- 1Department of Intensive Care Medicine, Eastern Hepatobiliary Surgery Hospital, The Third Affiliated Hospital of Naval Medical University, Shanghai, China
- 2Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Acute respiratory distress syndrome (ARDS) is a serious organ failure and postoperative complication. However, the incidence rate, early prediction and prevention of postoperative ARDS in patients undergoing hepatectomy remain unidentified.
Methods: A total of 1,032 patients undergoing hepatectomy between 2019 and 2020, at the Eastern Hepatobiliary Surgery Hospital were included. Patients in 2019 and 2020 were used as the development and validation cohorts, respectively. The incidence rate of ARDS was assessed. A logistic regression model and a least absolute shrinkage and selection operator (LASSO) regression model were used for constructing ARDS prediction models.
Results: The incidence of ARDS was 8.8% (43/490) in the development cohort and 5.7% (31/542) in the validation cohort. Operation time, postoperative aspartate aminotransferase (AST), and postoperative hemoglobin (Hb) were all critical predictors identified by the logistic regression model, with an area under the curve (AUC) of 0.804 in the development cohort and 0.752 in the validation cohort. Additionally, nine predictors were identified by the LASSO regression model, with an AUC of 0.848 in the development cohort and 0.786 in the validation cohort.
Conclusion: We reported the incidence of ARDS in patients undergoing hepatectomy and developed two simple and practical prediction models for early predicting postoperative ARDS in patients undergoing hepatectomy. These tools may improve clinicians’ ability to early estimate the risk of postoperative ARDS and timely prevent its emergence.
Introduction
The acute respiratory distress syndrome (ARDS) was first described in 1967, and specific diagnostic criteria were established in 1992 by an American-European consensus meeting (1, 2). ARDS is a common and serious complication that affects patients worldwide and often leads to a poor prognosis or even death (3). A prospective analysis of the ARDS incidence in the United States from 1999 to 2000 (4) estimated an annual incidence of 190,000 cases of ARDS in the United States, with a surprising hospital mortality rate of 38.5%. Another multicenter prospective study called LUNG-SAFE evaluated intensive care units (ICU) incidence and outcome of ARDS in 29,144 patients from 50 different counties (5). According to the findings, the prevalence of ARDS in ICU patients reached 10%, while it was identified in 23% of all patients receiving ventilation. In addition, the LUNG-SAFE study reported that the hospital mortality rate was 34.9% for patients with mild ARDS and 46.1% for those with severe ARDS. Furthermore, the global burden of ARDS is the highest in high-and upper-middle-income countries, with data indicating that the mean total hospitalization costs in Korea reached 12,336 United States dollars (USD) (6, 7). Therefore, the high morbidity and mortality of ARDS significantly reduce patients’ prognosis and place a heavy burden on global health.
There are many risk factors that contribute to ARDS development, such as pneumonia, non-pulmonary sepsis, major trauma, including surgical and accidental trauma, aspirations of gastric contents, and others (8, 9). In addition, other factors such as alcohol consumption, smoking, and hypoalbuminemia have all been linked to an increased risk of ARDS (10–12). Although great strides have been made in learning and treating ARDS over the last 50 years, effective preventive measures for postoperative ARDS still require further investigation (13, 14). Previous studies using administrative or perioperative data have established certain scoring models for predicting the risk of postoperative pneumonia, acute lung injury (ALI), or ARDS in patients (15–17). However, the majority of these studies built models based on either the general population or multiple types of surgery, and many of the predictors included in the models were inapplicable to patients undergoing specific type of surgery (16, 18, 19).
According to the most recent data, liver cancer is one of the most common cancers and the third leading cause of death among all cancers (20). Hepatectomy is a major surgical procedure that is commonly used for treating liver tumors (21). Despite improvements in hepatectomy safety over decades, the frequency of postoperative complications remains significant, ranging from 4.09 to 47.7% (22). Furthermore, data about the incidence of postoperative ARDS in patients undergoing hepatectomy is scarce. In addition, no predictors or prediction models for evaluating the possibility of developing ARDS in patients following hepatectomy were identified. Therefore, effective grades, predictive factors, or prediction models for early identifying the risk of developing ARDS in patients undergoing hepatectomy are extremely valuable.
Accordingly, by conducting a retrospective study in 1,032 patients undergoing hepatectomy between 2019 and 2020, we assessed potential predictors and constructed two prediction models to early predict the development of ARDS based on perioperative factors.
Materials and methods
Study design
This was a retrospective, single-center cohort study conducted at the Eastern Hepatobiliary Surgery Hospital, Shanghai, China. The study was approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (no. EHBHKY2021-K-011) and adhered to Helsinki Declaration and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria. Patients who signed the informed consent form and authorized permission for future research use of their medical records were included.
Participants
Patients were included between 1 January 2019, and 31 December 2020, based on the following criteria: (1) over the age of 18, (2) American Society of Anesthesiologists (ASA) Status I–III, (3) Child-Pugh class A or B, and (4) elective hepatectomy for liver cancer treatment, including hepatocellular carcinoma (HCC) and intrahepatic bile duct cancer. Patients who denied permission to utilize their health information for research purposes, as well as those with severe organ failure prior to surgery or a history of ALI or ARDS, were excluded from this study. It should be noted that liver failure was defined according to guidelines (23), heart failure as having a left ventricular ejection fraction less than 35%, respiratory failure as having an arterial oxygen partial pressure less than 60 mmHg, and renal failure as having a serum creatinine level greater than 442 μmol/L.
Variables and outcomes
The preoperative clinical characteristics of the patients were recorded. In addition, intraoperative and postoperative factors such as tumor-related data, surgical data, fluid transfusion, and postoperative complications were collected. Tumor size is expressed as the maximum tumor diameter or the sum of maximum diameters when the tumor number exceeds one. Furthermore, laboratory results of liver function, renal function, inflammatory, and other biomarkers were collected preoperatively and postoperatively within 24 h after surgery. All data were collected from the digital medical system or paper medical records by two trained researchers, checked, and entered into Excel or the EpiData system.
The outcome of this study was the development of ARDS within the first 7 days after hepatectomy. ARDS was evaluated and diagnosed by experienced ICU physicians according to the Berlin Definition (14). The diagnosis of ARDS could be listed as follow: (1) Within 1 week of a known clinical insult or new or worsening respiratory symptoms; (2) bilateral opacities of chest imaging which could not fully explained by effusions, lobar/lung collapse, or nodules; (3) respiratory failure which could not fully explained by cardiac failure or fluid overload based on the examination of electrocardiogram or echocardiogram; (4) hypoxemia which identified by the arterial blood gas analysis. (5) Comprehensive analysis based on the clinical symptom, the biochemical detection of blood and results of bacterial culture. Furthermore, ARDS prediction models were constructed using the aforementioned factors and tested in two cohorts.
Establishment and validation of prediction models
Patients with HCC or intrahepatic bile duct cancer who underwent hepatectomy in 2019 or 2020 were included in the development and validation cohorts, respectively. For prediction model construction, a logistic regression model and a least absolute shrinkage and selection operator (LASSO) model were used. Potential predictors with p-values of less than 0.05 in the multivariable logistic regression were selected for model construction. The effectiveness of the prediction model was validated in the validation cohort. Following that, a LASSO model was used to screen the most significant factors that contributed to the development of ARDS in patients. The risk score was calculated using the same method as described above, and the model was also validated in the validation cohort.
Due to the lack of an external data set for model validation, a 10-fold cross-validation method was used in both models. Furthermore, the nomogram was developed to offer a reliable and quantifiable method for immediately confirming the ARDS probability in two prediction models.
Statistical analysis
The statistical analyses were performed and graphics were designed using the R software, version 4.0.01 and the SPSS statistics software, version 23.0 (IBM SPSS Inc., Armonk, NY, USA). Categorical variables were presented as numbers and percentages, while continuous variables were expressed as mean and standard deviation or median (25% interquartile range, 75% interquartile range) based on their normality. Continuous variables were compared using the Student’s t-test or Mann-Whitney U test. Categorical variables were compared using the Chi-squared χ2 test. The receiver operator characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated to assess the model discrimination. Furthermore, calibration curves with C-index were made in two cohorts to test the prediction model stability. The optimal risk score cutoff point was determined by Youden’s index. The multiple imputation method was used to fill in the missing data for characteristics included in the prediction models, and all missing proportions were less than 10%. All statistical tests were two-sided, with p-values less than 0.05 considered statistically significant.
Results
Cohort characterization
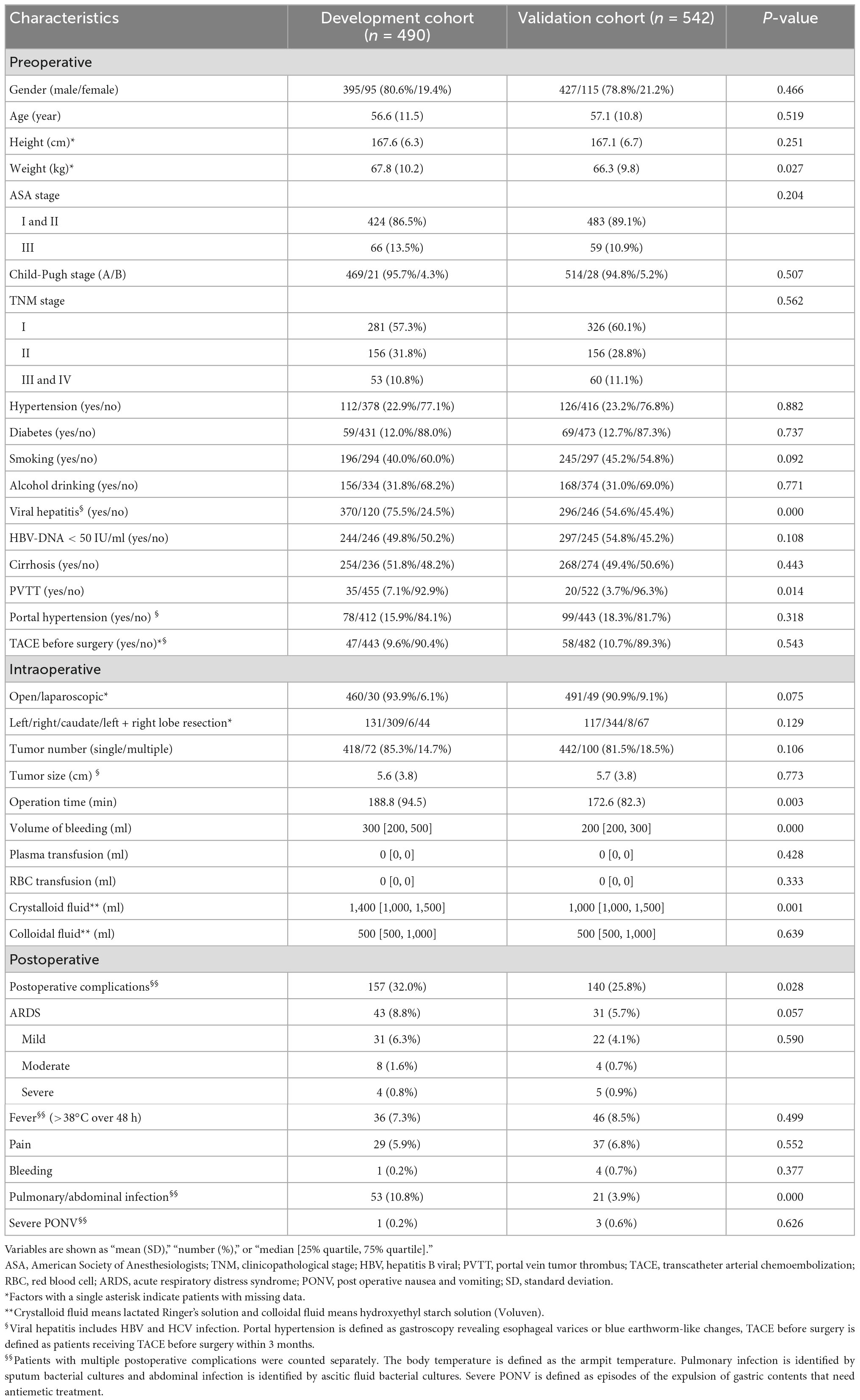
According to the inclusion criteria, 490 patients were included in the development cohort and 542 in the validation cohort (Table 1). The incidence rate of ARDS was 8.8% (43/490) in the development cohort and 5.7% (31/542) in the validation cohort. The overall incidence rate of ARDS in patients undergoing hepatectomy was 7.2% (74/1,032). Several characteristics, such as weight, viral hepatitis, portal vein tumor thrombus, operation time, and others, were significantly different between the two groups. The incidence rate of postoperative complications was higher in the development cohort (32.0%) than in the validation cohort (25.8%). In particular, the incidence rate of pulmonary or abdominal infections was higher in the development cohort (10.8%) than in the validation cohort (3.9%).
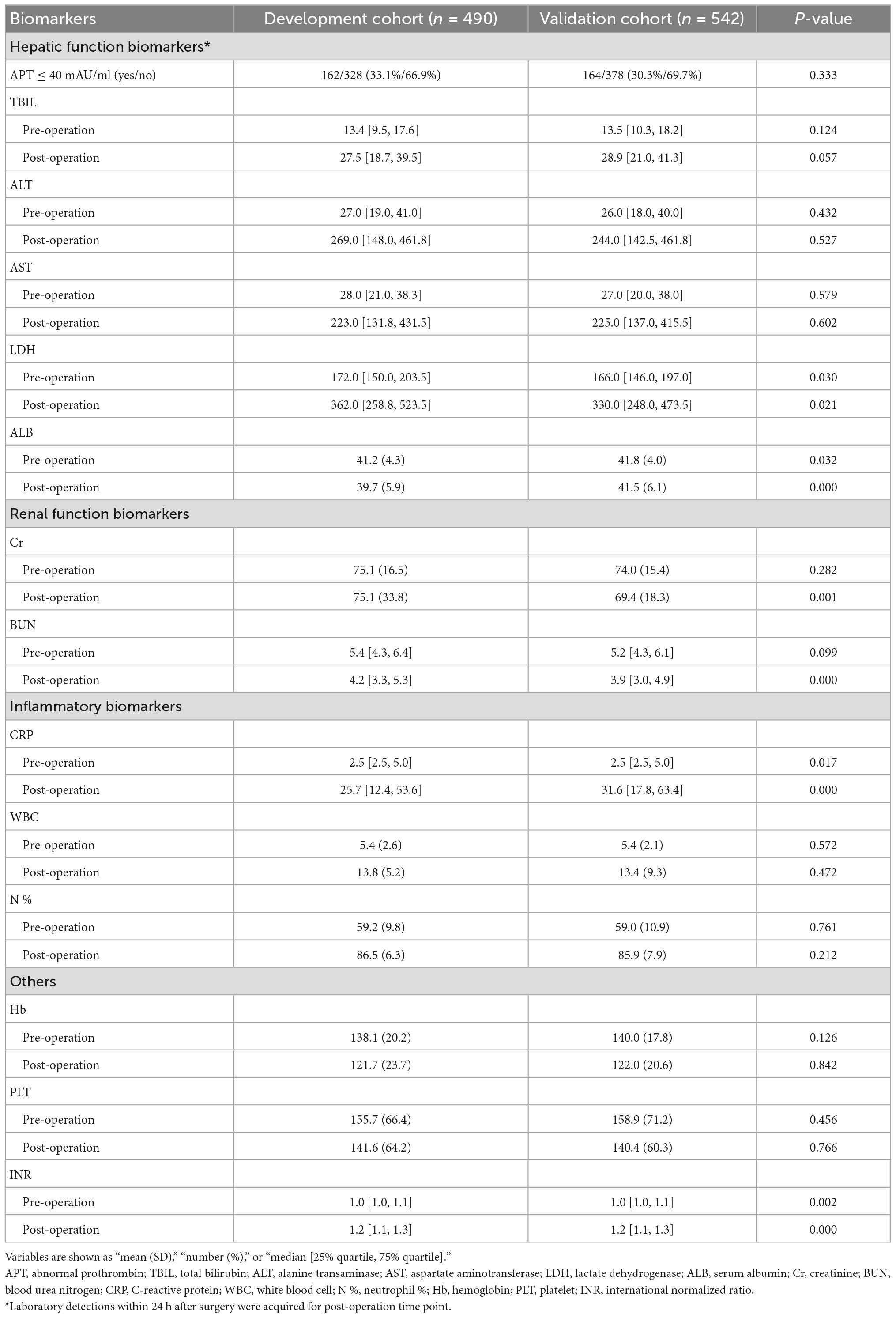
In addition, the laboratory results of 14 critical liver, renal, inflammatory, and coagulation-related biomarkers were collected preoperatively and postoperatively for model construction (Table 2). The levels of several biomarkers, such as lactate dehydrogenase (LDH), serum albumin (ALB), C-reactive protein, and others, were significantly different between the two cohorts.
Model construction and validation based on logistic regression
First, a logistic regression model was used to construct the prediction model. It included 18 covariates with p-values of less than 0.05 in the univariable logistic analysis (Supplementary Table 1). Three critical predictors; operation time, postoperative aspartate aminotransferase (AST), and postoperative hemoglobin (Hb); were identified and integrated into the prediction model (Supplementary Table 2). The risk score was calculated using the following formula:
The AUC was calculated using the ROC curve, and it was 0.804 (95% confidence interval (CI): 0.741–0.868) in the development cohort and 0.752 (95% CI: 0.660–0.844) in the validation cohort (Figure 1A). Furthermore, model validation using the 10-fold cross-validation method revealed similar discrimination with a mean C-index of 0.792 in the development cohort. Then, using raw data, a model was constructed, and a similar AUC was found in the two cohorts, with mean AUC values of 0.805 and 0.773 in the development and validation cohorts, respectively. Based on the logistic prediction model, a visual nomogram was developed to establish a reliable and quantitative method for assessing the probability of ARDS (Figure 1B).
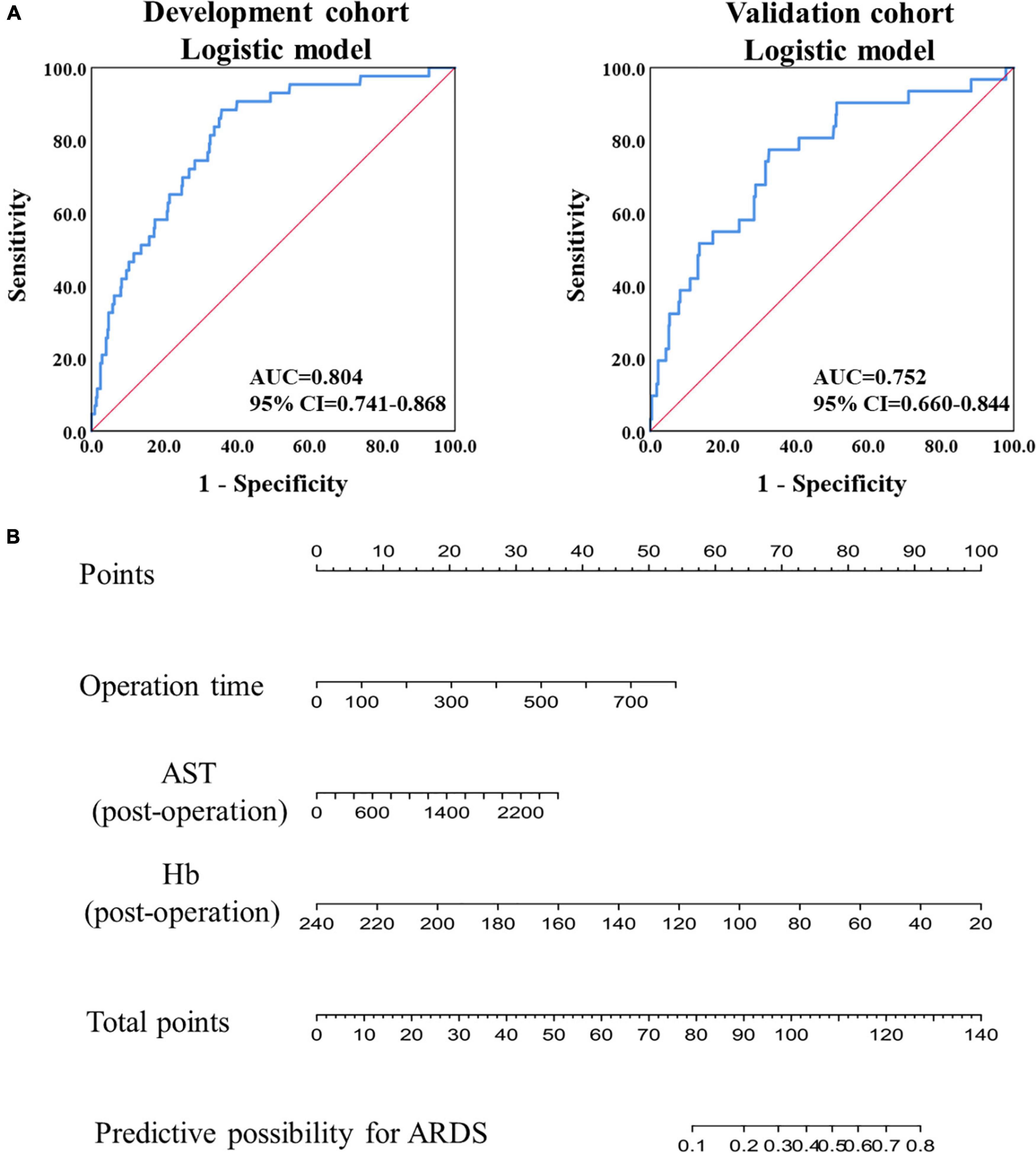
Figure 1. Model construction and validation based on logistic regression. (A) The ROC analysis of the prediction model in the development and validation cohorts. (B) The nomogram of prediction model based on the logistic regression in the development cohort.
Model construction and validation based on LASSO regression
A LASSO model was then used to construct another prediction model. Finally, 14 predictors were acquired, and nine predictors with coefficients greater than 0.001 were used in the final prediction model (Figure 2A). The risk score was calculated using the following formula:
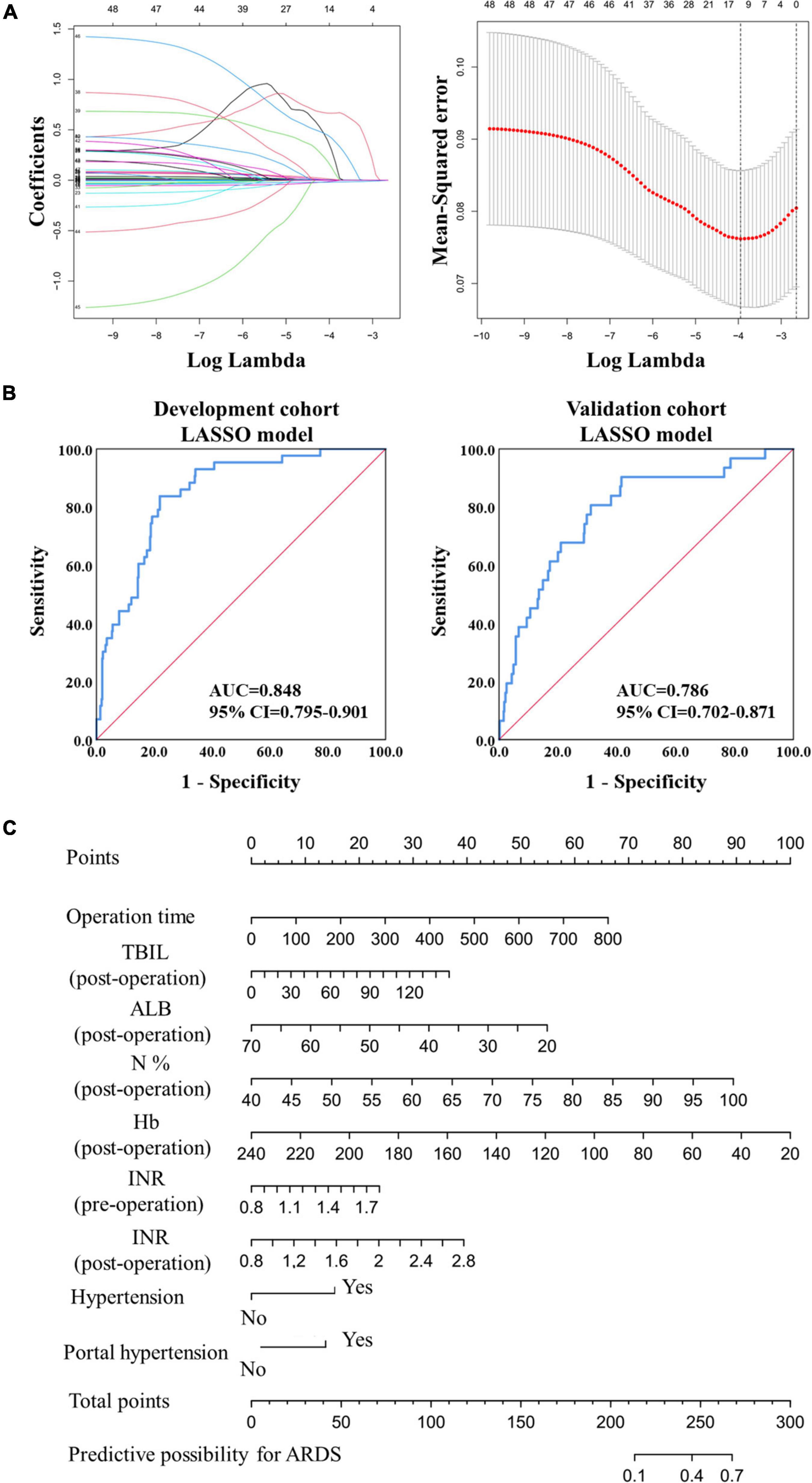
Figure 2. Model construction and validation based on LASSO regression. (A) The construction of prediction model based on the LASSO regression. (B) The ROC analysis of the prediction model in the development and validation cohorts. (C) The nomogram of the prediction model based on the LASSO regression in the development cohort.
The AUC value reached 0.848 (95% CI: 0.795–0.901) in the development cohort and 0.786 (95% CI: 0.702–0.871) in the validation cohort (Figure 2B), exceeding the logistic prediction model’s discriminating power. In addition, model validation using the 10-fold cross-validation method showed similar discrimination, with a mean C-index of 0.808 in the development cohort. Furthermore, a visual nomogram was developed to help visualize the prediction model and directly calculate the probability of ARDS (Figure 2C).
Furthermore, the calibration curves showed good fitting effects between predicted and observed outcomes for the logistic prediction model (Figure 3A) and the LASSO prediction model (Figure 3B) in two cohorts.
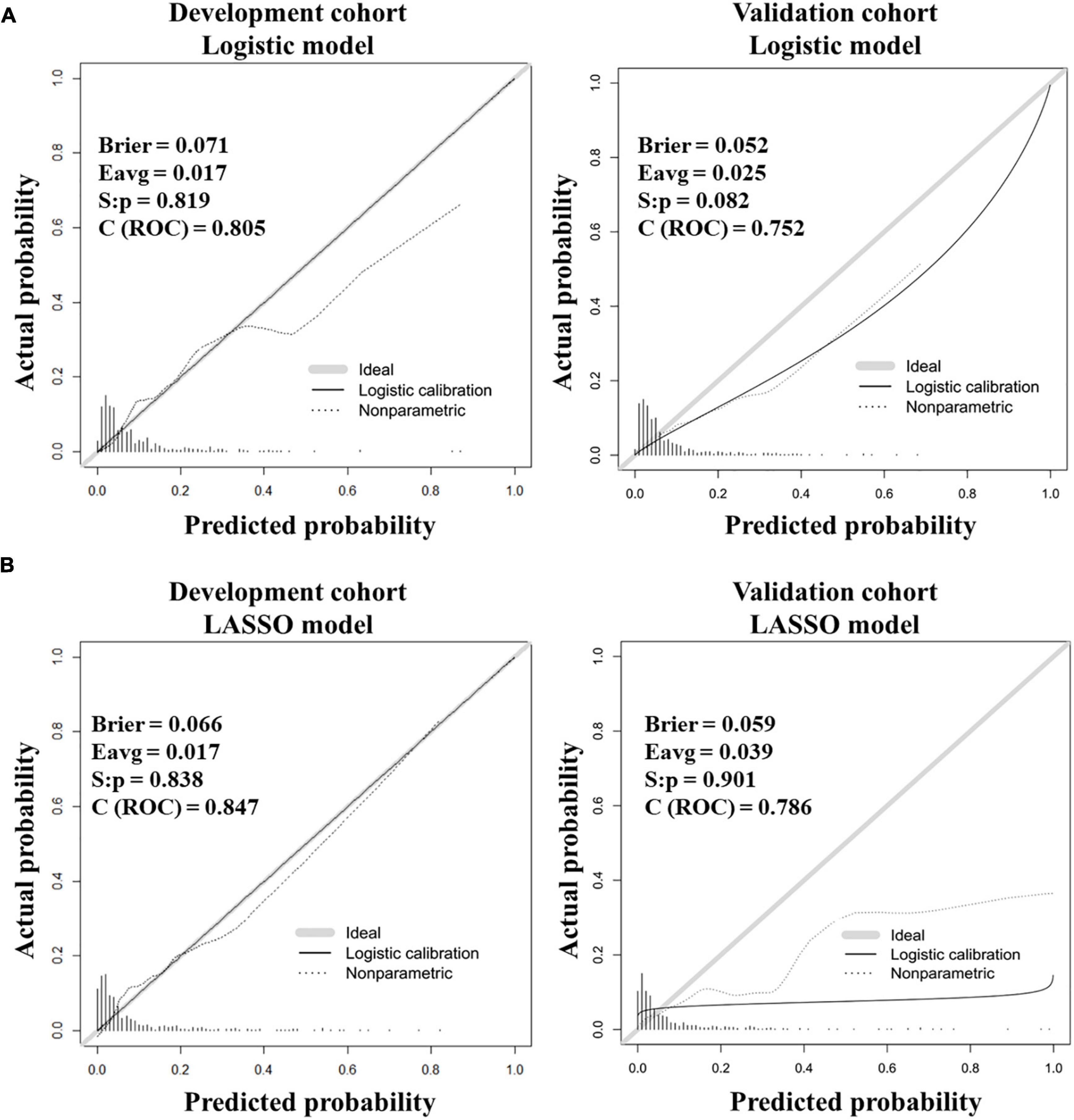
Figure 3. Calibration curves for testing the stability of prediction models in the development and validation cohorts. (A) Calibration curves for the logistic model. (B) Calibration curves for the LASSO model.
Discriminative effects of prediction models for ARDS by grading risk scores
To better evaluate the discrimination of our prediction models and simplify models for clinical application, risk scores were calculated based on formulas and graded to three degrees: low-risk, medium-risk, and high risk. According to Table 3, patients’ risk scores were distributed consistently in the development and validation cohorts, regardless of whether the logistic or the LASSO model was used, suggesting good robustness of our models. We were able to effectively classify patients into different risk groups by dividing the risk score into three degrees. Based on our cohorts, the incidence of ARDS was approximately 2% in the low-risk group, while it significantly increased in the medium-risk and high-risk groups in both cohorts (Table 3).
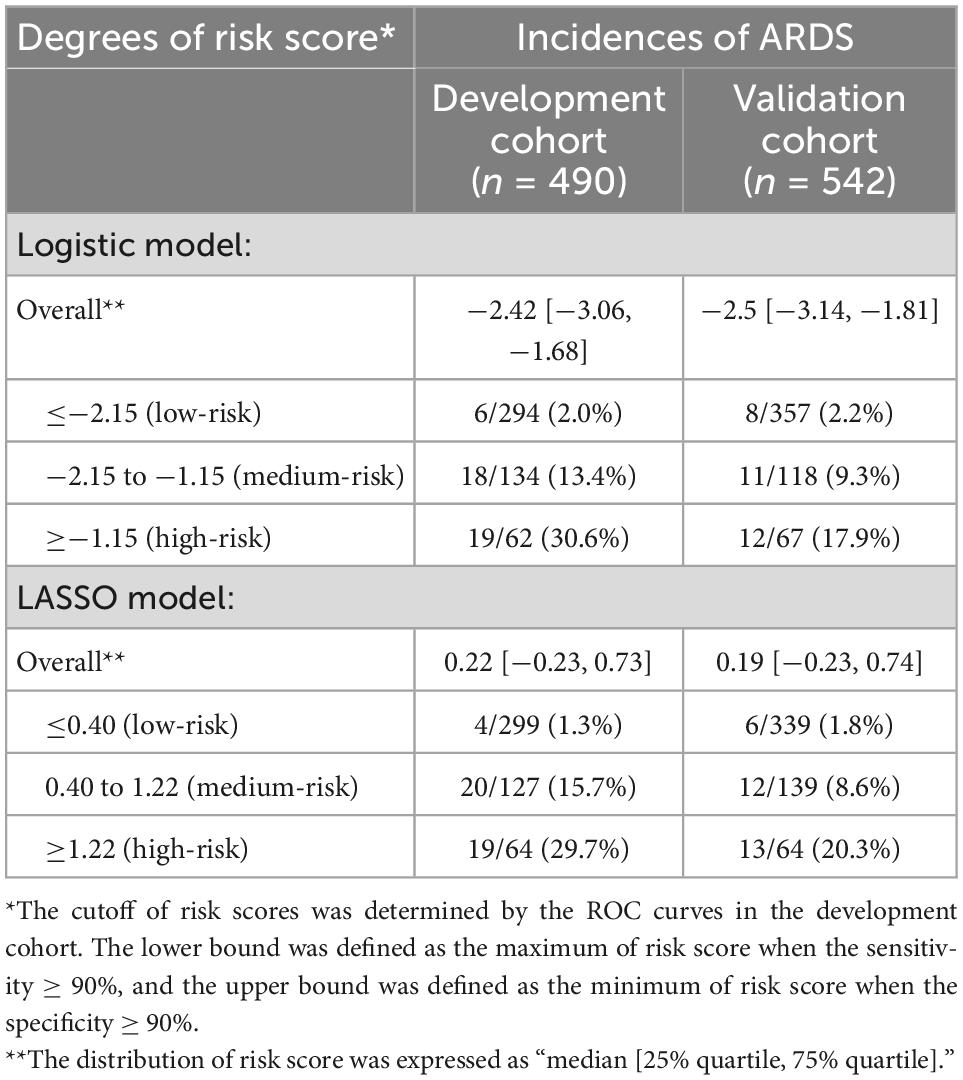
Table 3. Incidences of ARDS classified by risk scores in the development cohort and validation cohort.
In the development cohort using the optimal cutoff, the logistic model exhibited a sensitivity of 88.4% and a specificity of 64.2% (Supplementary Table 3), while the LASSO model had a sensitivity of 83.7% and a specificity of 78.1% (Supplementary Table 3), suggesting good predictive effects of our prediction models for ARDS.
Subgroup analysis of two prediction models in the development cohort
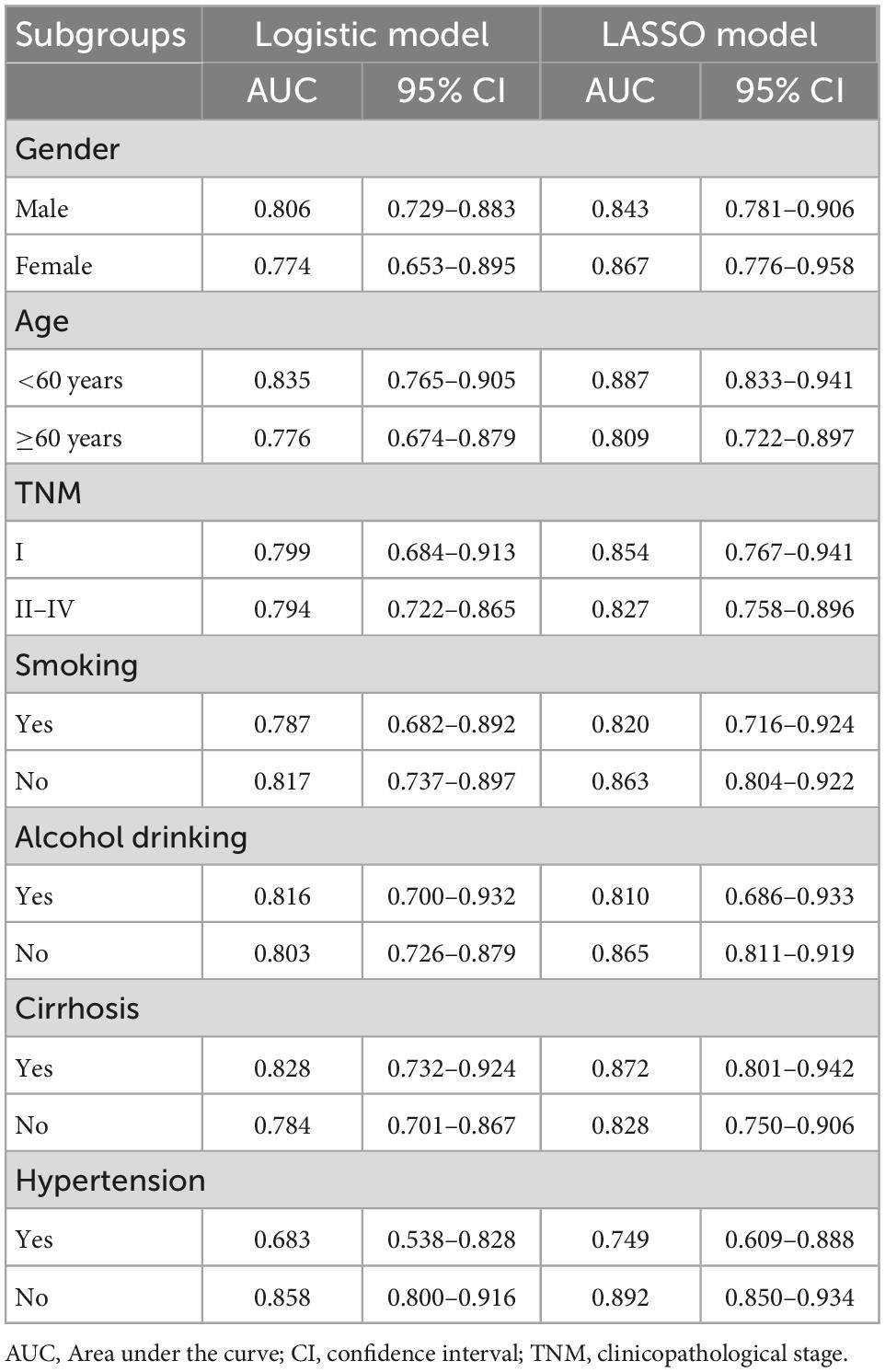
To verify the sensitivity of two models for predicting ARDS, subgroup analysis was performed in the development cohort. Table 4 shows that both prediction models had sustained predictive effects for ARDS in different subgroups. The AUC value reached 0.887 in the LASSO model in patients less than 60 years old. Furthermore, in the subgroup of patients with no hypertension, both logistic and LASSO models had superior predictive effects, with AUC of 0.858 and 0.892, respectively.
Discussion
Acute respiratory distress syndrome is one of the most devastating postoperative complications and significantly increased mortality. Early prediction and treatment of ARDS are critical for improving patients’ prognosis in clinical practice. To the best of our knowledge, our study was the first to report the incidence of ARDS in patients undergoing hepatectomy for liver cancer treatment, which was approximately 7.2%. In addition, we constructed two effective prediction models by integrating 48 perioperative covariates using logistic and LASSO regression. Both prediction models have good predictive effects, and the AUC exceeds 0.8 in the development cohort. Moreover, a number of major risk factors for postoperative ARDS have been identified. For instance, patients with a longer operation time, higher postoperative levels of AST, TBIL, and N %, and lower postoperative Hb and ALB levels are more likely to develop ARDS postoperatively. Furthermore, the risk of developing ARDS is effectively assessed by grading risk scores to three degrees. It enables clinicians to immediately identify patients at risk of ARDS.
Previously, a single-center retrospective study reported an acute lung injury (ALI) prediction model called the Lung Injury Prediction Score (LIPS) (24), and they developed a tool for evaluating the risk of ALI and ARDS in a population-based sample, which was validated in a multicenter study (16). Other studies, which focused on surgical lung injury, developed two scoring systems termed SLIP and SLIP-2 to predict the risk of early postoperative ALI and ARDS in patients undergoing elective surgery (17, 19). These studies supplied effective scoring systems for clinical practice.
Several predictors and prediction models for predicting the incidence of ARDS in patients with specific diseases or injuries have been reported (18, 25, 26). For instance, Majid et al. constructed a 3-variable model comprising total body surface area percent, inhalation injury, and von Willebrand factor-A2 to predict ARDS in patients with burn injuries, with an AUC of 0.90. In addition, Ning et al. developed a logistic model to predict the in-hospital incidence of ARDS in patients with acute pancreatitis (26). In addition, other studies reported the risk factors of ARDS in patients undergoing major surgery or extracorporeal membrane oxygenation (16, 18, 27–29). For instance, James et al. analyzed preoperative and intraoperative predictors of postoperative ARDS in a general surgical population (30). However, studies focusing on the incidence and early prediction models of ARDS in hepatobiliary surgery are scarce.
The logistic prediction model identified operation time, postoperative AST, and postoperative Hb as the most crucial predictors of ARDS. The operation duration reflects the complexity of the operation, and a longer operation duration usually means more trauma for the patients, as mentioned in another study on ARDS prediction (18). Furthermore, several studies found associations between liver function biomarkers (TBIL, AST, ALB, and international normalized ratio) and ARDS progression (31–33). Hypertension and portal hypertension also contributed to the development of ARDS based on the LASSO model (34, 35). It is possible to explain how portal hypertension can lead to ARDS as it is mainly caused by various forms of cirrhosis, which is closely associated with liver function and may aggravate ascites formation and hypoproteinemia. These predictors could be new biomarkers or targets for ARDS.
According to relevant studies, the incidence rate of ARDS following cardiac surgery ranges from 0.4 to 8.1% (17, 28, 36). Daryl et al. reported a 2.6% (113/4,366) incidence of ALI and ARDS in patients undergoing high-risk surgery, and 3.3% and 22% for patients undergoing spine surgery and high-risk vascular surgery, respectively (17, 19). In our study, the incidence of ARDS in patients undergoing hepatectomy was 7.2% (74/1,032), indicating a higher incidence rate of ARDS than in other types of surgery. Therefore, more attention should be paid to early prediction and prevention of ARDS development in patients undergoing hepatectomy.
In our study, two alternative prediction models were constructed, each with its own set of advantages. First, only three predictors were included in the logistic prediction model, making it easier and faster for clinicians to calculate risk scores and identify patients at risk for ARDS. Second, according to the AUC, the LASSO model with nine predictors outperforms the logistic model in predicting ARDS, with an AUC of 0.848 in the development cohort. However, predicting ARDS is more difficult for clinicians and requires more data from patients. Nevertheless, because only preoperative and postoperative (within 24 h after surgery) factors were included in the models, both models made it possible to early predict postoperative ARDS, saving time for pre-treatment of patients at high risk of ARDS.
There are some limitations to this study. First, due to the retrospective design of this study, there is unavoidably potential bias and confounding. Secondly, this is a single-center cohort, and prediction models are only validated using internal data. To compensate for this shortcoming, a 10-fold cross-validation method was employed to validate the robustness and accuracy of models simultaneously. Further studies are warranted to validate the results in more centers.
Conclusion
In conclusion, we reported the incidence of ARDS in hepatobiliary surgery and developed two simple and practical prediction models for predicting postoperative ARDS in patients undergoing hepatectomy. These tools may improve clinicians’ ability to early estimate the risk of postoperative ARDS and timely prevent its emergence.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XW, YL, and FW: study concept and design. XW, YL, HZ, and RZ: acquisition, analysis, and interpretation of data. XW and YL: statistical analysis, software, and drafting of the manuscript. FW and WY: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972574), the Shanghai Pudong New Area Municipal Commission of Health and Family Planning Funding (No. PWZxq2017-06), the Shanghai Municipal Key Clinical Specialty (No. shslczdzk03601), the Shanghai Engineering Research Center of Peri-operative Organ Support and Function Preservation (No. 20DZ2254200), and the National Natural Science Foundation of China Youth Training Project (No. 2021GZR003).
Acknowledgments
We thank Enago Company for native language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1025764/full#supplementary-material
Supplementary Table 1 | Univariable logistic regression analyses for ARDS in the development cohort.
Supplementary Table 2 | Multivariable logistic regression analyses for ARDS in the development cohort.
Supplementary Table 3 | Performance of two prediction models in the development and validation cohorts.
Abbreviations
ASA, American Society of Anesthesiologists; HBV, hepatitis B viral; PVTT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; RBC, red blood cell; ARDS, acute respiratory distress syndrome; PONV, post operative nausea and vomiting; APT, abnormal prothrombin; TBIL, total bilirubin; ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ALB, serum albumin; Cr, creatinine; BUN, blood urea nitrogen; CRP, C-reactive protein; WBC, white blood cell; N %, neutrophil %; Hb, hemoglobin; PLT, platelet; INR, international normalized ratio; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; LASSO, least absolute shrinkage and selection operator; HCC, hepatocellular carcinoma; ICU, intensive care unit; SD, standard deviation; ROC, receiver operator characteristic; AUC, area of under the curve.
Footnotes
References
1. Ashbaugh D, Bigelow D, Petty T, Levine B. Acute respiratory distress in adults. Lancet. (1967) 2:319–23. doi: 10.1016/S0140-6736(67)90168-7
2. Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, et al. The American-European consensus conference on ARDS. definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. (1994) 149(3 Pt. 1):818–24. doi: 10.1164/ajrccm.149.3.7509706
3. Beitler J, Thompson B, Baron R, Bastarache J, Denlinger L, Esserman L, et al. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med. (2022) 10:107–20. doi: 10.1016/S2213-2600(21)00157-0
4. Rubenfeld G, Caldwell E, Peabody E, Weaver J, Martin D, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. (2005) 353:1685–93. doi: 10.1056/NEJMoa050333
5. Bellani G, Laffey J, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care Units in 50 Countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
6. Oh T, Song I. Trends in mortality, treatment, and costs of management of acute respiratory distress syndrome in south korea: analysis of data between 2010 and 2019. Yonsei Med J. (2022) 63:452–60. doi: 10.3349/ymj.2022.63.5.452
7. Adhikari N, Fowler R, Bhagwanjee S, Rubenfeld G. Critical care and the global burden of critical illness in adults. Lancet. (2010) 376:1339–46. doi: 10.1016/S0140-6736(10)60446-1
8. Pham T, Rubenfeld G. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Med. (2017) 195:860–70. doi: 10.1164/rccm.201609-1773CP
9. Matthay M, Zemans R, Zimmerman G, Arabi Y, Beitler J, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. (2019) 5:18. doi: 10.1038/s41572-019-0069-0
10. Moss M, Bucher B, Moore F, Moore E, Parsons P. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. (1996) 275:50–4. doi: 10.1001/jama.275.1.50
11. Calfee C, Matthay M, Eisner M, Benowitz N, Call M, Pittet J, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. (2011) 183:1660–5. doi: 10.1164/rccm.201011-1802OC
12. Mangialardi R, Martin G, Bernard G, Wheeler A, Christman B, Dupont W, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in sepsis study group. Crit Care Med. (2000) 28:3137–45. doi: 10.1097/00003246-200009000-00001
13. Meyer N, Gattinoni L, Calfee C. Acute respiratory distress syndrome. Lancet. (2021) 398:622–37. doi: 10.1016/S0140-6736(21)00439-6
14. Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
15. Arozullah A, Khuri S, Henderson W, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Int Med. (2001) 135:847–57. doi: 10.7326/0003-4819-135-10-200111200-00005
16. Gajic O, Dabbagh O, Park P, Adesanya A, Chang S, Hou P, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. (2011) 183:462–70. doi: 10.1164/rccm.201004-0549OC
17. Kor D, Lingineni R, Gajic O, Park P, Blum J, Hou P, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology. (2014) 120:1168–81. doi: 10.1097/ALN.0000000000000216
18. Huang L, Song M, Liu Y, Zhang W, Pei Z, Liu N, et al. Acute respiratory distress syndrome prediction score: derivation and validation. Am J Crit Care. (2021) 30:64–71. doi: 10.4037/ajcc2021753
19. Kor D, Warner D, Alsara A, Fernández-Pérez E, Malinchoc M, Kashyap R, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. (2011) 115:117–28. doi: 10.1097/ALN.0b013e31821b5839
20. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
21. Marrero J, Kulik L, Sirlin C, Zhu A, Finn R, Abecassis M, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
22. Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. (2013) 19:7983–91. doi: 10.3748/wjg.v19.i44.7983
23. Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association, Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Guideline for diagnosis and treatment of liver failure]. Zhonghua Gan Zang Bing Za Zhi. (2019) 27:18–26.
24. Trillo-Alvarez C, Cartin-Ceba R, Kor D, Kojicic M, Kashyap R, Thakur S, et al. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. (2011) 37:604–9. doi: 10.1183/09031936.00036810
25. Afshar M, Burnham E, Joyce C, Gagnon R, Dunn R, Albright J, et al. Injury characteristics and von willebrand factor for the prediction of acute respiratory distress syndrome in patients with burn injury: development and internal validation. Ann Surg. (2019) 270:1186–93. doi: 10.1097/SLA.0000000000002795
26. Ding N, Guo C, Song K, Li C, Zhou Y, Yang G, et al. Nomogram for the prediction of in-hospital incidence of acute respiratory distress syndrome in patients with acute pancreatitis. Am J Med Sci. (2022) 363:322–32. doi: 10.1016/j.amjms.2021.08.009
27. Liu Y, Song M, Huang L, Zhu G. A nomogram to predict acute respiratory distress syndrome after cardiac surgery. Heart Surg Forum. (2021) 24:E445–50. doi: 10.1532/hsf.3809
28. Sanfilippo F, Palumbo G, Bignami E, Pavesi M, Ranucci M, Scolletta S, et al. Acute respiratory distress syndrome in the perioperative period of cardiac surgery: predictors, diagnosis, prognosis, management options, and future directions. J Cardiothorac Vasc Anesth. (2022) 36:1169–79. doi: 10.1053/j.jvca.2021.04.024
29. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus P, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. the respiratory extracorporeal membrane oxygenation survival prediction (RESP) score. Am J Respir Crit Care Med. (2014) 189:1374–82. doi: 10.1164/rccm.201311-2023OC
30. Blum J, Stentz M, Dechert R, Jewell E, Engoren M, Rosenberg A, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. (2013) 118:19–29. doi: 10.1097/ALN.0b013e3182794975
31. Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care. (2014) 2:32. doi: 10.1186/2052-0492-2-32
32. Harnisch L, Baumann S, Mihaylov D, Kiehntopf M, Bauer M, Moerer O, et al. Biomarkers of cholestasis and liver injury in the early phase of acute respiratory distress syndrome and their pathophysiological value. Diagnostics. (2021) 11:2356. doi: 10.3390/diagnostics11122356
33. Baranovskii D, Klabukov I, Krasilnikova O, Nikogosov D, Polekhina N, Baranovskaia D, et al. Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia. Curr Med Res Opin. (2021) 37:21–5. doi: 10.1080/03007995.2020.1853510
34. Contant C, Valadka A, Gopinath S, Hannay H, Robertson C. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. (2001) 95:560–8. doi: 10.3171/jns.2001.95.4.0560
35. Pranata R, Lim M, Huang I, Raharjo S, Lukito A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. (2020) 21:1470320320926899. doi: 10.1177/1470320320926899
Keywords: acute respiratory distress syndrome, liver cancer, hepatectomy, prediction model, organ failure, LASSO regression
Citation: Wang X, Zhang H, Zong R, Yu W, Wu F and Li Y (2023) Novel models for early prediction and prevention of acute respiratory distress syndrome in patients following hepatectomy: A clinical translational study based on 1,032 patients. Front. Med. 9:1025764. doi: 10.3389/fmed.2022.1025764
Received: 11 October 2022; Accepted: 14 December 2022;
Published: 09 January 2023.
Edited by:
Chenyang Duan, Chongqing Medical University, ChinaReviewed by:
Francesco Forfori, University of Pisa, ItalyLixin Xie, People’s Liberation Army General Hospital, China
Copyright © 2023 Wang, Zhang, Zong, Yu, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiran Li,  bGl5aXJhbmVoc2hAc2luYS5jb20=; Feixiang Wu,
bGl5aXJhbmVoc2hAc2luYS5jb20=; Feixiang Wu,  ZmVpeGlhbmd3dUBob3RtYWlsLmNvbQ==; Weifeng Yu,
ZmVpeGlhbmd3dUBob3RtYWlsLmNvbQ==; Weifeng Yu,  eXdmODA4QHllYWgubmV0
eXdmODA4QHllYWgubmV0
 Xiaoqiang Wang
Xiaoqiang Wang Hongyan Zhang1
Hongyan Zhang1 Weifeng Yu
Weifeng Yu Feixiang Wu
Feixiang Wu Yiran Li
Yiran Li