
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 29 November 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1034288
This article is part of the Research Topic Inflammation and Organic Damage in COVID-19: What Have We Learned 2 Years Into the Pandemic? View all 12 articles
 Stefano Brusa1
Stefano Brusa1 Daniela Terracciano1*
Daniela Terracciano1* Dario Bruzzese2
Dario Bruzzese2 Mariano Fiorenza1
Mariano Fiorenza1 Lucia Stanziola1
Lucia Stanziola1 Biagio Pinchera3
Biagio Pinchera3 Valeria Valente1
Valeria Valente1 Ivan Gentile3
Ivan Gentile3 Antonio Cittadini1
Antonio Cittadini1 Ilaria Mormile1
Ilaria Mormile1 Mauro Mormile3
Mauro Mormile3 Giuseppe Portella1
Giuseppe Portella1Background: Systemic biomarkers for severity of SARS-CoV-2 infection are of great interest. In this study, we evaluated a set of collagen metabolites and extracellular matrix remodeling biomarkers including procollagen type III amino terminal propeptide (PIIINP), tissue inhibitor of metalloproteinases 1 (TIMP-1) and hyaluronic acid (HA) as prognostic indicators in COVID-19 patients.
Methods: Ninety COVID-19 patients with the absence of chronic liver diseases were enrolled. Serum PIIINP, TIMP-1, and HA were measured and correlated with inflammatory indices and clinical variables. Patients were stratified for disease severity according to WHO criteria in two groups, based on the requirement of oxygen support.
Results: Serum TIMP-1, but not PIIINP and HA was significantly higher in patients with WHO score ≥5 compared to patients with WHO score <5 [PIIINP: 7.2 (5.4–9.5) vs. 7.1 (4.5–9.9), p = 0.782; TIMP-1: 298.1 (20.5–460) vs. 222.2 (28.5–452.8), p = 0.01; HA: 117.1 (55.4–193.7) vs. 75.1 (36.9–141.8), p = 0.258]. TIMP-1 showed moderate correlation with CRP (r = 0.312, p = 0.003) and with LDH (r = 0.263, p = 0.009). CRP and serum LDH levels were significantly higher in COVID-19 patients with WHO score ≥5 compared to the group of patients with WHO score < 5 [15.8 (9–44.5) vs. 9.3 (3.4–33.8), p = 0.039 and 373 (282–465) vs. 289 (218–383), p = 0.013, respectively].
Conclusion: In patients with COVID-19, circulating TIMP-1 was associated with disease severity and with systemic inflammatory index, suggesting that TIMP-1 could represent a promising non-invasive prognostic biomarker in COVID-19 patients. Interestingly, our results prompted that serum TIMP-1 level may potentially be used to select the patients for therapeutic approaches targeting matrix metalloproteases pathway.
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the infective agent responsible for Coronavirus Disease 2019 (COVID-19). SARS-CoV-2 stimulates the immune system leading to cytokine storm (1) with markedly increased levels of several cytokines as IL–1α, IL-1β, IL-6, and TNF-α (2). In addition, an increase of neutrophils count and decreased count of lymphocytes have been observed (3). COVID-19 infection also leads to ROS generation (4) and coagulation cascade favoring the risk of thrombosis (5). Some subjects infected by SARS-CoV-2 developed a broad range of pathologies including not only pneumonia, acute respiratory distress syndrome (ARDS), respiratory failure but also systemic inflammation and multiorgan failure (1). Severe COVID-19 was associated with massive alveolar damage with loss of lung architecture, leading to ventilatory failure. A recently published article (6) reported two types of lung fibrosis after COVID-19. The first with a diffuse fibrotic alveolar damage is characterized by extracellular matrix deposition resulting in fibrosis; these patients require intubation, mechanical ventilation and/or extracorporeal membrane oxygenation (ECMO). The second is the post-COVID pulmonary fibrosis, diagnosed by the combination of clinical, radiological, and pathological information. In about 25% of patients with severe COVID-19 disease (WHO Severity Grade 3 and 4), a restrictive ventilatory defect was revealed. Thus, there is a compelling clinical need to identify circulating fibrosis markers in COVID-19. Ideally, these markers should be non-invasive, able to mirror the extent of fibrosis and to reflect disease progression and therapeutic response. SARS coronavirus induced up-regulation of Type I collagen, leading to pulmonary pro-fibrotic responses (7). Thus, collagen metabolism plays a key role in COVID-19 clinical picture. Several blood parameters have been evaluated as predictors of COVID-19 severity. However, at present, still no validated biomarkers are reliably used in routine clinical practice.
Procollagen type III amino terminal propeptide is the peptide released during the biosynthesis and depositing of type III collagen (8). TIMP-1 is an inhibitor specific for extracellular matrix (ECM) degradation enzymes (9). HA is a glycosaminoglycan engaged in the formation of ECM (10).
Elevated serum levels of PIIINP, HA or TIMP-1 were found to be increased in other diseases, such as in patients with systemic sclerosis (SSc) (11). High levels of PIIINP and HA were demonstrated to be unfavorable predictors for survival in SSc suggesting that these markers could be useful to predict other fibrotic lesions (12).
In this study we investigated the potential role of PIIINP, HA and TIMP-1 as prognostic markers in COVID-19 patients.
We enrolled 90 adult hospitalized patients with a diagnosis of SARS-CoV-2 infection, confirmed by molecular analysis (RT-PCR) of the nasopharyngeal swab (13).
Patients were stratified for COVID-19 disease severity based on WHO scale (14). According to this classification patients were classified as: (1), asymptomatic, not hospitalized (2), symptomatic, not hospitalized, independent; (3), symptomatic, not hospitalized, assistance needed; (4), hospitalized, not requiring supplemental oxygen; (5), hospitalized, requiring oxygen by non-invasive mechanical ventilation (mask or nasal prongs); (6–9), hospitalized, requiring high-flow oxygenation and/or invasive mechanical ventilation; and 10, death.
Our study population was divided according to the severity of COVID-19 at the time of sampling into the following groups: (1) hospitalized COVID-19-positive patients requiring no respiratory support or oxygen support only (WHO ≤5); (2) hospitalized COVID-19-positive patients requiring invasive or non-invasive mechanical ventilation (WHO > 5).
The study was conducted in compliance with the Declaration of Helsinki. The protocol was approved by the Ethical Committee of the University Federico II of Naples (prot. no. 140/20). Informed consent was obtained from all individuals. At the time of sampling, laboratory parameters, clinical and demographic data were recorded.
Fasting blood samples were obtained. Sera were frozen and stored at −80°C until measurements. Samples were assayed in an automated analyzer that performs magnetic separation enzyme immunoassay tests (ADVIA Centaur; Siemens Healthcare Diagnostics, Tarrytown, NY, United States) for Hyaluronic acid (HA), amino-terminal propeptide of type-III-procollagen (PIIINP) and tissue inhibitor of metalloproteinase type-1 (TIMP-1).
All statistical analyses were performed using the R platform version 4.1.2. Standard descriptive statistics were used to describe the cohort: mean ± standard deviation (range) or median (25th; 75th percentile) (range) in case of numerical variables and absolute frequency with percentages for categorical factors. Accordingly, between-group comparisons were assessed using the t-test for independent samples, the Mann-Whitney U-test and the Chi-square test (or the Fisher exact test when appropriate). Median regression with bootstrapped standard errors was used to adjust the analysis for potential confounding factors.
A total of 90 COVID-19 patients (43 female and 47 male) were enrolled and classified for disease severity based on World Health Organization (WHO) stage. 68 (75.6%) COVID-19 patients with a WHO score <5 and 22 (24.4%) with a WHO score >5. Demographic and clinical features are showed in Table 1. Mean age was 58.6 ± 15.4 (range: 38–62) years, patients with WHO score >5 were significantly older than patients with WHO score <5 (p = 0.013); no differences in comorbidities at baseline were observed between the two groups. Median disease duration (time length to negativization) was 23 days (range 5–72 days) days with a longer disease duration in patients with a WHO score >5. In the overall cohort, 37 (41.6%) patients had a time length of negativization <21 days and 52 (58.4%) >21 days.
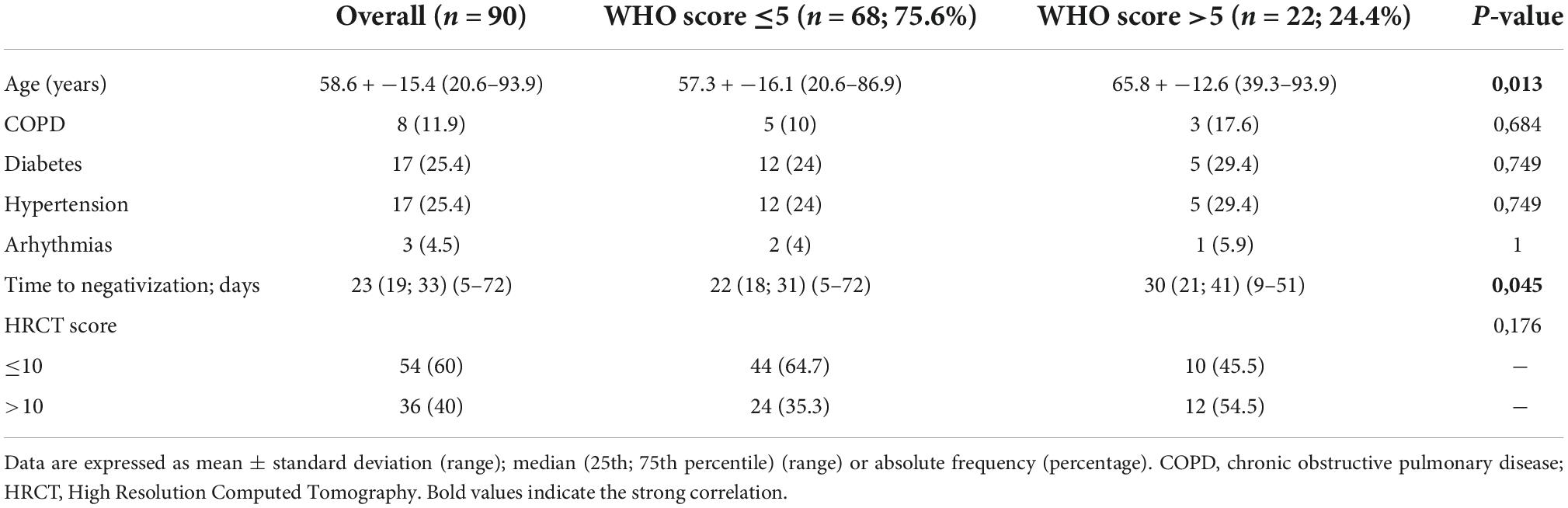
Table 1. Clinical and demographical characteristics of the study cohort stratified according to the World Health Organization (WHO) score at baseline.
Patients were classified also for High-Resolution Computed Tomography (HRCT) score, resulting in 54 subjects (60%) with a score <10 and 36 (40%) >10. Of note, lymphocyte number was significantly lower in patients with HRCT score >10 [670 (270–2540) vs. 880 (260–3350); p = 0.026).
Serum TIMP-1 levels were significantly higher in patients with WHO score >5 than in those with a WHO score ≤5 [TIMP-1: 222.2 (20.5–460) vs. 298.1 (28.5–452.8), p = 0.010] and the difference was confirmed after adjusting the analysis for the age of patients through median regression (p = 0.003). On the contrary, no statistically significant difference was observed in serum PIIINP and HA between patients with severe and mild disease [PIIINP: 7.2 (1.1–18.4) vs. 7.1 (1.2–47.5), p = 0.782; HA: 117.1(4.7–331.1) vs. 75.1 (8.3–1345.9), p = 0.258; Figure 1].
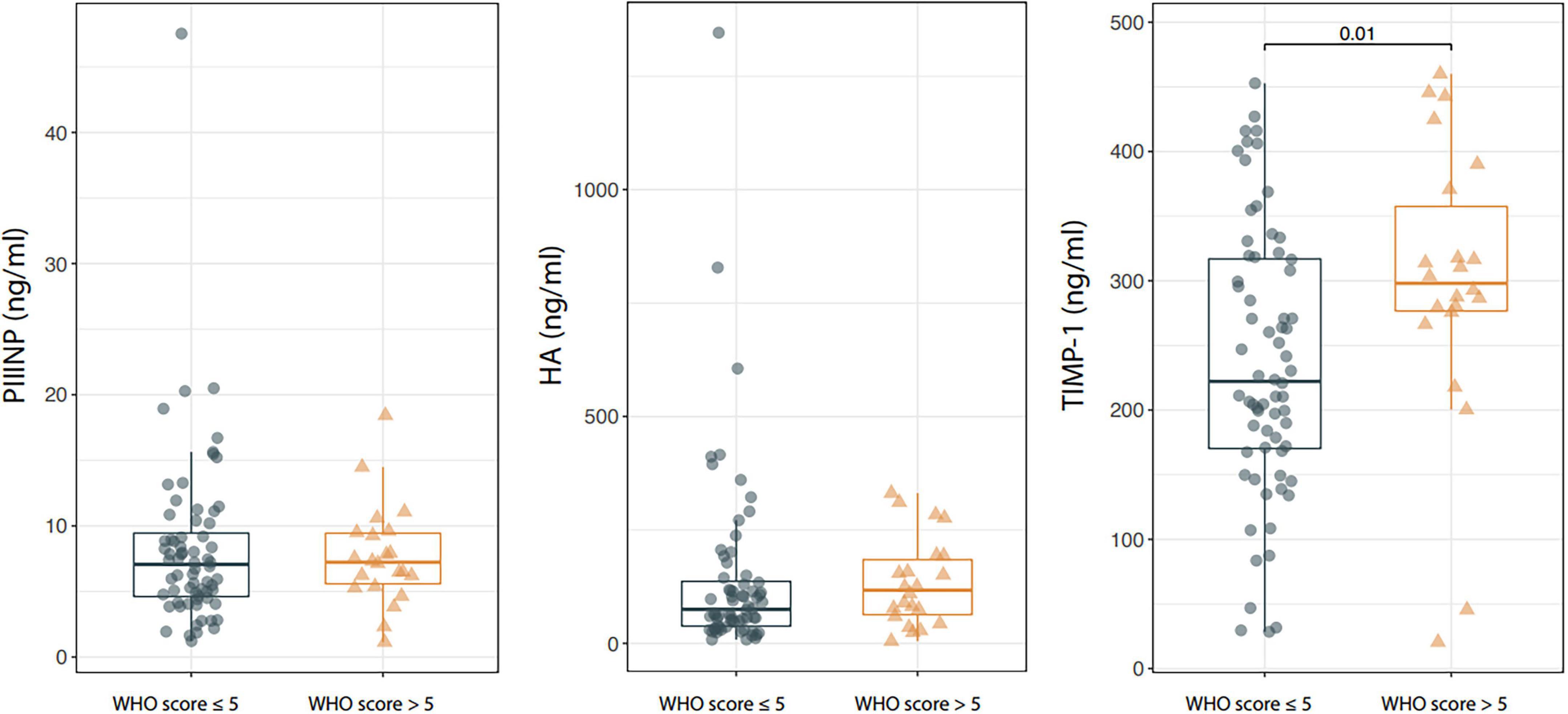
Figure 1. Box-plot showing serum procollagen type III amino terminal propeptide (PIIINP), Hyaluronic acid (HA), and tissue inhibitor of metalloproteinases 1 (TIMP-1) levels in patients with Coronavirus Disease 2019 (COVID-19) stratified according to the WHO score at baseline. Boxes are defined by Q1, Median (bold line) and Q3. Whiskers reach the minimum and the maximum of the distribution except for the presence of outliers, defined as data points below Q1–1.5*IQR or above Q3 + 1.5*IQR. To avoid overlapping a small amount of horizontal jitter was added. Q1, First quartile; Q3, Third quartile; IQR = Q3–Q1.
As shown in Table 2, LDH and CRP values were significantly higher in patients with severe disease [LDH: 373 (193–670) vs. 289 (111–741), p = 0.013; CRP: 15.8 (1.3–222.5) vs. 9.3 (0.3–132.6), p = 0.039]. Table 3 showed that serum PIIINP, HA and TIMP-1 positively correlated with LDH levels (PIIINP: r = 0.264, p = 0.009, HA: r = 0.267, p = 0.008; TIMP-1: r = 0.263, p = 0.009).

Table 2. Distribution of blood parameters in patients stratified according to their World Health Organization (WHO) score at baseline.

Table 3. Correlation among procollagen type III amino terminal propeptide (PIIINP), Hyaluronic acid (HA), and tissue inhibitor of metalloproteinases 1 (TIMP-1) with inflammatory markers.
Serum PIIINP and HA negatively correlated with albumin values (PIIINP: r = −0.362, p < 0.001; HA: r = −0.387, p < 0.001); circulating TIMP-1 levels positively correlated with CRP values (r = 0.312, p = 0.003).
Our study highlighted the significant positive correlation between changes of TIMP-1 and disease severity based on WHO classification, suggesting that TIMP-1 could serve as a non-invasive biomarker for prognosis in COVID-19.
Metzemaekers et al. (15) reported significantly higher levels of plasmatic tissue inhibitor of metalloproteinase 1 (TIMP-1) and of TIMP-1/MMP-9 complexes and significantly lower circulating total MMP activity in COVID-19 patients at intensive care unit (ICU) admission.
Our data showed that serum TIMP-1 in SARS-CoV-2 infected patients correlates with the WHO score and the CRP values, but not with HRCT score and time length of negativization.
These findings may reflect peculiar aspect of the involvement of TIMP-1 in the fibrotic process: TIMP-1 represent decreased collagen degradation and was a strong predictor of early fibrosis (16).
Considering the short disease duration and moderate disease severity of most of our study population, our data indicated that TIMP-1 could be a useful marker of fibrotic burden and disease prognosis in patients with COVID-19 at initial diagnosis. Several molecular mechanisms involving matrix metalloproteases pathway have been identified as relevant players in the clinical picture of COVID-19 (17). It has been recently shown that matrix metalloproteinase-9 (MMP-9) gene expression is increased in subjects infected with SARS-CoV-2 (18) and circulating MMP-9 levels were significantly associated with the risk of respiratory insufficiency (19) and with severity in COVID-19 patients (20). In fact, some authors previously demonstrated that metalloproteinases (MMPs) seem to play a key role in lung disease (21, 22). Severe COVID-19 shared many characteristics with sepsis (23) and plasma MMP-9 and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) have been also proposed as septic biomarkers (24, 25).
Other authors showed that periodontitis and diabetes have been associated with COVID-19 poor outcomes and both these diseases have been correlated with elevated MMP-8 levels (26, 27), further highlighting the role of MMPs as key players in COVID-19 risk and escalation.
Tissue damage during SARS-CoV-2 lung infection is associated with activation of members of the MMPs family (28, 29). Targeting MMPs pathway has been proposed as therapeutic strategy to counterbalance the host marked pro-inflammatory response to the SARS-CoV-2 infection (30). In addition of being MMP-inhibitor, TIMP-1 is independently proinflammatory and pro-growth-factor (31–33). Thus, the measurement of circulating TIMP-1 levels could be useful to assess the prognosis and to adopt a personalized treatment approach.
Serum PIIINP, TIMP-1, and HA are combined to calculate the Enhanced Liver Fibrosis (ELF) score, initially developed from a chronic liver disease cohort (34–36). Thus, it was expected that the algorithm was not readily applicable to COVID-19. Nevertheless, our results suggest the need to derive a COVID-specific algorithm based on the clinical performance of single analytes markers in SARS-CoV-2 infected subjects.
It should also be considered that serum collagen metabolites may be affected by age, diet and disease duration (16, 37). Thus, to prevent results misinterpretation, they could be better used for within-individual changes during follow-up.
This study has several limitations. First, the study population is small, second, data on the correlation of TIMP-1 levels and specific treatment are lacking, third, serial measurements to assess longitudinal modifications of serum collagen markers according to fibrotic changes are not available. Thus, larger samples are needed to obtain a better evaluation of TIMP-1 levels circulating levels as a prognostic biomarker in COVID-19 patients and to investigate its potential role in monitoring therapeutic response in different treatment subgroups of COVID-19 patients.
In conclusion, our study shed new light on the potential clinical utility of serum collagen metabolites and extracellular matrix remodeling as suitable markers of disease severity in COVID-19 patients. We unveil that changes in serum TIMP-1 significantly correlate with changes in clinical outcome. Collagen metabolites and extracellular matrix remodeling markers are worthy of further studies to assess their potential as prognostic and predictive biomarkers in COVID-19 patients. The identification of a COVID-specific index reflecting the fibrotic process in SARS-CoV-2 patients is strongly encouraged for its potential as a disruptive tool for clinical management.
The data that support the findings of this study are available from the corresponding author DT, (ZGFuaWVsYS50ZXJyYWNjaWFub0B1bmluYS5pdA==), upon reasonable request and with permission of AOU Federico II.
The studies involving human participants were reviewed and approved by the Ethical Committee of the University Federico II of Naples (prot. no. 140/20). The patients/participants provided their written informed consent to participate in this study.
DT and GP: conceptualization and writing—review and editing. SB, BP, LS, MF, VV, and IM: data curation. DB: formal analysis. GP: funding acquisition, methodology, supervision, and validation. SB, DT, MF, and LS: investigation. IG, GP, AC, and MM: resources. DT, IG, AC, MM, and GP: visualization. DT: writing—original draft. All authors have read and agreed to the published version of the manuscript.
We would like to thank Antonio D’Andrea for technical valuable contributions to our research. We appreciate technical support to our research-related activities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tang D, Comish P, Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. (2020) 16:e1008536. doi: 10.1371/journal.ppat.1008536
2. Cabaro S, D’Esposito V, Di Matola T, Sale S, Cennamo M, Terracciano D, et al. Cytokine signature and COVID-19 prediction models in the two waves of pandemics. Sci Rep. (2021) 11:20793. doi: 10.1038/s41598-021-00190-0
3. Kong M, Zhang H, Cao X, Mao X, Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. (2020) 148:e139. doi: 10.1017/S0950268820001557
4. Miripour ZS, Sarrami-Forooshani R, Sanati H, Makarem J, Taheri MS, Shojaeian F, et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens Bioelectron. (2020) 165:112435. doi: 10.1016/j.bios.2020.112435
5. Patell R, Bogue T, Bindal P, Koshy A, Merrill M, Aird WC, et al. Incidence of thrombosis and hemorrhage in hospitalized cancer patients with COVID-19. J Thromb Haemost. (2020) 18:2349–57. doi: 10.1111/jth.15018
6. King CS, Mannem H, Kukreja J, Aryal S, Tang D, Singer JP, et al. Lung transplantation for patients with COVID-19. Chest. (2022) 161:169–78. doi: 10.1016/j.chest.2021.08.041
7. Wang CY, Lu CY, Li SW, Lai CC, Hua CH, Huang SH, et al. SARS coronavirus papain-like protease up-regulates the collagen expression through non-samd TGF-beta1 signaling. Virus Res. (2017) 235:58–66. doi: 10.1016/j.virusres.2017.04.008
8. Lapiere CM, Lenaers A, Kohn LD. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci USA. (1971) 68:3054–8. doi: 10.1073/pnas.68.12.3054
9. Kikuchi K, Kadono T, Furue M, Tamaki K. Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol. (1997) 108:281–4. doi: 10.1111/1523-1747.ep12286457
10. Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. (2009) 284:9083–92. doi: 10.1074/jbc.M806989200
11. Young-Min SA, Beeton C, Laughton R, Plumpton T, Bartram S, Murphy G, et al. Serum TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis, primary raynaud’s phenomenon, and in normal controls. Ann Rheum Dis. (2001) 60:846–51.
12. Chen C, Wang L, Wu J, Lu M, Yang S, Ye W, et al. Circulating collagen metabolites and the enhanced liver fibrosis (ELF) score as fibrosis markers in systemic sclerosis. Front Pharmacol. (2022) 13:805708. doi: 10.3389/fphar.2022.805708
13. De Luca C, Gragnano G, Conticelli F, Cennamo M, Pisapia P, Terracciano D, et al. Evaluation of a fully closed real time PCR platform for the detection of SARS-CoV-2 in nasopharyngeal swabs: a pilot study. J Clin Pathol. (2021) 75:551–4. doi: 10.1136/jclinpath-2021-207516
14. WHO Working Group on the Clinical Characterisation and Management of Covid-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. (2020) 20:e192–7. doi: 10.1016/S1473-3099(20)30483-7
15. Metzemaekers M, Cambier S, Blanter M, Vandooren J, de Carvalho AC, Malengier-Devlies B, et al. Kinetics of peripheral blood neutrophils in severe coronavirus disease 2019. Clin Transl Immunol. (2021) 10:e1271. doi: 10.1002/cti2.1271
16. Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The enhanced liver fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. (2013) 59:236–42. doi: 10.1016/j.jhep.2013.03.016
17. Cacciola R, Gentilini Cacciola E, Vecchio V, Cacciola E. Cellular and molecular mechanisms in COVID-19 coagulopathy: role of inflammation and endotheliopathy. J Thromb Thrombolysis. (2021) 53:282–90. doi: 10.1007/s11239-021-02583-4
18. Hazra S, Chaudhuri AG, Tiwary BK, Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: a network-based meta-analysis. Life Sci. (2020) 257:118096. doi: 10.1016/j.lfs.2020.118096
19. Ueland T, Holter JC, Holten AR, Muller KE, Lind A, Bekken GK, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect. (2020) 81:e41–3. doi: 10.1016/j.jinf.2020.06.061
20. Gelzo M, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Scialo F, et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci Rep. (2022) 12:1212. doi: 10.1038/s41598-021-04677-8
21. Davey A, McAuley DF, O’Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J. (2011) 38:959–70. doi: 10.1183/09031936.00032111
22. Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, et al. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. (2006) 37:422–30. doi: 10.1016/j.humpath.2005.11.023
23. Beltran-Garcia J, Osca-Verdegal R, Pallardo FV, Ferreres J, Rodriguez M, Mulet S, et al. Sepsis and coronavirus disease 2019: common features and anti-inflammatory therapeutic approaches. Crit Care Med. (2020) 48:1841–4. doi: 10.1097/CCM.0000000000004625
24. Duda I, Krzych L, Jedrzejowska-Szypulka H, Lewin-Kowalik J. Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients. Open Med. (2020) 15:50–6. doi: 10.1515/med-2020-0008
25. Aguirre A, Blazquez-Prieto J, Amado-Rodriguez L, Lopez-Alonso I, Batalla-Solis E, Gonzalez-Lopez A, et al. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J Mol Med. (2017) 95:487–97. doi: 10.1007/s00109-017-1510-z
26. Gupta S, Mohindra R, Singla M, Khera S, Kumar A, Rathnayake N, et al. Validation of a noninvasive aMMP-8 point-of-care diagnostic methodology in COVID-19 patients with periodontal disease. Clin Exp Dent Res. (2022) 8:988–1001. doi: 10.1002/cre2.589
27. Gupta S, Saarikko M, Pfutzner A, Raisanen IT, Sorsa T. Compromised periodontal status could increase mortality for patients with COVID-19. Lancet Infect Dis. (2022) 22:314. doi: 10.1016/S1473-3099(22)00065-2
28. Ramirez-Martinez G, Jimenez-Alvarez LA, Cruz-Lagunas A, Ignacio-Cortes S, Gomez-Garcia IA, Rodriguez-Reyna TS, et al. Possible role of matrix metalloproteinases and TGF-beta in COVID-19 severity and sequelae. J Interferon Cytokine Res. (2022) 42:352–68. doi: 10.1089/jir.2021.0222
29. Gutman H, Aftalion M, Melamed S, Politi B, Nevo R, Havusha-Laufer S, et al. Matrix metalloproteinases expression is associated with SARS-CoV-2-induced lung pathology and extracellular-matrix remodeling in K18-hACE2 mice. Viruses. (2022) 14:1627. doi: 10.3390/v14081627
30. Hardy E, Fernandez-Patron C. Targeting MMP-regulation of inflammation to increase metabolic tolerance to COVID-19 pathologies: a hypothesis. Biomolecules. (2021) 11:390. doi: 10.3390/biom11030390
31. Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. (2008) 1:re6. doi: 10.1126/scisignal.127re6
32. Grunwald B, Schoeps B, Kruger A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol. (2019) 29:6–19. doi: 10.1016/j.tcb.2018.08.006
33. Schoeps B, Fradrich J, Kruger A. Cut loose TIMP-1: an emerging cytokine in inflammation. Trends Cell Biol. (2022). [Epub ahead of print]. doi: 10.1016/j.tcb.2022.08.005
34. Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. (2004) 127:1704–13. doi: 10.1053/j.gastro.2004.08.052
35. Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. (2010) 59:1245–51. doi: 10.1136/gut.2009.203166
36. Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced liver fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. (2011) 18:23–31. doi: 10.1111/j.1365-2893.2009.01263.x
Keywords: COVID-19, fibrosis, TIMP-1, collagen metabolites, extrcellular matrix remodelling biomarkers
Citation: Brusa S, Terracciano D, Bruzzese D, Fiorenza M, Stanziola L, Pinchera B, Valente V, Gentile I, Cittadini A, Mormile I, Mormile M and Portella G (2022) Circulating tissue inhibitor of metalloproteinases 1 (TIMP-1) at COVID-19 onset predicts severity status. Front. Med. 9:1034288. doi: 10.3389/fmed.2022.1034288
Received: 01 September 2022; Accepted: 15 November 2022;
Published: 29 November 2022.
Edited by:
Luis Garcia De Guadiana-Romualdo, Santa Lucía University General Hospital, SpainReviewed by:
Junfeng Jia, Fourth Military Medical University, ChinaCopyright © 2022 Brusa, Terracciano, Bruzzese, Fiorenza, Stanziola, Pinchera, Valente, Gentile, Cittadini, Mormile, Mormile and Portella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Terracciano, ZGFuaWVsYS50ZXJyYWNjaWFub0B1bmluYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.