
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 20 October 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1027586
 Josephine Braunsteiner1
Josephine Braunsteiner1 Dominik Jarczak1
Dominik Jarczak1 Christian Schmidt-Lauber2
Christian Schmidt-Lauber2 Olaf Boenisch1
Olaf Boenisch1 Geraldine de Heer1
Geraldine de Heer1 Christoph Burdelski1
Christoph Burdelski1 Daniel Frings1
Daniel Frings1 Barbara Sensen1
Barbara Sensen1 Axel Nierhaus1
Axel Nierhaus1 Elion Hoxha2
Elion Hoxha2 Tobias B. Huber2
Tobias B. Huber2 Dominic Wichmann1
Dominic Wichmann1 Stefan Kluge1
Stefan Kluge1 Marlene Fischer1†
Marlene Fischer1† Kevin Roedl1*†
Kevin Roedl1*†Background: Coronavirus disease 2019 (COVID-19) has resulted in high hospitalization rates worldwide. Acute kidney injury (AKI) in patients hospitalized for COVID-19 is frequent and associated with disease severity and poor outcome. The aim of this study was to investigate the incidence of kidney replacement therapy (KRT) in critically ill patients with COVID-19 and its implication on outcome.
Methods: We retrospectively analyzed all COVID-19 patients admitted to the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf (Germany) between 1 March 2020 and 31 July 2021. Demographics, clinical parameters, type of organ support, length of intensive care unit (ICU) stay, mortality and severity scores were assessed.
Results: Three-hundred critically ill patients with COVID-19 were included. The median age of the study population was 61 (IQR 51–71) years and 66% (n = 198) were male. 73% (n = 219) of patients required invasive mechanical ventilation. Overall, 68% (n = 204) of patients suffered from acute respiratory distress syndrome and 30% (n = 91) required extracorporeal membrane oxygenation (ECMO). We found that 46% (n = 139) of patients required KRT. Septic shock (OR 11.818, 95% CI: 5.941–23.506, p < 0.001), higher simplified acute physiology scores (SAPS II) (OR 1.048, 95% CI: 1.014–1.084, p = 0.006) and vasopressor therapy (OR 5.475, 95% CI: 1.127–26.589, p = 0.035) were independently associated with the initiation of KRT. 61% (n = 85) of patients with and 18% (n = 29) without KRT died in the ICU (p < 0.001). Cox regression found that KRT was independently associated with mortality (HR 2.075, 95% CI: 1.342–3.208, p = 0.001) after adjusting for confounders.
Conclusion: Critically ill patients with COVID-19 are at high risk of acute kidney injury with about half of patients requiring KRT. The initiation of KRT was associated with high mortality.
In late 2019 the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged and has spread worldwide since then, infecting millions of people (1). The clinical presentation of coronavirus disease 2019 (COVID-19) ranges from mild respiratory symptoms up to severe pneumonia with life-threatening complications, including acute respiratory distress syndrome (ARDS), multi-organ failure and subsequently death (2, 3). Around 20% of patients with SARS-CoV-2 infection have to be admitted to the hospital, and approximately 5% of all patients required treatment in the intensive care unit (ICU) (4–6). While the disease preferentially infects cells in the respiratory tract, there is evidence for involvement of other organ systems, particularly the kidneys (7, 8).
It has been suggested that acute kidney injury (AKI) is associated with COVID-19 disease severity and might be an indicator of poor prognosis (9, 10). Multiple pathogenic mechanisms of COVID-19 associated AKI have been proposed including local inflammation and cytokine release, possible viral invasion, endothelial dysfunction, exposure to nephrotoxins, hypovolemia, coagulopathy, rhabdomyolysis, and impact of mechanical ventilation on renal function (11–13). There is a large heterogeneity in the reported incidence of AKI (7–57%) owing to factors such as variations in clinical management, different definitions of AKI used in clinical research, geographical and socioeconomic differences, pre-existing comorbidities, and severity of disease (14–17). Requirement of kidney replacement therapy (KRT) was frequently reported in about 20–33% of critically ill patients with COVID-19 during the ICU stay (4, 9, 16). Of interest, patients with AKI associated with COVID-19 were more likely to require KRT than those without COVID-19 (16). Additionally, patients requiring both invasive ventilation and dialysis had the highest in-hospital mortality rate of 73% (5). A considerable number of patients with severe COVID-19 suffering from ARDS refractory to conservative management requires veno-venous extracorporeal membrane oxygenation (ECMO) as rescue therapy. In patients with ECMO support a high incidence of AKI (70 to 80%) and KRT was observed and a strong association with mortality was found (18). Currently, there is limited data of risk factors, use and outcome of KRT in critically ill patients with COVID-19. Furthermore, incidence and outcome of KRT in patients receiving ECMO due to severe COVID-19 associated ARDS has not been reported to date.
In the current study we aimed to identify the incidence, risk factors and outcome in critically ill patients with COVID-19 in a large tertiary care center in Germany.
We retrospectively analyzed consecutive COVID-19 patients admitted to the ICU of the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf (Germany) between 1 March 2020 and 31 July 2021. The study was approved by the Ethics Committee of the Hamburg Chamber of Physicians (No. 2021-300112-WF). Owing to the retrospective character of the study and anonymized data collection, the need for informed consent was waived. The primary endpoint of this study was requirement of KRT.
We included all consecutive adult patients (≥18 years) with confirmed and symptomatic COVID-19. Confirmed COVID-19 was defined as at least one positive result on reverse transcriptase polymerase chain reaction (PCR) obtained from nasopharyngeal swabs and/or bronchial secretions and typical symptoms including dyspnea, fever, or cough. Patients without confirmed COVID-19, ongoing ICU stay at the time of data censoring and patients aged <18 years were excluded.
Patient data was collected from the department’s electronical patient data management system (PDMS; Integrated Care Manager® (ICM), Version 9.1 – Draeger Medical, Luebeck, Germany). The data included positive SARS-CoV-2 PCR, gender, age, body mass index (BMI), comorbidities, admission diagnosis, length of ICU stay, organ support (mechanical ventilation, ECMO, vasopressor support, and KRT), medication, and laboratory test results.
Severity of illness was evaluated with the sequential organ failure assessment (SOFA) (19) and the simplified acute physiology scores II (SAPS II) (20). The Charlson Comorbidity Index (CCI) (21) was calculated for all patients. Clinical management was performed according to national and international guidelines, including prone positioning in moderate to severe ARDS and, restrictive fluid management following the initial resuscitation period (22). ARDS was defined according to the Berlin definition, using the PaO2/FiO2 ratio (Horowitz index) as marker for severity (23). Vasopressor support was initiated to maintain a mean arterial pressure (MAP) of 65 mmHg or higher using norepinephrine (22, 24). Patients with severe hypoxemic and/or hypercapnic respiratory failure in combination with severe respiratory acidosis refractory to adjunctive therapies received vv-ECMO. Criteria for the initiation of vv-ECMO support were based on the guidelines of the Extracorporeal Life Support Organization (ELSO) and national recommendations (22, 25). Severe AKI was diagnosed using urine output and/or serum creatinine following the KDIGO guidelines (26). Initiation of KRT followed the most recent Austrian/German recommendations (27, 28). Initiation of KRT was considered by the treating clinician in accordance with local standardized protocols in patients with severe metabolic acidosis (pH <7.2), anuria unresponsive to fluid resuscitation measures, hyperkalemia (serum potassium concentration exceeding 6.5 mmol/L), serum creatinine concentration above 3.4 mg/dl, presence of clinically significant organ edema (e.g., pulmonary edema), or uremic complications (27, 28). KRT in patients with and without vv-ECMO was performed via a separate central venous access. Patient survival was assessed at ICU discharge, after 28 and after 90 days. Last day of follow-up was 1 October 2021.
Data are presented as absolute numbers and relative frequency or median with interquartile range (IQR). Categorial variables were compared via Chi-square test or Fisher’s exact test, as appropriate. Continuous variables were compared via Mann–Whitney-U test. Survival function estimates were calculated using Kaplan–Meier method and were compared by log rank test. To assess factors associated with the requirement of KRT we performed a logistic regression analysis. Clinically relevant variables (age, BMI, gender, septic shock, SAPS II, CCI, ARDS, vasopressors, and ICU length of stay) were included in the initial model and were eliminated stepwise backward. The association between KRT and survival after 90 days was analyzed with a Cox regression model. Clinically relevant variables (age, BMI, gender, ARDS, vasopressors, and KRT) were included in the initial model and were eliminated stepwise backward. We performed an exploratory analysis. Statistical analysis was conducted using IBM SPSS Statistics Version 24.0 (IBM Corp., Armonk, NY, USA). The study protocol was prepared in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) recommendations (29).
Throughout the study period from 1 March 2020 until 31 July 2021, 316 critically ill patients with confirmed SARS-CoV-2 infection were admitted to our department. A total number of 300 patients were included in the study after exclusion of 16 patients with ongoing treatment at the end of the study period (Figure 1). Detailed demographics and baseline characteristics are reported in Table 1 and Supplementary Table 1.
Of 300 patients, 46% (n = 139) required KRT during their ICU stay. Chronic kidney disease prior to ICU admission was observed in 13% (n = 39) of the entire cohort. In 40% (56/139) of patients with KRT, KRT was started on the day of admission, in 9 patients KRT had been started in the referring hospital. The median time to start of KRT after ICU admission was 2 (1–8) days and the median duration of KRT on ICU was 13 (4–32) days. Indication for KRT was absolute in 73% (n = 102) and relative 27% (n = 37). At time of start of KRT there were more than one criterion (absolute or relative indication) present in 65% (n = 90) of patients. Primary KRT modality was continuous KRT in 96% (n = 133) and intermittent KRT in 4% (n = 6) patients. Of patients receiving ECMO (n = 91), 70% (n = 64) required concomitant KRT. Of patients surviving the ICU stay (n = 54) a majority (54%, n = 29) were dialysis dependent at time of ICU discharge. For detailed characteristics on indications KRT modality see Table 2. The primary cause of AKI was sepsis/septic shock triggered in 86% (n = 119), acute on chronic kidney injury in 12% (n = 16) and hemorrhagic shock in 3% (n = 4) of patients.
Clinical characteristics and details on ICU management of patients with and without KRT are reported in Table 3. Critically ill patients requiring KRT were generally older (KRT: median 62 vs. no-KRT: 60 years, p = 0.015), had a higher BMI (29.4 vs. 26.9, p < 0.001) and were more frequently male (71 vs. 61%, p = 0.076) compared to patients without KRT. Comorbidities, represented by the CCI were distributed equally between both groups. Pre-existing immunosuppression was found in 20% (n = 23) with and 16% (n = 22) patients without KRT (p = 0.951). Severity of illness represented by SAPS II (43 vs. 35 points, p < 0.001) and SOFA score (11 vs. 5 points, p < 0.001) on admission was significantly higher in patients with KRT. Overall, 78% (n = 235) of patients received vasopressor support during the ICU stay (86 vs. 61%, p < 0.001). In total 73% (n = 219) of patients received invasive mechanical ventilation. Placement of ECMO was performed in 46% (n = 64) with KRT and 17% (n = 27) without KRT in critically ill patients with severe ARDS accompanied by life-threatening hypoxia (p < 0.001).
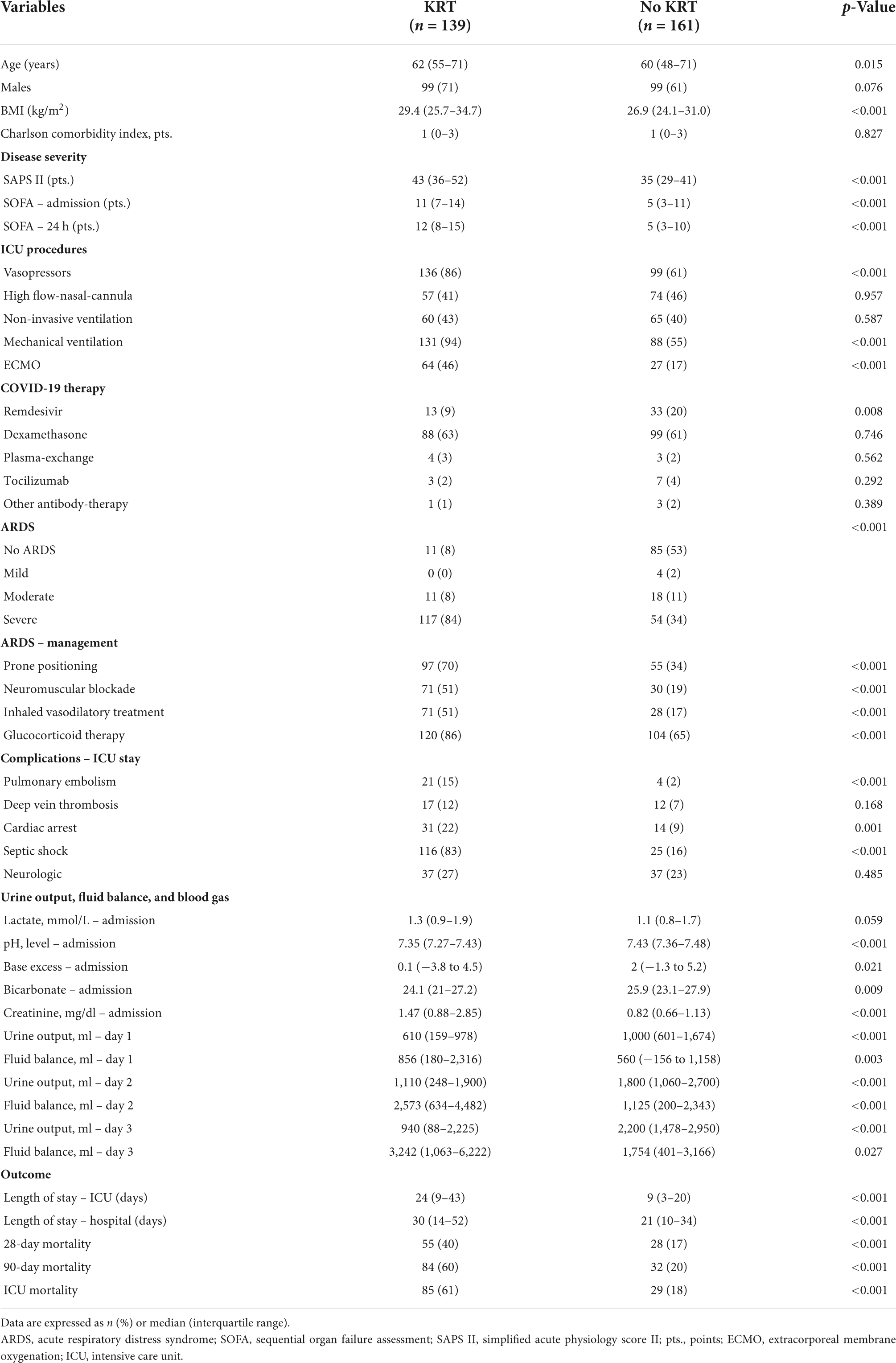
Table 3. Demographic and clinical characteristics of patients with and without kidney replacement therapy.
Complications during the ICU stay were frequent, pulmonary embolism and deep-vein thrombosis were found in 8% (15 vs. 2%, p < 0.001) and 10% (12 vs. 7%, p = 0.168), respectively. Overall, 15% suffered from cardiac arrest (22 vs. 9%, p = 0.001) and 47% from septic shock (83 vs. 16%, p < 0.001).
Detailed information about kidney function, urine output, and fluid balance of patients with and without KRT are reported in Tables 3, 4. Creatinine on admission was 1.47 (0.88–2.85) mg/dl in patients requiring KRT compared to 0.82 (0.66–1.13) in patients without KRT (p < 0.001). Further, the median pH level (7.35 vs. 7.43, p < 0.001), bicarbonate (24.1 vs. 25.9, p = 0.009) and base excess (0.1 vs. 2.0, p = 0.021) on admission were lower in patients requiring KRT. On admission and after 24 h inflammatory markers including leukocytes, procalcitonin, interleukin-6, ferritin, and C-reactive protein were significantly higher in patients with KRT (all p < 0.001). Further, we observed a significantly higher level of D-dimers on admission and after 24 h in patients with KRT compared to patients without KRT (both p < 0.001). Further differences in laboratory parameters on admission and after 24 h in patients with and without KRT can be found in Table 4 and Supplementary Table 2. Urine output during the first 3 days after ICU admission was significantly lower in patients requiring KRT (all p < 0.001). Cumulative fluid balance from day 1 to day 3 after admission was significantly higher in patients with KRT.
Multivariable regression analysis identified septic shock (OR 11.818, 95% CI: 5.941–23.506, p < 0.001), SAPS II (OR 1.048, 95% CI: 1.014–1.084, p = 0.006) and vasopressor therapy (OR 5.475, 95% CI: 1.127–26.589, p = 0.035) as factors significantly associated with the requirement of KRT initiation (Table 5A).
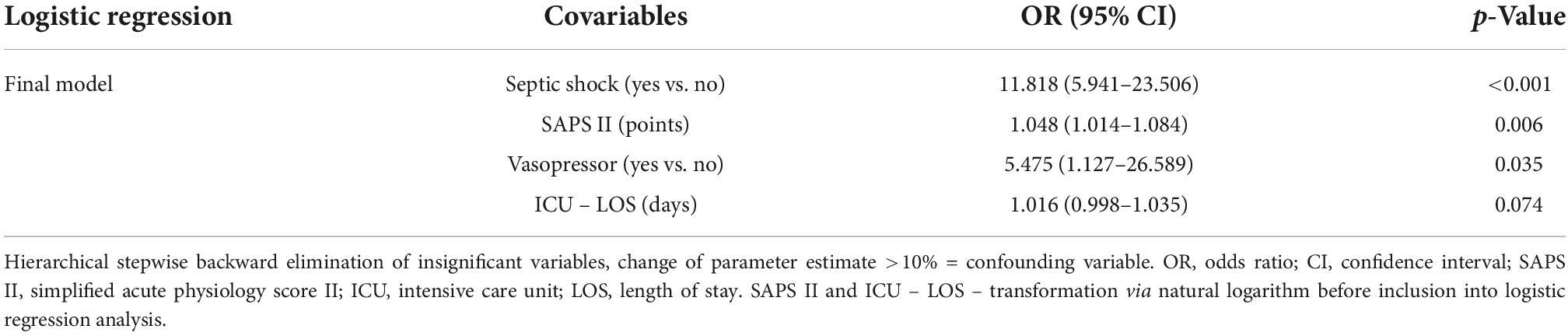
Table 5A. Logistic regression model for factors associated with requirement of kidney replacement therapy.
The median duration of ICU and hospital stay of patients with and without KRT was 24 (9–43) compared to 9 (3–20) days (p < 0.001) and 30 (14–52) compared to 21 (10–34) days (p < 0.001), respectively. Overall, a 28-day mortality of 28% (n = 83) and 90-day mortality of 39% (n = 116) was observed in our cohort. In patients with KRT we observed an ICU mortality of 61% (n = 85) compared to 29% (n = 18) in patients without KRT (p < 0.001). The 28- and 90-day mortality was 40% (n = 55) and 60% (n = 84) compared to 17% (n = 28) and 20% (n = 32), respectively (both p < 0.001). See also Kaplan–Meier survival estimates for 90-day mortality (Figure 2). In patients with ECMO the ICU mortality was 69% (n = 44) in patients with KRT compared to 56% (n = 15) in ECMO patients without KRT. Cox regression analysis identified ARDS (HR 4.658, 95% CI: 2.258–9.611, p < 0.001), KRT (HR 2.075, 95% CI: 1.342–3.208, p = 0.001), and age (HR 1.018, 95% CI: 1.002–1.034, p = 0.026) as factors significantly associated with 90-day mortality (see Table 5B).
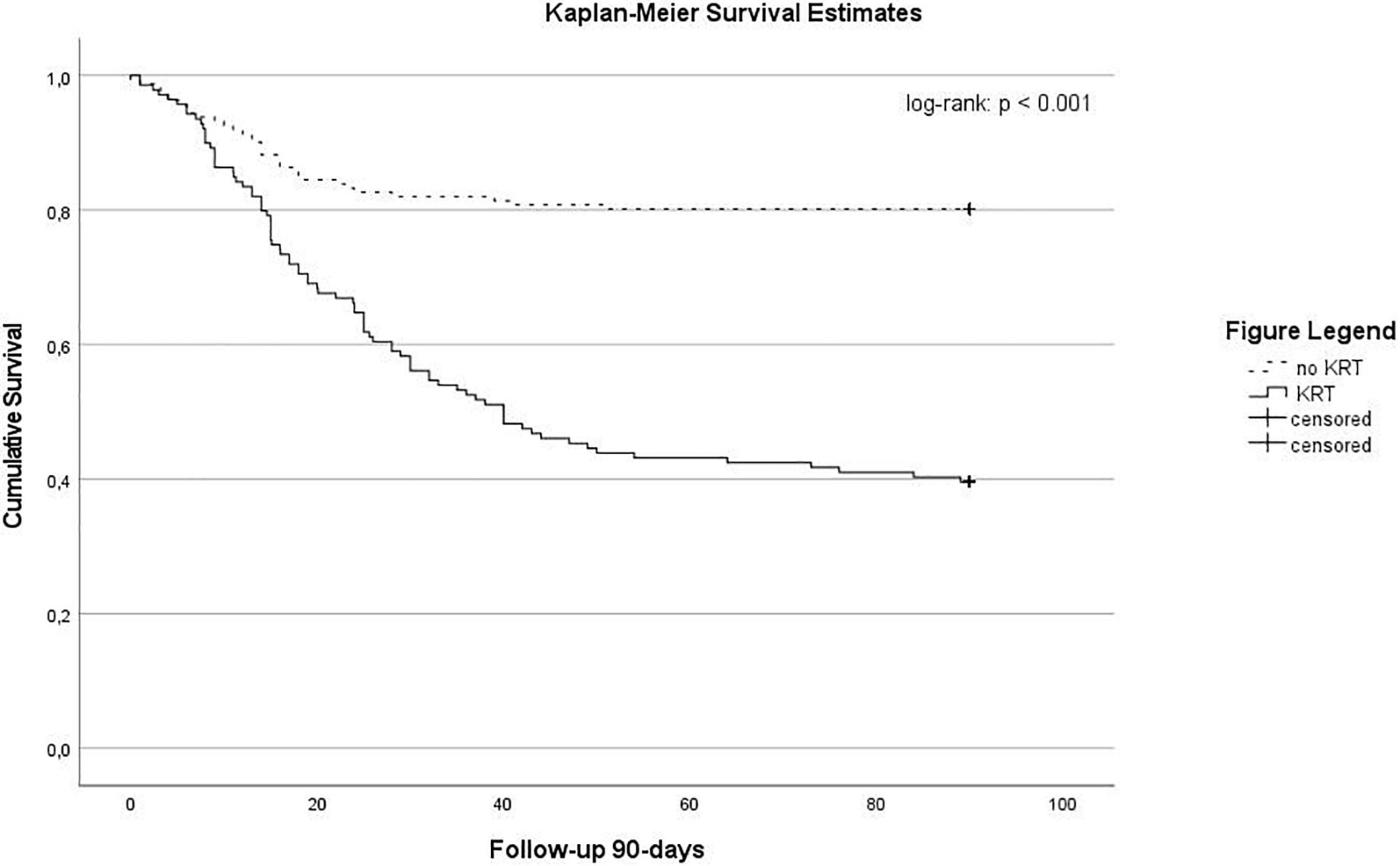
Figure 2. Kaplan-Meier survival estimates stratified according by the use of renal replacement therapy (log-Rank: p < 0.001).
In the present study, we investigated the incidence of KRT use in critically ill patients with COVID-19 admitted to a large tertiary care center. We found that almost half of the patients required KRT and initiation was independently associated with mortality. To our knowledge, this is the first study focusing exclusively on clinical characteristics and outcomes of patients requiring KRT in critically ill patients with COVID-19.
Requirement of KRT in critically ill patients with COVID-19 was reported in 20–33% (4, 9, 16). Of interest, one study in hospitalized patients showed that patients with COVID-19 were more likely to require KRT than those without (16). Overall, we observed a substantially higher incidence of KRT in our study population compared with previous observations (14). Interestingly the median time to start KRT after admission to ICU was 2 days, which appears quite low. However, this could be attributable to the very dynamic disease course especially in critically ill patients with COVID-19, a high number of septic shock and about one-third of patients requiring vv-ECMO where KRT is often used to facilitate fluid management. However, to date it is unclear if early or late initiation of KRT confers clinical benefits and this question is on an ongoing debate (30). Several factors may account for the higher incidence of KRT in our cohort. First, patients in our study presented with a high disease severity on admission (median SOFA score of 8 on admission). Further, 68% of patients developed ARDS which underlines the COVID-19 related disease burden of our study population. Second, our center is a referral hospital and it is specialized in the treatment of patients with ARDS. Therefore, the illness severity at baseline and the number of patients with multi-organ failure might be higher as compared with other centers. Third, a majority of patients requiring KRT suffered from septic shock and served as an independent predictor for KRT in our cohort. This probably explains the high requirement of KRT and is in line with other studies of patients with sepsis and septic shock (31–34). Of note, the high incidence of KRT may also be an expression of direct kidney involvement, as previously proposed, or complications during the ICU stay like pulmonary embolism, septic shock, or cardiac arrest (8). However, Hardenberg et al. suggest that severe AKI in COVID-19 is tightly intertwined with critical illness and systemic inflammation and is not observed in milder disease courses (7). This might point toward traditional mechanisms of AKI rather than a kidney-specific mechanism (7). Importantly, the incidence of COVID-19-associated AKI seems to be higher compared with other types of severe respiratory failure (35). About 50% of critically ill patients with H1N1 developed AKI that required KRT (16, 36–40). Furthermore, we investigated if the different waves of the pandemic included in this study could have had an impact on the initiation of KRT. We report from three waves of the pandemic in Germany (#1 – 03/2020 to 06/2020, #2 – 07/2020 to 12/2020, and #3 – 01/2021 to 08/2021). We observed numerical differences in KRT initiation, which did not reach statistical significance (49 vs. 45 vs. 46%, p = 0.881). We observed that half of the patients who required KRT were dialysis dependent on ICU discharge. This is higher than observed in other previous studies (41, 42). If this is attributable to COVID-19 or probably to an earlier referral to specialized rehabilitation facilities remains unclear and should be addressed in future studies.
Patients requiring KRT were significantly older and had a higher BMI, which is in line with previous studies in critically ill patients with COVID-19 (14). The logistic regression analysis identified that septic shock, SAPS II and use of vasopressors are associated with KRT requirement, which underlines the link between initiation of KRT and the severity of illness in the present cohort. Furthermore, patients requiring KRT had a substantially longer stay in the ICU and hospital. In general, the ICU mortality in our cohort was 38%. This is higher than previously reported in Germany (5). Partially, that can be explained by the severely ill population treated and the high number of patients in very critical condition referred to our center. Furthermore, also hemodynamic changes in severely ill patients alongside with mechanical ventilation, vasopressor therapy, and ARDS may be associated with higher KRT risk. We observed a substantially higher ICU mortality in patients with KRT compared to those without KRT. This is in line with several other studies in critically ill COVID-19 patients (43, 44). Further, we could demonstrate that KRT was an independent predictor of mortality in this cohort of critically ill patients with COVID-19.
Extracorporeal membrane oxygenation may be life-saving for patients with severe respiratory failure with potentially reversible causes. Over the past decade, the use of ECMO has increased substantially in ICUs (45). The pooled incidence of AKI and requirement of KRT in patients with ECMO therapy are 63% (AKI) and 45% (KRT), respectively (46). In the subgroup of patients with ECMO, we observed that 70% required KRT. Generally, risk factors for AKI in patients with ECMO are widespread and include older age, pre-existing comorbidities (e.g., cirrhosis), high lactate and, increased bilirubin (47). In patients with ECMO KRT is initiated to manage or prevent fluid overload, followed by AKI and electrolyte disturbances (47, 48). Previously reported 90-day mortality rates of patients with KRT while on ECMO were almost 69%, and the likelihood of dying for patients receiving KRT was reported to be three times higher than that of those without KRT (46). Of interest, we did not find a difference in mortality between ECMO patients with or without KRT. Complications like pulmonary embolism or septic shock that are known risk factors for KRT were significantly more frequent. Those probably concealed potential beneficial effects and lead to a similar outcome in both groups in our cohort. To date, it is unclear whether KRT directly increases mortality risk or whether it merely represents an epiphenomenon of disease severity (47, 49). We furthermore observed a high rate of septic shock and severe ARDS, one patients was placed on VA-ECMO due to cardiogenic shock during the ICU stay.
Our study has several limitations. First, we present the results of a single-center observational study with a high expertise in the management of critically ill patients with COVID-19. Thus, our results may not be generalizable to other cohorts (e.g., less experienced settings, patients with lower illness severity). Second, due to the retrospective design, pre-admission kidney laboratory tests were unknown, and a general sampling of urine, as well as fluid status or kidney sonography was not performed routinely. Third, only requirement of KRT was used to investigate renal failure (severe AKI) which could underestimate the incidence of less severe forms of AKI in this cohort. Further, due to missing pre-hospital data we could not investigate if AKI was community or hospital acquired which probably has an impact on outcome. Generally, our results may not be generalizable to other settings and have to be interpreted with caution. Fourth, we did not investigate the renal recovery during follow-up. This should be addressed in further prospective investigations. Fifth, changes in clinical practice over time may have influenced outcomes of critically ill patients with COVID-19. Sixth, residual confounding is a matter of concern and cannot be entirely excluded.
In conclusion, we found that critically ill patients with COVID-19 require KRT in about half of cases. Initiation of KRT is associated with high mortality. Septic shock and disease severity serve as independent predictors of KRT requirement. In a subgroup of patients requiring ECMO for refractory respiratory failure survival was independent from the presence of KRT, which warrants further investigation in future larger trials.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The study was approved by the local clinical institutional review board and complies with the Declaration of Helsinki. The study was registered with the Ethics Committee of the Hamburg Chamber of Physicians (No. 2021-300112-WF). Owing to the retrospective character of the study and its anonymized data collection, the need for informed consent was waived.
JB, KR, and MF participated in study conception and design and contributed to analysis and interpretation of data. JB, DJ, CS-L, OB, GH, CB, DF, BS, AN, EH, TH, SK, DW, MF, and KR were involved in acquisition of data. JB and KR drafted the manuscript. SK, MF, and DW were involved in critical revision of the manuscript for important intellectual content and participated in supervision. All authors read and approved the final manuscript.
Author SK received research support by Ambu, E.T.View Ltd., Fisher & Paykel, Pfizer, and Xenios, lecture honorarium from ArjoHuntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll, and consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios. Author DW received lecture honorarium from 3M, ADVANZ (previously Correvio), AMEOS, Gilead, Kite, Lilly, MSD, Pfizer, and Shionogi and consultation honorarium from Eumedica, EUSA-Pharm, Gilead, Kite, MSD, Novartis, Pfizer, and Shionogi. No other potential conflict of interest relevant to this article was reported. Author AN received research funds, lecture honoraria and travel reimbursement within the last 5 years from CytoSorbents Europe, Biotest AG, and Thermo Fisher Scientific. Author DF reports lecture honoraria within the last 5 years from Xenios AG. Author KR received travel reimbursement from Gilead within the last 5 years.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1027586/full#supplementary-material
2. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. (2020) 382:2327–36. doi: 10.1056/NEJMc2015312
3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
4. Roedl K, Jarczak D, Thasler L, Bachmann M, Schulte F, Bein B, et al. Mechanical ventilation and mortality among 223 critically ill patients with COVID-19 – a multicentric study in Germany. Aust Crit Care. (2020) 34:167–75. doi: 10.1016/j.aucc.2020.10.009
5. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. (2020) 8:853–62. doi: 10.1016/S2213-2600(20)30316-7
6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
7. Hardenberg JB, Stockmann H, Aigner A, Gotthardt I, Enghard P, Hinze C, et al. Critical Illness and Systemic Inflammation Are Key Risk Factors of Severe Acute Kidney Injury in Patients With COVID-19. Kidney Int Rep. (2021) 6:905–15. doi: 10.1016/j.ekir.2021.01.011
8. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
9. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
10. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
11. Nadim MK, Forni LG, Mehta RL, Connor MJ Jr., Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. (2020) 16:747–64.
12. Ostermann M, Lumlertgul N, Forni LG, Hoste E. What every Intensivist should know about COVID-19 associated acute kidney injury. J Crit Care. (2020) 60:91–5. doi: 10.1016/j.jcrc.2020.07.023
13. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 Pneumonia. J Am Soc Nephrol. (2020) 31:1157–65. doi: 10.1681/ASN.2020030276
14. Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. (2021) 11:123. doi: 10.1186/s13613-021-00914-5
15. Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, et al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with Coronavirus Disease 2019. Clin J Am Soc Nephrol. (2020) 15:1394–402. doi: 10.2215/CJN.04650420
16. Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol. (2020) 31:2145–57. doi: 10.1681/ASN.2020040509
17. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
18. Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. (2012) 7:1328–36. doi: 10.2215/CJN.12731211
19. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
20. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.270.24.2957
21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
22. Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. German recommendations for critically ill patients with COVID-19. Med Klin Intensivmed Notfallmed. (2020) 115(Suppl 3):111–4. doi: 10.1007/s00063-020-00689-w
23. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
24. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. (2020) 46:854–87. doi: 10.1007/s00134-020-06022-5
25. Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. (2021) 67:601–10. doi: 10.1097/MAT.0000000000001432
26. CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. (2013) 3:1–150.
27. Joannidis M, John S. Acute kidney injury and renal replacement therapy in critically ill patients in 2018 : Recommendations from the renal section of the DGIIN, ÖGIAIN and DIVI. Med Klin Intensivmed Notfallmed. (2018) 113:356–7. doi: 10.1007/s00063-018-0419-9
28. Investigators RRTS, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. (2009) 361:1627–38. doi: 10.1056/NEJMoa0902413
29. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. (2007) 4:e296. doi: 10.1371/journal.pmed.0040296
30. Bagshaw SM, Hoste EA, Wald R. When should we start renal-replacement therapy in critically ill patients with acute kidney injury: do we finally have the answer? Crit Care. (2021) 25:179. doi: 10.1186/s13054-021-03600-x
31. SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. (2016) 42:1980–9. doi: 10.1007/s00134-016-4504-3
32. Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. (2010) 303:2495–503. doi: 10.1001/jama.2010.851
33. Yébenes JC, Ruiz-Rodriguez JC, Ferrer R, Clèries M, Bosch A, Lorencio C, et al. Epidemiology of sepsis in Catalonia: analysis of incidence and outcomes in a European setting. Ann Intensive Care. (2017) 7:19. doi: 10.1186/s13613-017-0241-1
34. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. (2005) 294:813–8. doi: 10.1001/jama.294.7.813
35. Birkelo BC, Parr SK, Perkins AM, Greevy RA Jr., Hung AM, Shah SC, et al. Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States Veterans. Kidney Int. (2021) 100:894–905. doi: 10.1016/j.kint.2021.05.029
36. Hunt L, Knott V. Serious and common sequelae after Ebola virus infection. Lancet Infect Dis. (2016) 16:270–1. doi: 10.1016/S1473-3099(15)00546-0
37. Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. (2014) 29:301–6. doi: 10.1016/j.ijid.2014.09.003
38. Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. (2005) 67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x
39. Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, et al. Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian Province. Am J Kidney Dis. (2010) 55:848–55. doi: 10.1053/j.ajkd.2010.01.011
40. Abdulkader RCRM, Ho YL, de Sousa Santos S, Caires R, Arantes MF, Andrade L. Characteristics of Acute Kidney Injury in Patients Infected with the 2009 Influenza A (H1N1) Virus. Clin J Am Soc Nephrol. (2010) 5:1916–21. doi: 10.2215/CJN.00840110
41. De Corte W, Dhondt A, Vanholder R, De Waele J, Decruyenaere J, Sergoyne V, et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. (2016) 20:256. doi: 10.1186/s13054-016-1409-z
42. Conroy M, O’Flynn J, Marsh B. Mortality and long-term dialysis requirement among elderly continuous renal replacement therapy patients in a tertiary referral intensive care unit. J Intensive Care Soc. (2019) 20:138–43. doi: 10.1177/1751143718784868
43. Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically Ill Patients with COVID-19. J Am Soc Nephrol. (2021) 32:161–76.
44. Hsu CM, Gupta S, Tighiouart H, Goyal N, Faugno AJ, Tariq A, et al. Kidney recovery and death in critically Ill Patients With COVID-19-Associated acute kidney injury treated with dialysis: The STOP-COVID Cohort Study. Am J Kidney Dis. (2022) 79:404–16.
45. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. (2017) 63:60–7. doi: 10.1097/MAT.0000000000000475
46. Thongprayoon C, Cheungpasitporn W, Lertjitbanjong P, Aeddula NR, Bathini T, Watthanasuntorn K, et al. Incidence and impact of acute kidney injury in patients receiving extracorporeal membrane oxygenation: A meta-analysis. J Clin Med. (2019) 8:981. doi: 10.3390/jcm8070981
47. Ostermann M, Lumlertgul N. Acute kidney injury in ECMO patients. Crit Care. (2021) 25:313. doi: 10.1186/s13054-021-03676-5
48. Fleming GM, Askenazi DJ, Bridges BC, Cooper DS, Paden ML, Selewski DT, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. (2012) 58:407–14. doi: 10.1097/MAT.0b013e3182579218
Keywords: COVID-19, renal replacement therapy (RRT), AKI, multiple organ failure, ARDS, ECMO, SARS-CoV-2, kidney replacement therapy (KRT)
Citation: Braunsteiner J, Jarczak D, Schmidt-Lauber C, Boenisch O, de Heer G, Burdelski C, Frings D, Sensen B, Nierhaus A, Hoxha E, Huber TB, Wichmann D, Kluge S, Fischer M and Roedl K (2022) Outcomes of critically ill coronavirus disease 2019 patients requiring kidney replacement therapy: A retrospective cohort study. Front. Med. 9:1027586. doi: 10.3389/fmed.2022.1027586
Received: 25 August 2022; Accepted: 04 October 2022;
Published: 20 October 2022.
Edited by:
Savino Spadaro, University of Ferrara, ItalyReviewed by:
Marco Krasselt, University Hospital Leipzig, GermanyCopyright © 2022 Braunsteiner, Jarczak, Schmidt-Lauber, Boenisch, de Heer, Burdelski, Frings, Sensen, Nierhaus, Hoxha, Huber, Wichmann, Kluge, Fischer and Roedl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kevin Roedl, ay5yb2VkbEB1a2UuZGU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.