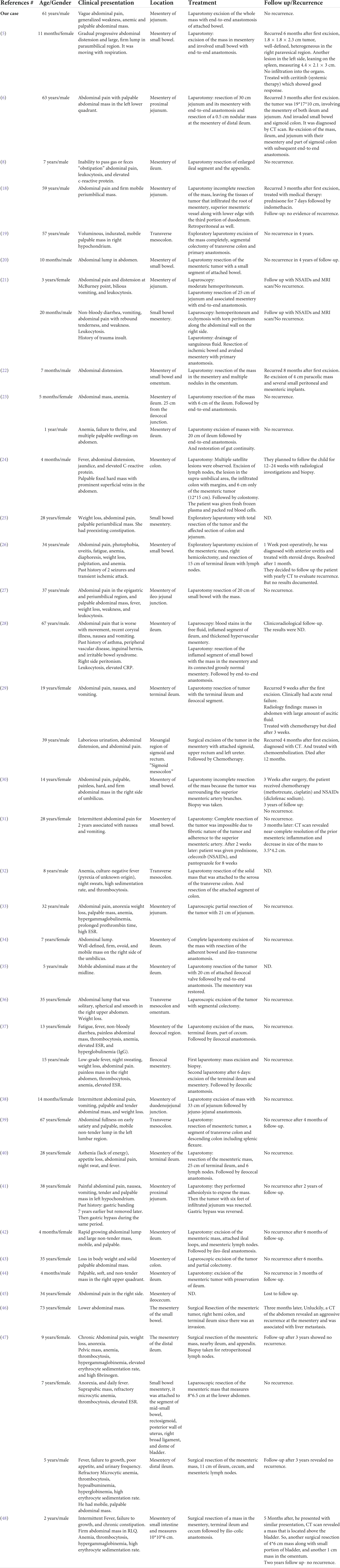
Table 1. Clinical features of the reported cases of inflammatory myofibroblastic tumor of the mesentery.
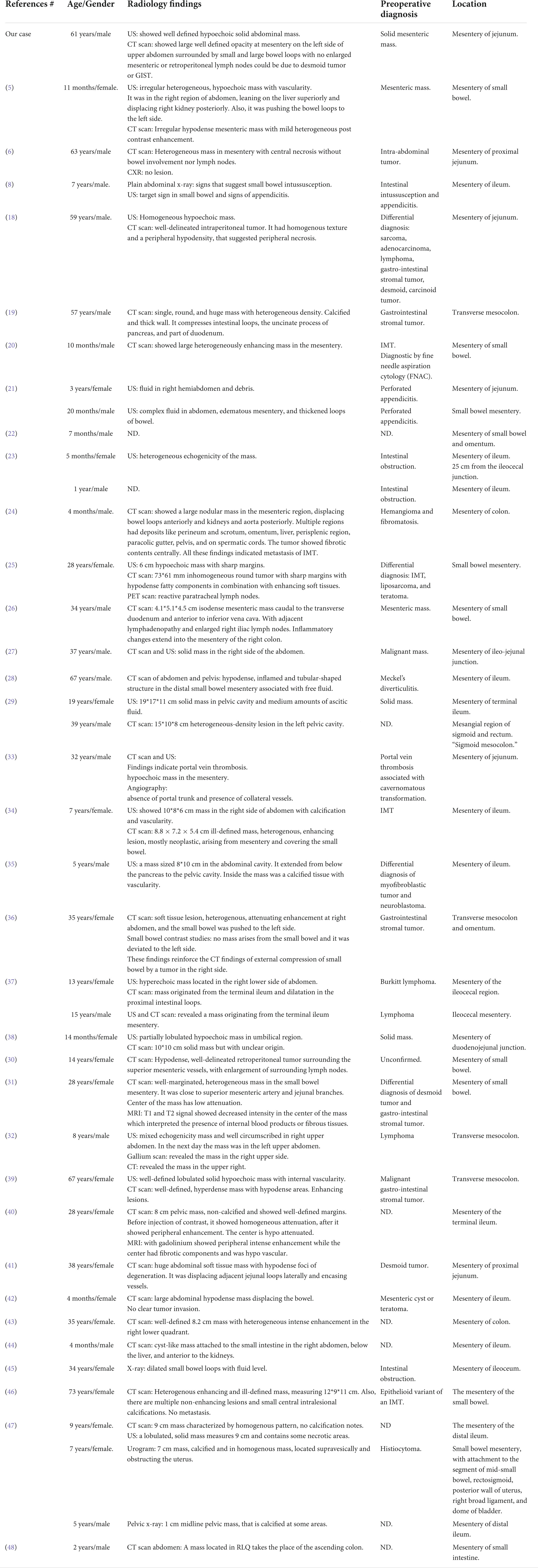
Table 2. Radiological features of the reported cases of inflammatory myofibroblastic tumor of the mesentery.

Table 3. Pathological features of the reported cases of inflammatory myofibroblastic tumor of the mesentery.
Clinical presentation of inflammatory myofibroblastic tumor
The mean age for the diagnosis of mesenteric IMT was 22.9 years (range: 0–73 years), and the median was 17 years old. The prevalence of IMT was 55.9% in males and 44.1% in females (male:female ratio = 1.27:1). Apparently, mesenteric IMT has a slight propensity to affect younger males than females. In the present case, the patient was 61-year-old male; hence, age was one of the rare characteristics of his clinical presentation.
Furthermore, we analyzed the most common clinical presentations of mesenteric IMT. A total of 24 patients presented with a palpable abdominal lump (67%, including the present case), 20 patients presented with abdominal pain (56%, including the present case), and 6 patients presented with elevated inflammatory markers (16.7%, including the present case). In some cases, there were several atypical presentations such as diarrhea (n = 3), difficult urination (n = 1), and obstipation (n = 1). In these cases, IMT developed as polyps in the mesentery of the small bowel, sigmoid colon, jejunum, and ileum, respectively. A previously published literature review demonstrated that patients with mesenteric IMT had various complaints. Notably, abdominal pain accounted for 33% of the clinical presentation, followed by a painless palpable abdominal lump. Occasionally, systemic symptoms might accompany those complaints, such as fever of unknown origin, weight loss, malaise, uveitis, anemia, and growth failure in children. Laboratory tests demonstrated abnormalities such as elevated ESR, hypergammaglobulinemia, and thrombocytosis (12).
Radiological features of inflammatory myofibroblastic tumor
There are no specific radiological features that discern mesenteric IMT from other tumors. The plain abdominal radiograph displays diffuse haziness of mesenteric IMT, and the barium studies show the displacement of the bowel due to the mass effect with possible narrowing of its lumen (12). Routinely, the initial diagnostic tool is the abdominal US. US can differentiate between ill- and well-defined tumor edges. Doppler US confirms an increased vascularity of mesenteric IMT (58). A CT scan with contrast exhibits various enhancement patterns by the mesenteric IMT mass ranging from non-enhancement, peripheral enhancement, and central enhancement. This variability reflects the extent of the fibrotic component of the lesion that delays the enhancement of the contrast (58). In addition, it defines the pattern of the lesion (heterogenous or homogenous), the anatomical site, the shape, and the adjacent structures. Magnetic resonance imaging (MRI) shows mesenteric IMT with a low signal intensity due to its fibrotic component. Fluorodeoxyglucose-positron emission tomography (FDG-PET) scan demonstrates various powers reflecting the complex cellular nature and biological behavior of mesenteric IMT (12, 59).
However, the most common radiological pattern was the heterogeneous pattern accounting for 33% of mesenteric IMT (e.g., our case). Only Diop et al. previously reported a homogeneous pattern by the US for mesenteric IMT (18). Mesenteric IMT often had a well-defined edge (19% of cases, including our case). Other documented characters varied between ill-defined, sharp, and irregular edges. Mesenteric IMT displaces the nearby organ (12). In 8% of the reported cases of mesenteric IMT, the tumor was extremely close to the superior mesenteric artery preventing a complete resection. Notably, 36% of the reported cases presented with hypoechoic (solid) mass on US, while few presented with hyperechoic mass. MRI with contrast showed good enhancement (n = 5) and mild enhancement (n = 2, including our case).
Two cases demonstrated low central but good peripheral enhancement of the tumor. Notably, 15% of cases emphasized the vascularity of this tumor. Infrequent features included a calcified thickened wall (19).
In conclusion, mesenteric IMT is a heterogeneous vascular hypoechoic mass. It has a well-defined edge and often causes displacement, but it may cause invasion in exceptional cases. Occasionally, IMT could be a distinctive lesion based on its intense enhancement.
Macroscopic features of inflammatory myofibroblastic tumor
It is extremely hard to have a definite pre-operative diagnosis of mesenteric IMT unless FNAC could be performed (20). Mesenteric IMT develops in both small and large bowel. The mesentery of the ileum (36%, n = 13) followed by the jejunal mesentery (17%; n = 6, including our case) was the most common locations. Only two patients developed mesenteric IMT in the ileo-jejunal junction. In 25% (n = 9) of mesenteric IMT cases, the location was in the small bowel mesentery without specific identification. The most common site in the colon was the transverse mesocolon (11%), while it rarely developed in the mesentery of the sigmoid colon.
Mesenteric IMT has various macroscopic features. They can be sessile or polypoid. They are unique with their white-to-yellow color and firm, fleshy, gelatinous, and whorled appearance. In the cut-sections, secondary changes may be present including hemorrhage, calcification, ulceration, necrosis, and ossification. A polypoid-shaped mass hides inside the intestinal lumen when mesenteric IMT seizes invasion power to penetrate the intestinal wall. Tumors present as a solitary mass ranging in size between 1 and 20 cm. Mesenteric IMT can present as multiple discrete lesions (12, 60, 61). In our literature review, the size of mesenteric IMT ranged from 0.5 to 20 cm. The largest mesenteric IMT size settled in the ileocecal mesentery. The median size tumor was 10 cm, and the mean was 10.8 cm. Occasionally, mesenteric IMT lesions presented as multicentric or multifocal masses [n = 5; Mazotas et al. (21), Ma et al. (22), Banerjee et al. (23), Kumar et al. (24)] and not a solitary mass. A total of eight (22%) cases presented as a well-circumscribed tumor, and four cases were encapsulated. Mesenteric IMT could have various surface contours such as lobulated (n = 4), nodular (n = 4), round (n = 4), multinodular (n = 2), and irregular (n = 1). Mesenteric IMT may have unusual consistencies such as rubbery (n = 2) or hard (n = 1). One case encountered a polypoid mass where mesenteric IMT was in the ileal mesentery (8). Mesenteric IMT appearance varied between myxomatous (n = 2), glistening (n = 1), and hyperemic (n = 1). Mesenteric IMT can block the blood supply of the attached bowel and causes ischemic (n = 4). Groenveld et al. (25), Choi et al. (26), and Koyuncuer et al. (27) reported no involvement of the attached bowel, while four cases reported partial involvement nearby bowel. For example, Mazotas et al. (21) documented the invasion of jejunal serosa, Papanikolas et al. (28) noticed the invasion of the muscular wall of the ileum, Chen et al. (6) reported the involvement of the muscular wall of the jejunum, and Telugu et al. (62) notified that the tumor embraced adventitia, muscular layer, and submucosa of the surrounding bowel.
We concluded that mesenteric IMT could be a well-circumscribed tumor with a myxomatous appearance. It presents diverse surface contours, but round, lobular, and nodular surfaces are the most repeated. Rarely, it may cause ischemia of the bowel.
Microscopic features of inflammatory myofibroblastic tumor
Although IMTs share histopathological similarities, certain histological differences may determine the tumor’s local growth, metastasis, and recurrence rate (63). IMT has three distinctive pathological presentations, namely, cellular, mixed, and fibrous types. The most common and conspicuous pathological findings are spindle-shaped cells and inflammatory cells including plasma cells and lymphocytes. Three histological patterns exist, of which the most common one is the fibromyxoid pattern representing fasciitis-like changes and containing myxoid and inflamed stroma. The proliferating pattern displays multiplying spindle cells sorted in a storiform or fascicular growth pattern accompanied by two processes, namely, inflammation and mitosis. Usually, this pattern is seen only in tumors with atypical features. The sclerosing pattern represents sclerosed desmoid-like areas that interfere with calcification. Undesirable prognosis results from high mitotic tendency, hypercellularity, and proliferation capacity, all of which are the characteristics of the proliferating pattern (64). Moreover, devastating prognostic characteristics include the presence of bizarrely shaped and round cells instead of spindle-shaped cells, nuclear pleomorphism, and infiltrating borders (12, 62).
Spindle-shaped myofibroblasts were the most evident cell type in mesenteric IMT (87%). Satellite and epithelioid myofibroblasts were also prominent. In all cases, lymphocytes, plasma cells, and occasionally neutrophils, histiocytes, macrophages, eosinophils, and monocytes were present. Giant cells (6), ganglion-shaped tumor cells (29), and cells with elongated and globular nuclei (65) were significantly correlated with the recurrence of IMT. Furthermore, pleomorphism contributed to IMT recurrence (5). Two patients died following the recurrence of mesenteric IMT; in both cases, ganglion-shaped tumor cells were present. Although mitosis was evident in 9, the tumor recurrence rate was only 44%. This may reflect that the extent of mitosis and not mitosis per se could determine the recurrence of IMT. The most evident histological pattern was the fibromyxoid/vascular pattern (77%). Only three patients with fibromyxoid patterns had a recurrence. Three cases (9.6%) were reported with fascicular/storiform growth-like patterns. Out of which, only one patient had recurrent tumors later when pleomorphism existed as well. Two patients presented with a sclerosing pattern without recurrent tumor. We concluded that the pathological features favoring a bad prognosis of mesenteric IMT include, but may be not limited to, pleomorphism, mitosis, ganglion-shaped tumor cells, fascicular/storiform growth-like pattern, and the presence of cells with elongated and globular nuclei.
Immunohistochemistry of inflammatory myofibroblastic tumor
The immunohistochemistry evaluation of mesenteric IMT appeared to be an equivocal approach. The assessment included smooth muscle actin (SMA), muscle-specific actin, ALK protein, desmin, calponin, cytokeratin, vimentin, factor VIII A, CD65, and CD117. Positive reaction to cytokeratin and desmin was different. Anaplastic large-cell lymphoma strongly resembles IMT in the presence of ALK (17). In contrast, the ALK gene discerns IMT from other spindle cell neoplastic tumors like the GIST, inflammatory leiomyoma, and congenital fibrosarcoma. ALK protein is present more often at a young age than in the elderly. ALK plays a vital role in the prognosis and diagnosis of IMT. ALK-positive mesenteric IMT had a better prognosis and a lower rate of recurrence (20).
Herein, we analyzed the results of the immunohistochemistry evaluation of mesenteric IMT. Five cases were excluded since no immunohistochemistry data were present. The most evident marker was the SMA (83.8%, n = 31, including our case). ALK protein and desmin were equally represented (48.3%, n = 15). Vimentin was present in 8 (25.8%) cases. Mesenteric IMT was inconstantly positive to CD117, DOG1, HHF-35, CD34, Ki-67, S100, CD3, CD2, RANBP-2, CD30, CD21, CK-4, cytokeratin, calponin, pan keratin, epithelial membrane antigen, CDK, COX-2, ki-67, BCL-2, CD-68, and p53. RANBP-2 is associated with unfavorable prognosis and high recurrence rate (67%, n = 3). In addition, HHF-35, Ki-67, and CD30 were also linked to unfavorable prognosis and tumor recurrence. In terms of the recurrence of mesenteric IMT, ALK-positive mesenteric IMT was slightly advantageous. Only 26% of the cases reported the recurrence of the tumor. We concluded that the highly assigned markers for mesenteric IMT were SMA, desmin, and ALK. Unfavorable markers with unfavorable prognosis or recurrence of IMT were RANBP-2, HHF-35, Ki-67, and CD30. ALK-positive mesenteric IMT has a lower recurrence rate.
Surgical treatment of inflammatory myofibroblastic tumor
The treatment of choice for mesenteric IMT is complete surgical resection where other remedies could be considered. Although IMT occasionally tends to be an aggressive tumor, complete excision, chemotherapy, and radiation are the recommended therapy (66–68). The risk of the recurrence of IMT is around 23%. Ill-defined edges and abdominopelvic occupation of the tumor are noticeably attributable factors for this risk (16, 17, 69). Metastatic cases are rare, accounting for less than 5% of our review cases (12). ALK rearrangement is one element that grants IMT a low metastatic feature. Therefore, inhibition of ALK could be a decent therapeutic approach. In fact, ALK-positive tumors have excellent crizotinib (ALK inhibitor) response (62, 70). Corticosteroids achieved a practical effect when used as part of adjuvant therapy and recurrence therapy of IMT (62, 71).
The most effective treatment for mesenteric IMT was complete resection with the attached bowel followed by an end-to-end anastomosis. However, four cases reported the recurrence of the tumor following this approach. Chen et al. reported tumor recurrence for large-sized lesions with unfavorable pathological characteristics and those presenting as two discrete masses rather than one (6). Tumor recurrence for 8 months following a complete resection of the tumor was previously attributed to the multicentric presentation rather than solitary mass (22). The treatment of recurrent IMT was re-excision with a significant bowel length. However, Mittal A. et al. documented that the treatment of recurrent mesenteric IMT with ceritinib showed good responses, which could be due to the positive reaction of the tumor to ALK (72). Other treatment modalities depend on several factors specified to the tumor. Complete surgical resection followed by chemotherapy was reported when tumor pathology was a risk factor for recurrence (12, 30). However, the consequences were unfavored. Other treatment strategies were incomplete surgical resection, NSAIDs, prednisone, and pantoprazole. Those approaches were practical when the fibrotic nature of the mass and adherence to the superior mesenteric artery hinder the complete excision. The use of celecoxib (NSAIDs) gave promising results in COX-2-positive mesenteric IMT (31). In another case, the tumor was partially excised but recurred 3 months postoperative. The patient then received prednisone for 7 days, followed by indomethacin, which gave significant results (18). The use of chemotherapy drugs and diclofenac sodium (NSAID) was also reported to be successful in a case of recurrent mesenteric IMT (30). For the sake of a comprehensive review of the topic, Tables 4–6 summarize the clinical, radiological, and pathological features of nine cases of IMT affecting the colon.
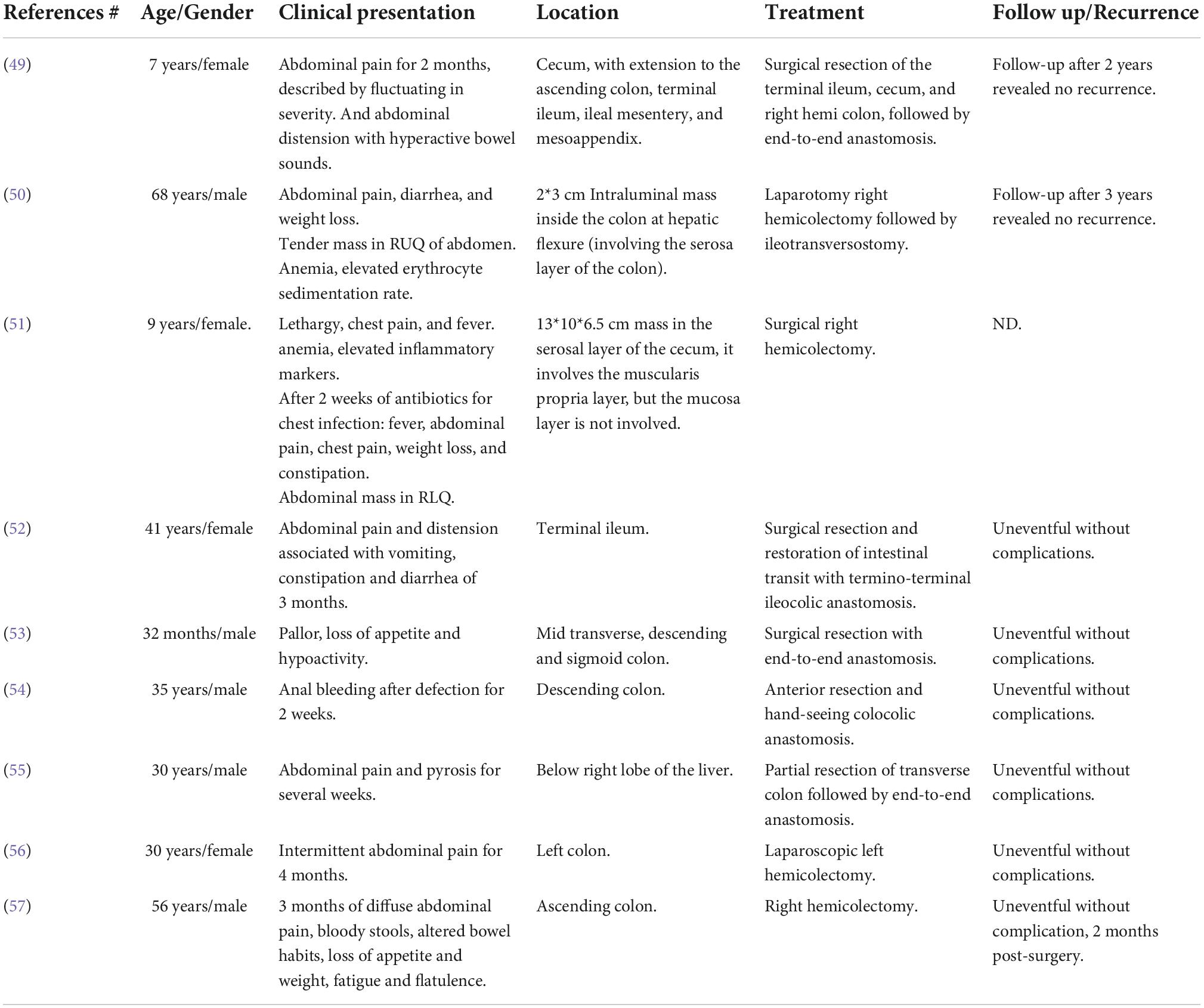
Table 4. Clinical features of the reported cases of inflammatory myofibroblastic tumor of the colon.
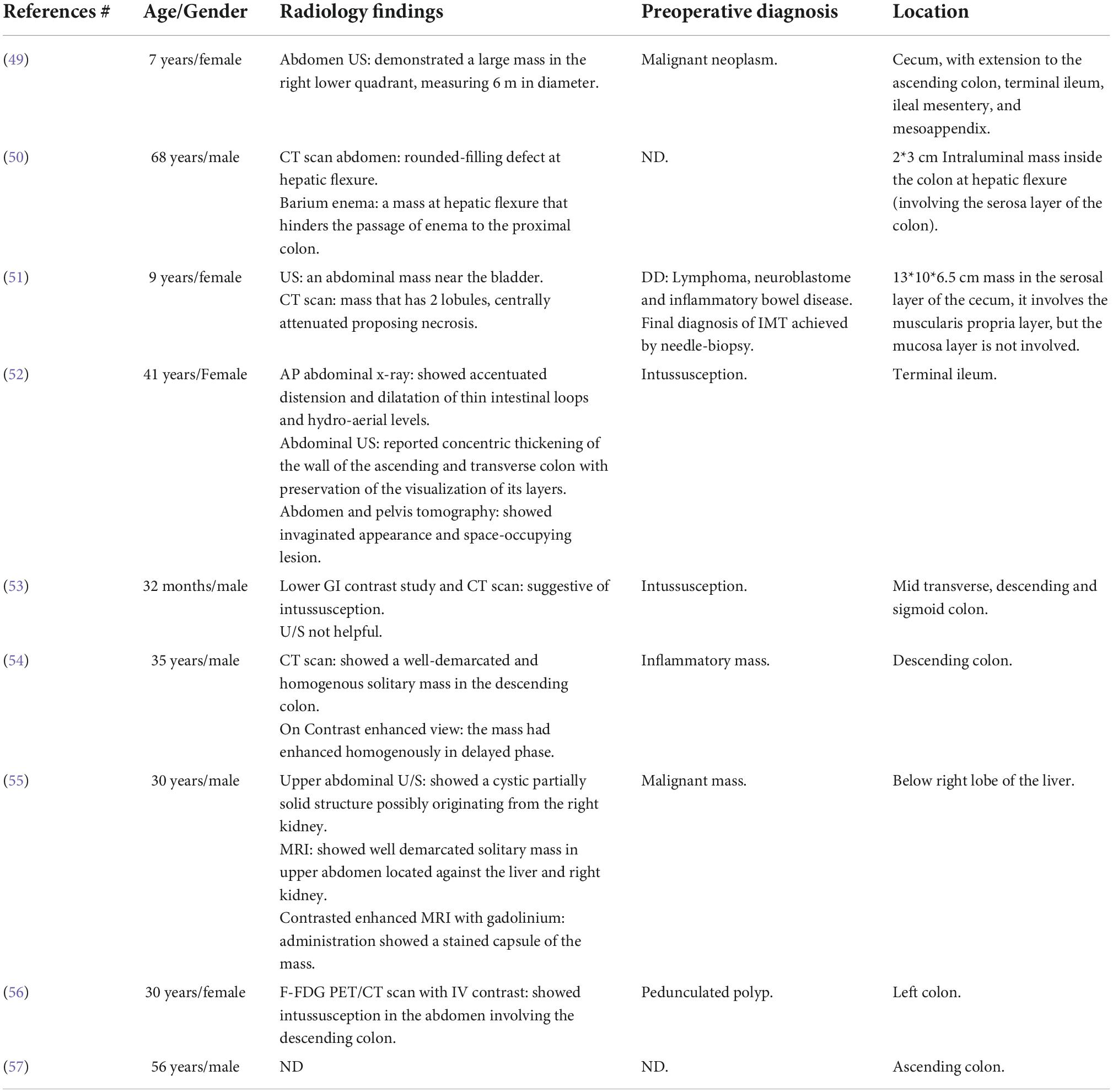
Table 5. Radiological features of the reported cases of inflammatory myofibroblastic tumor of the colon.
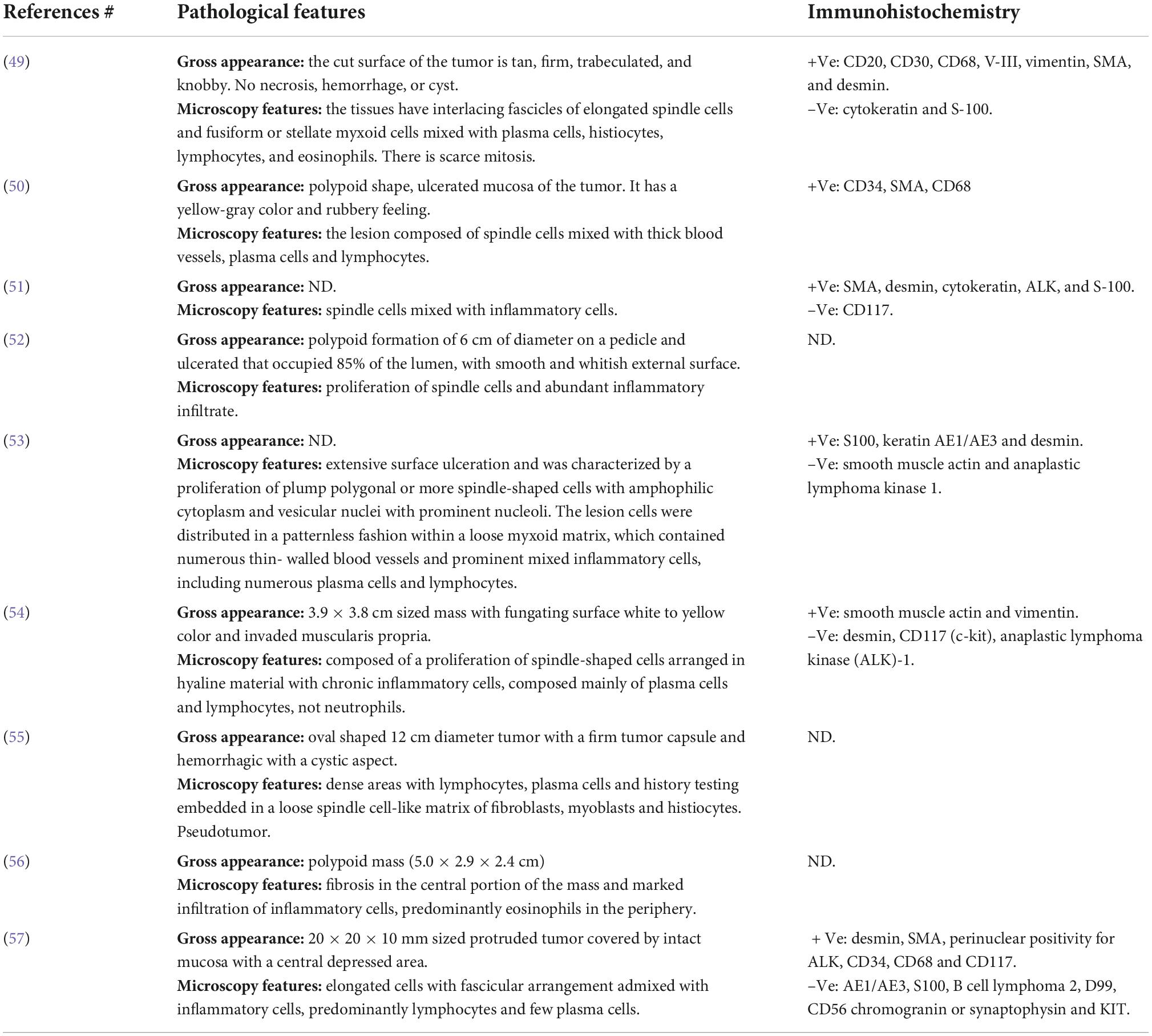
Table 6. Pathological features of the reported cases of inflammatory myofibroblastic tumor of the colon.
Conclusion
Mesenteric IMT demands vast effort to reveal the diagnosis due to its vagueness in the clinical presentation. Mesenteric IMT resembles each other in plenty of pathological and immunohistochemical characteristics. Unluckily, the final diagnosis is challenging to obtain before getting the sample for the histological report, after the operation, or using FNAC. To the best of our knowledge, the case reported here is the first case of jejunal malignant IMT occurring in an elderly male patient. Radical surgical resection seemed to be curative till the date of reporting.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Research and Ethics Committee (REC) College of Medicine and Medical Sciences, Arabian Gulf University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HA, SAA-S, and RY conceived and designed the study. HA, SAA-S, and NA performed the research process and collected the data. SAA-S performed the statistical analyses. HA, SKA, YN, and SAA-S wrote the original draft of the manuscript. HA, SAA-S, NA, FA-S, and KA-S prepared the figures and tables. HA, SKA, SAA-S, NA, YN, and RY edited and revised the manuscript. HA was the project manager. All authors approved the final version of the manuscript.
Acknowledgments
The authors would like to acknowledge the College of Medicine and Medical Sciences and the Arabian Gulf University for providing all the necessary resources. The authors would also like to acknowledge Shamil Naji Sarsam, Consultant Radiologist, for his generous help and effort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IMT, inflammatory myofibroblastic tumor; –Ve, negative; +Ve, positive; ND, not documented; SMA, smooth muscle actin; FISH, fluorescence in situ hybridization; ALK, anaplastic lymphoma kinase; RANBP1, Ran binding protein 2; NSAIDs, non-steroidal anti-inflammatory drug.
References
1. İçmeli ÖS, Alpay LA, Gündoğuş B, Türker H, Şen A. Inflammatory myofibroblastic tumor: a rare tumor of the lung. Eur Clin Respir J. (2014) 1. doi: 10.3402/ecrj.v1.25390
2. Mahajan P, Casanova M, Ferrari A, Fordham A, Trahair T, Venkatramani R. Inflammatory myofibroblastic tumor: molecular landscape, targeted therapeutics, and remaining challenges. Curr Probl Cancer. (2021) 45:100768. doi: 10.1016/j.currproblcancer.2021.100768
3. Han Q, He X, Cui L, Qiu Y, Li Y, Chen H, et al. Case report: early distant metastatic inflammatory myofibroblastic tumor harboring EML4-ALK fusion gene: study of two typical cases and review of literature. Front Med. (2022) 9:826705. doi: 10.3389/fmed.2022.826705
4. Cantera JE, Alfaro MP, Rafart DC, Zalazar R, Muruzabal MM, Barquín PG, et al. Inflammatory myofibroblastic tumours: a pictorial review. Insights Imaging. (2015) 6:85–96.
5. Gupta A, Sharma S, Mittal A, Barwad A, Rastogi S. Recurrent infantile inflammatory myofibroblastic tumor of mesentery—-Case report and review of imaging findings. Radiol Case Rep. (2021) 16:504–10. doi: 10.1016/j.radcr.2020.12.027
6. Chen S-S, Liu SI, Mok KT, Wang BW, Yeh MH, Chen YC, et al. Mesenteric inflammatory myofibroblastic tumors in an elder patient with early recurrence: a case report. World J Gastroenterol. (2007) 13:3645–8. doi: 10.3748/wjg.v13.i26.3645
7. Braham Y, Migaou A, Njima M, Achour A, Ben Saad A, Cheikh Mhamed S, et al. Inflammatory myofibroblastic tumor of the lung: a rare entity. Respir Med Case Rep. (2020) 31:101287–101287. doi: 10.1016/j.rmcr.2020.101287
8. Alabbas Z, Issa M, Omran A, Issa R. Mesenteric inflammatory myofibroblastic tumor as a rare cause of small intestinal intussusception. J Surg Case Rep. (2020) 2020:rjaa322. doi: 10.1093/jscr/rjaa322
9. Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. (2015) 39:957–67. doi: 10.1097/PAS.0000000000000404
10. Siraj F, Kaur M, Dalal V, Sonam J. Inflammatory myofibroblastic tumor of the breast mimicking malignancy in an elderly male. Ochsner J. (2017) 17:277–9.
11. Hameed T, Singh M, Nizam A, Bhatia R, Sawant G. Acute intestinal obstruction due to ileocolic intussusception in an adult; a rare presentation of inflammatory myofibroblastic tumor. Am J Case Rep. (2020) 21:e920438. doi: 10.12659/AJCR.920438
12. Chaudhary P. Mesenteric inflammatory myofibroblastic tumors. Ann Gastroenterol. (2015) 28:49–54.
13. Bettach H, Kaur M, Dalal V, Sonam J. Inflammatory myofibroblastic tumor of the spleen: a case report. Radiol Case Rep. (2021) 16:3117–9.
14. Da M, Qian B, Mo X, Xu C, Ha Wu, Jiang B, et al. Inflammatory myofibroblastic tumors in children: a clinical retrospective study on 19 cases. Front Pediatr. (2021) 9:543078. doi: 10.3389/fped.2021.543078
15. Teoh JY, Chan NH, Cheung HY, Hou SS, Ng CF. Inflammatory myofibroblastic tumors of the urinary bladder: a systematic review. Urology. (2014) 84:503–8. doi: 10.1016/j.urology.2014.05.039
16. Fu GX, Xu CC, Yao NF, Gu JZ, Jiang HL, Han XF. Inflammatory myofibroblastic tumor: a demographic, clinical and therapeutic study of 92 cases. Math Biosci Eng. (2019) 16:6794–804. doi: 10.3934/mbe.2019339
17. Zhang T, Yuan Y, Ren C, Du S, Chen J, Sun Q, et al. Recurrent inflammatory myofibroblastic tumor of the inguinal region: a case report and review of the literature. Oncol Lett. (2015) 10:675–80. doi: 10.3892/ol.2015.3297
18. Diop B, Konate I, Ka S, Sall I, Fall D, Dieng M, et al. Mesenteric myofibroblastic tumor: NSAID therapy after incomplete resection. J Visc Surg. (2011) 148:e311–4. doi: 10.1016/j.jviscsurg.2011.06.005
19. Lopes VN, Alvarez C, Dantas MJ, Freitas C, Pinto-de-Sousa J. Mesenteric inflammatory pseudotumor: a difficult diagnosis. Case report. Int J Surg Case Rep. (2017) 32:1–4.
20. Ghosh M, Islam N, Saha H, Mukhopadhyay M, Datta C, Saha K, et al. Cytodiagnosis of inflammatory myofibroblastic tumor: a report of three cases in infants. Diagn Cytopathol. (2018) 46:776–81. doi: 10.1002/dc.23950
21. Mazotas I, Hughes CD, Webster-Lake CA, Thaker S, Weiss R, Misra MV. Inflammatory myofibroblastic tumor masquerading as perforated appendicitis. J Pediatr Surg Case Rep. (2015) 3:517–20. doi: 10.5001/omj.2018.45
22. Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. (2003) 37:98–105. doi: 10.1002/gcc.10177
23. Banerjee M, Mukhopadhyay D, Gupta SD, Chatterjee U, Banerjee S. Intra-abdominal infantile inflammatory myofibroblastic tumors: a report of three cases. J Indian Assoc Pediatr Surg. (2014) 19:239–41. doi: 10.4103/0971-9261.142020
24. Kumar Jha A, Lipi L, Luthra M, Ahlawat K. Abdominal inflammatory myofibroblastic tumour in a 4 month old baby- a case report. Hematol Med Oncol. (2021) 6. doi: 10.15761/HMO.1000234
25. Groenveld RL, Raber MH, Oosterhof-Berktas R, Eijken E, Klaase JM. Abdominal inflammatory myofibroblastic tumor. Case Rep Gastroenterol. (2014) 8:67–71.
26. Choi AH, Bohn OL, Beddow TD, McHenry CR. Inflammatory myofibroblastic tumor of the small bowel mesentery: an unusual cause of abdominal pain and uveitis. J Gastrointest Surg. (2011) 15:584–8. doi: 10.1007/s11605-010-1408-3
27. Koyuncuer A. Inflammatory myofibroblastic tumor of the small-bowel mesentery: a case report of nonspecific clinical presentation and a review of the literature. Int J Surg Case Rep. (2014) 5:1214–7. doi: 10.1016/j.ijscr.2014.11.054
28. Papanikolas M, Chen M, Preda T. Inflammatory myofibroblastic tumour presenting with the acute abdomen: an uncommon cause of a mesenteric mass. ANZ J Surg. (2019) 90:1519–20. doi: 10.1111/ans.15617
29. Li J, Yin WH, Takeuchi K, Guan H, Huang YH, Chan JK. Inflammatory myofibroblastic tumor with RANBP2 and ALKgene rearrangement: a report of two cases and literature review. Diagn Pathol. (2013) 8:147. doi: 10.1186/1746-1596-8-147
30. Tao Y-L, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol. (2012) 18:7100–3.
31. Shatzel J, Wooten K, Ankola A, Cheney RT, Morrison CD, Skitzki JJ. Inflammatory myofibroblastic tumor of the mesentery: a clinical dilemma. Int J Clin Oncol. (2012) 17:380–4. doi: 10.1007/s10147-011-0297-0
32. Auringer ST, Scott MD, Sumner TE. Inflammatory pseudotumor: a gallium-avid mobile mesenteric mass. J Nucl Med. (1991) 32:1614–6.
33. Miyazaki H, Isada A, Ohira K, Masutani M, Okushiba S, Shimizu M, et al. Inflammatory pseudotumor of the mesentery causing portal venous thrombosis and cavernomatous transformation. Intern Med. (2002) 41:633–7. doi: 10.2169/internalmedicine.41.633
34. Shah H, Bothra J, Kumbhar V, Tiwari C. Pediatric inflammatory myofibroblastic tumor of the mesentery. Pediatr Oncall. (2016) 13:109.
35. Amouei A, Ehsania F, Vaghefib M, Tabatabaia SM, Anari PY. Inflammatory myofibroblastic tumor of the small intestine: a case report. Int J Surg Case Rep. (2016) 22:44–6.
36. Nakarmi R, Chen M-J, Ong K-H, Shrestha M, Maharjan S. Inflammatory myofibroblastic tumor in adult: a rare case. Nepal Med Coll J. (2021) 23:360–4.
37. Corapçioğlu F, Kargi A, Olgun N, Ozer E, Olguner M, Sarialioğlu F. Inflammatory myofibroblastic tumor of the ileocecal mesentery mimicking abdominal lymphoma in childhood: report of two cases. Surg Today. (2005) 35:687–91. doi: 10.1007/s00595-005-2992-9
38. Liaqat N, Tasneem S, Imran RM, Kanwal A, Gondal M, Jamil S. Inflammatory myofibroblastic tumor of jejunum in a child. J Pediatr Surg Case Rep. (2019) 51:101313.
39. Indusarath, Nayanar SK, Pareekutty NM, Satheesan B, Babu S. Inflammatory myofibroblastic tumour –an unusual tumour with intermediate malignant potential– report of three cases. J Clin Diagn Res. (2017) 11:ER01–03.
40. Kirchgesner T, Danse E, Sempoux Ch, Annet L, Dragean ChA, Trefois P, et al. Mesenteric inflammatory myofibroblastic tumor: MRI and CT imaging correlated to anatomical pathology. Jbr Btr. (2014) 97:301–2. doi: 10.5334/jbr-btr.1335
41. Al-Saeed M. Giant mesenteric inflammatory myofibroblastic tumor: a case report and review of the literature. Saudi J Health Sci. (2014) 3:56–8. doi: 10.1016/j.prp.2006.01.013
42. Batool S, Ahuja A, Chauhan DS, Bhardwaj M, Meena AK. Epithelioid inflammatory myofibroblastic sarcoma: the youngest case reported. Autops Case Rep. (2021) 11:e2021288. doi: 10.4322/acr.2021.288
43. Huang Y-H, Tian Y-F, Li C-F. Inflammatory myofibroblastic tumor with RANBP2 and ALK gene rearrangement with bland cytological features mimicking desmoid-type fibromatosis: a case report and review of the literature. Oncol Lett. (2016) 11:1429–34. doi: 10.3892/ol.2016.4082
44. Kim S-H, Cho YH, Kim H. Two cases of infantile intra-abdominal inflammatory myofibroblastic tumor. Pediatr Gastroenterol Hepatol Nutr. (2014) 17:116–20.
45. Ntloko S, Gounden A, Naidoo M, MaDIBA TE, Sing Y, Hadley GP. Intestinal inflammatory myofibroblastic tumour. South Afr J Surg. (2011) 49:190.
46. De Melio J, Mertens V, Gryspeerdt S. Inflammatory pseudotumor of the mesentery. J Belg Soc Radiol. (2021) 105:59.
47. Day DL, Sane S, Dehner LP. Inflammatory pseudotumor of the mesentery and small intestine. Pediatr Radiol. (1986) 16:210–5.
48. Souid AK, Ziemba MC, Stephen Dubansky A, Mazur M, Oliphant M, Deaver Thomas F, et al. Inflammatory myofibroblastic tumor in children. Cancer. (1993) 72:2042–8.
49. Cviko A, Milic Z, Cizmic A, Seiwerth S, Kruslin B. Inflammatory myofibroblastic tumor with extensive involvement of the bowel in a 7-year-old child. Croat Med J. (1999) 40:550–3.
50. Erkan N, Yildirim M, Yilmaz C, Yagci A. Inflammatory pseudo-tumour as an unusual cause of colonic obstruction: a case report. Acta Chir Belg. (2004) 104:462–4.
51. Saleem MI, Ben-Hamida MA, Barrett AM, Bunn SK, Huntley L, Wood KM, et al. Lower abdominal inflammatory myofibroblastic tumor-an unusual presentation-a case report and brief literature review. Eur J Pediatr. (2007) 166:679–83. doi: 10.1007/s00431-006-0305-y
52. Memba Ikuga R, Lamas Moure S, Ramos Rubio E, Climent Esteller MJ. Adult colocolic intussusception secondary to inflammatory myofibroblastic tumor. Cir Esp. (2007) 81:357–8. doi: 10.1016/s0009-739x(07)71342-8
53. Salameh M, Sultan I, Barbar M, Al Hussaini M, Jameel A, Ghandour K, et al. Inflammatory myofibroblastic tumor causing unexplained anemia in a toddler: a case report. J Med Case Rep. (2011) 5:1–4. doi: 10.1186/1752-1947-5-69
54. Kim EY, Lee IK, Lee YS, Yang N, Chung DJ, Yim KI, et al. Inflammatory myofibroblastic tumor in colon. J Korean Surg Soc. (2012) 82:45–9.
55. Aalbers AG, De Wilt JH, Zondervan PE, Ijzermans JN. A colon-derived inflammatory pseudotumor. Dig Dis Sci. (1999) 44:578. doi: 10.1023/a:1026665609461
56. Jeong JH, Cho IH, Kong EJ, Chun KA, Kim YJ, Kim JH. 18F-FDG PET/CT in inflammatory pseudotumor of the colon causing intussusception. Ann Nuclear Med. (2011) 25:447–50. doi: 10.1007/s12149-011-0481-3
57. Gurzu S, Bara T, Jung I. Inflammatory myofibroblastic tumor of the colon. J Clin Oncol. (2013) 31:e155–8.
58. Buzan MT, Wetscherek A, Rank CM, Kreuter M, Heussel CP, Kachelrieß M, et al. Delayed contrast dynamics as marker of regional impairment in pulmonary fibrosis using 5D MRI – a pilot study. Br J Radiol. (2020) 93:20190121–20190121. doi: 10.1259/bjr.20190121
59. Budylev A, Solar I, Kessner R, Aizic A. ROS1-positive Inflammatory myofibroblastic tumor of the small bowel causing obstruction: a case report. J Radiol Case Rep. (2022) 16:14–21. doi: 10.3941/jrcr.v16i1.3928
60. Mirshemirani A, Tabari AK, Sadeghian N, Shariat-Torbaghan S, Pourafkari M. Abdominal inflammatory myofibroblastic tumor: report on four cases and review of literature. Iran J Pediatr. (2011) 21:543–8.
61. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. (2008) 61:428–37.
62. Telugu RB, Prabhu AJ, Kalappurayil NB, Mathai J, Gnanamuthu BR, Manipadam MT. Clinicopathological Study of 18 cases of inflammatory myofibroblastic tumors with reference to ALK-1 expression: 5-year experience in a tertiary care center. J Pathol Transl Med. (2017) 51:255–63. doi: 10.4132/jptm.2017.01.12
63. Siemion K, Reszec-Gielazyn J, Kisluk J, Roszkowiak L, Zak J, Korzynska A. What do we know about inflammatory myofibroblastic tumors? – A systematic review. Adv Med Sci. (2022) 67:129–38. doi: 10.1016/j.advms.2022.02.002
64. Shah A, Pey E, Achonu JU, Bai JDK, Khan F. Inflammatory myofibroblastic tumor 12 years after treatment for synovial sarcoma: a case report. Orthop Res Rev. (2021) 13:163–9. doi: 10.2147/ORR.S333124
65. Iyer A, Radonic T, Heukamp LC, Thunnissen E, Daniels JMA. Inflammatory myofibroblastic tumour of the central airways: treatment and molecular analysis. ERJ Open Res. (2021) 7:00151–2020. doi: 10.1183/23120541.00151-2020
66. Dong Y, Zahid KR, Han Y, Hu P, Zhang D. Treatment of pediatric inflammatory myofibroblastic tumor: the experience from china children’s medical center. Children. (2022) 9:307.
67. Homaei-Shandiz F, Jafarzadeh-Esfehani R, Moazzen N, Amirabadi A. Inflammatory myofibroblastic tumor of salpinx: a very rare case treated with a less aggressive method. Iran J Cancer Prev. (2014) 7:244–7.
68. Inadomi K, Kumagai H, Takayoshi K, Ariyama H, Kusaba H, Nishie A, et al. Successful combination chemotherapy for metastatic inflammatory myofibroblastic tumor: a case report. Oncol Lett. (2015) 10:2981–5.
69. Wang X, Zhao X, Chin J, Zhu L, Wang Z, Zhong Z. Recurrent retroperitoneal inflammatory myofibroblastic tumor: a case report. Oncol Lett. (2016) 12:1535–8.
70. Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. (2010) 363:1727–33.
71. Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: a case report and review of the literature. Oncol Lett. (2013) 5:1672–6. doi: 10.3892/ol.2013.1260
Keywords: inflammatory, myofibroblastic, stromal, mesenchymal, tumor, jejunum, mesentery, mass
Citation: Al Shenawi H, Al-Shaibani SA, Al Saad SK, Al-Sindi F, Al-Sindi K, Al Shenawi N, Naguib Y and Yaghan R (2022) An extremely rare case of malignant jejunal mesenteric inflammatory myofibroblastic tumor in a 61-year-old male patient: A case report and literature review. Front. Med. 9:1042262. doi: 10.3389/fmed.2022.1042262
Received: 12 September 2022; Accepted: 18 October 2022;
Published: 08 November 2022.
Edited by:
Simona Gurzu, George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureş, RomaniaReviewed by:
Federico Pistoia, University of Genoa, ItalyLei Zhang, Southwest Medical University, China
Copyright © 2022 Al Shenawi, Al-Shaibani, Al Saad, Al-Sindi, Al-Sindi, Al Shenawi, Naguib and Yaghan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamdi Al Shenawi, aGFtZGltc0BhZ3UuZWR1LmJo; Yahya Naguib, eWFoeWFtbkBhZ3UuZWR1LmJo
 Hamdi Al Shenawi
Hamdi Al Shenawi Salamah A. Al-Shaibani2
Salamah A. Al-Shaibani2 Fedaa Al-Sindi
Fedaa Al-Sindi Noor Al Shenawi
Noor Al Shenawi Yahya Naguib
Yahya Naguib