
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 08 December 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1033980
This article is part of the Research Topic New Frontiers in Diagnosis and Treatment for Skin Diseases View all 13 articles
 Ayman Grada1*
Ayman Grada1* James Q. Del Rosso2,3
James Q. Del Rosso2,3 Angela Y. Moore4,5
Angela Y. Moore4,5 Linda Stein Gold6
Linda Stein Gold6 Julie Harper7
Julie Harper7 Giovanni Damiani8,9,10
Giovanni Damiani8,9,10 Katharina Shaw11
Katharina Shaw11 Sabine Obagi12
Sabine Obagi12 Raidah J. Salem13
Raidah J. Salem13 S. Ken Tanaka14
S. Ken Tanaka14 Christopher G. Bunick15,16*
Christopher G. Bunick15,16*Background: Vestibular side effects such as dizziness and vertigo can be a limitation for some antibiotics commonly used to treat acne, rosacea, and other dermatology indications.
Objective: Unlike minocycline, which is a second-generation tetracycline, sarecycline, a narrow-spectrum third-generation tetracycline-class agent approved to treat acne vulgaris, has demonstrated low rates of vestibular-related adverse events in clinical trials. In this work, we evaluate the brain-penetrative and lipophilic attributes of sarecycline in 2 non-clinical studies and discuss potential associations with vestibular adverse events.
Methods: Rats received either intravenous sarecycline or minocycline (1.0 mg/kg). Blood-brain penetrance was measured at 1, 3, and 6 h postdosing. In another analysis, the lipophilicity of sarecycline, minocycline, and doxycycline was measured via octanol/water and chloroform/water distribution coefficients (logD) at pH 3.5, 5.5, and 7.4.
Results: Unlike minocycline, sarecycline was not detected in brain samples postdosing. In the octanol/water solvent system, sarecycline had a numerically lower lipophilicity profile than minocycline and doxycycline at pH 5.5 and 7.4.
Conclusion: The reduced blood-brain penetrance and lipophilicity of sarecycline compared with other tetracyclines may explain low rates of vestibular-related adverse events seen in clinical trials.
Second-generation tetracycline-class antibiotics such as doxycycline and minocycline have been the mainstay treatment of moderate-to-severe acne vulgaris for over 50 years (1–3); however, the use of minocycline is often limited by vestibular side effects such as dizziness and tinnitus, leading to the inclusion of a warning for central nervous system side effects in the minocycline package insert (4). These side effects can impair an individual’s ability to perform daily tasks such as driving (4), thus contributing to the overall burden of managing acne vulgaris. In contrast, vestibular side effects are not typically associated with use of doxycycline (2). The higher lipophilicity of minocycline compared with doxycycline [e.g., distribution coefficient (logD) values of 1.11 (minocycline) vs. 0.95 (doxycycline) (5)] allows for greater penetration of the blood-brain barrier, thereby potentiating vestibular infiltration and by extension, dizziness and vertigo (2).
Sarecycline is a narrow-spectrum, 3rd generation tetracycline-class oral antibiotic approved by the US Food and Drug Administration (FDA) in 2018 for the treatment of moderate-to-severe acne vulgaris (6, 7). The efficacy and safety of sarecycline have been reported in two phase 3 randomized controlled trials [SC1401 (ClinicalTrials.gov identifier NCT02320149; N = 968) and SC1402 (ClinicalTrials.gov identifier NCT02322866); N = 1034], and its long-term safety was examined in a 40-week open-label extension study (ClinicalTrials.gov identifier NCT02413346; N = 490) (8, 9). Notably, low rates of vestibular-related adverse events (e.g., dizziness, motion sickness) were observed in these studies, and no events of vertigo or tinnitus were reported in patients receiving sarecycline (Figure 1). However, no clinical trials to date have compared sarecycline head-to-head with other tetracycline-class drugs (10), limiting the ability to make direct comparisons for safety and tolerability across therapies. Further, it remains unclear whether the biochemical properties of sarecycline (e.g., blood-brain barrier penetrance, lipophilicity) could be contributing to the low rates of vestibular adverse events in clinical trials.
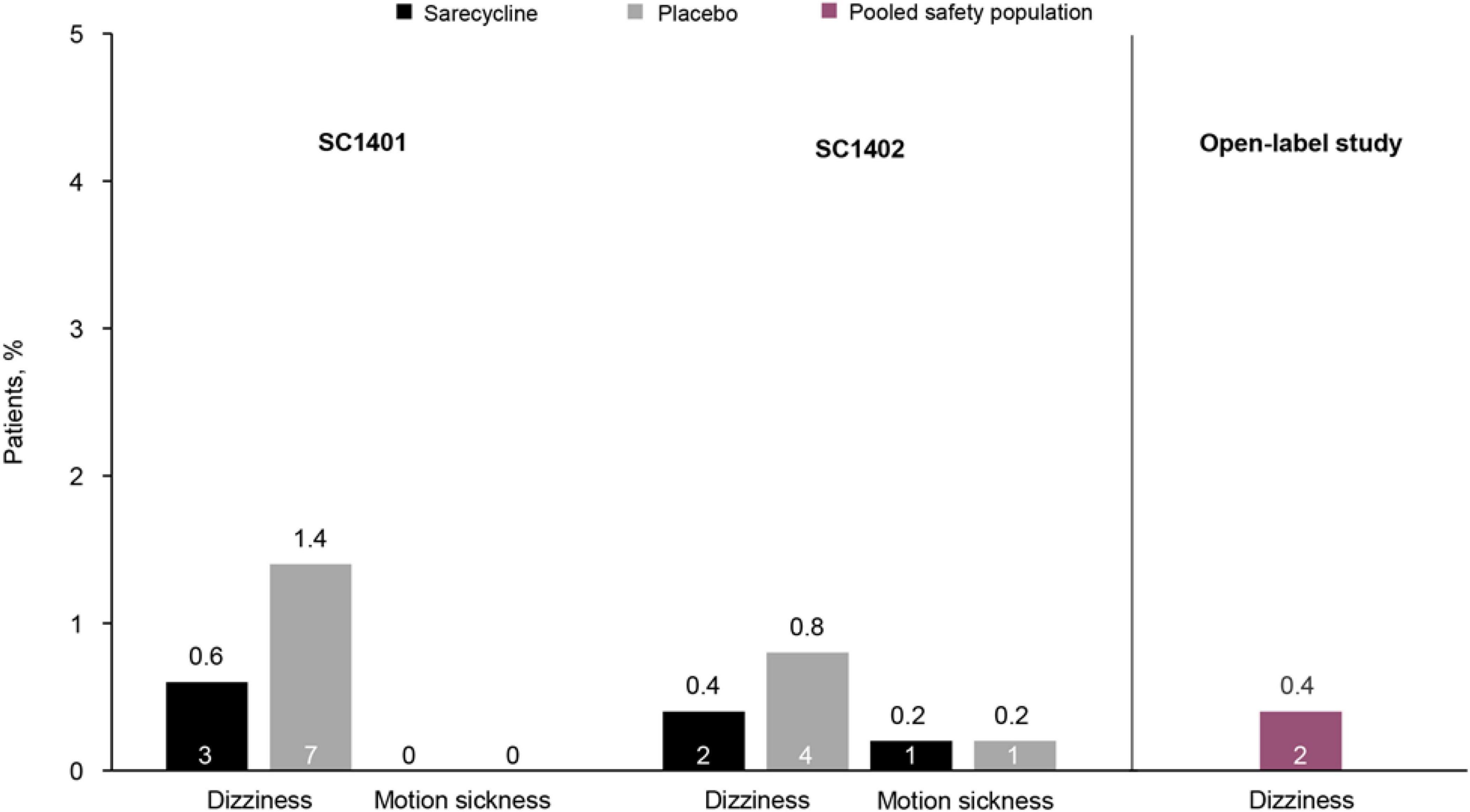
Figure 1. Rates of vestibular-related adverse events in two phase 3 placebo-controlled clinical trials of sarecycline [SC1401 (N = 964) and SC1402 (N = 1026); representing the safety population] and a phase 3 open-label extension study (N = 483) (8, 9). In the open-label study, rates of adverse events were pooled for patients who received placebo in the phase 3 clinical trials and then received sarecycline (n = 236) and those who received sarecycline in the phase 3 clinical trials and continued receiving sarecycline in the open-label study (n = 247). Motion sickness was not reported in the open-label study. The number of patients who experienced these adverse events is shown in each bar.
Here, we investigated the potential relationship between the brain-penetrative and lipophilic attributes of sarecycline from 2 preclinical in vivo and in vitro analyses. In the first analysis, we examined the ability of sarecycline to penetrate the blood-brain barrier relative to minocycline in a rat model study. In the second analysis, we determined the lipophilicity of sarecycline compared with minocycline and doxycycline.
All protocols involving animals were approved by the Institutional Animal Care and Use Committee (IACUC).
Six male Wistar rats (150–200 g; Charles River Laboratories, Wilmington, MA, USA) were used in this study. Each rat was pre-cannulated in the jugular vein. Rats were kept in individual cages, with water and feed ad libitum, and alternating 12 h light cycles. Before dosing, rats underwent an overnight fast (∼16 h) in metabolic cages and were weighed to determine dose volume (1.0 ml/kg). The rats were then intravenously (IV) dosed with either sarecycline (N = 3) or minocycline (N = 3) at a total dose of 1.0 mg/kg, and access to food was restored 2 h after dosing. Subsequently, rats were euthanized via CO2 and brain samples and whole blood, collected via heart puncture, were harvested from 2 rats at each of the following time points: 1, 3, and 6 h postdosing.
The endpoint of this analysis was concentration of sarecycline or minocycline in plasma (μg/ml) and in brain samples (μg/g) at 1, 3, and 6 h postdosing. Plasma and brain homogenate samples were prepared by protein precipitation with acetonitrile, followed by centrifugation. The samples were injected on an API 2000 mass spectrometer (Applied Biosystems, Foster City, CA, USA) and analyzed in positive ion mode using doxycycline as an internal standard. Values were calculated using Analyst 1.2 quantitation software (SciEx, Framingham, MA, USA). Linear through zero regression analysis with no weighting factor was used to determine the calculated concentrations of the injected samples.
Sarecycline, minocycline, and doxycycline were each prepared as 1.0 mg/ml stock solutions (4/8 ml) in 3 separate aqueous phase pH buffers (pH 3.5, 5.5, and 7.4). The pH of aqueous phase stock solutions was adjusted to within 0.1 of the desired pH before analysis; stock solutions had a further equilibration period of 2 h before pH adjustment as needed.
Aqueous phase stock solutions were quantified by high performance liquid chromatography (HPLC) before mixing with the organic phase to avoid any potential degradation effects. Next, 2 ml each of aqueous saturated octanol/chloroform and aqueous stock solution were combined and vortex-mixed to encourage interaction between phases. Each experimental condition was conducted in triplicate.
The mixtures were equilibrated at 25°C for 24 h at 750 rotations per minute to allow partitioning. At 2 intervals, the mixtures were vortex-mixed to ensure that an emulsion formed. Following equilibration, the mixtures were allowed to settle before aqueous and organic layers were separated into discrete HPLC vials.
Following 24-h equilibration and phase separation, final pH values of the aqueous phases were recorded. Sarecycline and doxycycline octanol and chloroform phases were diluted as needed with acetonitrile, and minocycline octanol and chloroform phases were diluted as needed with dimethyl sulfoxide. Phases were analyzed by HPLC.
The endpoint of this analysis was the calculation of logD values for each compound at pH 3.5, 5.5, and 7.4. LogD values were calculated by the ratio of the peaks found in the aqueous phase vs. the organic layer, with lower values indicating less lipophilicity and higher values indicating greater lipophilicity. Confirmation of recovery was calculated by determining the concentration of the aqueous stock solution.
Brief results of this study have been reported previously (10). Concentrations of sarecycline and minocycline in rat blood plasma and brain are included in Table 1 and illustrated in Figure 2. Concentrations of sarecycline and minocycline in rat plasma were similar to each other at each measured time point following IV administration [0.460, 0.217, 0.049 ug/ml (sarecycline) versus 0.333, 0.174 and 0.077 ug/ml (minocycline) at hours 1, 3, and 6, respectively]. However, while detectable concentrations of minocycline in the brain were observed at each measured time point (0.074, 0.139, and 0.068 μg/g at hours 1, 3, and 6, respectively), the level of sarecycline remained below the lower limit of quantitation (0.05 μg/g) in all brain samples at each measured time point.
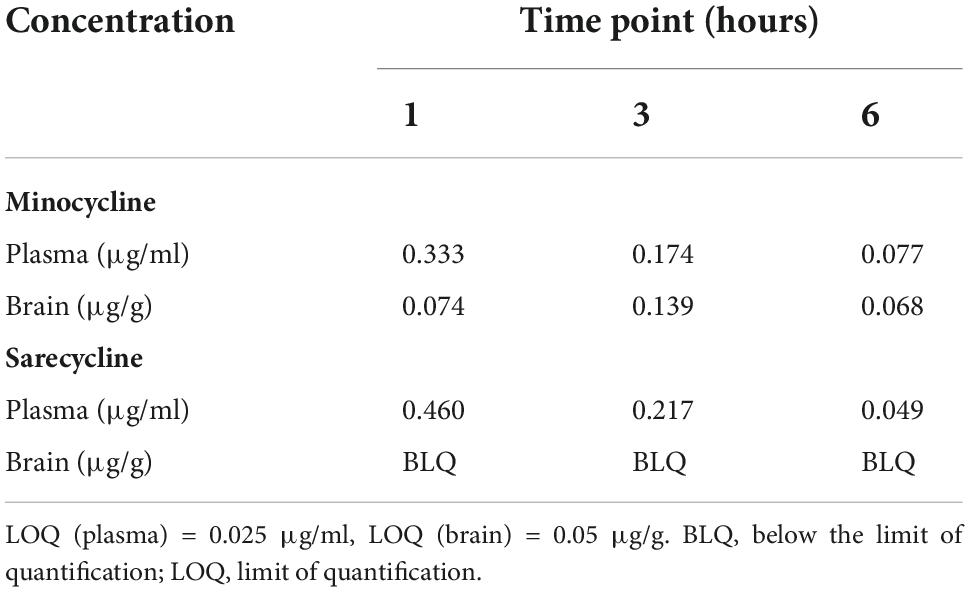
Table 1. Concentration of minocycline and sarecycline in plasma (μg/ml) and brain (μg/g) after intravenous administration.
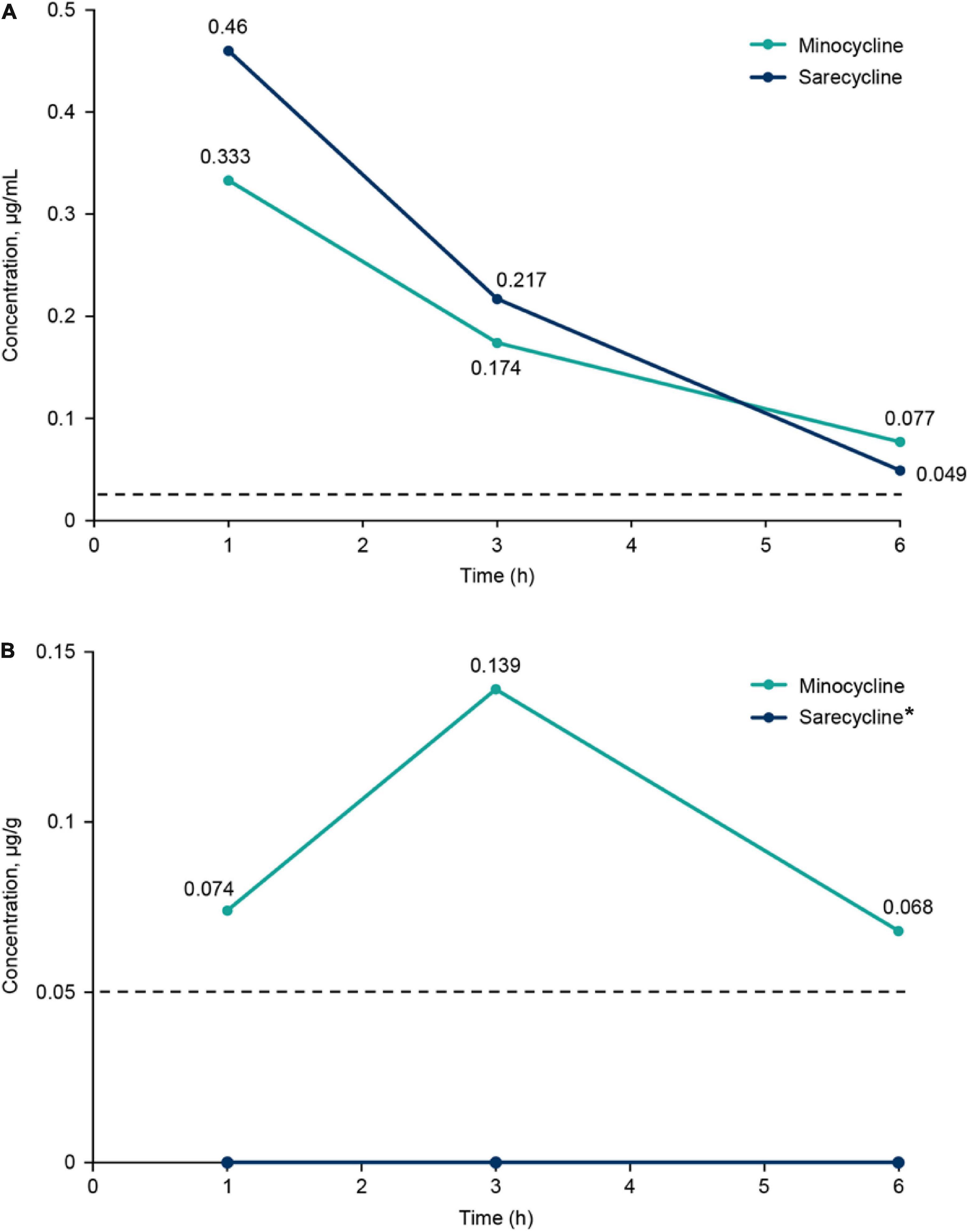
Figure 2. (A) Plasma concentrations of sarecycline and minocycline following intravenous administration in rats. (B) Brain concentrations of sarecycline and minocycline following intravenous administration in rats. Dashed line indicates limit of quantitation for plasma (0.025 μg/ml) and brain (0.05 μg/g). *Concentration of sarecycline in the brain was below the limit of quantitation at all time points.
The logD values of sarecycline, minocycline, and doxycycline in octanol/water and chloroform/water solvent systems at 25°C are reported in Table 2. In the octanol/water system, sarecycline was numerically less lipophilic than minocycline at pH 5.5 and 7.4 (−0.16 vs. 0.09 and −0.26 vs. 0.12, respectively) and numerically more lipophilic at pH 3.5 (−0.30 vs. −1.07, respectively). The lipophilicity of doxycycline was between that of sarecycline and minocycline at pH 5.5 and 7.4.
In the chloroform/water system, sarecycline was also numerically less lipophilic than minocycline at pH 7.4, was similarly lipophilic at pH 5.5, and was more lipophilic at pH 3.5. Overall, absolute values of logD were higher in the chloroform/water system than in the octanol/water system for sarecycline (pH 3.5, 1.14 vs. −0.30; pH 5.5, 1.48 vs. −0.16; and pH 7.4, 1.46 vs. −0.26, respectively) and minocycline (pH 3.5, 0.07 vs. −1.07; pH 5.5, 1.49 vs. 0.09; and pH 7.4, 1.65 vs. 0.12, respectively), whereas there was little difference in doxycycline logD values between the 2 solvent systems for each pH solution. Differences in logD values observed between the 2 solvent systems may be attributed to the unique hydrogen bonding capabilities of each system (11).
To explore potential mechanisms of action associated with the development of vestibular adverse events with certain oral tetracyclines, preclinical in vitro and in vivo analyses were performed to examine the biochemical properties of sarecycline relative to minocycline and doxycycline. In the analysis of in vivo blood-brain barrier penetrance, IV administered minocycline was detectable in the brain in rats, whereas sarecycline was not (10). In the analysis of in vitro lipophilicity, sarecycline had slightly lower logD values compared with doxycycline and minocycline at pH 5.5 and 7.4 in the octanol/water system. Taken together, these results suggest that the lower lipophilicity and reduced brain penetrance of sarecycline relative to minocycline could explain the lower incidence of vestibular adverse events (dizziness, tinnitus, vertigo) seen in sarecycline clinical trials vs. minocycline clinical trials.
In phase 3 clinical trials of sarecycline, overall rates of vestibular adverse events were low (Figure 1) (8, 9). In two identical, randomized, double-blind, placebo-controlled, phase 3 studies of sarecycline (SC1401 and SC1402), rates of the vestibular-related adverse events dizziness and motion sickness were low (≤ 1%) in 994 patients treated with sarecycline (SC1401 = 481; SC1402 = 513) over 12 weeks (8). Additionally, rates of dizziness were lower in patients receiving sarecycline (0.6 and 0.4%) compared with those receiving placebo (1.4 and 0.8%). In both studies, no events of vertigo or tinnitus were reported in either treatment group. Further, in a phase 3 open-label extension study of 483 patients who completed one of the two phase 3 placebo-controlled 12-week trials, dizziness occurred in 0.4% of patients during up to 40 additional weeks of sarecycline treatment (9). Similarly, no events of vertigo or tinnitus were reported in the extension study. Comparatively, in a placebo-controlled, dose-ranging, 12-week trial of extended-release minocycline in 233 patients with moderate to severe facial acne vulgaris, rates of acute vestibular adverse events (dizziness, vertigo, and ringing in the ears) were most commonly reported during the first 5 days of treatment (12, 13). Incidence of acute vestibular adverse events was dose dependent, occurring in 10.2, 23.7, and 28.3% of patients receiving extended-release minocycline 1, 2, or 3 mg/kg, respectively, versus 16.4% in the placebo group during the first 5 days of treatment. Similarly, in a pooled analysis of phase 3 trials of extended-release 1 mg/kg minocycline, rates of acute vestibular adverse events were 9–10.5% during the first 5 days of treatment (13). In a systematic review representing 226,019 pediatric and adult acne patients, Armstrong et al. reported adverse events associated with sarecycline, minocycline, doxycycline and tetracycline, and showed higher rates of acute vestibular events associated with minocycline (∼10%) (14).
In the current analysis, sarecycline was not detected in rat brain samples up to 6 h following IV dosing (10). In contrast, levels of minocycline were detected in rat brain up to 6 h after IV dosing. A limitation of this animal study is the small sample size, which may make it difficult to generalize. However, the trend seen in the results support previous reports that minocycline has high lipid solubility and, thus, may more readily cross the blood-brain barrier compared with other tetracyclines (15). For instance, a previous report in a canine model indicated that the blood-brain penetrance of minocycline was almost threefold higher than that of doxycycline after IV dosing (16). Although no human studies have definitively confirmed this association, the higher brain penetrance of minocycline is suspected to contribute to its higher rates of associated vestibular-related side effects relative to other tetracyclines (10, 16). Minocycline is considered unacceptable for military aviators and is completely restricted for use because of the risk for central nervous system side effects including vestibular side effects such as light-headedness, dizziness and vertigo (17). Conversely, the low rates of vestibular and phototoxic events seen with narrow-spectrum sarecycline make it an acceptable treatment option for the military population and individuals whose lifestyles and careers would suffer because of vestibular side effects.
Previously published logD values of tetracyclines, especially that of minocycline, are variable and inconsistencies permeate in the dermatology literature. For instance, it was previously reported that minocycline was fourfold more lipophilic than doxycycline and 10-fold more lipophilic than tetracycline at a pH of 5.5 (18, 19). Another analysis found that the logD values for minocycline and doxycycline at pH 5.6 were similar (1.11 and 0.95, respectively) (5). The current analysis indicates that in the octanol/water solvent system at pH 5.5 and 7.4, sarecycline (−0.16 and −0.26, respectively) was slightly less lipophilic than both minocycline (0.09 and 0.12, respectively) and doxycycline (0.00 and −0.08, respectively).
A limitation of this analysis is the interpretation of the lipophilicity results. Because octanol/water solvent systems are the most utilized format for lipophilicity analyses (20), this output was determined to provide the more meaningful result in the current analysis rather than the chloroform/water solvent system. However, caution has been advised for basing pharmacological behavior on partition coefficients, limiting the scope of these results (16). Nevertheless, a strength of the current analysis is that the low lipophilicity of sarecycline is supported by data demonstrating a lack of detectable brain penetrance in rats, which further validates the pharmacological profile of sarecycline.
The lower lipophilicity and decreased blood-brain barrier penetration observed for sarecycline ultimately must be explained by chemical structure differences between it and minocycline and doxycycline (21–23). While all three drugs share a common naphthacene four-ring core (21, 22), sarecycline is distinguished by a long C7 extension (7-[[methoxy(methyl)amino]methyl) that provides unique and enhanced ribosomal binding through mRNA contact (Figure 3) (21). The C7 moiety contains an oxygen atom (21), which functions as an acceptor of hydrogen bonds, thereby reducing lipophilicity (24, 25). Additional studies are warranted to investigate the mechanism by which sarecycline is associated with lower rates of vestibular adverse events relative to other tetracyclines, but the chemical structure points toward the C7 moiety oxygen. Thus, the new frontier in this work is specific alterations in the chemical properties of dermatologic drugs have potential to make a major impact on reducing real-world adverse events experienced by patients.
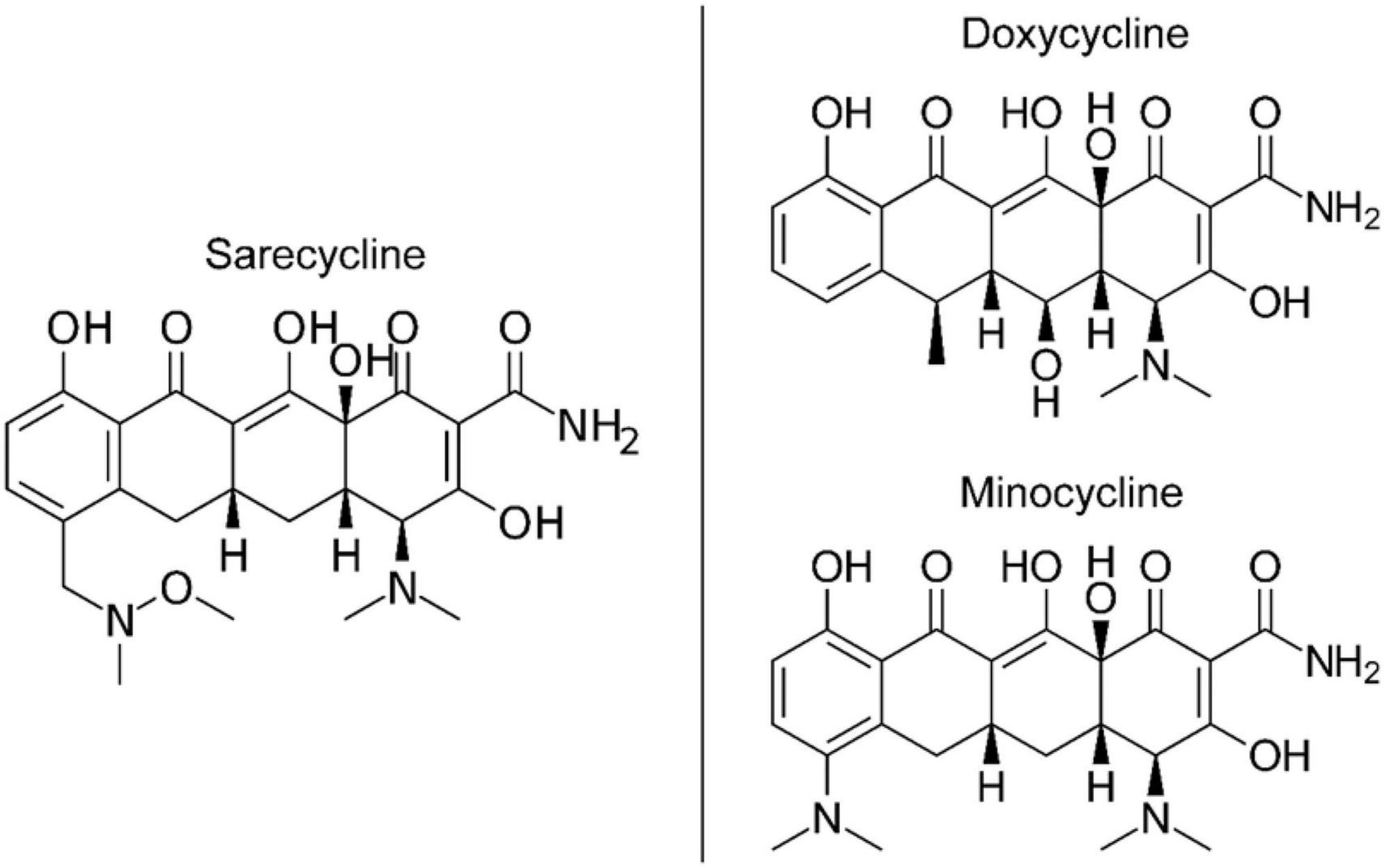
Figure 3. Chemical structures of tetracycline-class antibiotics (26).
While high drug lipophilicity in the skin is a desired attribute as it helps in penetrating and accumulating in the lipid-rich pilosebaceous unit—where acne vulgaris therapeutic target, Cutibacterium acnes, resides and proliferates (19)—the increased potential of minocycline to cross the blood-brain barrier compared with other tetracycline-class drugs has served as a purported explanation for the higher rates of vestibular side effects associated with systemic minocycline use in acne treatment, which typically requires a prolonged treatment duration (27–30). Although no confirmatory link has been established, the lack of detectable brain penetrance of sarecycline and its relatively low lipophilicity compared with minocycline as reported in these preclinical studies may correspond with the lower rates of vestibular adverse events observed in clinical trials of sarecycline.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
AG, CB, and ST: conceptualization. AG and ST: methodology. CB: validation. AG: formal analysis. AG and ST: investigation. AG, RS, and ST: resources. AG and LSG: writing—original draft preparation. AG, AM, LSG, CB, JH, GD, KS, RS, and ST: writing—review and editing. CB, SO, and RS: visualization. AG, RS, and ST: supervision. RS: project administration. All authors have read and agreed to the published version of the manuscript.
This study received funding from Almirall, LLC. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Editorial assistance was provided under the direction of the authors by Deirdre Rodeberg, Ph.D., CMPP, and Chris Lawrence, Ph.D., ELS, at MedThink SciCom.
AG was a former employee of Almirall, LLC. RS is an employee of Almirall, LLC. ST was an employee of Paratek Pharmaceuticals, Inc. AM has received research funds and honoraria from Almirall, Galderma, Mayne, and Vyne. JH has served as an advisor, investigator, speaker for Almirall, Cutera, EPI, Galderma, Sun, and Vyne. GD has received research funds and honoraria from Almirall, Galderma, Novartis, Amgen, and UCB. LSG has served as an advisor, investigator, and speaker for Almirall, Sun, Galderma, Ortho Derm, Cutera, and EPI Health. CB has served as an investigator for Almirall, a consultant for Abbvie, Almirall, LEO Pharma, Sanofi-Regeneron, UCB, a speaker for and received honoraria from Allergan, Almirall, LEO Pharma, and UCB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. (2016) 74:945–73.e33. doi: 10.1016/j.jaad.2015.12.037
2. Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. (2015) 8:19–26.
3. Graber EM. Treating acne with the tetracycline class of antibiotics: a review. Dermatol Rev. (2021) 2:321–30. doi: 10.1002/der2.49
5. Colaizzi JL, Klink PR. pH-Partition behavior of tetracyclines. J Pharm Sci. (1969) 58:1184–9. doi: 10.1002/jps.2600581003
7. Del Rosso JQ, Stein Gold L, Baldwin H, Harper JC, Zeichner J, Obagi S, et al. Management of truncal acne with oral sarecycline: pooled results from two phase-3 clinical trials. J Drugs Dermatol. (2021) 20:634–40.
8. Moore A, Green LJ, Bruce S, Sadick N, Tschen E, Werschler P, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. (2018) 17:987–96.
9. Pariser DM, Green LJ, Lain EL, Schmitz C, Chinigo AS, McNamee B, et al. Safety and tolerability of sarecycline for the treatment of acne vulgaris: results from a phase III, multicenter, open-label study and a phase I phototoxicity study. J Clin Aesthet Dermatol. (2019) 12:E53–62.
10. Moore AY, Del Rosso J, Johnson JL, Grada A. Sarecycline: a review of preclinical and clinical evidence. Clin Cosmet Investig Dermatol. (2020) 13:553–60. doi: 10.2147/CCID.S190473
11. el Tayar N, Tsai RS, Testa B, Carrupt PA, Leo A. Partitioning of solutes in different solvent systems: the contribution of hydrogen-bonding capacity and polarity. J Pharm Sci. (1991) 80:590–8. doi: 10.1002/jps.2600800619
12. Stewart DM, Torok HM, Weiss JS, Plott RT, Solodyn Phase 2 Study Group. Dose-ranging efficacy of new once-daily extended-release minocycline for acne vulgaris. Cutis. (2006) 78(Suppl.):11–20.
13. Fleischer AB Jr., Dinehart S, Stough D, Plott RT, Solodyn Phase 2 Study Group, Solodyn Phase 3 Study Group. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. (2006) 78(Suppl.):21–31.
14. Armstrong AW, Hekmatjah J, Kircik LH. Oral tetracyclines and acne: a systematic review for dermatologists. J Drugs Dermatol JDD. (2020) 19:s6–13.
15. Cunha BA, Baron J, Cunha CB. Similarities and differences between doxycycline and minocycline: clinical and antimicrobial stewardship considerations. Eur J Clin Microbiol Infect Dis. (2018) 37:15–20. doi: 10.1007/s10096-017-3081-x
16. Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother. (1975) 8:713–20. doi: 10.1128/AAC.8.6.713
17. Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. (2020) 106:18–20. doi: 10.12788/cutis.0057
18. Cunha BA, Garabedian-Ruffalo SM. Tetracyclines in urology: current concepts. Urology. (1990) 36:548–56. doi: 10.1016/0090-4295(90)80201-W
19. Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamic perspectives. J Clin Aesthet Dermatol. (2011) 4:40–7.
20. Giaginis C, Tsantili-Kakoulidou A. Alternative measures of lipophilicity: from octanol-water partitioning to IAM retention. J Pharm Sci. (2008) 97:2984–3004. doi: 10.1002/jps.21244
21. Batool Z, Lomakin IB, Polikanov YS, Bunick CG. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proc Natl Acad Sci U S A. (2020) 117:20530–7. doi: 10.1073/pnas.2008671117
22. Nguyen F, Starosta AL, Arenz S, Sohmen D, Donhofer A, Wilson DN. Tetracycline antibiotics and resistance mechanisms. Biol Chem. (2014) 395:559–75. doi: 10.1515/hsz-2013-0292
23. Dufès C. Chapter 6: Brain delivery of peptides and proteins. In: CVD Walle editor. Peptide and Protein Delivery. Cambridge, MA: Academic Press (2011). p. 105–22. doi: 10.1016/B978-0-12-384935-9.10006-9
24. Chikhale EG, Ng KY, Burton PS, Borchardt RT. Hydrogen bonding potential as a determinant of the in vitro and in situ blood-brain barrier permeability of peptides. Pharm Res. (1994) 11:412–9. doi: 10.1023/A:1018969222130
25. Czyrski A. Determination of the lipophilicity of ibuprofen, naproxen, ketoprofen, and flurbiprofen with thin-layer chromatography. J Chem. (2019) 2019:1–6. doi: 10.1155/2019/3407091
26. Bunick CG, Keri J, Tanaka SK, Furey N, Damiani G, Johnson JL, et al. Antibacterial mechanisms and efficacy of sarecycline in animal models of infection and inflammation. Antibiotics (Basel). (2021) 10:439. doi: 10.3390/antibiotics10040439
27. Brogden R, Speight T, Avery G. Minocycline: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs. (1975) 9:251–91. doi: 10.2165/00003495-197509040-00005
28. Zarzuelo A, Galvez J, Garrido-Mesa N, Garrido-Mesa N. Minocycline: far beyond an antibiotic. Br J Pharmacol. (2013) 169:337–52. doi: 10.1111/bph.12139
29. Somech R, Arav-Boger R, Assia A, Spirer Z, Jurgenson U. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatric Dermatol. (1999) 16:469–72. doi: 10.1046/j.1525-1470.1999.00106.x
Keywords: acne vulgaris, sarecycline, minocycline, dizziness, blood-brain barrier, lipophilicity, antibiotic
Citation: Grada A, Del Rosso JQ, Moore AY, Stein Gold L, Harper J, Damiani G, Shaw K, Obagi S, Salem RJ, Tanaka SK and Bunick CG (2022) Reduced blood-brain barrier penetration of acne vulgaris antibiotic sarecycline compared to minocycline corresponds with lower lipophilicity. Front. Med. 9:1033980. doi: 10.3389/fmed.2022.1033980
Received: 01 September 2022; Accepted: 22 November 2022;
Published: 08 December 2022.
Edited by:
Stefania Guida, Vita-Salute San Raffaele University, ItalyReviewed by:
Claudio Conforti, University of Trieste, ItalyCopyright © 2022 Grada, Del Rosso, Moore, Stein Gold, Harper, Damiani, Shaw, Obagi, Salem, Tanaka and Bunick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayman Grada, YXltYW4uZ3JhZGFAY2FzZS5lZHU=; Christopher G. Bunick, Y2hyaXN0b3BoZXIuYnVuaWNrQHlhbGUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.