
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 24 November 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1040441
This article is part of the Research Topic Case Reports in Pulmonary Medicine View all 17 articles
 Jiaming Liu
Jiaming Liu Yuan Gao*
Yuan Gao*Psittacosis is a zoonotic disease caused by Chlamydia psittaci. Systemic infections are mainly transmitted through the respiratory tract. The most common related disease is human atypical pneumonia, which is a rare pathogen of community-acquired pneumonia. Due to the difficulty of diagnosis, there have been few reports of C. psittaci pneumonia in the past. In recent years, with the widespread application of metagenomic next-generation sequencing (mNGS), the number of reported cases of C. psittaci has increased year by year. However, at present, most hospitals have little understanding of C. psittaci, especially for severe patients, and lack experience in diagnosis and treatment. Herein, we report the case of a 71-year-old woman with severe pneumonia that caused by C. psittaci. This patient was diagnosed through mNGS and was treated with tigecycline successfully. The level of IL-6 in the BALF was significantly increased. We discontinued tigecycline after mNGS of the blood was negative. In this review, we analyzed 53 cases to summarize the etiology, clinical manifestations, diagnosis and treatment strategies of severe C. psittaci pneumonia and hope to raise clinicians’ awareness of this disease.
Psittacosis is a zoonotic disease caused by Chlamydia psittaci (1). Humans are infected through the respiratory tract, and the most common related disease is human atypical pneumonia (2). It is a rare cause of community-acquired pneumonia, accounting for approximately 1% of community-acquired pneumonia. Some patients with psittacosis progress rapidly and can die if not treated in time. Due to the difficulty of diagnosis, there have been few reports of related cases in the past. In recent years, due to the widespread use of metagenomic next-generation sequencing (mNGS), reports of C. psittaci have been increasing year by year. This article reports a case of severe C. psittaci pneumonia who was diagnosed by mNGS and improved after treatment with tigecycline. There have been few cases of using tigecycline to treat C. psittaci pneumonia in the past, so it is necessary to summarize this case and review the literature.
The patient of a 71-year-old woman was admitted to our hospital because of fever for 10 days and dyspnea for 5 days on November 17, 2020. She presented with fever and chills 10 days before admission, and the maximum body temperature was 40°C, accompanied by fatigue and dry cough without sputum. Chest CT at the local hospital showed pneumonia in the inferior lobe of the left lung and mediastinal lymph node enlargement (Figure 1). Routine blood examination showed that the white blood cell count was 10.2 × 109/L and the CRP level was 139 mg/L. She went to a local clinic and applied cephalosporin antibiotics, but the above symptoms did not improve. Nausea and vomiting occurred 7 days prior, accompanied by diarrhea and watery stools, without abdominal pain. Then, she was hospitalized in the local hospital. Using ceftriaxone and moxifloxacin intravenously for 7 days showed no improvement. Dyspnea occurred 5 days prior and was aggravated after the event. One day before admission, the patient’s dyspnea was significantly aggravated, and re-examination of chest CT was significantly worse than before (Figure 1). The patient was treated with tracheal intubation and ventilator-assisted ventilation. She was sent immediately to Shengjing Hospital of China Midical University and was hospitalized in the Department of Pulmonary and Critiacl Care Medicine.

Figure 1. Patient’s chest CT scans: panels (A–C) show CT scans at the time of the patient’s first day of illness (November 7) in the local hospital. Panel (D–F) show CT scans on the 10th day of illness (November 17) in the local hospital. Panel (G–I) show CT scans on the 10th day after admission (November 27). Panel (J–L) show CT scans on the 26th day after admission (December 12).
The patient suffered from coronary atherosclerotic cardiopathy for 3 years, and she took aspirin intermittently. She had no hypertension and diabetes. She had no hepatitis and tuberculosis. She resides in Liaoning Province, northern China. She is a retired employee, has no smoking and drinking habits, and has no drug or food allergy.
When the patient was admitted to our hospital (day 1), her vital signs were as follows: body temperature 36.5°C, pulse rate 80 beats/min, respiratory rate 27 beats/min, blood pressure 117/69 mmHg, and pulse oxygen saturation 96% with a fraction of inspired oxygen (FiO2) of 0.80. Conscious, no yellowing of skin and sclera, no paleness of eyelid conjunctiva. Breath sounds in both lungs were rough, and wet rales were heard on auscultation. There was no audible murmur on cardiac auscultation. Tenderness in the abdomen and hepatosplenomegaly were not detected. No edema of both lower limbs.
The laboratory dates after admission to our hospital were as follows: arterial blood gas analysis showed a pH of 7.513, PaO2 of 98.6 mmHg, PaCO2 of 28.2 mmHg, HCO3- of 22.5 mmol/L and oxygenation index of 123.2. The white blood cell count was 18.5 × 109/L, with an elevated neutrophil ratio of 90.3%. The concentration of C-reactive protein (CRP) was 253.7 mg/L. The concentrations of procalcitonin (PCT, normal < 0.05 ng/ml) and interleukin-6 (IL-6, normal ≤5.4 pg/ml) were 2.91 ng/ml and 12.64 pg/ml, respectively. The D-dimer (normal 0–252 μg/L) was 2161 μg/L. Albumin level (normal 35–53 g/L) was 23.0 g/L. Alanine aminotransferase (ALT, normal 0–40 U/L) was 68 U/L, and aspartate aminotransferase (AST, normal 5–34 U/L) was 69 U/L. Bilirubin, creatinine, and electrolytes were within normal limits. The creatine kinase (CK, normal 29–200 U/L) was 75 U/L, and the creatine kinase isoenzyme (CK-MB, normal 0–24 U/L) was 17.4 U/L. The concentrations of B-type natriuretic peptide (BNP, normal 0–154.7 pg/mL) and cardiac troponin I (cTnI, normal 0–0.0116 U/L) were 300.8 pg/ml and 0.7743 μg/L, respectively. Mycoplasma pneumoniae IgM was positive, and IgG was negative. Chlamydia pneumoniae IgM was negative, and IgG was positive. The Epstein Barr virus DNA quantification was 8.15E + 03 copies/ml (normal < 1.0E + 03 copies/ml). Tests for cytomegalovirus, influenza A virus, influenza B virus, aenovirus and legionella bacteria were all negative. The β-D-glucan test and galacto Mannan test were both negative. T-SPOT-T and Tuberculin test were negative. The indicators for autoimmune diseases (anti-extractable nuclear antigen antibody, anti-nuclear antibody and anti-neutrophilic cytoplasmic antibody) were negative. The numbers of CD3 (normal 690–2,540/μl), CD8 (normal 190–1,140/μl) and CD4 (normal 410–1,590/μl) T lymphocytes in blood were 254, 41, and 165/μl, respectively. The concentrations of carcinoembryonic antigen (CEA, normal 0–5 ng/ml), cytokeratin 19 fragment (CYFRA21-1, normal 0.1–3.3 ng/ml) and neuron-specific enolase (NSE, normal 0–16.3 ng/ml) were 2.37, 6.30, and 17.32 ng/ml, respectively.
After admission, the patient continued to be treated with ventilator-assisted ventilation. Empirical anti-infective treatments, including meropenem, levofloxacin, ganciclovir, and arbidol, were used initially. After the initial treatment, the patient’s peak temperature decreased, but she still had fever. On the second day of admission (November 18), the patient underwent bronchoscopic alveolar lavage examination, and bronchoalveolar lavage fluid (BALF) was sent to Nanjing Difei Medical Laboratory Co., Ltd. for mNGS examination. The concentration of interleukin-6 (IL-6) in the BALF was 4,519.04 pg/ml (normal range 0–5.4 pg/ml). On the 4th day of admission (November 20), the result of mNGS of the BALF was reported, and 70 sequence reads corresponding to C. psittaci were identified and there was no sequence read corresponding to other pathogens. The patient had no history of direct contact with birds and poultry before the illness, but there were neighbors raising pigeons in the residential area, not except for indirect contact. According to the results of mNGS of BALF suggesting C. psittaci infection (Table 1), antibacterial agents were switched to tigecycline (100 mg intervenous drop infusion on day 4, then 50 mg q12 h intervenous drop infusion on days 4–25) combined with levofloxacin (0.5 g qd intervenous drop infusion, days 2–7), and meropenem was discontinued. The patient’s blood sample was sent to Guangzhou Weiyuan Gene Technology Co., Ltd. for mNGS testing again, and the results of the blood samples still identified 38 sequence reads corresponding to C. psittaci (Table 1). She was diagnosed with C. psittaci pneumonia. Her body temperature returned to normal on the 6th day of admission (Figures 1–4), and her dyspnea gradually eased. On the 7th day of admission (November 23), the patient’s sputum culture revealed Pseudomonas aeruginosa and Candida albicans. Considering the patient had a secondary infection. According to the results of drug sensitivity, the patient was administered piperacillin tazobactam (4.5 g q8 h intervenous drop infusion, days 8–25) while strengthening oral care. The patient had sinus bradycardia, and the electrocardiogram showed a prolonged QT interval, so levofloxacin was ceased. On the 10th day of admission (November 26), the patient’s tracheal intubation was removed. Re-examination of chest CT showed that lung inflammation was significantly absorbed (Figure 1). On the 24th day of admission (December 10), blood mNGS was performed again and was negative for C. psittaci (Table 1). Tigecycline and piperacillin tazobactam were discontinued. Re-examination of the chest CT (December 12) showed that the inflammation was well absorbed, and only a few fiber cord shadows were left. On the 29th day of admission (December 15), the patient was discharged from the hospital, and her status was close to premorbid condition.
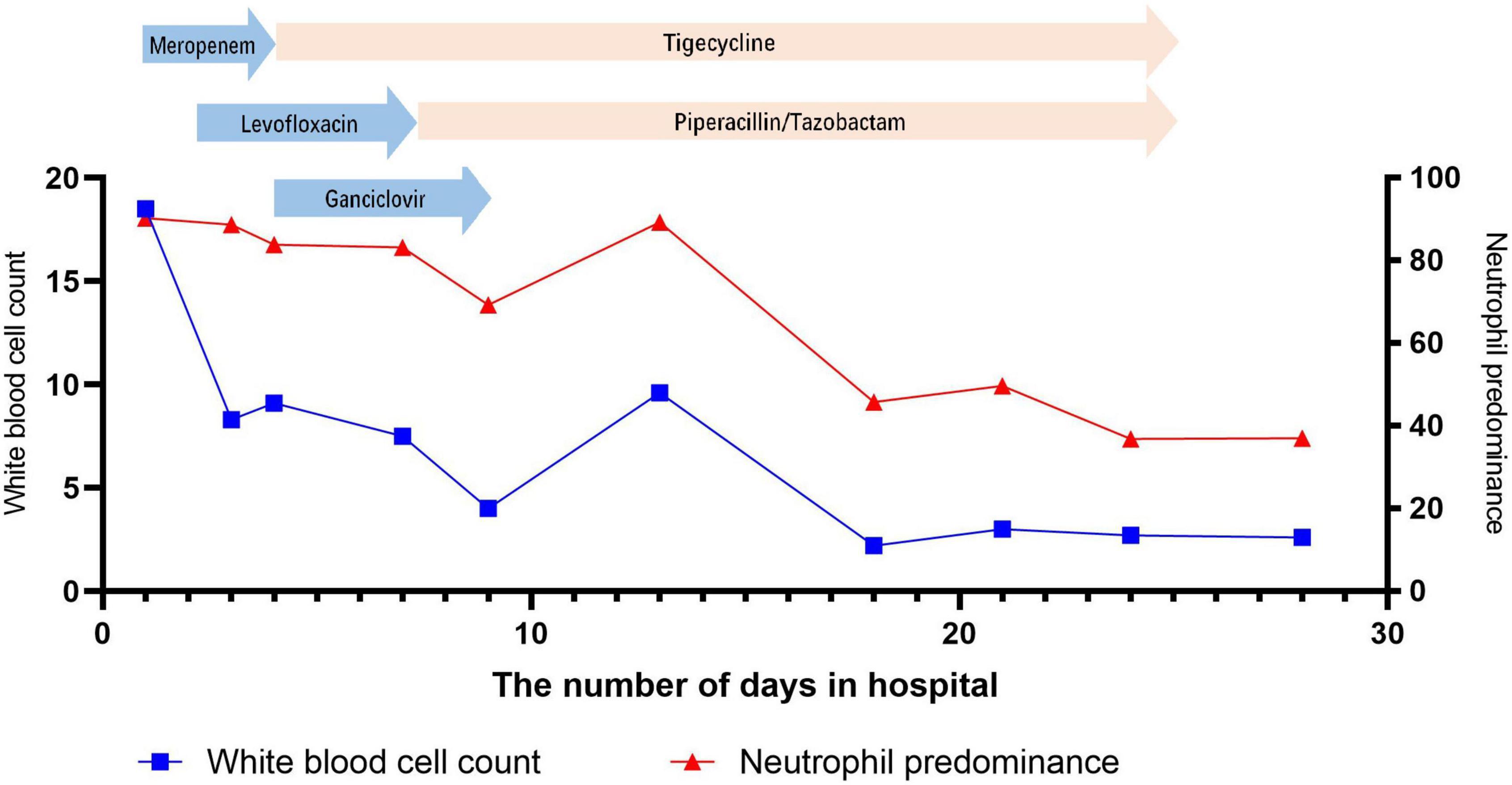
Figure 2. Change in white blood cell count and neutrophil predominance during hospitalization at the Shengjing Hospital of China Medical University.
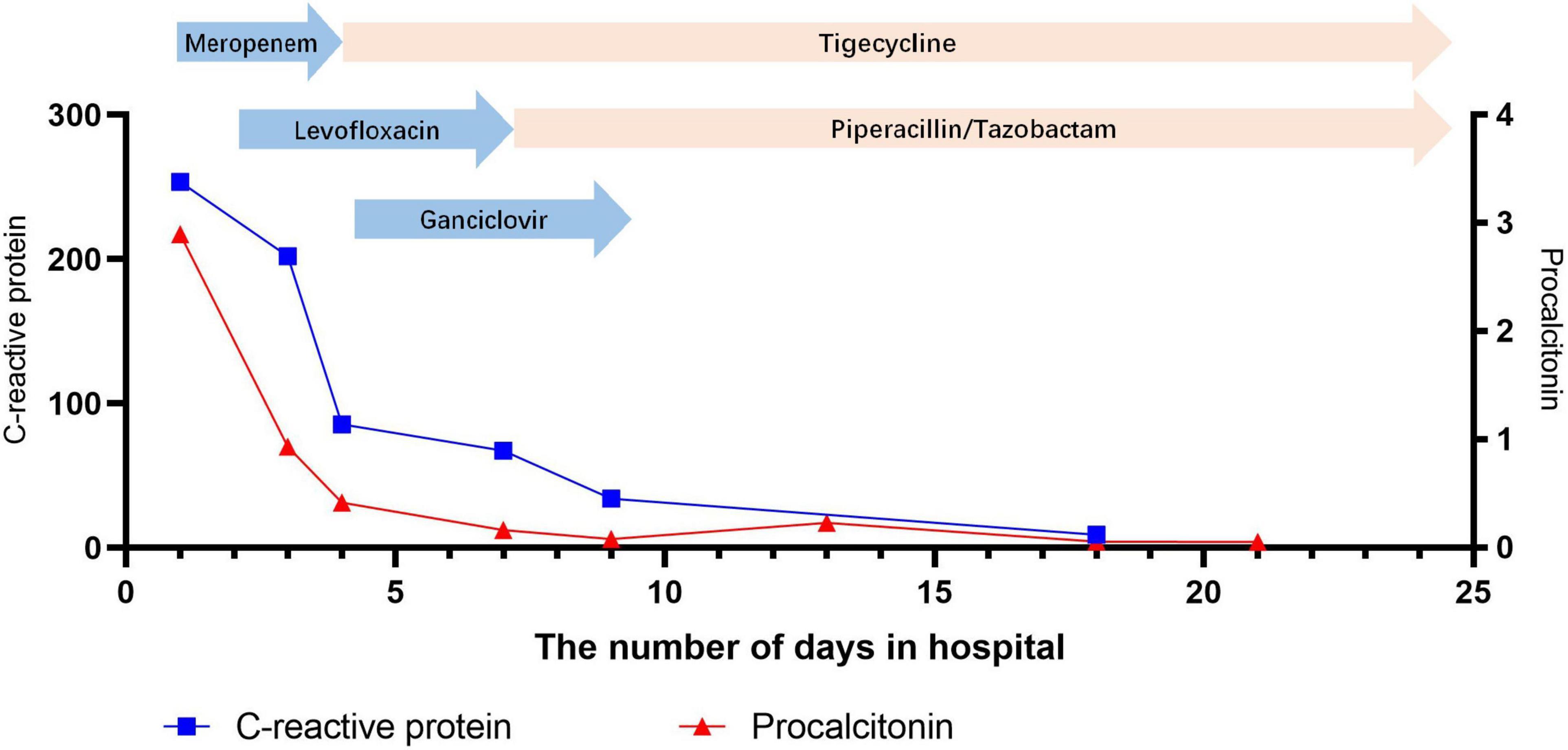
Figure 3. Change in C-reactive protein and procalcitonin during hospitalization at the Shengjing Hospital of China Medical University.

Figure 4. Body temperature and antimicrobial treatment during hospitalization at the Shengjing Hospital of China Medical University.
Other bacteria, including Propionibacterium acnes, Moraxella osloensis, Staphylococcus hominis, Staphylococcus warneri, and Malassezia globose, were also detected by mNGS in the patient’s blood samples (Table 1). These bacteria are all skin microecological flora and not considered as pathogenic bacteria.
We searched the PubMed database for articles on C. psittaci published before September 31, 2021. The search strategy was “Chlamydia psittaci” or “Chlamydia psittaci pneumonia”, and a total of 870 articles were found. Among them, there were 23 articles about severe C. psittaci infection, and 54 severe C. psittaci pneumonia patients were reported; 27 cases were treated with ventilators, and 7 cases died. A summary of the detailed information is shown in Table 2. Most cases had a history of direct or indirect exposure. Contact history included raising parrots, birds and poultry, etc., or going to a bird shop, passing through a poultry market. There were also a few patients who had been infected due to contact with pets or aborted sheep. There were also reports of human-to-human transmission. Most patients had underlying diseases. There were 2 pregnant women, and 1 of them died. Chest radiography or CT showed consolidation or ground glass-like changes, and some patients had pleural effusion.
Forty-one patients (54 cases in total) were treated with quinolones, including moxifloxacin, levofloxacin, and ciprofloxacin. Among them, 6 patients were improved by quinolones alone after diagnosis, and 29 patients were initially ineffective with quinolones. A total of 9 patients had been treated with macrolides, including azithromycin, erythromycin, and spiramycin. Five patients had no effect on initial treatment with macrolides. There was no successful case of using macrolides alone in severe patients. A total of 27 patients were treated with tetracycline (alone or in combination with quinolones and macrolides) after being diagnosed with psittacosis, including doxycycline and minocycline. Among them, 5 patients died of ineffective treatment, 1 patient improved but died after being discharged from the hospital, and the rest were cured. One pregnant patient had only used meropenem during the treatment and died. In 2 patients, the initial application of quinolones was not effective but improved after changing to tigecycline.
Chlamydia psittaci is an intracellular parasite and a gram-negative spherical pathogen that is the pathogen of epidemic avian chlamydia (1). The most common related disease is human atypical pneumonia, which is a rare cause of community-acquired pneumonia, accounting for approximately 1% of community-acquired pneumonia (2). A study on the detection rate of IgM antibodies to Mycoplasma and Chlamydia in infants and young children in Japan found that the positive rate of C. psittaci was approximately 2.2% (3). Spoorenberg et al. conducted PCR to detect C. psittaci in patients with community-acquired pneumonia in two hospitals in the Netherlands and found that the incidence of C. psittaci was much higher than that previously reported, approximately 4.8% (4). Among patients with severe pneumonia in the ICU, the incidence of C. psittaci could be as high as 8% (5). These findings suggest that the estimated incidence of C. psittaci in current epidemiological data may be underestimated, especially in critical patients.
The typical epidemiological data of C. psittaci are that humans are infected through contact with the eyes, beaks or intestinal feces of birds (1). Poultry (such as chickens, ducks, etc.) can also be infected with C. psittaci and then infect humans. The transmission routes include raising poultry or frequently visiting poultry markets (6). There were also reports in the that humans were infected through mammals, such as sheep and horses, but were rare (7, 8). There have also been reports of human-to-human transmission (9). Our patient had no history of direct contact with birds and poultry before the illness, but there were neighbors who raised birds in the patient’s residential area, not except for indirect contact.
The manifestations of C. psittaci include fever, headache, muscle aches, dry cough, dyspnea, etc., and some patients may have relative bradycardia. Severe cases may also involve other organs, including the heart, liver, skin, and central nervous system, and cause symptoms (10–14). Our patient had bradycardia, a prolonged Q-T interval, and elevated myocardial enzymological markers, which may be associated with C. psittaci involving the heart.
In most patients, the white blood cell counts were normal, the proportion of neutrophils was increased, the lymphocytes were decreased, C-reactive proteins (CRP) and erythrocyte sedimentation rates (ESR) were significantly increased, and the increase in procalcitonins was not obvious. In our patient, the initial white blood cell count did not increase significantly, but the CRP level increased significantly. After admission, the patient’s white blood cell count, CRP and procalcitonin were all increased significantly, which might be related to secondary infection (sputum culture suggests Pseudomonas aeruginosa).
Interleukin 6 is the main mediator of physiological, hematological, and immunological reactions during the acute phase of inflammation, especially regulating the synthesis of liver proteins in the acute phase. In rodent models of experimentally induced fever, the important role of IL-6 as a circulating endogenous pyrogen has been well established (15). Prohl et al. confirmed that after inoculating C. psittaci into the lungs of calves, the activity of IL-6 in BALF was significantly higher than that in blood serum and revealed no apparent relation between IL-6 in blood and body temperature, but they did reveal a relation between IL-6 and other markers of inflammation in BALF and concluded that a local inflammatory response in the lungs of infected calves caused fever (16). In our patient, IL-6 was also significantly increased in the BALF and was not significantly increased in the blood. This indicates that human infection with C. psittaci can also cause a strong local inflammatory response in the lungs and cause fever. The inflammatory response in the blood is mild and may have no relation with fever.
Chest CT of C. psittaci pneumonia showed ground glass-like changes or consolidation, and the lower lobes of the lung were often involved. This was occasionally accompanied by pleural effusion and mediastinal lymph node enlargement. Large shadows on the lung lobes and extensive bilateral pneumonia may also appear in severe cases (17). Chest CT of our patient showed consolidation of the lower lobe of the left lung initially and quickly progressed to extensive bilateral pneumonia, with hilar node enlargement, without pleural effusion. After the treatment was effective, the lung inflammation was significantly absorbed, only a few fiber cords remained, and the mediastinal lymph nodes were smaller than before.
There have been few reports of C. psittaci pneumonia in the past, on the one hand due to its low incidence and on the other hand due to the difficulty of diagnosis. Traditional pathogen culture is time-consuming, and C. psittaci needs to be cultured in cells, which requires very high laboratory conditions, so clinical application is rare. Serological testing is mainly used for retrospective studies, which has little value for the early diagnosis of severe patients, and most hospitals in China do not carry out such inspection items at present. PCR tests can quickly identify C. psittaci (18, 19), but this test has also not been carried out in Chinese hospitals. mNGS can quickly and accurately identify pathogens and has been widely used in the diagnosis of infectious diseases, especially to detect pathogens that cannot be detected by traditional methods (20). In recent years, with the widespread use of mNGS, an increasing number of cases of psittacosis have been diagnosed. We reviewed 23 articles, including 54 cases of severe Chlamydia psitsiti pneumonia. There were 12 articles using mNGS to confirm the diagnosis, all of which were reported in China.
In our patient, only C. psittaci was found in mNGS of BALF, with a sequence of 70 reads and without other background pathogens. We sent blood samples to other testing institutions for mNGS, and C. psittaci was also detected, with a sequence of 38 reads. These results indicate that the invasion of C. psittaci into the human body can not only cause lung infection but can also spread in the patient’s body and lead to explosive systemic disease, which may be related to the special infection mechanism of the intracellular bacteria. It has also been reported in the literature that C. psittaci first enters the reticuloendothelial cells of the liver and spleen to proliferate and then enters the lungs and other organs through the bloodstream (21, 22). Therefore, human psittacosis is a systemic infection, mainly respiratory infections (13, 23). Our patient was diagnosed with C. psittaci pneumonia. After symptomatic anti-infection treatment, the patient’s fever and dyspnea improved significantly, and the inflammatory indicators returned to normal. Re-examination of lung CT indicated that the lung inflammation was significantly absorbed. On the 24th day of hospitalization, we reexamined the patient’s blood mNGS, indicating that C. psittacis was not detected. Then, tigecycline was discontinued, and the total course of treatment was 21 days. During the follow-up, the patient was in good condition and returned closely to her premorbid condition. Currently, the specific course of treatment for C. psittaci is uncertain and is generally believed to be 10–21 days. Whether a prolonged course of treatment can prevent recurrence is still controversial. Whether the negative results of mNGS in BALF and blood can be used as evidence of drug withdrawal remains to be further clinically observed.
Tetracyclines are the first choice for the treatment of C. psittaci pneumonia, including doxycycline, tetracycline and minocycline. In the previous articles about severe C. psittaci infection, 22 patients (54 cases in total) responded to tetracycline therapy in combination with or alone after diagnosis, and 5 patients did not respond to the treatment and died. The deaths were thought to be related to the subsequent infections of other resistant bacteria, but the possibility of tetracycline resistance has not been determined.
When the use of tetracycline is contraindicated, macrolides (erythromycin, azithromycin, etc.) can be used instead, but it may not be effective in patients with severe disease or pregnancy (24). In previous studies, there were successful cases of using macrolides alone, but they were all mild patients. There was no successful case of severe C. psittaci pneumonia treated with macrolides alone. These results indicate that macrolides have a poor effect on severe C. psittaci and should be combined with other drugs.
The intracellular activity of quinolones against C. psittaci was lower than that of tetracycline and macrolides, but they showed activity against C. psittaci in vitro (25, 26). In previous studies, 41 cases (54 cases in total) of severe patients had been treated with quinolones, among which 29 cases had failed to be treated with quinolones initially, and 6 cases were improved by quinolones alone. These results indicate that quinolones are effective in the treatment of C. psittaci and can be used for severe C. psittaci pneumonia, but there may be drug resistance in some patients and treatment failure. Currently, the use of antibiotics in the poultry and pet bird industries is increasing, which may have contributed to the drug resistance of C. psittaci (27). Our patient had been treated with cephalosporin and moxifloxacin intravenously for 7 days in the local hospital but did not improve. After being transferred to our department, the patient was given meropenem combined with levofloxacin intravenously but still had intermittent fever. C. psittaci in this patient was resistant to quinolones. After the diagnosis of C. psittaci, the patient was treated with tigecycline and improved. Tigecycline is a new tetracycline antibiotic that is mainly used for the treatment of severe abdominal infection, lung infection and bloodstream infection. There have been few reports on the treatment of Chlamydia psittacosis pneumonia with tigecycline. Wen et al. (28) reported a case of severe C. psittaci pneumonia in which tigecycline was used for treatment, but the treatment effect was not described in detail. Wu et al. (29) reported that three patients received carbapenems, linezolid, or tigecycline in addition to doxycycline treatment after diagnosis, but the outcomes were not stated. Kong et al. (30) reported that two patients with severe C. psittaci pneumonia were not effective in initial treatment with moxifloxacin and were improved after using tigecycline. This demonstrates that tigecycline can be used as an alternative treatment for severe C. psittaci pneumonia, especially in patients with coexisting secondary infections, because of its broad antibacterial spectrum. Whether tigecycline is better than other tetracycline drugs in severe patients needs further large-sample clinical observation.
In conclusion, the manifestations of C. psittaci pneumonia are diverse and lack specificity. The overall prognosis is good, but severe patients may die if they are not treated in time. The mNGS is a promising detection method. For patients with severe infections, patients who were not efficacious with empirical anti-infection treatment, or patients who were possibly infected with special pathogens, mNGS testing should be performed as soon as possible.
YG contributed in diagnosing the disease, data collection, and data analysis. JL contributed to literature search and figures preparation. YG and JL were the main contributors to drafting the manuscript and performed the final manuscript review. Both authors read and approved the final manuscript.
This study was supported by grants from the National Natural Science Foundation of China (No. 82100020).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
mNGS, metagenomic next-generation sequencing; CT, computed tomography; BALF, bronchoalveolar lavage fluid; IL-6, interleukin-6; PCT, procalcitonin; CRP, C-reactive protein; FiO2, Fraction of inspired oxygen; pH, Pondus Hydrogenii; PaO2, partial arterial oxygen pressure; PaCO2, arterial partial pressure of carbon dioxide; PT, prothrombin time; APTT, activated partial thromboplastin time; ALT, alanine aminotransferase; CK-MB, creatine kinase isoenzyme; BNP, B-type natriuretic peptide; cTnI, cardiac troponin I; CEA, carcinoembryonic antigen; CYFRA, cytokeratin 19 fragment; NSE, neuron-specific enolase.
1. Balsamo G, Maxted AM, Midla JW, Murphy JM, Wohrle R, Edling TM, et al. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg. (2017) 31:262–82. doi: 10.1647/217-265
2. Hogerwerf L, De Gier B, Baan B, Van Der Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. (2017) 145:3096–105. doi: 10.1017/S0950268817002060
3. Numazaki K, Chiba S, Umetsu M. Detection of IgM antibodies to Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci from Japanese infants and children with pneumonia. In Vivo. (1992) 6:601–4.
4. Spoorenberg SM, Bos WJ, van Hannen EJ, Dijkstra F, Heddema ER, van Velzen-Blad H, et al. Chlamydia psittaci: a relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth J Med. (2016) 74:75–81.
5. Wu X, Li Y, Zhang M, Li M, Zhang R, Lu X, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. (2020) 9:1003–15. doi: 10.1007/s40121-020-00353-y
6. Hogerwerf L, Roof I, de Jong MJK, Dijkstra F, van der Hoek W. Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect Dis. (2020) 20:192. doi: 10.1186/s12879-020-4918-y
7. Janssen MJ, van de Wetering K, Arabin B. Sepsis due to gestational psittacosis: a multidisciplinary approach within a perinatological center–review of reported cases. Int J Fertil Womens Med. (2006) 51:17–20.
8. Johnson FW, Matheson BA, Williams H, Laing AG, Jandial V, Davidson-Lamb R, et al. Abortion due to infection with Chlamydia psittaci in a sheep farmer’s wife. Br Med J. (1985) 290:592–4. doi: 10.1136/bmj.290.6468.592
9. Lei JH, Xu Y, Jiang YF, Shi ZH, Guo T. Clustering cases of Chlamydia psittaci pneumonia in coronavirus disease 2019 screening ward staff. Clin Infect Dis. (2021) 73:e3261–e3265. doi: 10.1093/cid/ciaa1681
10. Lamáury I, Sotto A, Le Quellec A, Perez C, Boussagol B, Ciurana AJ. Chlamydia psittaci as a cause of lethal bacterial endocarditis. Clin Infect Dis. (1993) 17:821–2. doi: 10.1093/clinids/17.4.821
11. Samra Z, Pik A, Guidetti-Sharon A, Yona E, Weisman Y. Hepatitis in a family infected by Chlamydia psittaci. J R Soc Med. (1991) 84:347–8. doi: 10.1177/014107689108400614
12. Shi Y, Chen J, Shi X, Hu JR, Li H, Li X, et al. A case of Chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. (2021) 21:621. doi: 10.1186/s12879-021-06205-5
13. Meijer R, van Biezen P, Prins G, Boiten HJ. Multi-organ failure with necrotic skin lesions due to infection with Chlamydia psittaci. Int J Infect Dis. (2021) 106:262–4. doi: 10.1016/j.ijid.2021.03.091
14. Vande Weygaerde Y, Versteele C, Thijs E, De Spiegeleer A, Boelens J, Vanrompay D, et al. An unusual presentation of a case of human psittacosis. Respir Med Case Rep. (2018) 23:138–42.
15. Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. (2000) 526(Pt 3.):653–61.
16. Prohl A, Ostermann CH, Rummel CD, Roth J, Reinhold P. Circulating and broncho-alveolar interleukin-6 in relation to body temperature in an experimental model of bovine Chlamydia psittaci infection. PLoS One. (2017) 12:e0189321. doi: 10.1371/journal.pone.0189321
17. Su S, Su X, Zhou L, Lin P, Chen J, Chen C, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. (2021) 10:8051–60.
18. Hisada K, Hida Y, Kawabata N, Kawashima Y, Soya Y, Shimada A, et al. Development and evaluation of a novel quenching probe PCR (GENECUBE) assay for rapidly detecting and distinguishing between Chlamydia pneumoniae and Chlamydia psittaci. J Microbiol Methods. (2021) 184:106212. doi: 10.1016/j.mimet.2021.106212
19. McGovern OL, Kobayashi M, Shaw KA, Szablewski C, Gabel J, Holsinger C, et al. Use of real-time PCR for Chlamydia psittaci detection in human specimens during an outbreak of psittacosis - Georgia and Virginia, 2018. MMWR Morb Mortal Wkly Rep. (2021) 70:505–9. doi: 10.15585/mmwr.mm7014a1
20. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. (2018) 66:778–88. doi: 10.1093/cid/cix881
21. Zhang H, Zhan D, Chen D, Huang W, Yu M, Li Q, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. (2020) 8:401.
23. Knittler MR, Berndt A, Böcker S, Dutow P, Hänel F, Heuer D, et al. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int J Med Microbiol. (2014) 304:877–93. doi: 10.1016/j.ijmm.2014.06.010
24. Binet R, Maurelli AT. Frequency of development and associated physiological cost of azithromycin resistance in Chlamydia psittaci 6BC and C. trachomatis L2. Antimicrob Agents Chemother. (2007) 51:4267–75. doi: 10.1128/AAC.00962-07
25. Miyashita N, Fukano H, Yoshida K, Niki Y, Matsushima T. In-vitro activity of moxifloxacin and other fluoroquinolones against Chlamydia species. J Infect Chemother. (2002) 8:115–7. doi: 10.1007/s101560200019
26. Prohl A, Lohr M, Ostermann C, Liebler-Tenorio E, Berndt A, Schroedl W, et al. Enrofloxacin and macrolides alone or in combination with rifampicin as antimicrobial treatment in a bovine model of acute Chlamydia psittaci infection. PLoS One. (2015) 10:e0119736. doi: 10.1371/journal.pone.0119736
27. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. (2009) 15:11–7. doi: 10.1111/j.1469-0691.2008.02669.x
28. Wen W, Gu L, Zhao LW, Chen MY, Yang WQ, Liu W, et al. [Diagnosis and treatment of Chlamydia psittaci pneumonia: experiences of 8 cases]. Zhonghua Jie He He Hu Xi Za Zhi. (2021) 44:531–6.
29. Wu HH, Feng LF, Fang SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. (2021) 21:300. doi: 10.1186/s12890-021-01673-6
30. Kong CY, Zhu J, Lu JJ, Xu ZH. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. (2021) 134:353–5.
31. Chen X, Cao K, Wei Y, Qian Y, Liang J, Dong D, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. (2020) 48:535–42. doi: 10.1007/s15010-020-01429-0
32. Wang Y, Lu H, Shao R, Wang W. [Clinical characteristics analysis of patients with pneumonia infected by Chlamydia psittaci]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2020) 32:1388–90.
33. Katsura D, Tsuji S, Kimura F, Tanaka T, Eguchi Y, Murakami T. Gestational psittacosis: a case report and literature review. J Obstet Gynaecol Res. (2020) 46:673–7.
34. Zhang Q, Li S, Zhou W, Zheng L, Ren Y, Dong L, et al. Application of metagenomic next-generation sequencing (mNGS) combined with rapid on-site cytological evaluation (ROSCE) for the diagnosis of Chlamydia psittaci pneumonia. Int J Clin Exp Pathol. (2021) 14:389–98.
35. Yuan Y, Zhang X, Gui C. Detection of Chlamydia psittaci in both blood and bronchoalveolar lavage fluid using metagenomic next-generation sequencing: a case report. Medicine. (2021) 100:e26514. doi: 10.1097/MD.0000000000026514
36. Teng XQ, Gong WC, Qi TT, Li GH, Qu Q, Lu Q, et al. Clinical analysis of metagenomic next-generation sequencing confirmed Chlamydia psittaci pneumonia: a case series and literature review. Infect Drug Resist. (2021) 14:1481–92. doi: 10.2147/IDR.S305790
37. Qi YF, Huang JL, Chen JH, Huang CP, Li YH, Guan WJ. [Chlamydia psittaci pneumonia complicated with rhabdomyolysis: a case report and literature review]. Zhonghua Jie He He Hu Xi Za Zhi. (2021) 44:806–11.
38. Arenas-Valls N, Chacón S, Pérez A, Del Pozo R. Atypical Chlamydia Psittaci pneumonia. four related cases. Arch Bronconeumol. (2017) 53:277–9. doi: 10.1016/j.arbres.2016.10.006
39. Fraeyman A, Boel A, Van Vaerenbergh K, De Beenhouwer H. Atypical pneumonia due to Chlamydophila psittaci: 3 case reports and review of literature. Acta Clin Belg. (2010) 65:192–6. doi: 10.1179/acb.2010.040
40. Bourne D, Beck N, Summerton CB. Chlamydia psittaci pneumonia presenting as acute generalised peritonism. Emerg Med J. (2003) 20:386–7. doi: 10.1136/emj.20.4.386
41. Soni R, Seale JP, Young IH. Fulminant psittacosis requiring mechanical ventilation and demonstrating serological cross-reactivity between Legionella longbeachae and Chlamydia psittaci. Respirology. (1999) 4:203–5. doi: 10.1046/j.1440-1843.1999.00176.x
42. Verweij PE, Meis JF, Eijk R, Melchers WJ, Galama JM. Severe human psittacosis requiring artificial ventilation: case report and review. Clin Infect Dis. (1995) 20:440–2. doi: 10.1093/clinids/20.2.440
43. Shapiro DS, Kenney SC, Johnson M, Davis CH, Knight ST, Wyrick PB. Brief report: Chlamydia psittaci endocarditis diagnosed by blood culture. N Engl J Med. (1992) 326:1192–5. doi: 10.1056/NEJM199204303261805
44. Villemonteix P, Agius G, Ducroz B, Rouffineau J, Plocoste V, Castets M, et al. Pregnancy complicated by severe Chlamydia psittaci infection acquired from a goat flock: a case report. Eur J Obstet Gynecol Reprod Biol. (1990) 37:91–4. doi: 10.1016/0028-2243(90)90101-6
Keywords: psittaci, Chlamydia psittaci pneumonia, severe pneumonia, tigecycline, metagenomic nextgeneration sequencing (mNGS)
Citation: Liu J and Gao Y (2022) Tigecycline in the treatment of severe pneumonia caused by Chlamydia psittaci: A case report and literature review. Front. Med. 9:1040441. doi: 10.3389/fmed.2022.1040441
Received: 09 September 2022; Accepted: 10 November 2022;
Published: 24 November 2022.
Edited by:
Suzana Erico Tanni, São Paulo State University, BrazilReviewed by:
Ke Cao, Nanjing Drum Tower Hospital, ChinaCopyright © 2022 Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Gao, Z2FveTJAc2otaG9zcGl0YWwub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.