- 1Department of Hematology, National Center for Cancer Care and Research (NCCCR), Hamad Medical Corporation (HMC), Doha, Qatar
- 2Pharmacy Department, Heart Hospital, Hamad Medical Corporation (HMC), Doha, Qatar
Tyrosine kinase inhibitors (TKIs) have significantly improved the prognosis of chronic myeloid leukemia (CML) since their approval. Although safe in general, TKIs carry concerns about cardiovascular adverse events. Hypertension, diabetes, and dyslipidemia are among the most common baseline comorbidities among CML patients. Guidelines for the management of the existing comorbidities or those related to TKI therapy are lacking. This paper will review hypertension, hyperglycemia and hyperlipidemia reported in CML patients or associated with TKI therapy and then propose a simple guide on their management.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm caused by a fusion gene that encodes for the oncoprotein BCR-ABL, with constitutive tyrosine kinase activity (1); the target for tyrosine kinase inhibitor(s) [TKI(s)] (2). The introduction of the first generation TKI (imatinib) in 2001, substantially improved CML prognosis with reported survival figures comparable to that of the general population (1, 3). The striking results continued with the availability of the second (bosutinib, dasatinib, nilotinib) and third (ponatinib) generation TKIs, by obtaining faster and more effective deep molecular responses, but at the expense of the toxicity profile (1). TKIs are generally safe, and most patients experience mild and transient adverse events (3). However, there are major concerns about the reported cardiovascular adverse events and toxicity in randomized and observational trials (1). CML patients usually have baseline comorbidities or experience cardiovascular events that precluded them from enrolling in the CML landmark trials (4, 5). The evidence for the medical management of the existent or new comorbidities is scarce (3), and dedicated guidelines are lacking. Herein, this review will discuss some of the most common comorbidities reported in CML patients or associated with their TKI therapy and draft a practical guide on their management in the daily clinical practice.
Baseline comorbidities and on-TKI incidence of cardiovascular events
Patients with CML usually have baseline comorbidities or develop new morbidities when starting TKI therapy. An analysis of data from the United States (n = 1,639) reported an incidence of cardiovascular diseases (e.g., myocardial infarction, angina, stroke, peripheral arterial disease, atherosclerosis, and heart failure) of 33% and cardiovascular risk factors (e.g., diabetes mellitus, hypertension, smoking and high blood cholesterol) of 77.7% in CML patients at five-year follow-up. At 1 year follow up, the age and gender standardized annual rates of cardiovascular conditions for CML patients were significantly higher by a factor of 1.3 to 3.5 compared to the general population. Similarly, the standardized annual rates for hypertension, diabetes, and obesity were higher by 20–40% (p < 0.001) (6). Another retrospective analysis from the MarketScan Commercial and Medicare databases (n = 2,296) demonstrated that 41% of those who newly started TKI therapy had at least one comorbid disease. The most prevalent comorbidity among patients with CML was heart disease. Its prevalence (45% among Medicare-insured and 23% among commercially insured individuals) was higher than that among the United States population which ranged from 12% (age 47–64) to 37% (age ≥75). Diabetes comes second with a prevalence of 24% among Medicare-insured and 16% among commercially insured CML patients that is also higher than that of the general population (13% for individual aged 45–64 and 20% for those aged ≥75 years) (7). Elderly patients are more likely to have multiple comorbidities; 56% of patients with a median age of 78.5 years at the time of diagnosis had two or three comorbidities (8). Interestingly, although the presence of comorbidities did not affect the success of CML treatment, it has negatively impacted the overall survival. This was demonstrated in the analysis of the CML Study IV (n = 1,519) that examined the impact of comorbidities, using the Charlson Comorbidity Index (CCI), at the CML diagnosis on remission rate and overall survival. There was a significant negative association between comorbidities and overall survival. The CCI of 2, 3–4, 5–6, and ≥7 was associated with 93.6, 89.4, 77.6, and 46.4% probabilities of eight-year overall survival, respectively. In a multivariate analysis, CCI was the strongest predictor of the overall survival. The benefit from imatinib therapy was significantly higher in those with multiple comorbidities. However, comorbidities had no influence on remission rates or disease progression. Thus, comorbidities may be a determinant for survival as opposed to the CML disease itself (9).
A study which investigated the incidence of cardiovascular and arteriothrombotic events in CML patients treated with TKIs (n = 531), found that 45 and 9% of patients developed cardiovascular and arteriothrombotic adverse events, respectively. The most common morbidity was hypertension (33%) (10). With regard TKI therapy, the second generation TKIs (at least nilotinib and dasatinib) have 1.5–2 times higher cardiovascular risk than with imatinib. The incidence of myocardial infarction in patients treated with dasatinib or nilotinib increased by 2.4 or 3.6 times in comparison with imatinib (3). Whereas ponatinib, the most potent and third generation TKI, had the highest incidence ratios for cardiovascular [40.7; 95% confidence interval (CI): 27.9–59.4] and arteriothrombotic (9.0; 95% CI: 4.1–20.1) adverse events, moreover, treatment with ponatinib was associated with significantly higher incidence rate ratios for cardiovascular (4.62; 95% CI: 2.7–7.7; p < 0.0001) and arteriothrombotic (6.38; 95% CI: 1.8–21.8; p < 0.0001) adverse events compared with imatinib when using a multivariable analysis. Overall, newer TKIs, especially ponatinib, may carry higher risk for cardiovascular adverse events (10). Given the lack of head-to-head comparison between various TKIs and inconsistency in the reporting of adverse events, precise quantitation of this increased risk is not possible. For the same reason, using data from the randomized trial that compared bosutinib, dasatinib, or nilotinib with imatinib may allow an indirect comparison between the second-generation drugs (3). The cardiovascular toxicities of bosutinib are considered rare. In the BELA (Bosutinib Efficacy and Safety in Newly Diagnosed CML) trial, cardiotoxicities (including heart failure) were similar between bosutinib and imatinib arms (11). A meta-analysis of 10 randomized trials did not show statistically significant difference in the risk of vascular occlusive complications between bosutinib and imatinib (12).
Mechanism of cardiovascular morbidities or adverse events
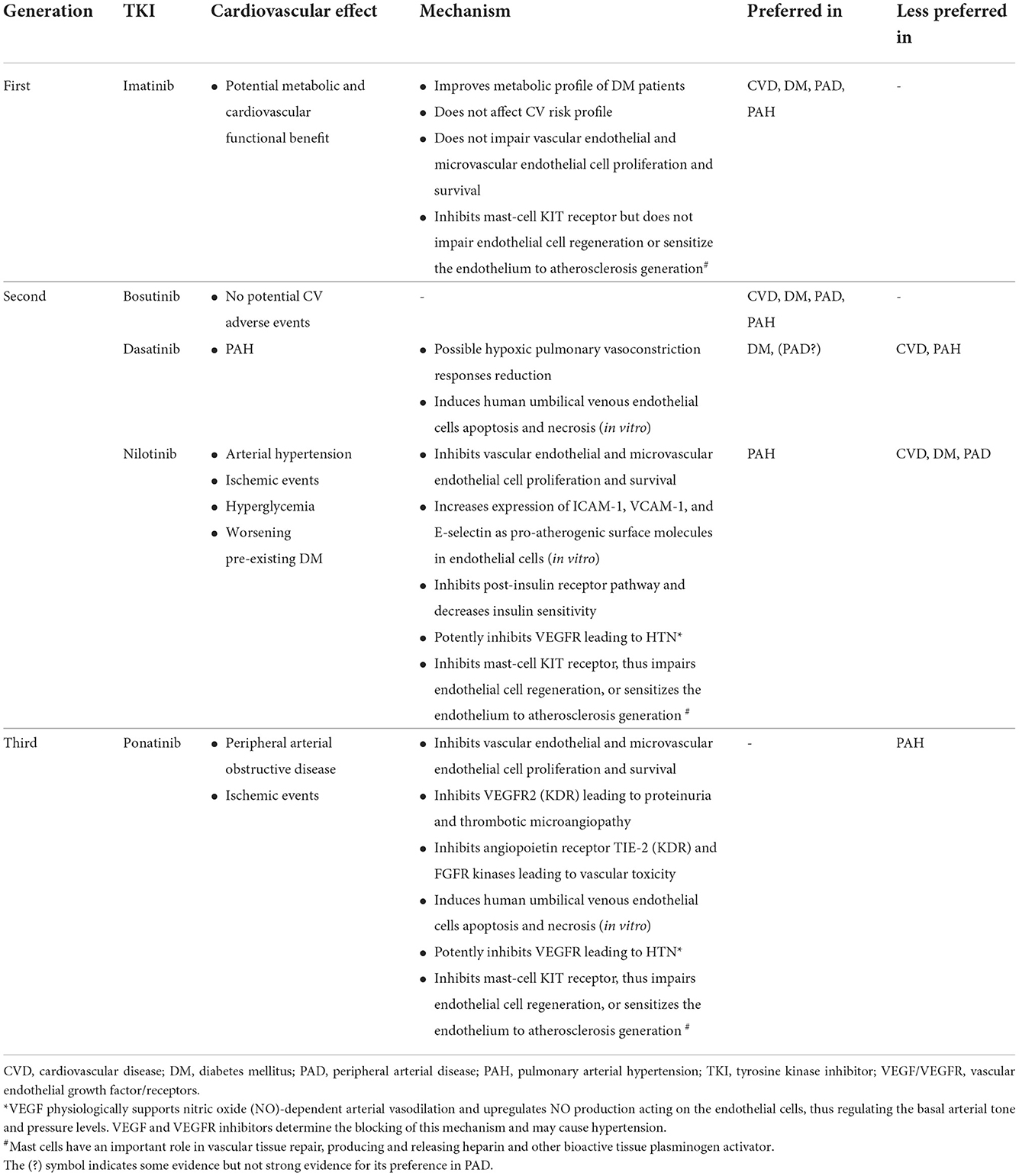
The mechanisms of the TKIs adverse effects are related to inhibiting the main BCR-ABL1 tyrosine kinase (i.e., on-target effects) and other kinases that are not involved to the CML pathogenesis (off-target effects) (1). The activity and potency of the approved TKIs against non-BCR-ABL kinases varies and targets vascular endothelial growth factor receptors (VEGFR) 1–3, angiopoietin receptor TIE-2, fibroblast growth factor receptors, and platelet-derived growth factor receptors A and B (2). The resultant off-target effects are believed to increase cardiovascular risk. The spectrum of cardiovascular adverse effects of each TKI also vary and can be influenced by patient's age, gender, comorbidities, and the existence of other cardiovascular risk factors such as smoking, diabetes, dyslipidemia, obesity, drug-drug interaction and so forth. The mechanism of cardiovascular adverse effects caused by TKIs is not fully explained but it might be multifactorial. Different factors may lead to endothelial damage and atherosclerosis, hypertensive effect, metabolic impairment, and mast-cell disruption (1). TKIs can also affect the platelets activities such inhibiting their activation, spreading, and aggregation (2). Table 1 summarizes cardiovascular adverse effects of TKIs and their potential mechanisms (1–3).
Hypertension
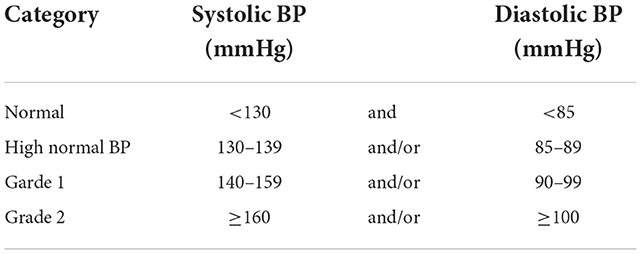
The International Society of Hypertension/American Heart Association's definition of hypertension is shown in Table 2 (13). The available evidence showed that TKIs are associated with an increased hypertension incidence of varying degrees, specifically with ponatinib which is approved for the treatment of resistant CML cases particularly those carrying the T315I mutation. This can be explained by the potent inhibitory effect of ponatinib on VEGFR-2 leading to a decrease in nitric oxide production (i.e., a potent vasodilator) and an increase in endothelin production (2). Hypertension was the most frequent adverse event (33%) among 531 patients treated with four TKIs, imatinib (in two doses, 400 and 800 mg), nilotinib, dasatinib, and ponatinib. New-onset or worsening hypertension rates were observed in 15% and 18% of patients. The readings of blood pressure measurements, both systolic and diastolic, increased significantly after starting therapy for all TKIs. The change in blood pressure measurement before and after therapy did not differ among the studied TKIs (10). The incidence of hypertension with ponatinib can reach up to 68% of patients (14). A dose dependent pattern of increase in cardiovascular risk was also noted in ponatinib trials especially in older patients with history of diabetes or previous ischemic events (2). Dasatinib was associated with an increase in pulmonary hypertension by 5% at 5 years of follow-up (15).
Hyperlipidemia
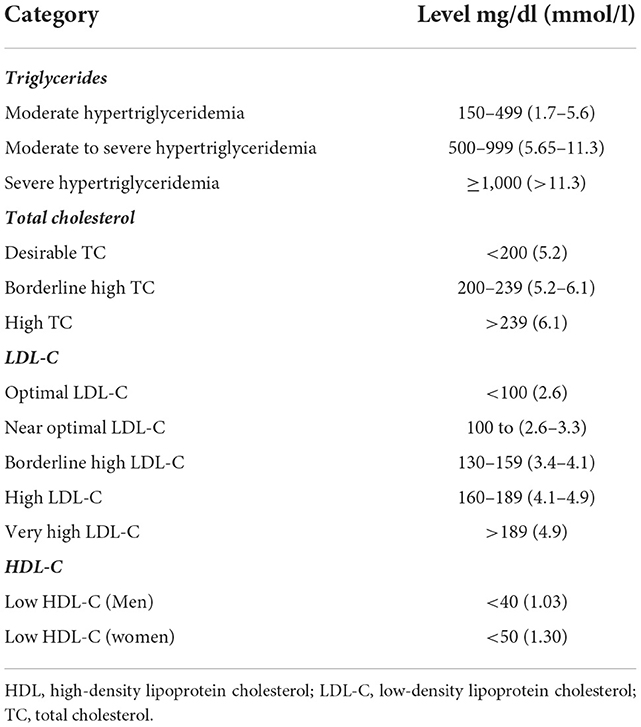
There are variable classifications of hyperlipidemia, an example is shown in Table 3 (16). The incidence of hyperlipidemia in CML patients on dasatinib or nilotinib as first or second line therapy was reported as 46.4 per 1,000 Patient-year (95% CI: 33.00–63.45) for dasatinib and 74.6 per 1,000 Patient-year (95% CI: 50.70–105.94) for nilotinib. The latter had higher rate of incidence (hazard ratio 1.75; 95% CI: 1.07–2.87) (17). A study evaluating plasma lipid profile and cardiovascular risk in a series of 27 CML patients at 1 year of follow-up found that nilotinib significantly increased total, low- and high-density lipoprotein cholesterol (TC, LDL-C and HDL-C) after 3 months of therapy initiation (18). As a result, the percentage of patients with less-than-optimal LDL-C moved up from 48.1 to 88.9% at 12 months of therapy with 22.2% of the patients needed to start cholesterol lowering treatment. This was reflected in an 11% increase in global cardiovascular risk. Thus, due to the known atherogenic potential of LDL-C, the authors suggested frequent monitoring of lipid profile along with life-style modifications and/or drug therapy if indicated once initiating treatment with nilotinib. In a recent retrospective study, which evaluated the long-term effect of imatinib on the glycemic and lipid profiles of CML patients, significant reductions in LDL-C [17.8 mg/dL, interquartile range (IQR) −1.3–34.0; p < 0.001] and triglycerides (25.0 mg/dL, IQR −2.3–58.3; p < 0.001) levels were recorded at 12 months of treatment independent of patient demographics or usage of statin therapy (19).
Hyperglycemia
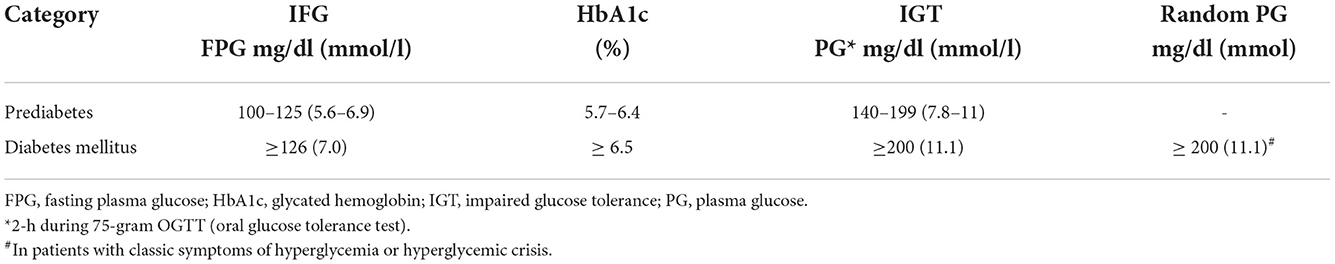
The criteria for the diagnosis of hyperglycemic conditions as endorsed by the American Diabetes Association are presented in Table 4 (20). A study investigated the effect of the first and second TKI generations on glucose metabolism in CML patients without previous hyperglycemic disorder, found that diabetes mellitus or impaired fasting glucose were identified in 25% of imatinib- and dasatinib-treated patients, and in 33% in nilotinib cohort (p = 0.39 vs. imatinib and p = 0.69 vs. dasatinib). Metabolic syndrome was seen in 42.4% of imatinib-treated, 37.5% of dasatinib-treated, and 36.1% of nilotinib-treated patients (p = 0.46 vs. imatinib and p = 0.34 vs. dasatinib). The authors concluded that although nilotinib does not seem to induce hyperglycemic disorders to a significantly higher extent than dasatinib or imatinib, it has a worse glucometabolic profile (21). At a long-term follow-up of a phase III trial on the efficacy and safety of nilotinib vs. imatinib in newly diagnosed CML patients in chronic phase, hyperglycemia was reported in 50% of patients on nilotinib 300 mg twice daily, 53% of those on nilotinib 400 mg twice daily compared with 31% of patients treated with imatinib 400 mg per day (22). Despite the previously mentioned rates, there is no real-life data that accurately estimates the incidence of diabetes, impaired fasting glucose, or metabolic syndrome in CML patients on TKI therapy. In a recent retrospective study, which evaluated the long-term effect of initiating imatinib on glycemic and lipid profiles in CML patients (n = 611), significant reductions in HbA1c (0.53%, IQR 0.09–1.19; p < 0.001) and fasting blood glucose (10.2 mg/dL, IQR −3.5–32.2; p < 0.001) were observed in patients with diabetes (n = 118) during the first year of therapy independent of anti-diabetic medications use or patients' demographics. The reductions persisted through the second year of treatment. The authors concluded that imatinib is associated with a sustained metabolic benefit (19).
Guide to management of comorbidities
Prevention
Cardiovascular risk can be reduced through adopting lifestyle modifications and preventing risk factors occurrence (1, 23). Improving modifiable cardiovascular risk factors (e.g., hypertension, dyslipidemia, cigarette smoking, inactivity, obesity) after the diagnosis of CML is preferrable. The preventative strategy starts with baseline patient evaluation and risk assessment (e.g., SCORE charts, Framingham risk score), appropriate choice of TKI treatment, and proper therapy for primary and secondary prevention of cardiovascular diseases (1). The “ABCDE” approach has been recommended for the primary and secondary prevention of cardiovascular diseases in the general population (3, 23). It was proposed for CML patients treated with TKIs as well, especially those with comorbidities (2, 3). ABCDE refers to assessment of risk, antiplatelet therapy, blood pressure management, cholesterol management, cigarette/tobacco cessation, diet and weight management, diabetes prevention and treatment, and exercise (Figure 1) (23).
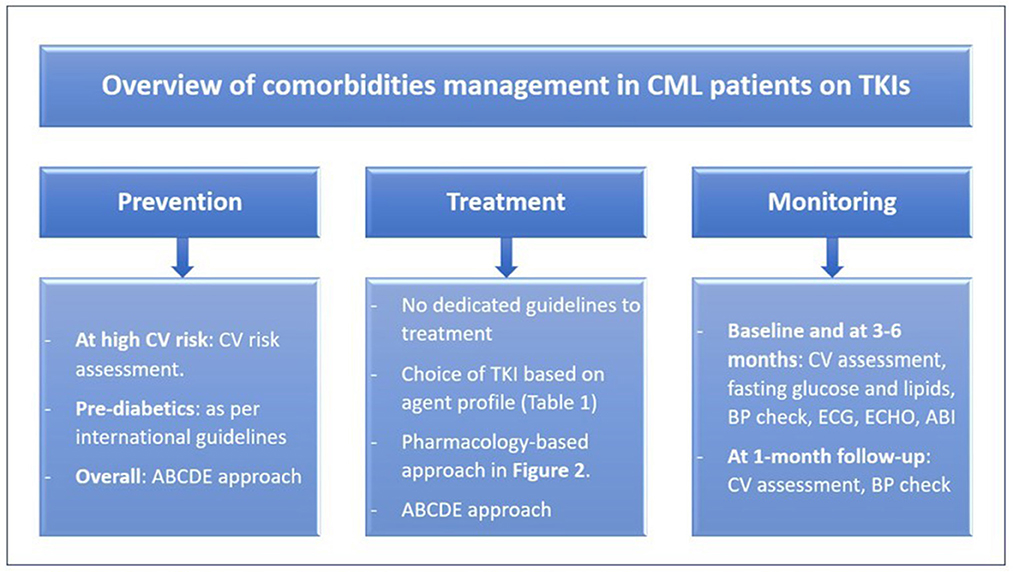
Figure 1. Overview of comorbidities management in CML patients on TKIs. ABCDE, assessment of risk, antiplatelet therapy, BP management, cholesterol management, cigarette/tobacco cessation, diet and weight management, diabetes prevention and treatment, and exercise; ABI, ankle-brachial index; BP, blood pressure; CML, chronic myeloid leukemia; CV, cardiovascular; ECG, electrocardiogram; ECHO, echocardiogram; TKIs, tyrosine kinase inhibitors.
Selection of TKI based on comorbidities
The treatment of patients with existent or new comorbidity remains empiric. It should target two elements: selecting the most appropriate TKI (Table 1) and aggressive management of the comorbidities (3, 23) (Figure 2). The selection of a TKI depends on the expected adverse effect, comorbidities, disease characteristics, and patient preference (2). For instance, as strongly recommended by the 2020 European LeukemiaNet, ponatinib and nilotinib should be avoided in patients with arterial vascular disease (24), due to the associated high rate of vascular events with their use (2). Furthermore, nilotinib causes hyperlipidemia and hyperglycemia, thus it is not suitable for CML patients having such morbidities (3).
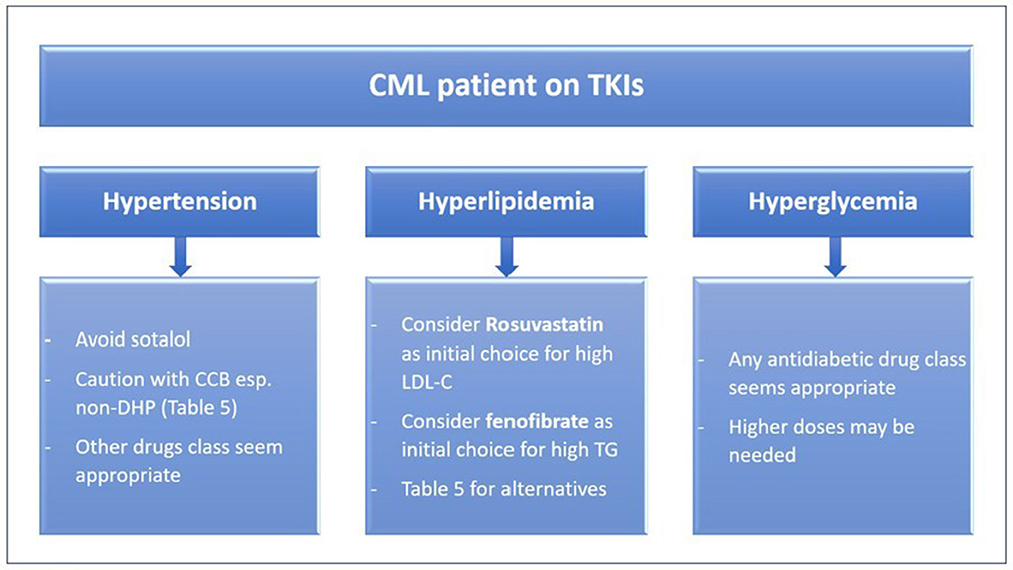
Figure 2. Pharmacology based approach to treating comorbidities in CML patients on TKIs. CCB, calcium channel blockers; CML, Chronic myeloid leukemia; LDL-C, low-density lipoprotein cholesterol; non-DHP, non-dihydropyridine, TG, triglycerides, TKIs, tyrosine kinase inhibitors.
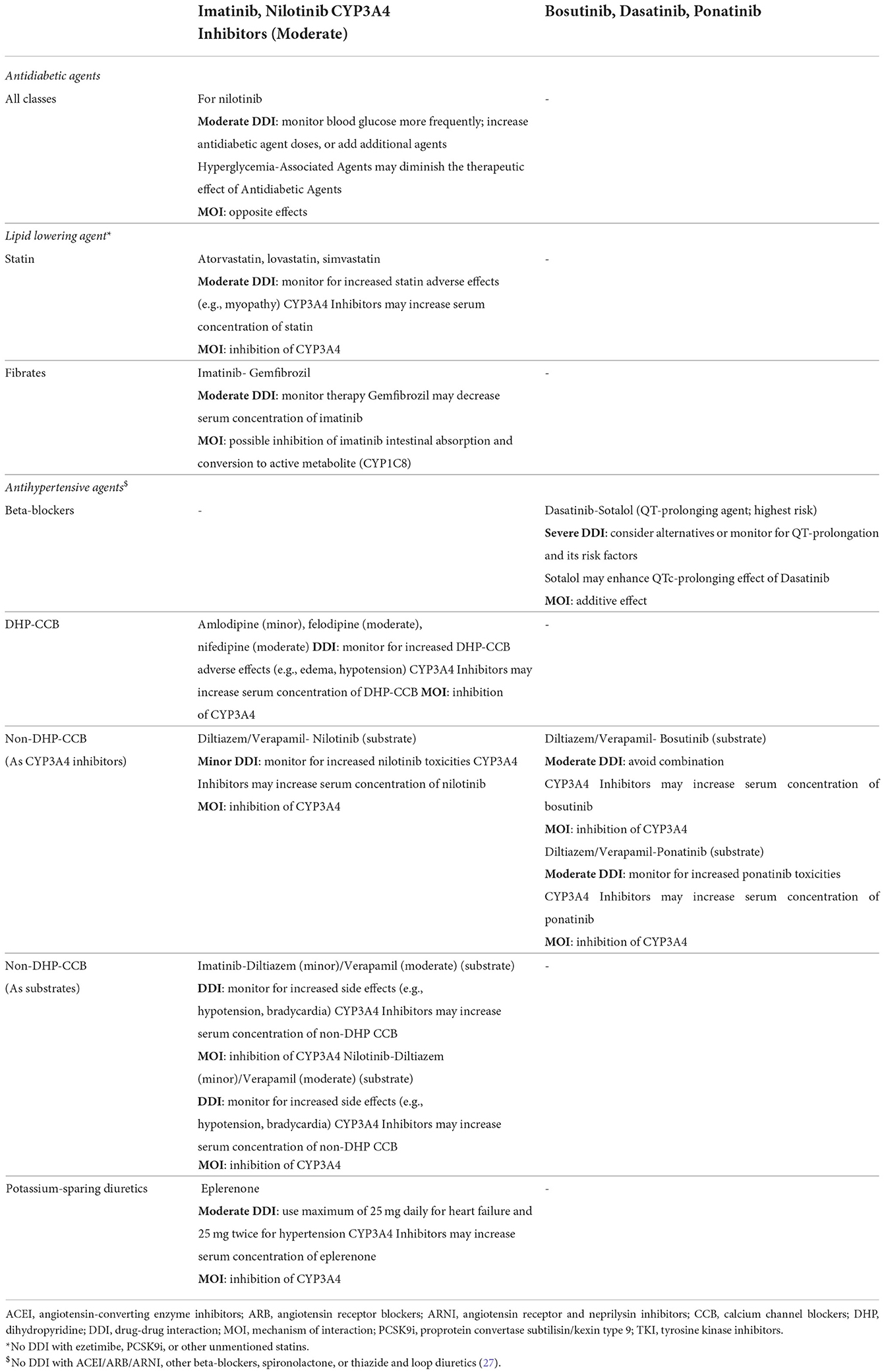
To reduce the dose-dependent cardiovascular adverse events, minimized doses of second and third generation TKIs can be considered to maintain the therapeutic benefit in those who attained adequate response after starting TKI therapy with full-dose regimen as suggested by observational studies (1, 2). A five-year analysis of the DASISION (Dasatinib vs. Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients) trial demonstrated that reducing TKI dose for any reason did not reduce major molecular response (MMR) (i.e., efficacy was not affected) with no safety concern (25). In patients newly diagnosed with CML in chronic phase, dasatinib approved standard dose (i.e., 100 mg daily) can cause pleural effusion and myelosuppression. Naqvi et al. found that the use of a lower dose (i.e., 50 mg daily) is safe and effective in early therapy for CML in chronic phase (n = 60). Complete cytogenetic response was attained in 86% of patients at 6 months of therapy, with faster and higher rates than that with the standard dose (73%) or imatinib (59%). The MMR rate was 79% at 12 months. Overall, the therapy was well-tolerated, only one patient (1%) experienced pleural effusion, which necessitated dose reduction to 20 mg daily. Treatment interruption for 7–14 days was unavoidable in only 12% of the patients (26). Interruption of TKI therapy is possible and safe in certain cases (1). Another important factor to consider is the drug-drug interactions between TKIs and drugs prescribed to treat the comorbidities. For example, pravastatin and rosuvastatin are less interactive with TKIs than atorvastatin or simvastatin (3, 27) (Table 5).
Monitoring and follow-up
High risk patients, based on the selected TKI or present comorbidities, require monitoring and regular follow-up (Figure 1), because risk factors accumulate over time and eventually lead to adverse cardiovascular events (2). A multidisciplinary approach can offer a good opportunity to optimize management. Involvement of a cardio-oncologist or having a cardio-oncology service may offer an individualized approach to ensure TKI therapy benefit, cardiovascular risk prevention, and optimal comorbidity treatment (1, 2).
Conclusion
Adverse cardiovascular events are considered a major concern of TKI therapy for the treatment of CML. The underlying mechanism is not fully understood but it is related to the inhibition of the non-BCR-ABL kinases. Patients with CML usually have baseline comorbidities which can impact survival. Hypertension, diabetes, and dyslipidemia are among the most common comorbidities. The existing or TKI-associated comorbidities necessitate treatment. The “ABCDE” approach is a concise guide for the primary and secondary prevention of cardiovascular adverse events in the absence of dedicated guidelines. Monitoring and regular follow-up with cardio-oncology service should be an integral part of management.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by the academic health system-Hamad Medical Corporation. We would like to thank them for their help in publishing this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Acronym, Explanation; BELA, Bosutinib Efficacy and Safety in Newly Diagnosed CML; CCI, Charlson Comorbidity Index; CI, Confidence interval; CML, Chronic myeloid leukemia; DASISION, Dasatinib vs. Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients; HDL-C, High-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, Low-density lipoprotein cholesterol; MMR, major molecular response; TC, Total cholesterol; TKI(s), Tyrosine kinase inhibitor(s); VEGFR, vascular endothelial growth factor receptors.
References
1. Santoro M, Mancuso S, Accurso V, Di Lisi D, Novo G, Siragusa S. Cardiovascular issues in tyrosine kinase inhibitors treatments for chronic myeloid leukemia: a review. Front Physiol. (2021) 12:675811. doi: 10.3389/fphys.2021.675811
2. Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. (2015) 33:4210–18. doi: 10.1200/JCO.2015.62.4718
3. Cortes J. How to manage CML patients with comorbidities. Blood. (2020) 136:2507–12. doi: 10.1182/blood.2020006911
4. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. (2010) 362:2260–70. doi: 10.1056/NEJMoa1002315
5. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. (2010) 362:2251–9. doi: 10.1056/NEJMoa0912614
6. Coutinho AD, Makenbaeva D, Farrelly E, Landsman-Blumberg PB, Lenihan D. Elevated cardiovascular disease risk in patients with chronic myelogenous leukemia seen in community-based oncology practices in the United States. Clin Lymphoma Myeloma Leuk. (2017) 17:676–83. doi: 10.1016/j.clml.2017.06.011
7. Jabbour E, Makenbaeva D, Lingohr-Smith M, Lin J. Use of real-world claim databases to assess prevalence of comorbid conditions relevant to the treatment of chronic myelogenous leukemia based on national comprehensive network treatment guidelines. Clin Lymphoma Myeloma Leuk. (2015) 15:797–802. doi: 10.1016/j.clml.2015.09.008
8. Crugnola M, Castagnetti F, Breccia M, Ferrero D, Trawinska MM, Abruzzese E, et al. Outcome of very elderly chronic myeloid leukaemia patients treated with imatinib frontline. Ann Hematol. (2019) 98:2329–38. doi: 10.1007/s00277-019-03767-y
9. Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood. (2015) 126:42–9. doi: 10.1182/blood-2015-01-617993
10. Jain P, Kantarjian H, Boddu PC, Nogueras-González GM, Verstovsek S, Garcia-Manero G, et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after frontline TKIs. Blood Adv. (2019) 3:851–61. doi: 10.1182/bloodadvances.2018025874
11. Brümmendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillié L, Pavlov D, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. (2015) 168:69–81. doi: 10.1111/bjh.13108
12. Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:625–632. doi: 10.1001/jamaoncol.2015.5932
13. Carey RM, Whelton PK. 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med. (2018) 168:351–8. doi: 10.7326/M17-3203
14. Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. (2017) 70:2552–565. doi: 10.1016/j.jacc.2017.09.1095
15. Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. (2014) 123:494–500. doi: 10.1182/blood-2013-06-511592
16. Parhofer KG, Laufs U. The diagnosis and treatment of hypertriglyceridemia. Dtsch Arztebl Int. (2019) 116:825–32. doi: 10.3238/arztebl.2019.0825
17. Franklin M, Burns L, Perez S, Yerragolam D, Makenbaeva D. Incidence of type 2 diabetes mellitus and hyperlipidemia in patients prescribed dasatinib or nilotinib as first- or second-line therapy for chronic myelogenous leukemia in the US. Curr Med Res Opin. (2018) 34:353–60. doi: 10.1080/03007995.2017.1399870
18. Rea D, Mirault T, Cluzeau T, Gautier JF, Guilhot F, Dombret H, et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. (2014) 99:1197–203. doi: 10.3324/haematol.2014.104075
19. Markovits N, Kurnik D, Friedrich C, Gueta I, Halkin H, David S, et al. Effects of imatinib on glycemic and lipid profiles: a retrospective cohort study. Leuk Lymphoma. (2022) 1–9. doi: 10.1080/10428194.2022.2068003
20. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. (2021) 44(Suppl 1):S15–S33. doi: 10.2337/dc21-S002
21. Iurlo A, Orsi E, Cattaneo D, Resi V, Bucelli C, Orofino N, et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients: a real clinical problem? Oncotarget. (2015) 6:33944–51. doi: 10.18632/oncotarget.5580
22. Larson RA, Kim DW, Issaragrilsil S, Coutre P, Llacer PED, Etienne G. Efficacy and safety of nilotinib (NIL) vs Imatinib (IM) in Patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): Long-Term Follow-Up (f/u) of ENESTnd. Blood. (2014) 124:4541. doi: 10.1182/blood.V124.21.4541.4541
23. Hsu S, Ton VK, Dominique Ashen M, Martin SS, Gluckman TJ, Kohli P, et al. A clinician's guide to the ABCs of cardiovascular disease prevention: the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease and American College of Cardiology Cardiosource Approach to the Million Hearts Initiative. Clin Cardiol. (2013) 36:383–93. doi: 10.1002/clc.22137
24. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34:966–84. doi: 10.1038/s41375-020-0776-2
25. Cortes JE, Hochhaus A, Kantarjian HM, Guilhot F, Kota VK, Hughes TP, et al. Impact of dose reductions on 5-year efficacy in newly diagnosed patients with chronic myeloid leukemia in chronic phase (CML-CP) from DASISION. J Clin. Oncol. (2017) 35 (suppl):7051. doi: 10.1200/JCO.2017.35.15_suppl.7051
26. Naqvi K, Jabbour E, Skinner J, Yilmaz M, Ferrajoli A, Bose P, et al. Early results of lower dose dasatinib (50 mg daily) as frontline therapy for newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. (2018) 124:2740–7. doi: 10.1002/cncr.31357
27. Interactions. Lexi-Drugs. Hudson, OH: Lexicomp. Available online at: http://online.lexi.com/lco/action/interact (accessed February 28, 2022).
Keywords: chronic myeloid leukemia, hypertension, hyperglycemia, hyperlipidemia, dasatinib, nilotinib, ponatinib, tyrosine kinase
Citation: Ahmed K, Kaddoura R and Yassin MA (2022) A practical guide to managing hypertension, hyperlipidemia, and hyperglycemia in patients with chronic myeloid leukemia. Front. Med. 9:1025392. doi: 10.3389/fmed.2022.1025392
Received: 22 August 2022; Accepted: 21 November 2022;
Published: 07 December 2022.
Edited by:
Ahmet Emre Eskazan, Istanbul University-Cerrahpasa, TurkeyReviewed by:
Mervat Mattar, Cairo University, EgyptIbrahim C. Haznedaroglu, Hacettepe University Hospital, Turkey
Copyright © 2022 Ahmed, Kaddoura and Yassin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khalid Ahmed, tinbish@gmail.com; Mohamed A. Yassin, yassinmoha@gmail.com
†These authors have contributed equally to this work
 Khalid Ahmed
Khalid Ahmed