- 1Department of Nephrology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
- 2The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 3Department of Nephrology, The First People’s Hospital of Hangzhou Lin’an District, Hangzhou, China
Tripterygium wilfordii—a traditional Chinese herbal medicine—is used to treat several diseases, including chronic kidney disease, rheumatic autoimmune disorder, and skin disorders. With the development of modern pharmacology, scientists have gradually realized that T. wilfordii has side effects on several organs and systems of the human body, including the liver, kidney, reproductive system, hematopoietic system, and immune system. Our understanding of its toxicity remains unclear. The incidence of problems in the hematopoietic system is not low but few related studies have been conducted. The serious consequences need to be of concern to clinicians and scientists. To ensure the safety of patients, it is important to elucidate the mechanism underlying the damage to the hematopoietic system caused by T. wilfordii and strategies to reduce its toxicity. Routine blood and biochemical tests should be conducted when administering T. wilfordii, and in case of any abnormality, the medication should be terminated in time along with a comprehensive symptomatic treatment. Herein, we report the case of a 50-year-old Chinese female with end-stage renal disease (ESRD) who developed severe bone marrow suppression after taking a short-term normal dose of a T. wilfordii-containing decoction. She died of sepsis and septic shock, although timely therapeutic measures (e.g., stimulating hematopoiesis, anti-infection treatment, and hemodialysis) were administered. To the best of our knowledge, this is the first report of death by T. wilfordii-induced myelosuppression from a short term, conventional dose in an adult female with ESRD. Although the underlying mechanism remains unclear, this case contradicts the notion that side effects on the hematopoietic system are non-lethal.
Introduction
A 50-year-old female patient with end-stage renal disease (ESRD) who was not on renal replacement therapy took a Tripterygium wilfordii-containing decoction for 11 days, following which she developed obvious fatigue and scattered multiple subcutaneous ecchymoses on her lower limbs. The results of laboratory tests revealed abnormal coagulation function and peripheral hypocytosis mainly indicated by deceased leukocytes and platelets. Repeated bone marrow puncture results suggested acute suppression caused by medicinal ingredients. Later, the patient suffered a serious pulmonary infection. During her hospitalization, timely therapeutic measures were undertaken, including stopping the decoction usage, preventing bleeding, stimulating hematopoiesis, blood transfusion, anti-infection treatment, and hemodialysis. However, there was no change in the patient’s condition owing to persistent bone marrow suppression. Finally, she died of sepsis and septic shock after 2 months due to a serious infection.
To the best of our knowledge, this is the first report of death in an adult female patient with ESRD who developed severe bone marrow suppression after taking a short-term normal dose of T. wilfordii-containing decoction. Although the underlying mechanism remains unclear, it contradicts the notion that side effects on the hematopoietic system are non-lethal. The safety of administering T. wilfordii to patients with ESRD needs further evaluation, and a more detailed study on the mechanism of its toxic effects is essential.
Case presentation
First hospitalization
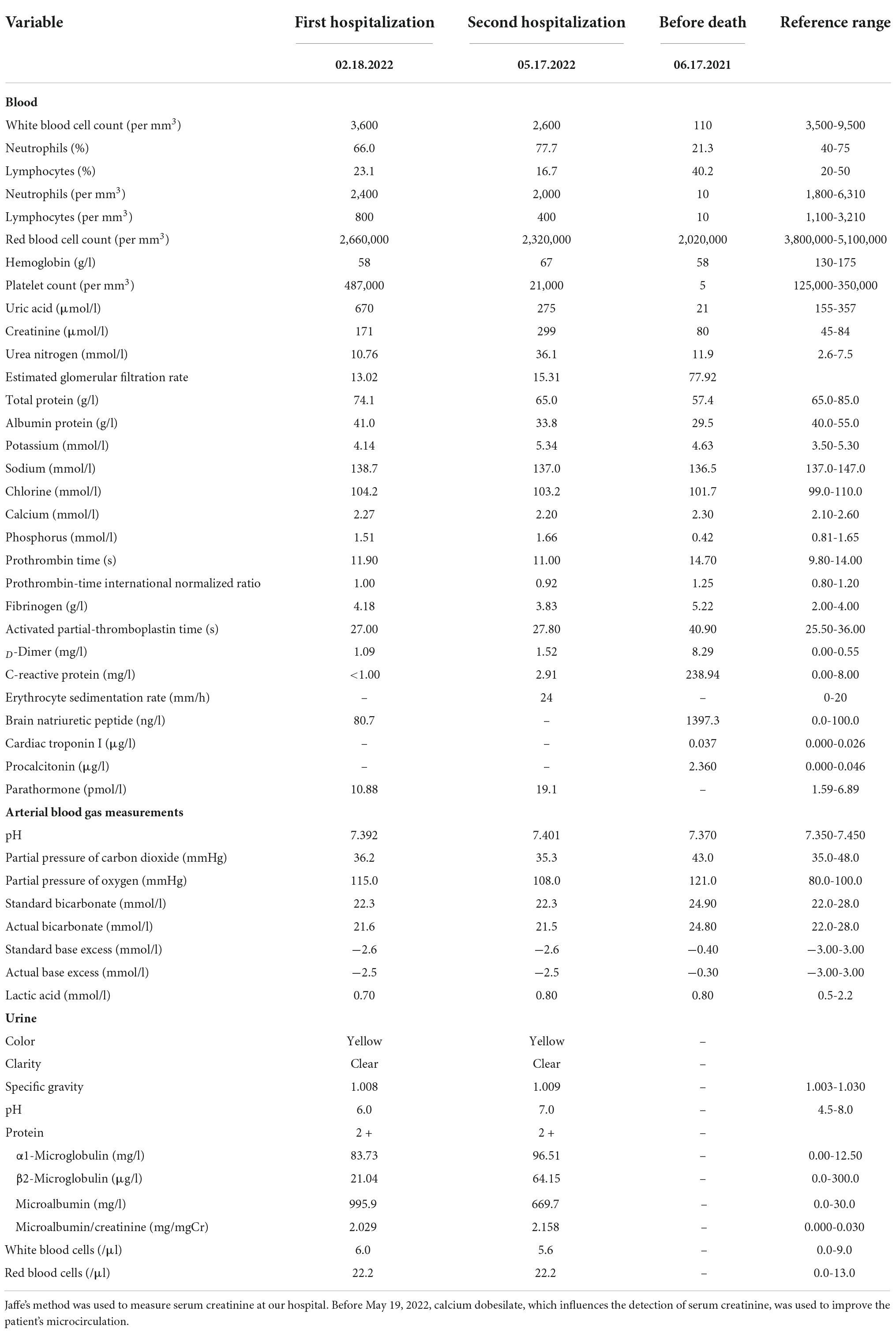
On February 17, 2022, a 50-year-old Chinese female patient was admitted to Zhejiang University of Traditional Chinese Medicine First Affiliated Hospital in China with stage 5 chronic kidney disease, hypertension, renal anemia, and hyperuricemia. She had suffered from chronic kidney disease for more than 10 years, which developed into stage 5 approximately 2 years previously. Her renal pathological diagnosis was unclear. The patient was admitted for backache, nausea, and vomiting, and underwent a battery of routine tests (Table 1). Her body mass index (BMI) was 22.9. Physical examination was negative. Other examinations suggested that immunoglobulin G4, tumor markers, light chain test results, and thyroid function were normal. The antinuclear antibody spectrum showed a titer of 1:80; anti-Sjogren’s syndrome antigen A/Ro antibodies were positive, but the patient denied the relevant suspected clinical manifestations. Computed tomography (CT) of the chest showed normal images (Figure 1A). Emission computed tomography (ECT) of the kidneys revealed that the estimated renal plasma flow (left kidney: 13.08 ml/min; right kidney: 32.31 ml/min) and glomerular filtration (left kidney: 2.13 ml/min; right kidney: 2.42 ml/min) rates were low. We advised renal replacement therapy to the patient, but she refused it and asked for conservative treatment. We formulated the following treatment plan: compound α-ketoacid 3.78 g–3 times per day, roxadustat 120 mg–3 times per week, felodipine 5 mg–twice a day, calcium dobesilate 0.5 g–3 times per day, sodium bicarbonate 1 g–3 times per day, febuxostat 40 mg once daily, and beraprost sodium 40 μg–3 times per day. The patient was discharged, and her follow-up was scheduled as a nephrology outpatient.

Figure 1. Chest imaging. (A) Normal computed tomography (CT) image taken on February 18, 2022. (B) Normal CT image taken on May 18, 2022. (C) The CT image taken on June 10, 2022 shows scattered large high-density shadows in both lungs.
Second hospitalization
On May 04, a test of the patient’s urine confirmed persistent proteinuria, and the patient agreed to be prescribed T. wilfordii-containing decoction per day to preserve residual renal function, but she requested an active treatment plan, so the dosage of T. wilfordii was set as 12 g per day, which was the maximum dose within the safe range, for 14 days. The patient was required to decoct T. wilfordii for 2 h first. We advised the patient to consult a nephrologist if she experienced any discomfort, including fever, subcutaneous ecchymosis, nausea, and vomiting; if not, routine blood and biochemical tests should be conducted after 2 weeks. The patient gave informed consent. 11 days later, the patient showed obvious fatigue and subcutaneous ecchymoses for the first time and discontinued the Chinese medication. On May 17, she visited our hospital and underwent relevant examinations (Table 1). Her BMI did not change. Physical examination was negative except for multiple scattered subcutaneous ecchymoses on her lower limbs. ECT of the patient’s kidneys revealed a lower glomerular filtration rate (left kidney: 1.16 ml/min; right kidney: 2.52 ml/min) than before and estimated renal plasma flow was not detected. CT images of the chest were normal (Figure 1B). We admitted the patient to the intensive care unit. The patient showed obvious bone marrow suppression indicated by the deceased leukocytes and platelets, accompanied by abnormal coagulation function. However, she denied any previous hematopoietic system-related diseases. We prescribed dexamethasone, avatrombopag, recombinant human granulocyte colony-stimulating factor, recombinant human erythropoietin, and recombinant human thrombopoietin to stimulate hematopoiesis; carbazochrome sodium sulfonate to prevent bleeding; intravenous immunoglobulin to gain passive immunity; and repeated blood transfusion of red blood cells, albumin, human fibrinogen, platelets, and plasma. The patient consented to hemodialysis through a deep vein catheter. To clarify the cause of this condition, the hematology department was called upon for multidisciplinary combination therapy, and repeated bone marrow aspiration and biopsy were suggested (Figures 2A–C). The results showed that the patient’s hematopoietic functions were seriously inhibited. A Coombs test excluded autoimmune hemolytic anemia. Considering her history, we diagnosed acute bone marrow hematopoietic stagnation caused by drugs.
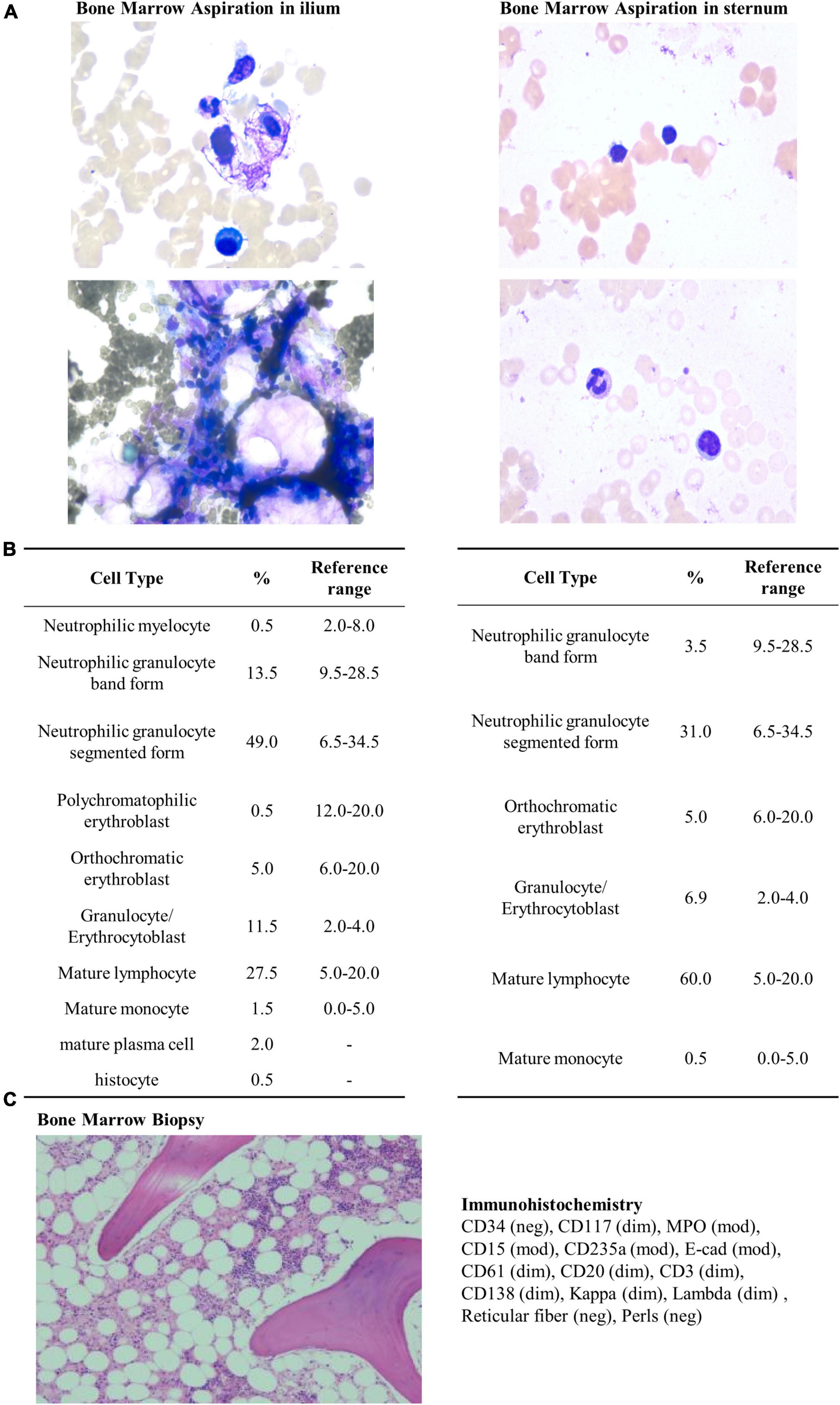
Figure 2. Bone marrow aspiration, biopsy, and immunohistochemistry. Bone marrow aspiration was performed using Wright’s staining technique. Bone marrow biopsy and immunohistochemistry were performed using staining techniques involving hematoxylin, Giemsa, acid fuchsin, reticular fiber, and Prussian blue stains. (A) Bone marrow aspiration in ilium performed on May 20, 2022. The results reveal low proliferation of nucleated cells without abnormality in morphology. (B) Bone marrow aspiration in sternum performed on May 24, 2022. The results reveal low proliferation of nucleated cells without abnormality in morphology. (C) Bone marrow biopsy and immunohistochemistry performed on May 20, 2022. Hematopoietic elements are substantially reduced (30%), and bone marrow space is replaced with adipose tissue (70%). Granulocyte and erythrocyte development are normal.
The therapeutic schedule of this patient remained unaltered before and after the appearance of abnormal hematopoietic function, except for the addition of T. wilfordii to the patient’s decoction. To the best of our knowledge, serious side effects related to the hematopoietic system have never been reported for the other herbal compounds in the decoction, based on “Buyang Huanwu Decoction” (1, 2), which was administered to the patient previously without any side effects on the hematopoietic system, for approximately 2 years. The composition formula is displayed in the Supplementary Table.
Combining with the results of blood routine tests before administering T. wilfordii, we believe that T. wilfordii caused bone marrow suppression (Figures 3A–C). Unfortunately, bone marrow suppression persisted throughout her second hospitalization. On May 31, the patient presented symptoms of dyspnea, cough, expectoration, and oxygen desaturation. CT images of the chest showed scattered large high-density shadows in both lungs, which suggested lung infection (Figure 1C). The results of sputum and blood culture suggested multidrug-resistant Enterobacter cloacae and carbapenem-resistant Acinetobacter Baumannii infection. Therefore, we administered several antibiotics including meropenem, polymyxin, tigecycline, cefoperazone sodium, and sulbactam sodium successively to treat the infection.
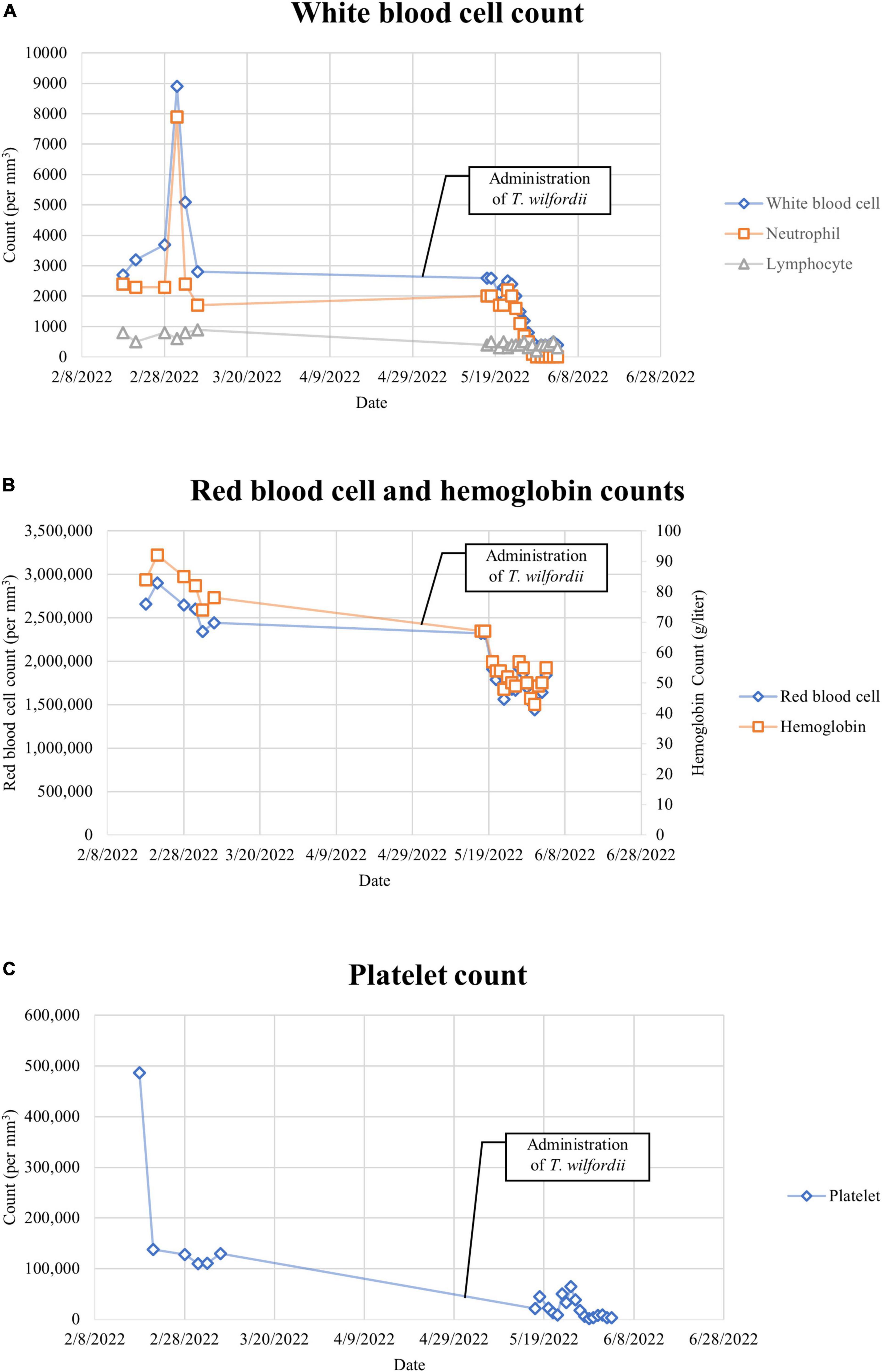
Figure 3. (A) White blood cell count. The normal reference ranges of the above data are listed in Table 1. (B) Red blood cell and hemoglobin counts. The normal reference ranges of the above data are listed in Table 1. (C) Platelet count. The normal reference ranges of the above data are listed in Table 1.
Results
After more than 1 month of treatment, on June 18, the patient died of sepsis and septic shock.
Discussion
Efficacy of Tripterygium wilfordii
Tripterygium wilfordii Hook, belonging to Tripterygium of Celastraceae, has been used as a traditional Chinese medicine for hundreds of years. It is widely used to treat various diseases and shows remarkable curative effects. T. wilfordii is an antirheumatic Chinese medicinal herb. The earliest record that systematically summarized its efficacy in China is in “Ben Cao Gang Mu Shi Yi,” a book dating back nearly 300 years. It is used to treat various diseases including chronic renal disease, rheumatic immune disease, and skin disease (3). A meta-analysis of the treatment of chronic renal disease with T. wilfordii polycoride has shown that T. wilfordii can alleviate proteinuria and delay the progression of chronic renal disease (4).
Modern pharmacology suggests that T. wilfordii exerts anti-tumor, anti-inflammatory, and immunosuppressive effects (5, 6), and its main active ingredients are triptolide, celastrol, and total alkaloids of T. hypoglaucum (7). Its excellent curative effect is accompanied by some side effects; thus, scientists have conducted several studies to identify its toxic components and side effects and develop strategies to reduce them (8, 9). The toxic components and active components of T. wilfordii are largely overlapping. For example, triptolide, a diterpenoid epoxide in T. wilfordii, is a medicinal and noxious compound (7, 10).
Methods of Tripterygium wilfordii drug delivery
At present, T. wilfordii is used clinically in two ways in China. One way is to use it in decoctions. Some studies have shown that combining it with other traditional Chinese medicines, such as licorice, silymarin, and ginseng, can help reduce the toxicity of T. wilfordii (11–13). The reference dose is different among Chinese pharmacopoeias and herbal guidelines. For example, the Traditional Chinese Pharmacology stipulated a dosage of 1–3 g of T. wilfordii per prescription in the formula and decoction time of 45–60 min before adding other herbs (14). In contrast, the Chinese Materia Medica recommended a dosage of 10–12 g of T. wilfordii per prescription in the formula and decoction time of 1–2 h (15). Our hospital suggests that the initial dosage of T. wilfordii should be different according to the patient’s weight. For adult patients weighing less than 60 kg, the dosage is 3 g per day; for those weighing more than or equal to 60 kg, it is 5 g per day. If there is no adverse reaction, the dose is increased to 12 g per day at most. The herb should be decocted for 2 h first. Considering that not all toxic substances have therapeutic effects, another method is to extract the effective components of T. wilfordii and convert them into a patented Chinese medicine to reduce toxicity. Among several preparations, T. wilfordii polycoride is the most convenient and widely used preparation, containing diterpene lactones, alkaloids, and triterpenoids (16). According to the Chinese Pharmacopoeia and National Standards published in 2010, the T. wilfordii lactone content should be not less than 0.1 mg/g/tablet; the recommended dose is 1.0–1.5 mg/kg/day, administered three times a day after meals (17).
In recent years, new methods of drug delivery have been proposed. Wang et al. attempted a transdermal microemulsion drug delivery system for T. wilfordii Hook f. to ameliorate its toxic effects on the male reproductive system (18). Xue et al. reported the protective effects of Tripterygium glycoside-loaded solid lipid nanoparticles against toxicity to the male reproductive system (19). However, these drug delivery methods neither demonstrate protection of the kidney or hematopoietic system nor are widely used for now.
Tripterygium wilfordii toxicity
Tripterygium wilfordii affects various systems and organs, causing reproductive toxicity (20), liver damage (21), and kidney damage (22). Relevant studies have focused on these aspects (23–25). The toxicity of T. wilfordii is generally considered to be related to its dose and duration, and most of the side effects are reversible (26). However, T. wilfordii sometimes causes damage to the hematopoietic system, which usually manifests as leukopenia and aplastic anemia (27). The incidence of this side effect is lower than that of liver injury, but not uncommon, and its mechanism is unclear (4, 27, 28). According to Kusy et al., celastrol, an important component of T. wilfordii, specifically impairs the development of B cells and erythrocytes in the peripheral blood, bone marrow, spleen, and peritoneal cavity, but in mature lineages, the adverse effects are transient, as recovery is complete 4 weeks after the removal of the drug (29). Pyatt et al. suggested that T. wilfordii directly blocks the ability of very early multilineage as well as lineage-specific committed hematopoietic progenitor cells to form colonies in a dose-dependent way, which might be related to nuclear factor-kappa B signaling (30). These studies cannot fully explain the conditions found in this case.
Wu et al. (31), Feng et al. (32), and Liu et al. (33) reported several severe cases of bone marrow suppression caused by excessive doses or long-term use of T. wilfordii. The medicine was terminated in these cases and blood transfusion was performed to stimulate the hematopoietic system. The patients were eventually rescued and bone marrow suppression was eliminated.
Case characteristics
To the best of our knowledge, this is the first report of death in an adult female patient with ESRD caused by severe bone marrow suppression after taking a short-term normal dose of a T. wilfordii-containing decoction. This finding contradicts the prevailing belief that side effects on the hematopoietic system are non-lethal. The patient had no previous hematopoietic system-related diseases, dosage of T. wilfordii decoction complied with the specifications, consumption duration was short, and medication was stopped immediately after symptoms were detected. Therefore, the cause of severe bone marrow suppression was unclear. Subsequent treatment continued for nearly 2 months; however, the patient did not recover and finally died of serious infection. It is unknown whether ESRD was involved in the occurrence of bone marrow suppression. From this case study, it is reasonable to conclude that T. wilfordii side effects are probably not only limited to toxic accumulation, but also related to the immune system, thereby triggering hematopoietic cell destruction. Moreover, we cannot exclude the possibility of rare idiosyncratic drug reactions (34). However, owing to the lack of relevant basic research, further studies are required to confirm these conjectures.
Limitations
This case report has some limitations. First, owing to the complex ingredients of T. wilfordii decoction, we could not detect the blood concentration of T. wilfordii. Thus, it is difficult to directly confirm whether bone marrow suppression was caused by the toxic accumulation of T. wilfordii. Second, according to the recommendations of the Chinese Materia Medica and Traditional Chinese Pharmacology, T. wilfordii is toxic and needs to be decocted for a long time to reduce toxicity before adding other herbs (14, 15). Although the patient was instructed to decoct T. wilfordii for 2 h, we could not determine whether this was strictly performed. Finally, the reference dosage of T. wilfordii varies greatly among guidelines. We may not propose the most effective and safe reference dosage for patients with chronic kidney disease due to the lack of relevant study.
Future research directions
Proposals for novel methods of drug delivery to alleviate T. wilfordii toxicity are essential. At present, the content of TWHF in T. wilfordii polyglycoside tablets varies among manufacturers (35). It is necessary to quantify blood drug concentration in clinical settings. Pharmacokinetic studies and safety evaluation of T. wilfordii should be continued. Genetic testing may verify whether the severe side effects of T. wilfordii are related to heredity. Detailed medication guidelines should be prepared for patients with liver or kidney damage, pregnant women, and the elderly population. The mechanisms underlying the toxic effects should be studied in detail to avoid bone marrow suppression, and a systematic treatment plan to prevent side effects should be prepared.
Conclusion
Although T. wilfordii has been used for hundreds of years, our understanding of its toxic effects remains incomplete, and the mechanism remains unclear. In addition to reproductive toxicity and liver and kidney injuries, hematopoietic system problems are possible. These serious consequences deserve clinicians’ attention. When using T. wilfordii, the initial dose should be small and routine blood and biochemical tests should be conducted regularly. In case of abnormalities, the medicine should be stopped in time and symptomatic treatments should be provided. The safety of T. wilfordii in patients with ESRD requires detailed evaluation. Elucidating the mechanism of T. wilfordii-induced hematopoietic system damage and seeking new methods to reduce its toxicity are necessary for clinical applications.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ and XL researched data and wrote the manuscript. CX, LH, HM, XW, and PZ reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1036422/full#supplementary-material
References
1. Yu K-Y, Huang X-H, Li W-L, Liu B, Qiu H-Z. Treatment of refractory thrombocytopenic purplegia in children with integrated traditional chinese and western medicine. Jilin J Chin Med. (2002) 22:43–4. doi: 10.13463/j.cnki.jlzyy.2002.02.039
2. Li S-K, Li Y. Treatment of 68 cases of chronic aplastic anemia with buyang huanwu decoction. Jilin J Chin Med. (2004) 24:18. doi: 10.13463/j.cnki.jlzyy.2004.03.015
3. Cui J, Chen X, Su J-C. Advanced progress of main pharmacology activities of triptolide. China J Chin Materia Med. (2017) 42:2655–8. doi: 10.19540/j.cnki.cjcmm.20170609.011
4. Guo Y-L, Gao F, Dong T-W, Bai Y, Liu Q, Li R-L, et al. Meta-analysis of clinical efficacy and safety of Tripterygium wilfordii polyglycosides tablets in the treatment of chronic kidney disease. Evid Based Complement Alternat Med. (2021) 2021:6640594. doi: 10.1155/2021/6640594
5. Shui G-X, Wan Y-G, Jiang C-M, Zhang H-L, Chen P, Wang C-J, et al. Progress in Tripterygium wilfordii and its bioactive components in the field of pharmacodynamics and pharmacology. China J Chin Materia Med. (2010) 35:515–20. doi: 10.4268/cjcmm20100425
6. Liu P, Zhang J, Wang Y, Shen Z, Wang C, Chen D-Q, et al. The active compounds and therapeutic target of Tripterygium wilfordii hook. F. In attenuating proteinuria in diabetic nephropathy: a review. Front Med. (2021) 8:747922. doi: 10.3389/fmed.2021.747922
7. Lv H, Jiang L, Zhu M, Li Y, Luo M, Jiang P, et al. The genus tripterygium: a phytochemistry and pharmacological review. Fitoterapia. (2019) 137:104190. doi: 10.1016/j.fitote.2019.104190
8. Zhao X-M, Gong M, Dong J-M, Wang J-B, Xiao X-H, Zhao K-J, et al. Preliminary research on effect of licorice-processed Tripterygium wilfordii on reducing liver toxicity. China J Chin Materia Med. (2017) 42:119–24. doi: 10.19540/j.cnki.cjcmm.20161222.020
9. Wang J, Wang C, Wu J, Li Y, Hu X, Wen J, et al. Oral microemulsion based delivery system for reducing reproductive and kidney toxicity of tripterygium glycosides. J Microencapsul. (2019) 36:523–34. doi: 10.1080/02652048.2019.1631402
10. Li X-J, Jiang Z-Z, Zhang L. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. (2014) 155:67–79. doi: 10.1016/j.jep.2014.06.006
11. Zhang W, Lu C, Liu Z, Yang D, Chen S, Cha A, et al. Therapeutic effect of combined triptolide and glycyrrhizin treatment on rats with collagen induced arthritis. Planta Med. (2007) 73:336–40. doi: 10.1055/s-2007-967136
12. Wang L, Huang Q-H, Li Y-X, Huang Y-F, Xie J-H, Xu L-Q, et al. Protective effects of silymarin on triptolide-induced acute hepatotoxicity in rats. Mol Med Rep. (2018) 17:789–800. doi: 10.3892/mmr.2017.7958
13. Zhang B-Y, Zhang Q-C, Liu M-Z, Zhang X-L, Shi D-L, Guo L-W, et al. Increased involvement of panax notoginseng in the mechanism of decreased hepatotoxicity induced by Tripterygium wilfordii in rats. J Ethnopharmacol. (2016) 185:243–54. doi: 10.1016/j.jep.2016.03.027
14. Zhong G-S. Traditional Chinese Pharmacology. Beijing: China Press of Traditional Chinese Medicine (2016).
15. Song L-R, Wu Y-G, Hu L, Zhang G-Z. Chinese Materia Medica (Zhonghua Bencao). Shanghai, China: Shanghai Science & Technology Press (1999). p. 206–15.
16. Wang Y-D, Wang Q, Zhang J-B, Dai Z, Lin N, Wu X-F, et al. Research progress on chemical constituents and quality control of Tripterygium wilfordii preparations. China J Chin Materia Med. (2019) 44:3368–73. doi: 10.19540/j.cnki.cjcmm.20190606.501
17. Chinese Pharmacopoeia Committee [CPC]. Pharmacopoeia of the People’s Republic of China (English version). Beijing: China Medical Science and Technology Press (2010). p. 1.
18. Wang X, Xue M, Gu J, Fang X, Sha X. Transdermal microemulsion drug delivery system for impairing male reproductive toxicity and enhancing efficacy of Tripterygium wilfordii hook f. Fitoterapia. (2012) 83:690–8. doi: 10.1080/13543776.2018.1519025
19. Xue M, Jiang Z-Z, Wu T, Yan M, Liu J-P, Mu X-M, et al. Protective effects of tripterygium glycoside-loaded solid lipid nanoparticles on male reproductive toxicity in rats. Arzneimittelforschung. (2011) 61:571–6. doi: 10.1055/s-0031-1300555
20. Xu Y, Fan Y-F, Zhao Y, Lin N. Overview of reproductive toxicity studies on Tripterygium wilfordii in recent 40 years. China J Chin Materia Med. (2019) 44:3406–14. doi: 10.19540/j.cnki.cjcmm.20190524.401
21. Tian Y-G, Su X-H, Liu L-L, Kong X-Y, Lin N. Overview of hepatotoxicity studies on Tripterygium wilfordii in recent 20 years. China J Chin Materia Med. (2019) 44:3399–405. doi: 10.19540/j.cnki.cjcmm.20190527.408
22. Luo H-M, Gu C-Y, Liu C-X, Wang Y-M, Wang H, Li Y-B. Plasma metabolic profiling analysis of strychnos nux-vomica linn. and Tripterygium wilfordii hook f-induced renal toxicity using metabolomics coupled with uplc/q-tof-ms. Toxicol Res. (2018) 7:1153–63. doi: 10.1039/c8tx00115d
23. Li M, Hu T, Tie C, Qu L, Zheng H, Zhang J. Quantitative proteomics and targeted fatty acids analysis reveal the damage of triptolide in liver and kidney. Proteomics. (2017) 17:1700001. doi: 10.1002/pmic.201700001
24. Duan X-Y, Ma R-J, Hsiao C-D, Jiang Z-Z, Zhang L-Y, Zhang Y, et al. Tripterygium wilfordii multiglycoside-induced hepatotoxicity via inflammation and apoptosis in zebrafish. Chin J Nat Med. (2021) 19:750–7. doi: 10.1016/S1875-5364(21)60078-X
25. Guo J, Huang Y, Lei X, Zhang H, Xiao B, Han Z, et al. Reproductive systemic toxicity and mechanism of glucosides of Tripterygium wilfordii hook. F.(gtw). Ann Clin Lab Sci. (2019) 49:36–49.
26. Chang Z, Qin W, Zheng H, Schegg K, Han L, Liu X, et al. Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat Commun. (2021) 12:1253. doi: 10.1038/s41467-021-21517-5
27. Li Z, Ma D, Yang X, Sun F, Yu K, Zhan S. Meta-analysis of blood system adverse events of Tripterygium wilfordii. China J Chin Materia Med. (2015) 40:339–45.
28. Ren D, Zuo C, Xu G. Clinical efficacy and safety of Tripterygium wilfordii hook in the treatment of diabetic kidney disease stage iv: a meta-analysis of randomized controlled trials. Medicine. (2019) 98:e14604.
29. Kusy S, Ghosn EE, Herzenberg LA, Contag CH. Development of b cells and erythrocytes is specifically impaired by the drug celastrol in mice. PLoS One. (2012) 7:e35733. doi: 10.1371/journal.pone.0035733
30. Pyatt DW, Yang Y, Mehos B, Le A, Stillman W, Irons RD. Hematotoxicity of the chinese herbal medicine Tripterygium wilfordii hook f in cd34-positive human bone marrow cells. Mol Pharmacol. (2000) 57:512–8. doi: 10.1124/mol.57.3.512
31. Wu L-Y, Fu W-J, Jia Q. A case of severe myelosuppression caused by Tripterygium wilfordii polyglycoside tablets. Chin J Clin Ration Drug Use. (2019) 12:78–9.
32. Feng G-A, Guo N-J, Dong X-B, Hong S-C, Chang Y-L, Chen Y, et al. Aplastic anemia induced by multiglycosidore triptergii: a report of 3 cases and literature review. J Clin Hematol. (2005) 1:42–4.
33. Liu G-X. Drug induced myelosuppression caused by Tripterygium wilfordii: a report of 2 cases. J Chengdu Univ Tradition Chin Med. (1997) 3:44–5.
34. Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med. (1997) 336:1365–72. doi: 10.1056/NEJM199705083361906
Keywords: traditional Chinese medicine, Tripterygium wilfordii, myelosuppression, end-stage renal disease, case report
Citation: Zhang W, Liu X, Xia C, He L, Ma H, Wang X and Zhang P (2022) Case report: A rare case of death due to end-stage renal disease caused by Tripterygium wilfordii-induced myelosuppression. Front. Med. 9:1036422. doi: 10.3389/fmed.2022.1036422
Received: 04 September 2022; Accepted: 11 November 2022;
Published: 30 November 2022.
Edited by:
Sree Bhushan Raju, Nizam’s Institute of Medical Sciences, IndiaReviewed by:
Duorui Nie, Hunan University of Chinese Medicine, ChinaMei-Yao Wu, China Medical University, Taiwan
Copyright © 2022 Zhang, Liu, Xia, He, Ma, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoran Wang, emhhbmd3ZW4wNTI0QDEyNi5jb20=; Peipei Zhang, emhhbmdwZWlwZWluanVAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Wen Zhang
Wen Zhang Xinyin Liu
Xinyin Liu Cong Xia1†
Cong Xia1† Xiaoran Wang
Xiaoran Wang Peipei Zhang
Peipei Zhang