
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 11 January 2023
Sec. Geriatric Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1027503
Introduction: Geriatric syndrome (GS) increases risk of disability and mortality in older adults. Sarcopenia is a predominant illness of GS and accelerate its progression. This study aimed to investigate associations between mortality, emergency department (ED) re-visits and GS-related illnesses among older adults who visited the ED.
Method: This retrospective observational study enrolled elderly patients who visited the ED in our hospital between January 2018 and October 2020. Patients were evaluated for potential sarcopenia, which was defined by both low handgrip strength and calf circumference. Follow-up was at least 6 months. Data of age, gender, mortality, ED re-visits, and GS-related illnesses were collected and analyzed for associations.
Results: A total of 273 older adults aged 74 years or older were included, of whom 194 were diagnosed with possible sarcopenia. Older adults with possible sarcopenia also had significantly lower body mass index (BMI); a higher proportion needed assistance with daily activities; more had malnutrition, frailty, and history of falls (all p < 0.001) and acute decline in activities of daily living (p = 0.027). Multivariate analysis showed that possible sarcopenia [adjusted hazard ratio, aHR): 9.89, 95% confidence interval (CI): 1.17–83.81, p = 0.036], living in residential institutions (aHR: 2.85, 95% CI: 1.08–7.50, p = 0.034), and frailty (aHR: 7.30, 95% CI: 1.20–44.62, p = 0.031) were associated with mortality. Aged over 85 years (adjusted odds ratio: 2.44, 95% CI: 1.25–4.80, p = 0.02) was associated with ED re-visits.
Conclusion: Sarcopenia is associated with mortality among older adults who visit ED. Initial screening for sarcopenia and relevant risk factors among older adults in the ED may help with early intervention for those at high-risk and may improve their prognosis.
Geriatric syndromes (GS) are multifactorial illnesses prevalent in geriatric populations (1, 2). Patients with GS may present with various physiological or psychiatric illnesses, including delirium, dementia, cognitive impairment, urinary incontinence, frailty, and mobility impairment (3). These disorders reduce the mobility of patients with GS, obstruct their connection to communities they belong, and subsequently increase their risk of disability and cognitive impairment (4, 5). Furthermore, those with GS need additional treatments frequently, including medications, nursing, hospitalization, or residential care, and may limit budgets and government funds for people of other ages in individual households (6–8). Therefore, monitoring the dynamic trends of GS may assist healthcare administrators or government policy-makers to implement policies to reduce the disadvantages of GS-associated illnesses among older adults.
To understand the dynamic of GS in a given population, it is necessary to comprehensively investigate the incidence of GS-associated illnesses and analyze their crosstalk. However, the progression of GS is multifactorial, and the interaction between GS-associated illnesses is complicated (1). Nevertheless, several pivotal factors are identified that contribute to GS, such as malnutrition, sarcopenia, and chronic diseases (9, 10). Sarcopenia describes a chronic loss of muscle mass and function for which diagnostic criteria include handgrip strength lower than 28 kg in men and 16 kg in women, calf circumference less than 34 cm in men and 33 cm in women, and 6-m walking speed less than 0.8 m per second in both genders(11). Sarcopenia obstructs mobility and impairs the balance of older adults, which correlates directly with the incidence of social isolation, frailty, and disability in the geriatric population (12, 13). Of note, sarcopenia involves the progression of GS but the level of involvement varies between ethnic groups and countries (14–16). Previous studies showed that 9% elders in Yilan county, Taiwan had sarcopenia (17) and the prevalence of sarcopenia was about 10% in the world (18). Because of the aging population, the dynamics of GS are monitored consistently in Taiwan (19). However, the association between GS and age-related clinical outcomes is still unclear. This study aimed to investigate the associations between mortality, ED re-visits, level of cytokines, and GS-related illnesses among older adults who visited the ED.
In this retrospective observational study, elderly patients who visited the ED of our hospital between January 2018 and October 2020 were enrolled. Inclusion criteria were: (1) over the age of 74 years; (2) with follow-up for at least 6 months. Those with missing information on grip strength or calf circumference were excluded.
The European and Asian Sarcopenia Working Groups have proposed that muscle mass, muscle strength, and physical performance are three indicators used for the evaluation of sarcopenia (20, 21). Therefore, diagnosis of sarcopenia in this study was based on the following three procedures: muscle strength by handgrip strength lower than or equal to 28 kg in males or 16 kg in females, calf circumference less than 34 cm in males or 33 cm in females, and 6-m walking speed < 0.8 m/s in both males and females, as previously described (22, 23). We defined subjects diagnosed with sarcopenia and severe sarcopenia as “possible” sarcopenia. Other subjects were defined as subjects without sarcopenia (non-sarcopenia).
The primary outcomes of interest were mortality and re-visits to the ED within 6 months during follow-up.
Grading of frailty, depression, malnutrition, and Charlson’s comorbidity index (CCI) were used for analysis in this study. Depression was graded by a five-item Geriatric Depression Scale (24). Participants had grade ≥ 2 were considered as depression. Mini-Nutrition assessment-short form was used for evaluating malnutrition (25). Participants had score lower than 12 were considered as malnutrition. Frailty score was determined by the Cardiovascular Health Study (26). CCI was evaluated following Age-adjusted Charlson’s comorbidity index (27).
Blood was collected into a BD Vacutainer™ EDTA Blood Collection Tubes (8 mL) and kept on ice until processing. The blood samples were centrifugated at 3,000 rpm for 10 min and the supernatants were frozen in –20°C until analysis.
Human TNF-α ELISA kit (ARG80120, Arigo biolaboratories, Hsinchu city, Taiwan) and Human IL-6 ELISA kit (ARG80110, Arigo biolaboratories) were used to analyze the concentration of TNF-α and IL-6 in the serum, respectively. The procedures suggested by the manufacturer was followed to determine the cytokine concentrations.
The Shapiro–Wilk test was used for continuous data with non-normal distribution, including grip strength, calf circumference, and BMI, which are presented as medians (interquartile: 25–75th percentile, IQR). Categorical variables were estimated as frequency and percentage, including gender, age, education level, marital status, living status, daily activities, Charlson comorbidity index (CCI), ED discharge destination, and GS components. Hazard ratios (HRs) were estimated using the Cox proportional-hazard model to present the effects of covariates on mortality. Logistic regression analysis was used to explore associations between covariates and ED re-visits. Odds ratios (ORs) were used to present the associations between high cytokine levels, the presence of possible sarcopenia at ED, and mortality based on the logistic regression model. All statistics were two-sided and all statistical analysis was performed using SAS statistical software (version 9.4, SAS, Cary, NC, USA). A P-value < 0.05 was established as statistical significance.
In total, 320 patients were enrolled in this study. After excluding, 273 patients were including in the cohort. Unfortunately, 25 patients were lost of follow-up due to death in the study period and the follow-up rate was 90.84% (248/273) (Figure 1). Among the subjects, 194 patients were identified as possible sarcopenias. The prevalence of possible sarcopenia in our study population is 71.06%. Table 1 demonstrates the demographic and anthropometric characteristics of the study population as well as clinical signs of GS. Significant differences were found between possible sarcopenia and non-sarcopenia groups, including lower median grip strength [males: 17.3 (13.50–21.10) vs. 26.2 (19.40–26.20), females: 10.0 (7.90–12.80) vs. 16.50 (13.00–16.50)] and calf circumference [males: 30.0 (27.5–32.0) vs. 35.0 (34.0–36.50), females: 28.5 (26.5–31.0) vs. 33.00 (30.25–34.25)] (all p < 0.001). The proportion of possible sarcopenia was comparable in both genders (males 70.9% vs. females 71.22%, p = 0.952). Subjects with possible sarcopenia had significantly lower BMI [23.19 (20.96–25.39) vs. 26.79 (24.13–29.76), p < 0.001]; higher percentages of those needing assistance with daily activities (80.95% vs. 19.05%; p < 0.001), and acute ADL decline (72.98% vs. 27.02%, p = 0.027); malnutrition (MNASF < 12) (79.33% vs. 20.67%, p < 0.001), frailty (Cardiovascular Health Study ≥ 3) (84.88% vs. 15.12%, p < 0.001) (26), and history of falls (85.42% vs. 14.58%, p < 0.001).
Table 2 presents the associations between study variables and mortality. Univariate Cox PH model showed that mortality was associated with possible sarcopenia (crude HR = 9.75, 95% CI: 1.32–72.21, p = 0.026), living in residential institutions (crude HR = 3.17, 95% CI: 1.23–8.14, p = 0.017), frailty (crude HR = 5.88, 95% CI: 1.38–25.07, p = 0.017), cognitive impairment (crude HR = 3.14, 95% CI: 1.42–6.93, p = 0.005), and defecation problems (crude HR = 2.79, 95% CI: 1.26–6.19, p = 0.012).
In the multivariate model, living in residential institutions [adjusted HR (aHR): 2.85, 95% CI: 1.08–7.50, p = 0.034] was an independent risk factor for mortality after adjusting for variables with p-value < 0.1, including possible sarcopenia, frailty, cognitive impairment, and defecation problems (Model 1). On the other hand, frailty (aHR: 7.30, 95% CI: 1.20–44.62, p = 0.031) was an independent risk factor for mortality after adjusting for all variables (Model 2). Possible sarcopenia (aHR = 9.89; 95% CI: 1.17–83.81, p = 0.036; Figure 2) was an independent risk factor for mortality after adjusting for all variables except frailty, daily activities, living status, and cognitive impairment (Model 3). Compared to males, females had lower risk for mortality (Model 2, aHR: 0.18, 95% CI: 0.05–0.59, p = 0.005; Model 3, aHR: 0.29, 95%CI: 0.1–0.89, p = 0.03).
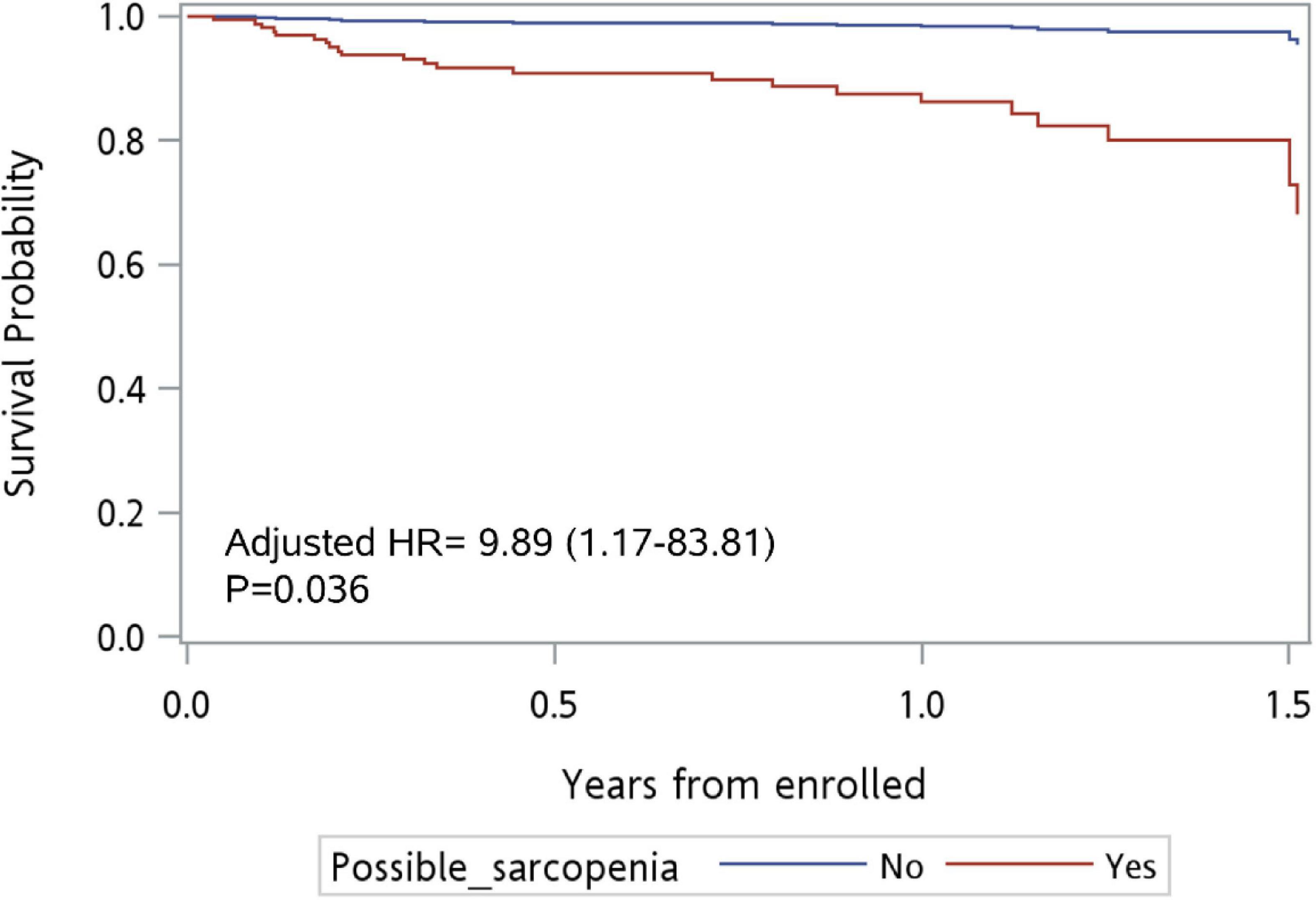
Figure 2. Survival curve for patients with possible sarcopenia vs. no sarcopenia at ED (n = 273). Adjusted for all variables except frailty, daily activity, living status, and cognitive impairment.
Table 3 presents the associations between study variables and ED re-visits. Univariate logistic regression model showed ED re-visits were associated with age group 85–100 years (compared to 75–79 years group: crude OR = 2.56; 95% CI: 1.33–4.92, p = 0.02) and malnutrition (crude OR = 1.83; 95% CI: 1.05–3.18, p = 0.032). The multivariate model showed that age group 85–100 years was an independent risk factor for ED re-visits (adjusted OR: 2.44, 95% CI: 1.25–4.80, p = 0.02) after adjusting for possible sarcopenia, variables with p-value < 0.1, including marital status, malnutrition, and frailty (Model 1).
No associations were found between the level of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) and the presence of possible sarcopenia in ED patients (Table 4). Nor did mortality associate with cytokine level or cytokine level plus possible sarcopenia (Table 5).
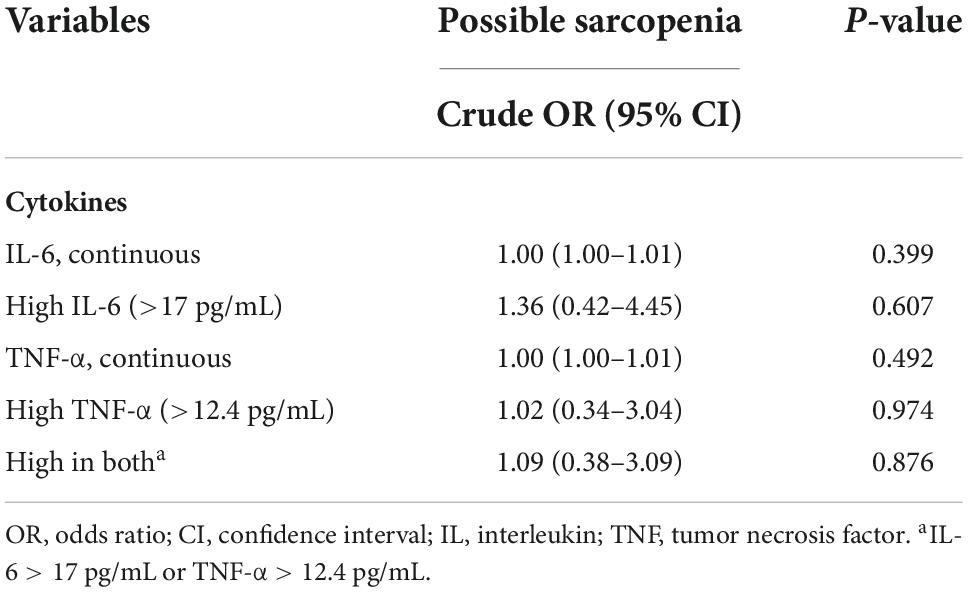
Table 4. Associations between high cytokine levels and presence of possible sarcopenia at ED (n = 68).
The present study found that older adults with sarcopenia also had lower BMI, higher percentages of acute ADL decline, malnutrition, frailty, and history of falls. Among these older adults who visited the ED, mortality was associated with gender, possible sarcopenia, living in residential institutions, and frailty. ED re-visits were associated with age older than 85 years. Of note, elderly ED patients with sarcopenia did not exhibit higher concentrations of IL-6 and TNF-α, which indicated that possible sarcopenia did not correlate with systemic inflammation.
Xu et al. reported that sarcopenia was associated with 3-month and 1-year mortality in geriatric rehabilitation inpatients (28). They also did a meta-analysis and reported that sarcopenia was associated with significantly higher risks of mortality in adults independent of population (29). Although the target subjects in our study are not specified in their comparison, our conclusion can be strengthened by their analysis. It is apparent that sarcopenia affects mortality in non-critical elderly patients visiting the emergency department.
In the present study, possible sarcopenia, frailty, and living in a residential institution contributed to increased geriatric mortality. Sarcopenia and frailty are known to elevate the risk of hospitalization among older adults (30, 31). Appetite decline causes malnutrition and frailty in older adults and further exacerbates sarcopenia and osteoporosis due to the decline in daily activities (12, 32–34). Furthermore, the decline in daily activities is also linked to cognitive impairment and increased mortality in the geriatric population (35, 36). Therefore, the positive relationship between sarcopenia, frailty, and risk of hospitalization among older adults is foreseeable. Interestingly, nursing home or long-term care residents exhibited a higher risk of mortality than those who lived at home. Previous studies revealed that nursing home residents do not need to take care of their daily life so they spend more time doing nothing or watching TV than those living at home or a private dwelling (37–39). This suggests that the negative impact on the health status of older adults living in residential institutions may be the result of the decline in daily activities.
Multivariate regression analysis also revealed that mortality in men was significantly higher than that in women. Gender difference have been observed as well in life expectancy (40). However, the pivotal factors causing gender differences vary between different countries (40). Elderly men in China have a higher willingness to utilize long-term care institutions than women (41). Also, frailty is more prevalent among elderly men in China than among women (42). Bellettiere et al. and Chen et al. both reported that elderly men had fewer daily activities than women; the demand for assistance with daily activities was also stronger in men than that in women (43, 44. These studies revealed that the potential cause of gender differences in mortality in China may be associated with the frailty that results from reduced daily activities in long-term care institutions. However, two investigations of older adults in Spain and Italy showed that elderly women had a higher risk of frailty and disability than men due to differences in mechanical and psychological patterns (45, 46). Despite that the life expectancy of women in these two populations is higher than that of men (47), mechanical and psychological patterns and disability do not seem to be the critical factors in gender differences in life expectancy. The present study preliminarily exhibited lower mortality in elderly women than that in elderly men but further studies are needed to identify the underlying factors.
Age older than 85 years was an independent risk factor for ED re-visits in the present study. This is the first study to report a correlation between age and frequency of ED revisits. An observational study in Turkey reported that the ED-visiting rate, or frequency, was negatively associated with age and elders aged between 65 and 74 years constituted the ED-visiting population with the most frequent visits, largely due to upper respiratory tract infections and chest pain (48). A similar trend was also observed in clinical investigations in America and Australia (49, 50). Of note, an observational trial in Finland revealed a positive correlation between the frequency of ED-visiting and the age of elders (51). The predominant diseases that cause older adults to visit the ED frequently are cardiovascular diseases such as heart failure and atrial fibrillation (51). Although the correlation between age and frequency of ED visits differs between countries, the complaints that contribute to ED visits are universal. In the present investigation, we discovered a positive correlation between age and ED re-visits, but the predominant complaint was unclear. Further investigations are needed to evaluate the causes of frequent visits to the ED, and results may help hospital and government policy-makers develop appropriate policies to address the complaints and thereby reduce ED visits.
Surprisingly, older adults with possible sarcopenia did not concurrently exhibit a higher level of IL-6 and TNF-α than non-sarcopenia subjects in our study. IL-6 and TNF-α are both inflammatory cytokines. Previous studies had shown that inflammatory cytokines were involved with muscle wasting and high level of those cytokines were negatively related to muscle strength and mass (52–56). Bian et al. also reported that the emergence of sarcopenia was accompanied by increased levels of TNF-α and IL-6 in elderly (57). Our study had completely opposite conclusion as their reports. In the study of Bian et al. (57) elders aged more than 60 years old and can walk by themselves or stand for 5 min with other auxiliaries were included. The percentage of subjects identified as sarcopenia was 17.9% (79/441). On the contrary, our subjects were recruited when they were admitted in the emergency department and they were older than 75 years old and the ratio of sarcopenia was 71.06%. Thus, subjects in our study were older, weaker, and having less muscle mess and strength.
In recent knowledge, pivotal links between sarcopenia and inflammatory cytokines include gut dysbiosis, malnutrition, and reduced daily activities. Gut dysbiosis, the imbalance of gut microbiota, affects nutrition absorption in the entericus and causes malnutrition to some extent (58, 59). Increased daily activities or physical exercises improve the appetite, which may reduce the risk of sarcopenia (60, 61). Moreover, exercises can trigger gut microbiota secreting short-chain fatty acids with anti-inflammatory effects, reduce oxidative stress, and maintain muscle mass (62–64). Older adults having pro-inflammatory diets may potentiate the incident risk of sarcopenia (65). More extensive study is needed to clarify the underlying mechanisms.
This study has a few limitations, mainly lying in the inherent restrictions of analyzing retrospective data, including that additional follow-up data were not available. Results of the single-center study in Taiwan also cannot be easily generalized to other populations or locations. However, observational studies focusing on a narrow topic may help to reduce the inherent limitations to a minimum. Results of the appendicular skeletal muscle mass index (direct evidence of sarcopenia) were missing for all patients, which would have fully demonstrated sarcopenia (66), providing a more accurate measure of possible sarcopenia in ED patients. Besides, cytokine levels were evaluated only in the minority of patients. This let it is impossible to apply the results to general geriatric population.
Sarcopenia is highly associated physical condition, like BMI, frailty, and living in residential institutions, rather than systemic inflammation in the elders. And the mortality of ED-visiting elders is associated with gender and possible sarcopenia.
Findings of the present study may serve as a reference to guide ED management and to develop an appropriate ED examination protocol for geriatric emergency visits, including a preliminary assessment of GS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Y-TC: concept, design, acquisition, analysis, interpretation of data, and supervision. M-CL: drafting of the manuscript. C-CY and Y-TL: statistical analysis. F-DH: administrative, technical, or material support. All authors contributed to the article and approved the submitted version.
.
This research was supported by the project “A preliminary study on the comprehensive assessment of the elderly emergency patients” (project #VGHKS18-CT8-04) from Kaohsiung Veterans General Hospital & Veterans Affairs Council, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. (2007) 55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x
2. Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
3. Takayama S, Tomita N, Arita R, Ono R, Kikuchi A, Ishii T. Kampo medicine for various aging-related symptoms: a review of geriatric syndrome. Front Nutr. (2020) 7:86. doi: 10.3389/fnut.2020.00086
4. Lane NE, Stukel TA, Boyd CM, Wodchis WP. Long-term care residents’ geriatric syndromes at admission and disablement over time: an observational cohort study. J Gerontol A Biol Sci Med Sci. (2019) 74:917–23. doi: 10.1093/gerona/gly151
5. Ong M, Pek K, Tan CN, Chew J, Lim JP, Yew S, et al. Social frailty and executive function: association with geriatric syndromes, life space and quality of life in healthy community-dwelling older adults. J Frailty Aging. (2022) 11:206–13. doi: 10.14283/jfa.2021.43
6. Huo C, Xiao G, Chen L. The crowding-out effect of elderly support expenditure on household consumption from the perspective of population aging: evidence from China. Front Bus Res China. (2021) 15:1–20. doi: 10.1186/s11782-021-00099-5
7. Lane NE, Wodchis WP, Boyd CM, Stukel TA. Disability in long-term care residents explained by prevalent geriatric syndromes, not long-term care home characteristics: a cross-sectional study. BMC Geriatr. (2017) 17:49. doi: 10.1186/s12877-017-0444-1
8. Picco L, Achilla E, Abdin E, Chong SA, Vaingankar JA, McCrone P, et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res. (2016) 16:173. doi: 10.1186/s12913-016-1421-7
9. Lynch DH, Spangler HB, Franz JR, Krupenevich RL, Kim H, Nissman D, et al. Multimodal diagnostic approaches to advance precision medicine in sarcopenia and frailty. Nutrients. (2022) 14:1384. doi: 10.3390/nu14071384
10. Vetrano DL, Foebel AD, Marengoni A, Brandi V, Collamati A, Heckman GA, et al. Chronic diseases and geriatric syndromes: The different weight of comorbidity. Eur J Intern Med. (2016) 27:62–7.
11. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307e302. doi: 10.1016/j.jamda.2019.12.012
12. Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol. (2019) 10:255. doi: 10.3389/fendo.2019.00255
13. Jahn K, Freiberger E, Eskofier BM, Bollheimer C, Klucken J. Balance and mobility in geriatric patients : assessment and treatment of neurological aspects. Z Gerontol Geriatr. (2019) 52:316–23.
14. Alex D, Fauzi AB, Mohan D. Online multi-domain geriatric health screening in Urban community dwelling older Malaysians: a pilot study. Front Public Health. (2020) 8:612154. doi: 10.3389/fpubh.2020.612154
15. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
16. Wu X, Li X, Xu M, Zhang Z, He L, Li Y. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS One. (2021) 16:e0247617. doi: 10.1371/journal.pone.0247617
17. Kuo Y-H, Wang T-F, Liu L-K, Lee W-J, Peng L-N, Chen L-K. Epidemiology of sarcopenia and factors associated with it among community-dwelling older adults in Taiwan. Am J Med Sci. (2018) 357:124–33.
18. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21.
19. Chiu CJ, Cheng YY. Utility of geriatric syndrome indicators for predicting subsequent health care utilization in older adults in Taiwan. Int J Environ Res Public Health. (2019) 16:456. doi: 10.3390/ijerph16030456
20. Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian working group for sarcopenia. J Am Med Dir Assoc. (2016) 17:e761–7. doi: 10.1016/j.jamda.2016.05.016
21. Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing. (2014) 43:748–59. doi: 10.1093/ageing/afu115
22. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31.
23. Kawakami R, Murakami H, Sanada K, Tanaka N, Sawada SS, Tabata I, et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. (2015) 15:969–76. doi: 10.1111/ggi.12377
24. Hoyl MT, Alessi CA, Harker JO, Josephson KR, Pietruszka FM, Koelfgen M, et al. Development and testing of a five-item version of the geriatric depression scale. J Am Geriatr Soc. (1999) 47:873–8.
25. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. (2009) 13:782–8.
26. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56.
27. Qu WF, Zhou PY, Liu WR, Tian MX, Jin L, Jiang XF, et al. Age-adjusted charlson comorbidity index predicts survival in intrahepatic cholangiocarcinoma patients after curative resection. Ann Transl Med. (2020) 8:487. doi: 10.21037/atm.2020.03.23
28. Xu J, Reijnierse EM, Pacifico J, Wan CS, Maier AB. Sarcopenia is associated with 3-month and 1-year mortality in geriatric rehabilitation inpatients: RESORT. Age Ageing. (2021) 50:2147–56. doi: 10.1093/ageing/afab134
29. Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. (2022) 68:361–76.
30. Chang SF, Lin HC, Cheng CL. The Relationship of Frailty and Hospitalization Among Older People: Evidence From a Meta-Analysis. J Nurs Scholarsh. (2018) 50:383–91.
31. Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. (2018) 18:188. doi: 10.1186/s12877-018-0878-0
32. Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People. (2015) 27:29–35.
33. Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, Sherrington C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Act. (2020) 17:150. doi: 10.1186/s12966-020-01040-4
34. Roberts HC, Lim SER, Cox NJ, Ibrahim K. The challenge of managing undernutrition in older people with frailty. Nutrients. (2019) 11:808.
35. Li CL, Chiu YC, Shyu YL, Stanaway FF, Chang HY, Bai YB. Does physical activity protect older persons with frailty and cognitive impairment from excess all-cause mortality? Arch Gerontol Geriatr. (2021) 97:104500. doi: 10.1016/j.archger.2021.104500
36. Orgeta V, Abbey CE, Orrell M. Physical activity for improving cognition in older people with mild cognitive impairment. Cochrane Database Syst Rev. (2018) 2018:CD008198.
37. Chang SH, Wung SF, Crogan NL. Improving activities of daily living for nursing home elder persons in Taiwan. Nurs Res. (2008) 57:191–8.
38. den Ouden M, Bleijlevens MH, Meijers JM, Zwakhalen SM, Braun SM, Tan FE, et al. Daily (In)activities of nursing home residents in their wards: an observation study. J Am Med Dir Assoc. (2015) 16:963–8.
39. Hopman-Rock M, van Hirtum H, de Vreede P, Freiberger E. Activities of daily living in older community-dwelling persons: a systematic review of psychometric properties of instruments. Aging Clin Exp Res. (2019) 31:917–25. doi: 10.1007/s40520-018-1034-6
40. Carmel S. Health and well-being in late life: gender differences worldwide. Front Med. (2019) 6:218. doi: 10.3389/fmed.2019.00218
41. Qian Y, Chu J, Ge D, Zhang L, Sun L, Zhou C. Gender difference in utilization willingness of institutional care among the single seniors: evidence from rural Shandong China. Int J Equity Health. (2017) 16:77. doi: 10.1186/s12939-017-0577-z
42. Shi J, Tao Y, Meng L, Zhou B, Duan C, Xi H, et al. Frailty status among the elderly of different genders and the death risk: a follow-up study. Front Med. (2021) 8:715659. doi: 10.3389/fmed.2021.715659
43. Bellettiere J, Carlson JA, Rosenberg D, Singhania A, Natarajan L, Berardi V, et al. Gender and age differences in hourly and daily patterns of sedentary time in older adults living in retirement communities. PLoS One. (2015) 10:e0136161. doi: 10.1371/journal.pone.0136161
44. Chen N, Li X, Deng M, Wang CQ, Zhou C. Gender difference in unmet need for assistance with activities of daily living among disabled seniors in China : a cross-sectional study. BMJ Open. (2021) 11:e044807. doi: 10.1136/bmjopen-2020-044807
45. Abad-Diez JM, Calderon-Larranaga A, Poncel-Falco A, Poblador-Plou B, Calderon-Meza JM, Sicras-Mainar A, et al. Age and gender differences in the prevalence and patterns of multimorbidity in the older population. BMC Geriatr. (2014) 14:75. doi: 10.1186/1471-2318-14-75
46. Corbi G, Cacciatore F, Komici K, Rengo G, Vitale DF, Furgi G, et al. Inter-relationships between gender, frailty and 10-year survival in older Italian adults: an observational longitudinal study. Sci Rep. (2019) 9:18416. doi: 10.1038/s41598-019-54897-2
47. Pinho-Gomes AC, Vassallo A, Carcel C, Peters S, Woodward M. Gender equality and the gender gap in life expectancy in the European Union. BMJ Glob Health. (2022) 7:e008278. doi: 10.1136/bmjgh-2021-008278
48. Gulacti U, Lok U, Celik M, Aktas N, Polat H. The ED use and non-urgent visits of elderly patients. Turk J Emerg Med. (2016) 16:141–5.
49. Kent T, Lesser A, Israni J, Hwang U, Carpenter C, Ko KJ. 30-day emergency department revisit rates among older adults with documented dementia. J Am Geriatr Soc. (2019) 67:2254–9. doi: 10.1111/jgs.16114
50. Street M, Berry D, Considine J. Frequent use of emergency departments by older people: a comparative cohort study of characteristics and outcomes. Int J Qual Health Care. (2018) 30:624–9.
51. Ukkonen M, Jamsen E, Zeitlin R, Pauniaho SL. Emergency department visits in older patients: a population-based survey. BMC Emerg Med. (2019) 19:20. doi: 10.1186/s12873-019-0236-3
52. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5.
53. Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. (2015) 12:22–6. doi: 10.11138/ccmbm/2015.12.1.022
54. Jo E, Lee SR, Park BS, Kim JS. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. (2012) 24:412–22.
55. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. (2006) 119:.e529–517.
56. Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. (2002) 57:M326–32.
57. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. (2017) 22:25. doi: 10.1186/s40001-017-0266-9
58. Kumar M, Ji B, Babaei P, Das P, Lappa D, Ramakrishnan G, et al. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: lessons from genome-scale metabolic modeling. Metab Eng. (2018) 49:128–42. doi: 10.1016/j.ymben.2018.07.018
59. Nardone OM, de Sire R, Petito V, Testa A, Villani G, Scaldaferri F, et al. Inflammatory bowel diseases and sarcopenia: the role of inflammation and gut microbiota in the development of muscle failure. Front Immunol. (2021) 12:694217. doi: 10.3389/fimmu.2021.694217
60. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. (2012) 15:12–22.
61. Hubner S, Boron JB, Koehler K. The effects of exercise on appetite in older adults: a systematic review and meta-analysis. Front Nutr. (2021) 8:734267. doi: 10.3389/fnut.2021.734267
62. Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev. (2009) 37:165–70.
63. Rodziewicz-Flis EA, Kawa M, Flis DJ, Szaro-Truchan M, Skrobot WR, Kaczor JJ. 12 weeks of physical exercise attenuates oxidative stress, improves functional tests performance, and reduces fall risk in elderly women independently on serum 25(OH)D concentration. Front Physiol. (2022) 13:809363. doi: 10.3389/fphys.2022.809363
64. Vijay A, Kouraki A, Gohir S, Turnbull J, Kelly A, Chapman V, et al. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. (2021) 13:1997559. doi: 10.1080/19490976.2021.1997559
65. Bagheri A, Soltani S, Hashemi R, Heshmat R, Motlagh AD, Esmaillzadeh A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr J. (2020) 19:129.
Keywords: sarcopenia, geriatric syndrome, systemic inflammation, elderly emergency, prognosis
Citation: Liao M-C, Yen C-C, Lin Y-T, Huang F-D and Chang Y-T (2023) Sarcopenia is associated with mortality in non-critical elderly patients visiting the emergency department. Front. Med. 9:1027503. doi: 10.3389/fmed.2022.1027503
Received: 25 August 2022; Accepted: 14 November 2022;
Published: 11 January 2023.
Edited by:
Bagher Larijani, Tehran University of Medical Sciences, IranReviewed by:
Vahid Rashedi, University of Social Welfare and Rehabilitation Sciences, IranCopyright © 2023 Liao, Yen, Lin, Huang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Te Chang, dmdoa3MxMTA5QHlhaG9vLmNvbS50dw==, eXV5dGNoYW5nQHZnaGtzLmdvdi50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.