
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 08 December 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1036793
This article is part of the Research TopicReviews in GastroenterologyView all 10 articles
Enteral nutrition (EN) is a diet-remission therapy for inflammatory bowel disease (IBD) that plays a more important role in children than adults. EN includes exclusive enteral nutrition (EEN), partial enteral nutrition (PEN), and maintenance enteral nutrition (MEN). However, EEN remains an unstandardized treatment for pediatric IBD. The types and methods of EN differ around the world. The current study reviewed the EN literature on children with IBD. A total of 12 survey studies were identified that analyzed the current state of EN use, including clinical opinions, implementation methods, treatment course, EEN formula, IBD classification, progress, dietary reintroduction, and patient feedback. The findings revealed that EEN has a strong effect on mild to moderate Crohn’s disease (CD). The usage rates of this treatment in different sites were ileum/colon (Paris classification L3) > ileum (L1) > upper digestive tract (L4) > colon (L2) > perianal disease (P) > ulcerative colitis (UC) > extraintestinal lesions. The polymeric formula was the most used EN formulation. New EN diets include a CD exclusion diet (CDED), a specific carbohydrate diet (SCD), and a CD treatment-with-eating (CD-TREAT) diet. Children with IBD responded similarly to EEN administered orally or using a feeding tube. Most guidelines recommended 6–8 weeks of EEN treatment to induce remission. Many clinicians preferred to combine drug medications during EEN and recommended that MEN accounts for at least 25–35% of daily caloric intake. EN remains an unstandardized therapy that requires teamwork across disciplines.
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD), ulcerative colitis (UC), and unclassified inflammatory bowel disease (IBD-U). Enteral nutrition (EN), which includes exclusive enteral nutrition (EEN), partial enteral nutrition (PEN), and maintenance enteral nutrition (MEN), is a food-induced therapy for IBD remission. Due to its safety profile, this treatment is commonly used in children. EEN is recommended as the first line of treatment for CD remission, especially among children with active luminal CD (1). This review summarizes the results of 12 survey studies and provides an update on the global status of EN use for pediatric IBD to standardize the treatment.
PubMed, Embase, Cochrane Library, CNKI, and CBM databases were searched for global studies on EN treatment of pediatric IBD. Studies published from the establishment of each database to December 2021 were included in the search. A combination of subject headings and free words, including inflammatory bowel disease, Crohn’s disease, ulcerative colitis, enteral nutrition, and children, were used as the search terms. Studies were included if the subjects were children with IBD who were ≤18 years of age, the intervention included EN, the outcome measures included patient attitudes and implementation of EEN among pediatric patients with IBD, and the study was survey-based. Studies were excluded if they were duplicate reports, articles from which the original text could not be obtained, or articles lacking the required information. Two researchers independently screened the literature and cross-checked the data. Disagreements were resolved by discussion or consultation with a third party. Literature screening was performed by first reading the title and excluding irrelevant literature. Further reading of the abstract and full text was then performed to determine whether the study should be included. If necessary, the original authors were contacted by mail or telephone to obtain unidentified information. Several variables were extracted, including research title, first author, publication journal, publication period, survey country or region, and key results. A total of 1,243 relevant studies were obtained during the initial inspection. After a layer-by-layer screening, 12 survey studies were considered highly relevant and selected for further analysis (Study flow diagram in Figure 1). These studies reflected the implementation status of pediatric EN, including regional variation, time evolution, clinical opinions, and patient attitudes. Analysis results of the 12 studies (ART12Q) are summarized in Table 1.
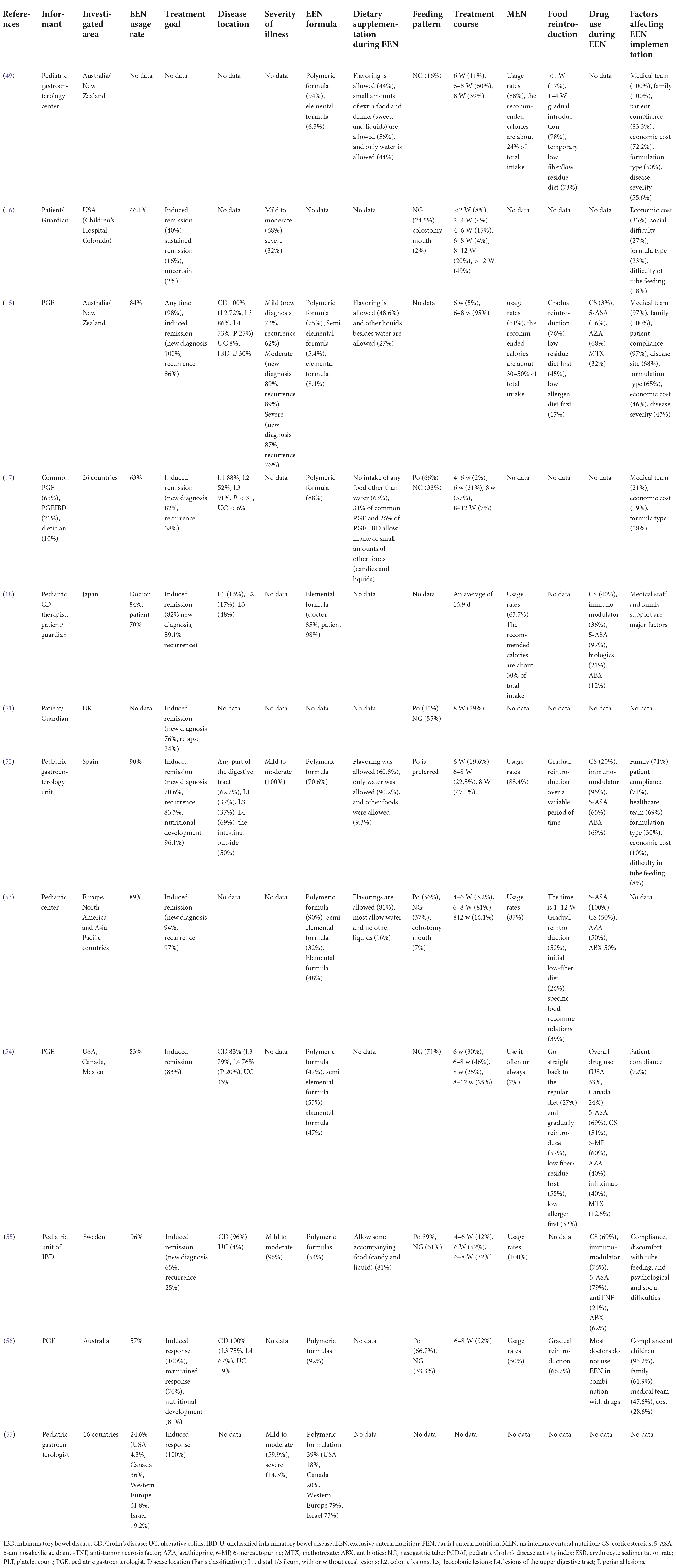
Table 1. Global questionnaire survey on exclusive enteral nutrition (EEN) implementation of inflammatory bowel disease (IBD) children.
Exclusive enteral nutrition is effective at inducing the remission of intraluminal CD (1); however, only a few studies recommend the use of this treatment for active perianal lesions and pediatric UC (2), and the supporting data are insufficient. There is also little evidence to support the use of EEN for isolated extra-gastrointestinal lesions and isolated oral lesions (3). ART12Q found that the use of EEN differed by lesion site, with ileal/colonic lesions > ileal lesions > upper gastrointestinal lesions > colonic lesions > perianal lesions. However, EEN-induced remission is not significantly associated with the lesion site (1), thus it is not necessary to consider this variable in the treatment of children with CD.
Exclusive enteral nutrition is primarily used to induce IBD remission. Some meta-analyses and prospective studies have shown that EEN is as effective as corticosteroids (4, 5) and biologics (infliximab) (6) at promoting pediatric CD remission (7–10), and more effective than corticosteroids at inducing mucosal healing (11, 12). Frivolt et al. reported a 92% response rate after the first course of EEN therapy. In several retrospective studies, the rate of remission was 58.3–80% after the second course (13). ART12Q found that most clinicians agreed that EEN was effective at inducing the remission of new-onset disease and used this treatment to induce the remission of recurrent cases in some areas (Figure 2A). Indeed, the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) (14) concluded that EEN treatment can be revisited in cases of recurrence. While EEN is effective at maintaining remission (3), ART12Q found that it was not widely adopted by doctors (Figure 2A) and that patient compliance was extremely low. A total of 11 studies reported the rate of recurrence after EEN-induced remission (14), and these values ranged from 2 to 67% within 1 year and 58–68% within 2 years with a median recurrence time of 6.5–12.7 months. Thus, EEN is recommended as the first-line therapy for inducing remission of newly diagnosed CD in children and is suggested for use in treating recurrent cases and maintaining remission as needed.
Analysis results of the 12 studies found that most pediatricians approved the efficacy of EEN to treat mild-moderate IBD, but showed that EEN efficacy against severe CD was relatively low (Figure 2B). ESPGHAN (14) identified that EEN could promote the mucosal healing of pediatric CD and transmural healing in some patients. EEN was also shown to have a partial effect on severe penetrating injury associated with pediatric CD. The use of EEN treatment for severe CD has gradually increased, which may be related to the low compliance of pediatric patients with mild-to-moderate diseases (15) and a change in clinician attitudes toward the treatment.
The duration of EEN treatment varied from <2 weeks to >12 weeks in different countries (16), with treatment in North America > Western Europe (17) > Japan, where the duration was only 2 weeks ± (18) (Figure 2D). Clinical symptoms usually began to resolve several days after initiating EEN, and the median time to clinical remission was 11 days to 2.5 weeks (19). Inflammatory markers were usually reduced in 1 week (19), while improvement in inflammation and nutrition took several weeks. Endoscopic and histological studies also showed that mucosal healing required about 8 weeks of EEN (9, 20). Thus, the 2020 consensus guideline of the European Crohn’s and Colitis Organization (ECC) and ESPGHAN (1) recommended 6–8 weeks of treatment for EEN-induced remission.
Exclusive enteral nutrition is significantly better at relieving symptoms in children with active CD than PEN (6, 21) and is associated with a stronger decline in the pediatric CD activity index (PCDAI) than PEN (47% of total energy) after 6 weeks of treatment (22). More EN consumption was also associated with a higher remission rate in adults (23, 24). Thus, the stringency of execution correlates with the efficacy of EEN. ART12Q showed that EEN strictness varied by country. Some minor reforms were made to improve the compliance of children, including adding flavorings to reduce taste fatigue, creating high-energy-density formulas with small volumes, and permitting the consumption of small amounts of other beverages.
Partial enteral nutrition is not often used alone to induce remission but can supplement the induction of remission or be used in patients with mild disease and a low risk of recurrence. Sigall-Boneh et al. reported that a 50% PEN diet plus a structured exclusion diet was associated with a 70% remission rate in children with mild-to-moderate CD (25). A retrospective study by Wilschanski et al. found that consumption of a normal diet during the day and PEN at night (through continuous nasogastric tube feeding) could prolong remission and improve linear growth (26). Thus, PEN is a useful substitute for inducing remission in children with mild-to-moderate CD who cannot strictly adhere to EEN (27).
Exclusive enteral nutrition formulations include element formula (EF; amino acid type), semi-element formula (SEF; oligopeptide type), and polymeric formula (PF; integral protein type). ART12Q found that PF, at a concentration of 1 kcal/ml, was the most used. While clinicians in Western Europe, Oceania, and Israel all prefer PF, doctors and patient families in Japan are willing to adopt EF. However, meta-analyses (28) and clinical research studies (8, 29) found no difference in the efficacy of EF, SEF, and PE in treating CD. There was also no evidence that dietary protein sources would affect treatment success. Thus, except for special cases, such as patients with a milk protein allergy, standard PF with a moderate fat content is recommended by ESPGHAN (14) due to its palatability and low cost.
The exclusive enteral nutrition formula is designed to reduce the complex interaction between diet and host immunity. However, different formulations, including low-fat, high-fat; supplementation with medium-chain triglycerides (MCT) (30), monounsaturated fatty acids (MUFA) (31); or anti-inflammatory substances [glutamine (32), transforming growth factor-β (33), and omega-3 (34)] are not found to cause significant clinical improvements. The addition of probiotics, prebiotics, and dietary fiber also requires further verification using randomized controlled trials (RCT). A specific carbohydrate diet (SCD) (35) was shown to have therapeutic value in treating IBD, but whether excessive carbohydrate levels are beneficial to children remains to be determined. While CDED (36) and CD-TREAT formulations are designed to mimic EEN by excluding certain components found in common foods (37), these are still immature protocols. For example, the low fermentable oligo-, di-, monosaccharide, and polyol (FODMAP) diet was shown to be effective against adult IBD but has not been fully studied in children (38, 39). In addition, the lactose-free diet (LFD) (40) may cause vitamin D deficiency and low calcium. The paleolithic, vegan, gluten-free, and food-specific IgG4 antibody-guided exclusion diets all had some effect on IBD (36), but no substantial progress in their development has been made. Since exclusion/restrictive diets may affect nutrition, psychology, and quality of life, ESPGHA does not recommend them for the treatment of children and adolescents with IBD, unless the potential benefits are higher than the risks. Research on novel formulations is promising, but findings will need to be verified by adequate RCT.
Adherence is the biggest issue associated with EEN, especially with the poor-tasting EF and SEF formulas. Feeding through a nasogastric tube (NG) or gastrointestinal stoma is often used to ensure adequate intake. While retrospective studies found no difference in the efficacy of EEN between oral and tube feeding (7), oral intake of <120% of the total daily calorie requirements may affect EEN effectiveness (41). Tube feeding may be more effective in adults because they are less receptive to single-taste diets than children, who still lack experience with rich flavors (7). ART12Q found that most children with IBD choose oral administration, potentially because of taste improvements in EEN formulations. Thus, ESPGHAN has recommended attempting oral administration first and then transitioning to NG feeding if oral intake remains inadequate.
Analysis results of the 12 studies showed that most gastroenterologists believe that combining drugs such as 5-ASA, 6-MP, AZA, CS, or infliximab with EEN achieves better remission and often prescribe these combinations for their patients. During glucocorticoid-induced remission, the early introduction of immunomodulators is beneficial for the maintenance of remission in patients with moderate to severe CD (42). However, the clinical benefits of early drug combinations during EEN-induced remission have not been confirmed. In addition, side effects, such as nausea, that are associated with immunomodulators may adversely affect EEN treatment.
Both invasive and non-invasive methods are used to evaluate the efficacy of EEN to induce remission. Endoscopic evaluation following EEN-induced remission can help achieve mucosal healing, reduce the risk of long-term complications (1), and extend the remission period to 3 years (43). However, ART12Q found that most clinicians still use non-invasive indicators to evaluate EEN efficacy, including clinical PCDAI score, CRP, erythrocyte sedimentation rate (ESR), fecal calprotectin, nutrition score, blood cell count, biochemical indicators, and imaging. Invasive evaluations such as endoscopy and biopsy are only used in about 50% of cases. A comprehensive score combining fecal calprotectin, clinical score, and CRP is currently considered the most suitable non-invasive evaluation method for pediatric CD (1). While an evaluation of EEN induced-remission is typically recommended after 6–8 weeks, many medical centers suggest evaluating its effects after 2–3 weeks.
Analysis results of the 12 studies found that most medical centers gradually introduced low-fat, low-fiber, and low-allergen foods after EEN-induced remission. A retrospective study showed that the rate of recurrence and the maintenance of remission at 1 year was similar regardless of whether the food was reintroduced within 5 weeks or 3 days (44). An exclusion diet guided by food-specific antibodies appears to help maintain EEN-induced remission (45). While food intolerance was not common after the reintroduction of conventional foods, the necessity of low-allergen foods was not confirmed (46). Since most EEN formulas do not contain fiber (44), many doctors recommend a short-term low-fiber diet for the reintroduction of food to children; however, there is little evidence to support this. Given the lack of data required to form a standard plan for food reintroduction, ESPGHAN (14) recommends gradually reintroducing regular foods and reducing EEN use within 2–3 weeks. Fiber restriction is not suggested for children with IBD who have no evidence of gastrointestinal stenosis.
Either MEN or PEN treatment is usually initiated after EEN-induced remission. MEN was developed to maintain remission, improve nutrition, and promote growth and weight gain. ART12Q found that PF was the most used MEN formulation, and almost all dietitians used dietary energy reference values to estimate pediatric energy requirements (47). Gkikas et al. reported that MEN, which accounts for 35% of the daily energy requirement, is sufficient to improve clinical remission (48). ART12Q found that 89% of nutritionists recommend MEN to fulfill 25–30% of their daily energy needs. However, the use of MEN after EEN has not been recommended as a standard protocol, especially in children without malnutrition. The optimal time for MEN treatment is also unclear. Some dietitians suggest using this therapy for as long as possible, while others suggest stopping treatment when maintenance drugs start to show effect, growth is stable and appropriate, and an ideal weight has been reached (49) (Figure 2C).
While EEN is associated with minimal side effects, nausea, vomiting, diarrhea, abdominal distension, and abdominal discomfort can occur (2). Clinicians need to be aware of the risk of refeeding syndrome in severely malnourished children (50). In this patient population, it is necessary to gradually reduce intake of the normal diet by about 25% of the resting caloric intake needed per day and slowly increase the volume and concentration of EEN over several days until electrolyte levels are balanced (14).
Analysis results of the 12 studies identified many barriers to the successful implementation of EN, including EEN exclusiveness, compliance of the children and their families, health care resources, and cost-effectiveness (Figure 2E). These issues can be resolved by establishing a standardized EN program, personalizing adjustment, assuring effective doctor–patient communication, and solving social EN restrictions. Most patients and families expect dietary guidance and psychological support to become an integral part of IBD treatment (10, 41). Thus, an ideal EN management model should include education and training as well as a complete management team that includes gastroenterologists, dietitians, psychologists, nursing staff, and social workers. While insurance reimbursement to the national health system and private companies will need to be improved to reduce the burden of IBD on families.
Enteral nutrition is a safe but underused treatment for children with IBD. However, there are still significant gaps in the global understanding and implementation of EN. This review evaluated recent survey studies and summarized the current status of EN treatment. The findings can be used to develop a standardized EN therapy for children with IBD.
Y-MX and JL designed the research, performed the research, analyzed the data, and wrote the manuscript. MW, J-GZ, and L-LH analyzed the data. All authors contributed to the article and approved the submitted version.
This research was funded by the key research and development project of the Sichuan Provincial Science and Technology Program (No. 2019YFG0165) and the Fundamental Research Funds for the Central Universities (SCU2022F4080).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. (2020) 15:jjaa161. doi: 10.1093/ecco-jcc/jjaa161
2. Wong S, Lemberg DA, Day AS. Exclusive enteral nutrition in the management of perianal Crohn’s disease in children. J Dig Dis. (2010) 11:185–8. doi: 10.1111/j.1751-2980.2010.00434.x
3. Sigall-Boneh R, Levine A, Lomer M, Wierdsma N, Allan P, Fiorino G, et al. Research gaps in diet and nutrition in inflammatory bowel disease. A topical review by D-ECCO working group [dietitians of ECCO]. J Crohns Colitis. (2017) 11:1407–19. doi: 10.1093/ecco-jcc/jjx109
4. Manguso MT, Coruzzo A, D’Armiento F, Romeo EF, Cucchiara S. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. (2006) 38:381–7. doi: 10.1016/j.dld.2005.10.005
5. Hojsak I, Pavić AM, Mišak Z, Kolaček S. Risk factors for relapse and surgery rate in children with Crohn’s disease. Eur J Pediatr. (2014) 173:617–21. doi: 10.1007/s00431-013-2230-1
6. Lee D, Baldassano RN, Otley AR, Albenberg L, Griffiths AM, Compher C, et al. Comparative effectiveness of nutritional and biological therapy in North American children with active Crohn’s disease. Inflamm Bowel Dis. (2015) 21:1786–93. doi: 10.1097/MIB.0000000000000426
7. Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Datab Syst Rev. (2018) 4:CD000542. doi: 10.1002/14651858.CD000542.pub3
8. Grogan JL, Casson DH, Terry A, Burdge GC, El-Matary W, Dalzell AM. Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: a double-blind randomized controlled trial with two years follow-up. Inflamm Bowel Dis. (2012) 18:246–53. doi: 10.1002/ibd.21690
9. Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther. (2011) 33:1332–9. doi: 10.1111/j.1365-2036.2011.04662.x
10. Levine A, Turner D, Pfeffer Gik T, Amil Dias J, Veres G, Shaoul R, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflamm Bowel Dis. (2014) 20:278–85. doi: 10.1097/01.MIB.0000437735.11953.68
11. Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. (2006) 4:744–53. doi: 10.1016/j.cgh.2006.03.010
12. Pigneur B, Lepage P, Mondot S, Schmitz J, Goulet O, Doré J, et al. Mucosal healing and bacterial composition in response to enteral nutrition vs steroid-based induction therapy-a randomised prospective clinical trial in children with Crohn’s disease. J Crohns Colitis. (2019) 13:846–55. doi: 10.1093/ecco-jcc/jjy207
13. Frivolt K, Schwerd T, Werkstetter KJ, Schwarzer A, Schatz SB, Bufler P, et al. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: predictors of efficacy and outcome. Aliment Pharmacol Ther. (2014) 39:1398–407. doi: 10.1111/apt.12770
14. Miele E, Shamir R, Aloi M, Assa A, Braegger C, Bronsky J, et al. Nutrition in pediatric inflammatory bowel disease: a position paper on behalf of the Porto inflammatory bowel disease group of the European society of pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:687–708. doi: 10.1097/MPG.0000000000001896
15. Ho S, Day AS. Exclusive enteral nutrition in children with inflammatory bowel disease: physician perspectives and practice. JGH Open. (2018) 3:148–53. doi: 10.1002/jgh3.12121
16. Mehta P, Pan Z, Furuta GT, Kim DY, de Zoeten EF. Parent perspectives on exclusive enteral nutrition for the treatment of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. (2020) 71:744–8. doi: 10.1097/MPG.0000000000002847
17. Lawley M, Wu JW, Navas-López VM, Huynh HQ, Carroll MW, Chen M, et al. Global variation in use of enteral nutrition for pediatric Crohn disease. J Pediatr Gastroenterol Nutr. (2018) 67:e22–9. doi: 10.1097/MPG.0000000000001946
18. Ishige T, Tomomasa T, Tajiri H, Yoden A. Japanese study group for pediatric Crohn’s disease. Japanese physicians’ attitudes towards enteral nutrition treatment for pediatric patients with Crohn’s disease: a questionnaire survey. Intest Res. (2017) 15:345–51. doi: 10.5217/ir.2017.15.3.345
19. Otley AR, Russell RK, Day AS. Nutritional therapy for the treatment of pediatric Crohn’s disease. Expert Rev Clin Immunol. (2010) 6:667–76. doi: 10.1586/eci.10.37
20. Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. (2000) 14:281–9. doi: 10.1046/j.1365-2036.2000.00707.x
21. Terry A, Grogan JL, Casson DH, Dalzell AM, El-Matary W. Tube feeding therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther. (2011) 34:260–1. doi: 10.1111/j.1365-2036.2011.04720.x
22. Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. (2006) 55:356–61. doi: 10.1136/gut.2004.062554
23. Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther. (2006) 24:1333–40. doi: 10.1111/j.1365-2036.2006.03120.x
24. Verma S, Kirkwood B, Brown S, Giaffer MH. Oral nutritional supplementation is effective in the maintenance of remission in Crohn’s disease. Dig Liver Dis. (2000) 32:769–74. doi: 10.1016/s1590-8658(00)80353-9
25. Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis. (2014) 20:1353–60. doi: 10.1097/MIB.0000000000000110
26. Wilschanski M, Sherman P, Pencharz P, Davis L, Corey M, Griffiths A. Supplementary enteral nutrition maintainsremission in paediatric Crohn’s disease. Gut. (1996) 38:543–8. doi: 10.1136/gut.38.4.543
27. Duncan H, Buchanan E, Cardigan T, Garrick V, Curtis L, McGrogan P, et al. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: supplemental enteral nutrition is better than nothing! BMC Gastroenterol. (2014) 14:50. doi: 10.1186/1471-230X-14-50
28. Akobeng AK, Thomas AG. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Datab Syst Rev. (2007) 18:CD005984. doi: 10.1002/14651858.CD005984.pub2
29. Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B. Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr. (2004) 93:327–35.
30. Sakurai T, Matsui T, Yao T, Takagi Y, Hirai F, Aoyagi K, et al. Short-term efficacy of enteral nutrition in the treatment of active Crohn’s disease: a randomized, controlled trial comparing nutrient formulas. JPEN J Parenter Enteral Nutr. (2002) 26:98–103. doi: 10.1177/014860710202600298
31. Gassull MA, Fernández-Bañares F, Cabré E, Papo M, Giaffer MH, Sánchez-Lombraña JL, et al. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn’s disease: results of a double blind randomisedmulticentre European trial. Gut. (2002) 51:164–8. doi: 10.1136/gut.51.2.164
32. Turner D, Steinhart AH, Griffiths AM. Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Datab Syst Rev. (2007) 3:CD006443. doi: 10.1002/14651858.CD006443.pub2
33. Hartman C, Berkowitz D, Weiss B, Shaoul R, Levine A, Adiv OE, et al. Nutritional supplementation with polymeric diet enriched with transforming growth factor-b 2 for children with Crohn’s disease. Isr Med Assoc J. (2008) 10:503–7.
34. Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Mäkitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. (2012) 91:121–30. doi: 10.1007/s00223-012-9617-2
35. Britto S, Kellermayer R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease. J Crohns Colitis. (2019) 13:942–8. doi: 10.1093/ecco-jcc/jjz011
36. Matuszczyk M, Kierkus J. Nutritional therapy in pediatric Crohn’s disease-are we going to change the guidelines? J Clin Med. (2021) 10:3027. doi: 10.3390/jcm10143027
37. Svolos V, Hansen R, Nichols B, Quince C, Ijaz UZ, Papadopoulou RT, et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology. (2019) 156:1354–67.e6. doi: 10.1053/j.gastro.2018.12.002
38. Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. (2009) 3:8–14. doi: 10.1016/j.crohns.2008.09.004
39. Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (Low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2016) 22:1129–36. doi: 10.1097/MIB.0000000000000708
40. Vernia P, Loizos P, Di Giuseppantonio I, Amore B, Chiappini A, Cannizzaro S. Dietary calcium intake in patients with inflammatory bowel disease. J Crohns Colitis. (2014) 8:312–7. doi: 10.1016/j.crohns.2013.09.008
41. Critch J, Day AS, Otley A, King-Moore C, Teitelbaum JE, Shashidhar H, et al. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012; 54(2):298-305. Erratum J Pediatr Gastroenterol Nutr. (2012) 54:573. doi: 10.1097/MPG.0b013e318235b397
42. Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis. (2014) 20:861–71. doi: 10.1097/MIB.0000000000000023
43. Lafferty L, Tuohy M, Carey A, Sugrue S, Hurley M, Hussey S. Outcomes of exclusive enteral nutrition in paediatric Crohn’s disease. Eur J Clin Nutr. (2017) 71:185–91. doi: 10.1038/ejcn.2016.210
44. Faiman A, Mutalib M, Moylan A, Morgan N, Crespi D, Furman M, et al. Standard versus rapid food reintroduction after exclusive enteral nutritional therapy in paediatric Crohn’s disease. Eur J Gastroenterol Hepatol. (2014) 26:276–81. doi: 10.1097/MEG.0000000000000027
45. Wang G, Ren J, Li G, Hu Q, Gu G, Ren H, et al. The utility of food antigen test in the diagnosis of Crohn’s disease and remission maintenance after exclusive enteral nutrition. Clin Res Hepatol Gastroenterol. (2018) 42:145–52. doi: 10.1016/j.clinre.2017.09.002
46. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. (2013) 341:1237439. doi: 10.1126/science.1237439
47. Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug-Sales M, Jackson R, et al. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. (2006) 21:1609–14. doi: 10.1111/j.1440-1746.2006.04294.x
48. Cameron FL, Gerasimidis K, Papangelou A, Missiou D, Garrick V, Cardigan T, et al. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment Pharmacol Ther. (2013) 37:622–9. doi: 10.1111/apt.12230
49. Burgess D, Herbison K, Fox J, Collins T, Landorf E, Howley P. Exclusive enteral nutrition in children and adolescents with Crohn disease: dietitian perspectives and practice. J Paediatr Child Health. (2021) 57:359–64. doi: 10.1111/jpc.15220
50. Akobeng AK, Thomas AG. Refeeding syndrome following exclusive enteral nutritional treatment in Crohn disease. J Pediatr Gastroenterol Nutr. (2010) 51:364–6. doi: 10.1097/MPG.0b013e3181e712d6
51. Svolos V, Gerasimidis K, Buchanan E, Curtis L, Garrick V, Hay J. Dietary treatment of Crohn’s disease: perceptions of families with children treated by exclusive enteral nutrition, a questionnaire survey. BMC Gastroenterol. (2017) 17:14. doi: 10.1186/s12876-016-0564-7
52. Navas-López VM, Martín-de-Carpi J, Segarra O, García-Burriel JI, Díaz-Martín JJ, Rodríguez A, et al. PRESENT; PREScription of enteral nutrition in pediaTric Crohn’s disease in Spain. Nutr Hosp. (2014) 29:537–46. doi: 10.3305/nh.2014.29.3.7184
53. Whitten KE, Rogers P, Ooi CY, Day AS. International survey of enteral nutrition protocols used in children with Crohn’s disease. J Dig Dis. (2012) 13:107–12. doi: 10.1111/j.1751-2980.2011.00558.x
54. Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr. (2011) 52:38–42. doi: 10.1097/MPG.0b013e3181e2c724
55. Gråfors JM, Casswall TH. Exclusive enteral nutrition in the treatment of children with Crohn’s disease in Sweden: a questionnaire survey. Acta Paediatr. (2011) 100:1018–22. doi: 10.1111/j.1651-2227.2011.02178.x
56. Day AS, Stephenson T, Stewart M, Otley AR. Exclusive enteral nutrition for children with Crohn’s disease: use in Australia and attitudes of Australian paediatric gastroenterologists. J Paediatr Child Health. (2009) 45:337–41. doi: 10.1111/j.1440-1754.2009.01498.x
Keywords: inflammatory bowel disease, enteral nutrition, children, implementation status, questionnaire
Citation: Luo J, Xie Y-M, Wu M, Zhao J-G and Hu L-L (2022) Global attitudes on and the status of enteral nutrition therapy for pediatric inflammatory bowel disease. Front. Med. 9:1036793. doi: 10.3389/fmed.2022.1036793
Received: 05 September 2022; Accepted: 14 November 2022;
Published: 08 December 2022.
Edited by:
Enis Kostallari, Mayo Clinic, United StatesReviewed by:
Ying Fang, Xi’an Children’s Hospital, ChinaCopyright © 2022 Luo, Xie, Wu, Zhao and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Mei Xie, bWF5Lnh5bUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.