
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 24 November 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1039954
 Yin-Shui Miao1,2†
Yin-Shui Miao1,2† Yuan-Yuan Li1†
Yuan-Yuan Li1† Bo-Wen Cheng1,2
Bo-Wen Cheng1,2 Yan-Fang Zhan1,2
Yan-Fang Zhan1,2 Sheng Zeng3
Sheng Zeng3 Xiao-Jiang Zhou1
Xiao-Jiang Zhou1 You-Xiang Chen1
You-Xiang Chen1 Nong-Hua Lv1
Nong-Hua Lv1 Guo-Hua Li1*
Guo-Hua Li1*Background: Endoscopic retrograde cholangiopancreatography (ERCP) has become an important method to diagnose and treat biliary-pancreatic diseases. Perforations are infrequent but serious complications can occur during ERCPs. However, it is unclear which patients are suitable for surgery and when these patients should receive surgery.
Aim: To analyze the outcome of 45 patients with endoscopic retrograde cholangiopancreatography (ERCP) related perforation.
Materials and methods: We retrospectively reviewed all 45 patients with ERCP-related perforation between January 2003 and December 2017, and observed the location and causes of perforation, treatment strategies, and mortality.
Results: Twenty thousand four hundred and seventy-nine patients received ERCP procedures from January 2003 to December 2017 in our digestive endoscopy center. Forty-five patients suffered from ERCP-related perforations. The incidence rate of ERCP-related perforations was 0.22%. Twenty-six patients suffered from periampullary perforations, 15 patients suffered from duodenal wall perforations, 1 patient suffered from a fundus perforation, 1 patient suffered from a residual gallbladder duct perforation, 1 patient suffered from a papillary diverticulum perforation, and 1 patient suffered from an intrahepatic bile duct perforation. Six patients with duodenal perforations underwent surgery, and the other patients received conservative treatment. One patient with a duodenal perforation and ERCP-related pancreatitis died of heart failure, and all the other patients recovered. The mortality rate was 2.2%.
Conclusion: Endoscopic closure is seen as the first method for treating Stapfer type I perforations in the early phase, and surgery is seen as a remedial method when local treatment was failed. The Stapfer type II to type IV perforations can recover by conservative treatment.
Endoscopic retrograde cholangiopancreatography (ERCP) has become an important method to diagnose and treat biliary-pancreatic diseases. Perforations are infrequent but serious complications can occur during ERCPs. Multiple case series have shown the overall risk of perforation during ERCP to be < 1%, with a mortality range of 7.8–9.9% (1–5). Many patients with ERCP-related perforations recovered by undergoing surgery or conservative therapy (6–10). However, it is unclear which patients are suitable for surgery and when these patients should receive surgery. In this study, we evaluated our experiences in the management of ERCP-related perforations at our digestive endoscopy center. We now report the results we found.
We collected cases at our endoscopy center (The Digestive Endoscopy Center of Jiangxi Province) from January 2003 to December 2017. We retrospectively reviewed all cases in this period. The patients’ demographics, including age, sex, and comorbidities, such as coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD), chronic renal failure, and malignancies, were investigated. The indication for ERCP, clinical presentation of perforation, and management were also recorded and analyzed.
Before the ERCP, a routine preoperative blood examination, electrocardiography, chest X-ray, and echocardiography were conducted. On the day of the ERCP, the patient took medicine that treats hypertension and coronary heart disease in the morning before the operation. The fasting blood glucose of diabetic patients was controlled at 8–10 mmol/L. The patients fasted for 8 h before surgery and signed consent for the ERCP. Intravenous anesthesia was administered with propofol, a TJF-240, or JF-240(Olympus, Tokyo, Japan). Duodenoscope was used for endoscopy, and third-generation visualization was achieved with a contrast agent (iodide injection, Guerbet).
Angiography was performed after selective intubation to understand the nature of the biliary and pancreatic duct lesions with different processing methods. For cases with a combination of clinical circumstances, such as a diagnosis of common bile duct stones in the lining of the duodenal papilla sphincter incision (EST), operations such as balloon lithotomy were performed on the stones; in cases of inflammatory bile duct stenosis or ampullary tumors, endoscopic biliary stent implantation (ERBD), or nasobiliary drainage (ENBD) procedure was performed. For Oddi sphincter dysfunction, a duodenal papillary sphincterotomy was performed.
Postoperative treatment included fasting for 24 h after surgery, acid inhibition, rehydration, other symptomatic treatments, and if needed, antibiotic treatment. Abdominal pain, haematemesis, melena, fever, and other conditions were observed, and blood amylase tests and routine blood tests were performed at 3 and 24 h after surgery.
A total of 20,479 ERCPs were performed at our endoscopy center. A total of 45 cases with ERCP-related perforations (0.22%) were identified. The 45 cases with ERCP-related perforations (45/20,479) were identified by X-ray and/or duodenoscopy during the ERCP. The average age of the patients was 67 ± 12.6 years old (from 25 to 88 years old); the patients included 18 male patients and 27 female patients. The incidence of ERCP-related perforations was 0.22%. The demographical characteristics and clinical data of these patients are presented in Table 1.
Among the 45 perforations, 26 patients experienced peri-ampullary perforations, 15 patients experienced duodenal wall perforations, including 3 afferent limb perforations, and the other patients experienced peri-ampullary diverticulum perforations, small bile duct perforations on the liver surface, residual duct of gallbladder perforations, and fundus perforations. The fundus perforation and 15 duodenal wall perforations resulted from duodenoscopy; the peri-ampullary diverticulum perforation, the residual duct of gallbladder perforation, and five peri-ampullary perforations resulted from the stone extraction basket; 15 peri-ampullary perforations resulted from papillotomy; 4 peri-ampullary perforations resulted from precut surgical methods; 2 peri-ampullary perforations resulted from balloon catheter dilation; and the small bile duct perforation on the liver surface resulted from the guide wire. The Stapfer types, scope, and etiology of perforations are listed in Table 2.
All ERCP-related perforations had been diagnosed during the ERCP procedure. The presentation of a retroperitoneal perforation showed skin emphysema and a clear kidney shadow in fluoroscopy X-ray even individual cases developing pneumoscrotum (the gas could reach the scrotum by tracking along the transversalis fascia, which forms the innermost covering layer of the spermatic cord) (11), and the presentation of a peritoneal perforation showed a free gas shadow under the diaphragm in fluoroscopy X-ray, a visible gastrointestinal wall lesion under the endoscope, and signs of peritonitis. Most duodenal wall perforations had signs of peritoneal perforations and most peri-ampullary perforations had signs of retroperitoneal perforations. However, two peri-ampullary perforations had both intraperitoneal and retroperitoneal perforation manifestations, and a duodenal perforation and peri-ampullary diverticulum perforation had only retroperitoneal perforation manifestations (Figures 1, 2).
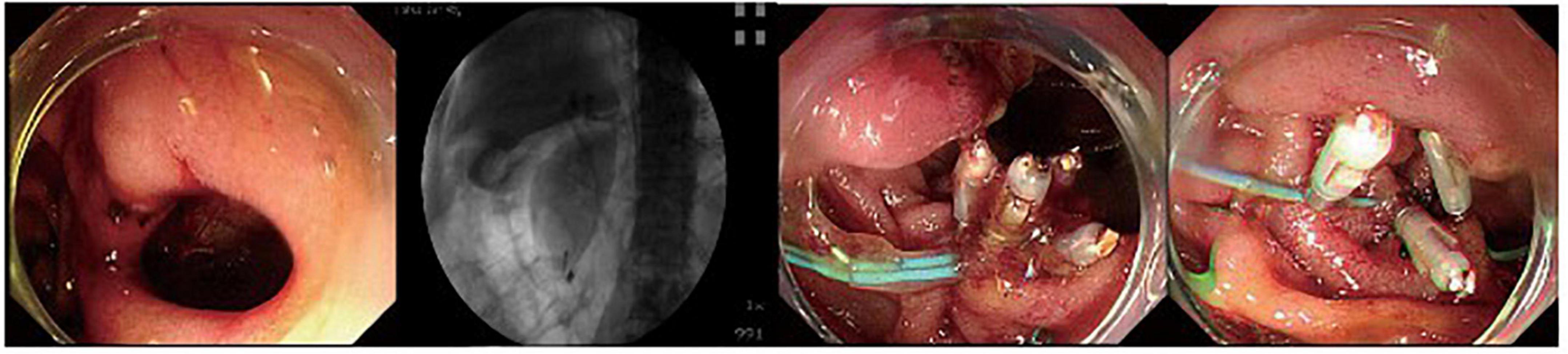
Figure 1. A duodenal wall perforation closed by clips and nylon rope under a single cavity forward-viewing endoscope. The perforation had only signs of retroperitoneal perforation.
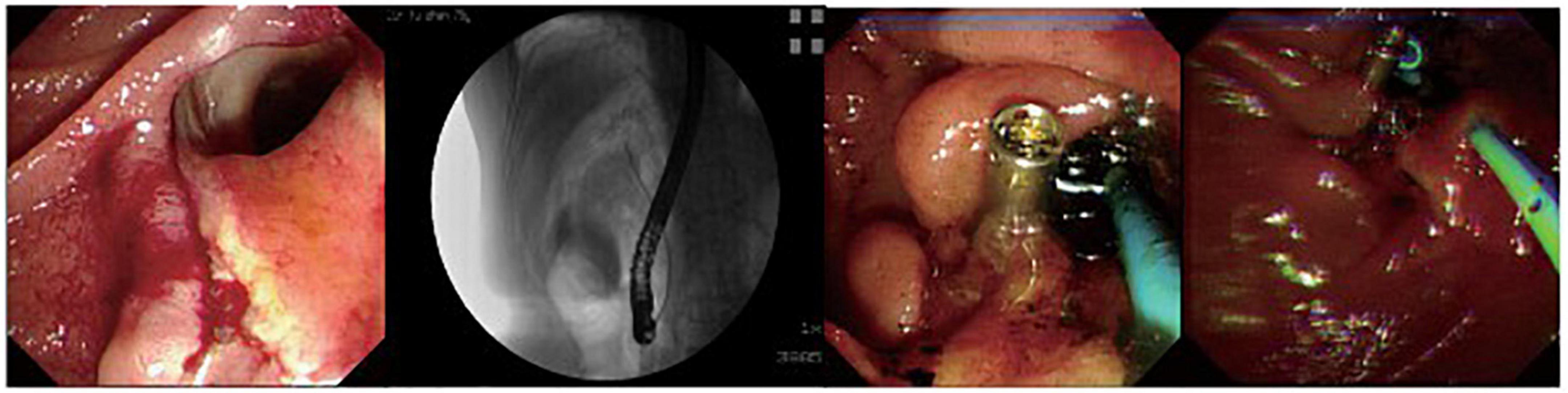
Figure 2. A patient with peri-ampullary perforation received ERBD and ERPD. The perforation had signs of retroperitoneal and peritoneal perforation.
The fundus perforation, the peri-ampullary diverticulum perforation, and eight duodenal perforations were treated by closing the lesion, performing endoscopic nasobiliary drainage (ENBD) or endoscopic retrograde biliary drainage (ERBD), conducting gastrointestinal decompression, and using proton pump inhibitor (PPI), somatostatin (SS), and broad-spectrum antibiotics for 5–7 days. Each lesion was closed by clips, purse string sutures, or over-the-scope-clip (OTSC) (Figures 1–3). Three afferent limb perforations and three duodenal wall perforations were treated through surgery. The small bile duct perforation on the liver surface, the residual duct of gallbladder perforation, and the 26 peri-ampullary perforations were healed through nasobiliary drainage or biliary stenting drainage, gastrointestinal decompression, and using PPI, SS, and broad-spectrum antibiotics for 5–7 days. Biliary stents are typically 8.5 Fr × 7 cm in size, whereas pancreatic stents are typically 5 Fr × 5 cm in size. If no unusual conditions exist, the stents will be removed after 1 month of satisfactory drainage. Three patients received endoscopic retrograde pancreatic drainage (ERPD) at the same time. The 81-year-old female patient with a duodenal wall perforation, which had been closed with OTSC, died of heart failure and post-ERCP pancreatitis 3 days after the ERCP procedure. The other patients recovered successfully (Figure 4). Management outcomes of the 45 patients were summarized in Table 3. The mortality was 2.2% (1/45).
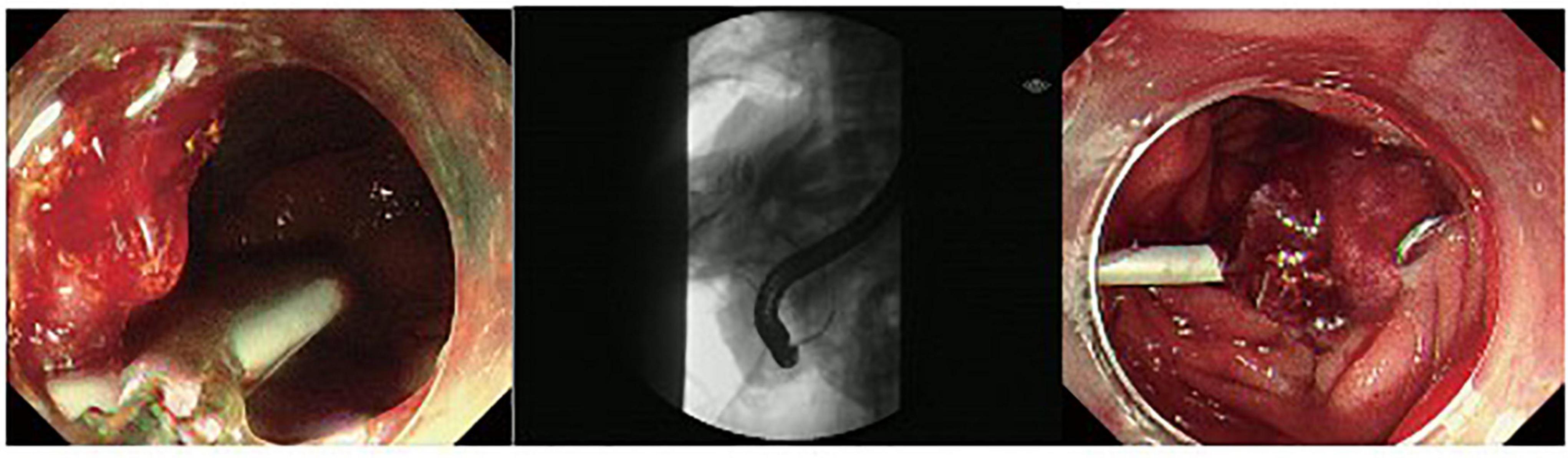
Figure 3. A duodenal wall perforation closed by OTSC. The patient received ERBD. The perforation per had signs of peritoneal perforation.
Perforation related to ERCP is the most serious complication to avoid because it can potentially threaten the life of patients. Consequently, studies regarding this complication should be necessary; however, there have been only a few reports with a limited number of cases, mainly owing to its rarity. Table 4 is a compilation of relevant material produced since the 20th century (a MEDLINE search was performed from 2000–2022 using the keywords “perforation”, “ERCP”, and “endoscopic sphincterotomy”). Reviewing 17 studies, including 140,588 patients, the incidence was 0.33% (95% CI: 0.30–0.36). The overall mortality was 8.8% (95% CI: 6.21–11.35). According to Stapfer classification, type I counted 27.6%, type II counted 47.5%, and type III counted 19.1%. In our study, 16 perforations resulted from duodenoscopy injuries, 15 peri-ampullary perforations resulted from papillotomy, 4 peri-ampullary perforations resulted from precut surgical methods, 7 perforations resulted from inserting the basket into the common bile duct (CBD) after papillotomy while removing the stone, 2 peri-ampullary perforations resulted from papilla balloon dilation, and 1 resulted from the guide wire passing through the liver surface. The mechanism of injury is mentioned in 573 patients from 20 studies (Table 5). Endoscopic sphincterotomy was responsible for 41.1% of perforations, insertion, and manipulations of the endoscope for 25.2%, guide wires for 14.6%, dilation of strictures for 2.9%, other instruments for 6.8%, stent insertion or migration for 1.9%, and in 7.6% of cases, the etiology was unknown. This showed that endoscopic sphincterotomy (EST) was the most prevalent cause of ERCP-related perforations, followed by endoscopic insertion and guide wire.
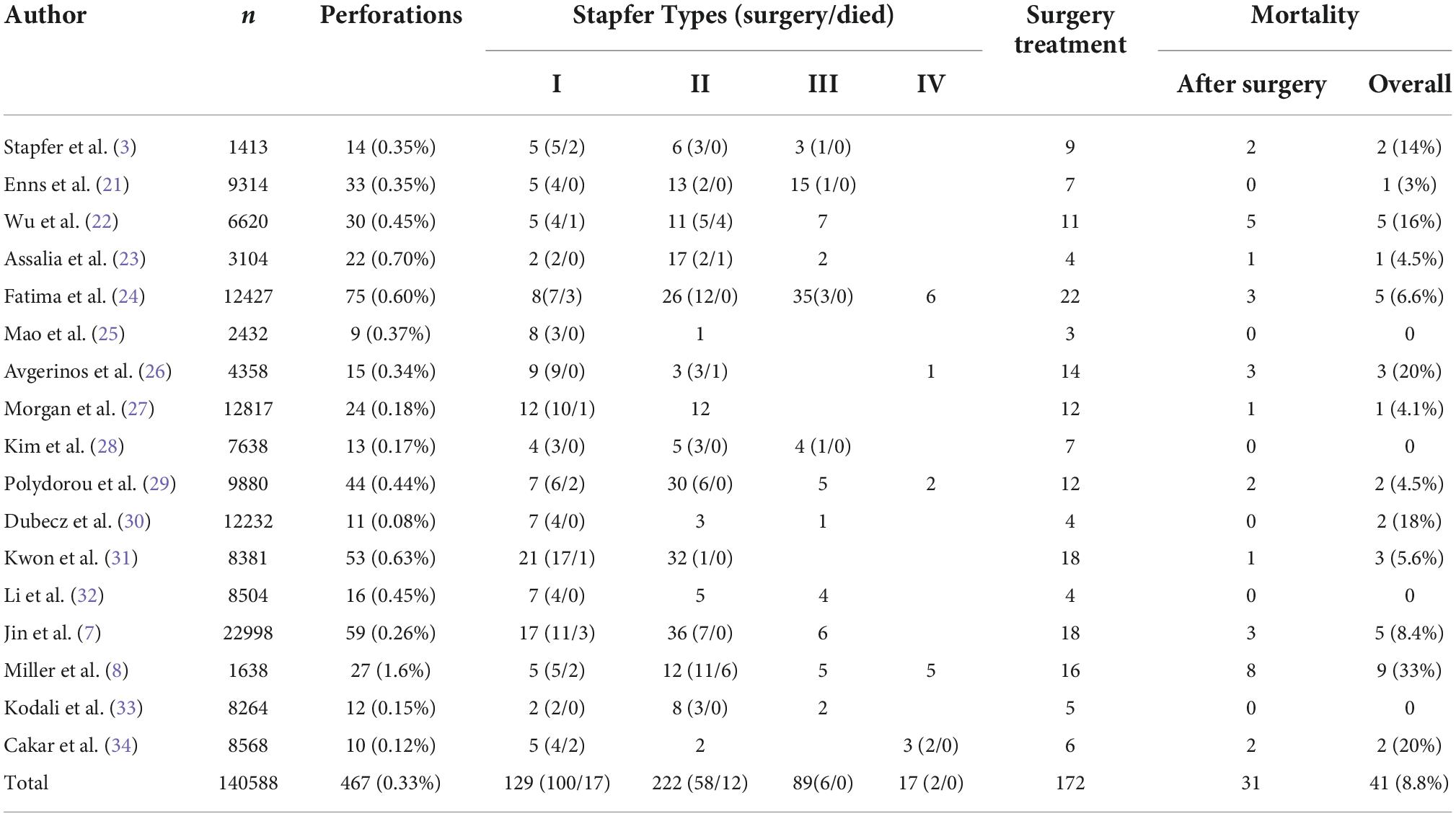
Table 4. In 17 papers, the incidence of ERCP-related perforation, overall mortality, and treatment were reviewed.
According to the AGA 2021 updated expert review, delayed recognition of a perforation more than 6 h after ERCP is associated with an increased length of hospital stay and mortality and may result in a more complicated surgical intervention (5, 7, 9). Thus, the early diagnosis of the complication is very important. We should especially note the signs of retroperitoneal and peritoneal perforations (12). In our study, all perforations were diagnosed during the ERCP procedure by X-ray fluoroscopy and/or endoscopy. We typically performed fluoroscopy for each patient before and after the ERCP procedure to determine whether a perforation occurred during the procedure. This habit helped us to diagnose these perforations early, which may be the reason for a lower mortality rate in our study.
After the recognition of an ERCP-related perforation, the first dilemma is conservative treatment or surgery (6, 13, 14). A total of 172 of the 467 perforations underwent surgery, with 31 deaths, for a surgical rate of 36.8%, and a postoperative mortality rate of 18%, with surgical deaths accounting for 75.6% of total deaths. Non-operative therapy was given to 295 patients, with 10 fatalities and a mortality rate of 3.4%, accounting for 24.3% of all deaths.
For Stapfer type I perforations, there were 117 cases in the 17 papers we reviewed. Of these cases, 100 underwent surgery and 17 died. The postoperative mortality rate of 17% remains still high. In our study, one fundus perforation and nine duodenal perforations (including one peri-ampullary diverticulum perforation) were closed successfully by clips, purse string sutures, or OTSC and treated by conservative treatment. Seven duodenal perforations (including three afferent limb perforations) were treated by surgery. An 81-year-old female patient with COPD and coronary heart disease had a duodenal perforation, which was closed by OTSC, and ERCP-related pancreatitis and died of heart failure 3 days after the ERCP procedure. The other patients recovered successfully. This outcome is consistent with the AGA expert review’s conclusion that for patients who do undergo successful endoscopic closure, the chance of clinical successful recovery without surgery is > 90% (5, 15). Therefore, we recommend endoscopic closure is seen as the first method for treating Stapfer type I perforations in the early phase, and surgery is seen as a remedial method when local treatment fails (16, 17).
For Stapfer type II perforations, most were successfully treated conservatively, with only 58 cases undergoing surgery and 12 postoperative deaths. The operative rate was 26.1% and the postoperative mortality rate was 20.7%. In our study, all peri-ampullary perforations in the early phase recovered successfully with conservative treatment, including nasobiliary or biliary stenting drainage, gastrointestinal decompression, fasting, intravenous nutrition, and using PPI, SS, and broad-spectrum antibiotics for 5–7 days. Our experiences suggest that these peri-ampullary perforations could recover with these conservative treatment methods in the early phase, which may be because (i) the peri-ampullary perforations were small perforations; and (ii) conservative management in the early phase could alleviate the stimulation and secretion of gastric acid, bile, and pancreatic liquid. This result was in line with the recent statement by ESGE and AGA that a majority of patients with Stapfer type II perforations can be managed non-surgically, with emergency surgery indicated only in rare cases where a major contrast leakage is insufficiently sealed (2, 5).
The perforations that are classified as Stapfer types III and IV should be treated by conservative treatment because all patients with type III or type IV perforations recovered by conservative treatment in recent reports (8–10, 12, 15, 18–20). Moreover, effective therapy should also include preventing or treating infections using broad-spectrum antibiotics.
In our study, we have minimal experience treating ERCP-related perforations in the late phase, which can present large fluid exudation and infection in the retroperitoneal space and peritoneal cavity. According to the statement issued by ESGE in 2020, regional management of drained collections is required. This can be performed through percutaneous access or during surgery, which also allows the evacuation of debris (2). However, the statement does not specify which patients require surgery and when these patients should receive surgery. In general, a surgical operation might increase the risk of trauma or death and should be applied cautiously. When a large lesion could not be closed or the fluid exudation and infection in the retroperitoneal space and peritoneal cavity could not be adequately drained, surgery may be necessary (16, 17). In addition, percutaneous drainage merits additional investigation.
Endoscopic sphincterotomy (EST) was the most prevalent cause of ERCP-related perforations, followed by endoscopic insertion and guide wire. Endoscopic closure is seen as the first method for treating Stapfer type I perforations in the early phase, and surgery is seen as a remedial method when local treatment fails. Patients with Stapfer type II to type IV perforations could recover by undergoing conservative treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of The First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Y-SM and Y-YL designed the study, performed the data analysis, wrote the manuscript, and interpreted the results. X-JZ, Y-XC, and SZ extracted the data and revised the manuscript. N-HL and Y-FZ designed the study and interpreted the results. G-HL and B-WC were involved in intellectual content, designed the study, and interpreted the results. All authors read and approved the final manuscript.
This study was supported by Science and Technology Plan of Health and Family Planning Commission, Jiangxi, China (number: 20185079) and Science and Technology Research Project of Education Department of Jiangxi Province (number: GJJ200104).
The content of this manuscript has been presented in part at the ESGE and AGA statements (2, 5).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. (2007) 102:1781–8. doi: 10.1111/j.1572-0241.2007.01279.x
2. Paspatis GA, Arvanitakis M, Dumonceau J-M, Barthet M, Saunders B, Turino SY, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) position statement–update 2020. Endoscopy. (2020) 52:792–810. doi: 10.1055/a-1222-3191
3. Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. (2000) 232:191–8. doi: 10.1097/00000658-200008000-00007
4. Nakamura K, Yamaguchi Y, Hasue T, Higa K, Tauchi M, Toki M, et al. The usefulness and safety of carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography in elderly patients: a prospective, double-blind, randomized, controlled trial. Hepatogastroenterology. (2014) 61:2191–5.
5. Lee JH, Kedia P, Stavropoulos SN, Carr-Locke DJCG. AGA clinical practice update on endoscopic management of perforations in gastrointestinal tract: expert review. Clin Gastroenterol Hepatol. (2021) 19:2252–61. doi: 10.1016/j.cgh.2021.06.045
6. Vezakis A, Fragulidis G, Polydorou A. Endoscopic retrograde cholangiopancreatography-related perforations: diagnosis and management. World J Gastrointest Endosc. (2015) 7:1135–41. doi: 10.4253/wjge.v7.i14.1135
7. Jin Y-J, Jeong S, Kim JH, Hwang JC, Yoo BM, Moon JH, et al. Clinical course and proposed treatment strategy for ERCP-related duodenal perforation: a multicenter analysis. Endoscopy. (2013) 45:806–12. doi: 10.1055/s-0033-1344230
8. Miller R, Zbar A, Klein Y, Buyeviz V, Melzer E, Mosenkis BN, et al. Perforations following endoscopic retrograde cholangiopancreatography: a single institution experience and surgical recommendations. Am J Surg. (2013) 206:180–6. doi: 10.1016/j.amjsurg.2012.07.050
9. Kumbhari V, Sinha A, Reddy A, Afghani E, Cotsalas D, Patel YA, et al. Algorithm for the management of ERCP-related perforations. Gastrointest Endosc. (2016) 83:934–43. doi: 10.1016/j.gie.2015.09.039
10. Artifon EL, Minata MK, Cunha MAB, Otoch JP, Aparicio DP, Furuya CK, et al. Surgical or endoscopic management for post-ERCP large transmural duodenal perforations: a randomized prospective trial. Rev Gastroenterol Peru. (2017) 35:313–7.
11. Milone M, Manigrasso M, Di Lauro K, Manzo B, Maione F, Milone F, et al. Pneumoscrotum after ERCP-related duodenal perforation. Endoscopy. (2016) 48(S 01):E295. doi: 10.1055/s-0042-116428
12. Keswani RN, Qumseya BJ, O’Dwyer LC, Wani SJCG. Association between endoscopist and center endoscopic retrograde cholangiopancreatography volume with procedure success and adverse outcomes: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2017) 15:1866–75. doi: 10.1016/j.cgh.2017.06.002
13. Alfieri S, Rosa F, Cina C, Tortorelli AP, Tringali A, Perri V, et al. Management of duodeno-pancreato-biliary perforations after ERCP: outcomes from an Italian tertiary referral center. Surg Endosc. (2013) 27:2005–12. doi: 10.1007/s00464-012-2702-9
14. Machado NO. Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP. (2012) 13: 18–25.
15. Park SMJCE. Recent advanced endoscopic management of endoscopic retrograde cholangiopancreatography related duodenal perforations. Clin Endosc. (2016) 49:376–82. doi: 10.5946/ce.2016.088
16. Booth FVM, Doerr RJ, Khalafi RS, Luchette FA, Flint LM Jr. Surgical management of complications of endoscopic sphincterotomy with precut papillotomy. Am J Surg. (1990) 159:132–6.
17. Cirocchi R, Kelly MD, Griffiths EA, Tabola R, Sartelli M, Carlini L, et al. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon. (2017) 15:379–87. doi: 10.1016/j.surge.2017.05.004
18. Ercan M, Bostanci EB, Dalgic T, Karaman K, Ozogul YB, Ozer I, et al. Surgical outcome of patients with perforation after endoscopic retrograde cholangiopancreatography. J Laparoendosc Adv Surg Tech A. (2012) 22:371–7. doi: 10.1089/lap.2011.0392
19. Dixon P, Kowdley GC, Cunningham SC. The role of surgery in the treatment of endoscopic complications. Best Pract Res Clin Gastroenterol. (2016) 30:841–51.
20. Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. (2016) 30:793–805. doi: 10.1016/j.bpg.2016.10.007
21. Enns R, Eloubeidi M, Mergener K, Jowell P, Branch M, Pappas T, et al. ERCP-related perforations: risk factors and management. Endoscopy. (2002) 34: 293–8.
22. Wu HM, Dixon E, May GR, Sutherland FRJH. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): a population-based review. HPB (Oxford). (2006) 8:393–9. doi: 10.1080/13651820600700617
23. Assalia A, Suissa A, Ilivitzki A, Mahajna A, Yassin K, Hashmonai M, et al. Validity of clinical criteria in the management of endoscopic retrograde cholangiopancreatography–related duodenal perforations. Arch Surg. (2007) 142:1059–64. doi: 10.1001/archsurg.142.11.1059
24. Fatima J, Baron TH, Topazian MD, Houghton SG, Iqbal CW, Ott BJ, et al. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: diagnosis and management. Arch Surg. (2007) 142:448–55. doi: 10.1001/archsurg.142.5.448
25. Mao Z, Zhu Q, Wu W, Wang M, Li J, Lu A, et al. Duodenal perforations after endoscopic retrograde cholangiopancreatography: experience and management. J Laparoendosc Adv Surg Tech A. (2008) 18:691–5. doi: 10.1089/lap.2008.0020
26. Avgerinos DV, Llaguna OH, Lo AY, Voli J, Leitman IM. Management of endoscopic retrograde cholangiopancreatography: related duodenal perforations. Surg Endosc. (2009) 23:833–8. doi: 10.1007/s00464-008-0157-9
27. Morgan KA, Fontenot BB, Ruddy JM, Mickey S, Adams DBJTAS. Endoscopic retrograde cholangiopancreatography gut perforations: when to wait! When to operate! Am Surg. (2009) 75:477–84.
28. Kim BS, Kim I-G, Ryu BY, Kim JH, Yoo KS, Baik GH, et al. Management of endoscopic retrograde cholangiopancreatography-related perforations. J Korean Surg Soc. (2011) 81:195–204. doi: 10.4174/jkss.2011.81.3.195
29. Polydorou A, Vezakis A, Fragulidis G, Katsarelias D, Vagianos C, Polymeneas G. A tailored approach to the management of perforations following endoscopic retrograde cholangiopancreatography and sphincterotomy. J Gastrointest Surg. (2011) 15:2211–7. doi: 10.1007/s11605-011-1723-3
30. Dubecz A, Ottmann J, Schweigert M, Stadlhuber RJ, Feith M, Wiessner V, et al. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg. (2012) 55:99. doi: 10.1503/cjs.027110
31. Kwon W, Jang J-Y, Ryu JK, Kim Y-T, Yoon YB, Kang MJ, et al. Proposal of an endoscopic retrograde cholangiopancreatography-related perforation management guideline based on perforation type. J Korean Surg Soc. (2012) 83:218–26. doi: 10.4174/jkss.2012.83.4.218
32. Li G, Chen Y, Zhou X, Lv N. Early management experience of perforation after ERCP. Gastroenterol Res Pract. (2012) 2012:657418. doi: 10.1155/2012/657418
33. Kodali S, Monkemuller K, Kim H, Ramesh J, Trevino J, Varadarajulu S, et al. ERCP-related perforations in the new millennium: A large tertiary referral center 10-year experience. United Eur Gastroenterol J. (2015) 3:25–30. doi: 10.1177/2050640614560784
34. Cakar E, Gurbulak B, Colak S, Duzkoylu Y, Bektas U. ERCP-Related perforations: treatment options, conservative approach and timing of surgery. Acta Medica Mediterr. (2019) 35:3063–70.
35. Kayhan B, Akdoǧan M, Şahin B. ERCP subsequent to retroperitoneal perforation caused by endoscopic sphincterotomy. Gastrointest Endosc. (2004) 60:833–5. doi: 10.1016/s0016-5107(04)02171-6
36. Knudson K, Raeburn CD, McIntyre RC Jr., Shah RJ, Chen YK, Brown WR, et al. Management of duodenal and pancreaticobiliary perforations associated with periampullary endoscopic procedures. Am J Surg. (2008) 196:975–82. doi: 10.1016/j.amjsurg.2008.07.045
37. Krishna RP, Singh RK, Behari A, Kumar A, Saxena R, Kapoor VKJST. Post-endoscopic retrograde cholangiopancreatography perforation managed by surgery or percutaneous drainage. Surg Today. (2011) 41:660–6. doi: 10.1007/s00595-009-4331-z
Keywords: endoscopic retrograde cholangiopancreatography (ERCP), evaluation, perforation, strategy, duodenal perforations
Citation: Miao Y-S, Li Y-Y, Cheng B-W, Zhan Y-F, Zeng S, Zhou X-J, Chen Y-X, Lv N-H and Li G-H (2022) Clinical analysis of 45 cases of perforation were identified during endoscopic retrograde cholangiopancreatography procedure. Front. Med. 9:1039954. doi: 10.3389/fmed.2022.1039954
Received: 08 September 2022; Accepted: 02 November 2022;
Published: 24 November 2022.
Edited by:
Muhammad Aziz, University of Toledo Medical Center, United StatesReviewed by:
Martin Freeman, University of Minnesota Twin Cities, United StatesCopyright © 2022 Miao, Li, Cheng, Zhan, Zeng, Zhou, Chen, Lv and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Hua Li, bGlndW9odWE5OEBzb2h1LmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.