- 1Mother and Child Health, Interactive Research School for Health Affairs, Bharati Vidyapeeth (Deemed to be University), Pune, India
- 2Gupte Hospital and Research Centre, Pune, India
- 3Department of Obstetrics and Gynecology, Bharati Medical College and Hospital, Bharati Vidyapeeth (Deemed to be University), Pune, India
- 4Department of Pediatrics, Bharati Medical College and Hospital, Bharati Vidyapeeth (Deemed to be University), Pune, India
- 5Division of Reproductive, Biology, Maternal and Child Health (RBMCH) and Nutrition, Indian Council of Medical Research, New Delhi, India
- 6MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom
- 7Department of Pediatrics and Clinical Epidemiology, Sitaram Bhartia Institute of Science and Research, New Delhi, India
Objective: To determine the trimester specific gestational weight gain (GWG) in a population of pregnant women from Western India and compare it with the Intergrowth–21st international and an Indian reference (GARBH–Ini cohort—Group for Advanced Research on BirtH outcomes).
Study design: A prospective longitudinal observational study was undertaken in Pune, West India and data for gestational weight gain was collected [the REVAMP study (Research Exploring Various Aspects and Mechanisms in Preeclampsia)]. Generalized Additive Models for Location, Scale and Shape method (GAMLSS model) were used to create GWG centile curves according to gestational age, stratified by BMI at recruitment (n = 640) and compared with Intergrowth-21st reference and GARBH–Ini cohort. Multivariable regression analysis was used to evaluate the relationship between GWG and antenatal risk factors.
Results: The median GWG was 1.68, 5.80, 7.06, and 11.56 kg at gestational ages 18, 26, 30, and 40 weeks, respectively. In our study, pregnant women gained less weight throughout pregnancy compared to Intergrowth-21st study, but more weight compared to the GARBH–Ini cohort centile curves in all the BMI categories. GWG in overweight/obese women (BMI ≥ 25) was significantly lower (<0.001) as compared to underweight (BMI < 18.5), or normal weight women (BMI ≥ 18.5 and <25). The median GWG at 40 weeks in underweight, normal and overweight/obese women was 13.18, 11.74, and 10.48 kg, respectively. Higher maternal BMI, older maternal age, higher parity and higher hemoglobin concentrations were associated with lower GWG, while taller maternal height was associated with greater GWG.
Conclusion: GWG of Indian women is lower than the prescriptive standards of the Intergrowth charts.
Introduction
Appropriate gestational weight gain (GWG) is essential for optimal fetal growth and birth outcome (1, 2). It is also a measure of maternal nutrition status (3, 4). Suboptimal GWG is associated with unfavorable delivery outcomes (5, 6) including an increased risk of intrauterine growth restriction, low birth weight, and preterm birth (7, 8). Excessive GWG is associated with an increased risk of gestational diabetes, gestational hypertension, preeclampsia, preterm birth, cesarean delivery, macrosomia, infant mortality, postpartum weight retention, and childhood obesity (8–12). Recent reports indicate a high prevalence of inadequate gestational weight gain in South Asia, in particular in India (13, 14). Appropriate GWG is usually evaluated in comparison to two international standards – those of the Institute of Medicine (IOM) and International Fetal and Newborn Growth-21st (Intergrowth-21st) (9, 15).
Studies from Asian countries have evaluated the total GWG until the end of pregnancy (5, 16–23). However, this does not indicate weight gain during different trimesters of gestation (24). Based on the comparison of total and trimester specific GWG, it has been suggested that the IOM standards are not appropriate for Asian women, who are shorter or thinner than the population used to construct these references (25–27).
A multi-ethnic, international standard of GWG, known as Intergrowth-21st, derived from data collected in eight countries, including India offers a prescriptive GWG reference chart (9). This study selected healthy women who were at low risk of adverse maternal and perinatal outcomes and provided centiles of GWG for each week of gestation. In India, maternal malnutrition is highly prevalent (28) and there is a high burden of inadequate GWG (13). Despite these challenges, and the association between GWG and optimal fetal growth, there are only few studies in Asia that have explored weight gain in different trimesters of pregnancy and compared against global references (Intergrowth-21st) (2, 29). In India, there is only one study, from Haryana, that has recently established a GWG reference for gestational age. This study demonstrates that pregnant women from the GARBH-Ini cohort (Group for Advanced Research on BirtH outcomes) gained less weight during early pregnancy (at 18 weeks) when compared with the Intergrowth–21st reference (24). This study questions the use of western estimates to identify an appropriate GWG. The related editorial emphasizes a need for establishing local references (state level) for GWG (30). India is a vast country with substantial regional differences in diet and other determinants of GWG. It is therefore useful to examine GWG in various populations across India, which could inform country specific guidelines, and be of use for the clinicians for better monitoring of GWG.
The current study examines GWG in a population of pregnant women attending ante-natal clinics in two hospitals in Western India and compares the weight gain across different periods of gestation with Intergrowth–21st reference and GARBH–Ini cohort. We also compare the centile curves across various BMI categories.
Materials and methods
Study site and population selection
The current study is a part of the Indian Council of Medical Research (ICMR) funded Center for Advanced Research project on “Investigating mechanisms leading to preeclampsia” at IRSHA, Bharati Vidyapeeth University, Pune (5/7/1069/13-RCH), which has established a cohort of pregnant women who were followed from early pregnancy until delivery [the REVAMP study (Research Exploring Various Aspects and Mechanisms in Preeclampsia)].
This study was initiated in March 2017 in the city of Pune, in Maharashtra State, India, at two urban hospitals -Bharati Hospital, and Gupte Hospital. The primary objective of this study was to examine the associations of maternal LCPUFA (Long-chain polyunsaturated fatty acids) and one carbon micronutrient status in early gestation with clinical outcome in preeclampsia and to understand the operative biochemical and molecular mechanisms. The protocol of REVAMP study has been previously published (31). In brief, pregnant women planning to give birth in Bharati and Gupte hospitals were recruited at their first antenatal visit (11–14 weeks’ gestation) and followed up subsequently at 18–22 weeks, 26–28 weeks, and at delivery. These time points were dependent on the methods of the primary study (REVAMP). The World Health Organization (32) recommends an ultrasound scan <24 weeks of gestation as part of the routine antenatal care, mid-trimester anomaly scan at 18–22 weeks of gestation is more optimal timing for it (33). According to Diabetes in Pregnancy study Group India (DIPSI) criteria, testing for GDM detection should be done at 24–28 weeks of gestation (34, 35). Hence, 18–22 and 26–28 weeks, was selected as the two most common antenatal visits for the pregnant women also keeping in mind the logistic and financial constraints.
Study participants
This study used data from all the participants (n = 1154) who enrolled between March 2017 and March 2022 in the REVAMP cohort. The study analysis included only those women who: (1) visited the hospitals for antenatal care <14 weeks’ gestation; (2) were aged 18–45 years; (3) had singleton pregnancies; and (4) were free of chronic diseases (n = 1096). The study aimed to recruit 100 women with preeclampsia and 200 normotensive women from early pregnancy, to give 87% power to detect a difference of the same magnitude when alpha is kept at 0.05 (36). To get a sample size of 100 women with preeclampsia we followed 1096 women longitudinally across pregnancy.
In order to obtain a ‘healthy’ sub-set, women with adverse pregnancy outcomes including gestational diabetes, hypertensive disorders, preterm births and low birth weight, were excluded from the study, leaving 672. Amongst these 672 women, information on GWG was not available for 32 women (out of the 4 visits of the study, 28 women had only 3 weight measurements and 4 women had only 2 weight measurements) and were excluded to generate smoothed centile curves of maternal weight across pregnancy. This resulted in a primary analytic sample of 640 participants for GWG trajectory analysis (Figure 1). In addition, we also selected a low-risk population, analogous to the Intergrowth-21st inclusion criteria (Supplementary Table 1) which included 15.9% (102/640) participants in our cohort.
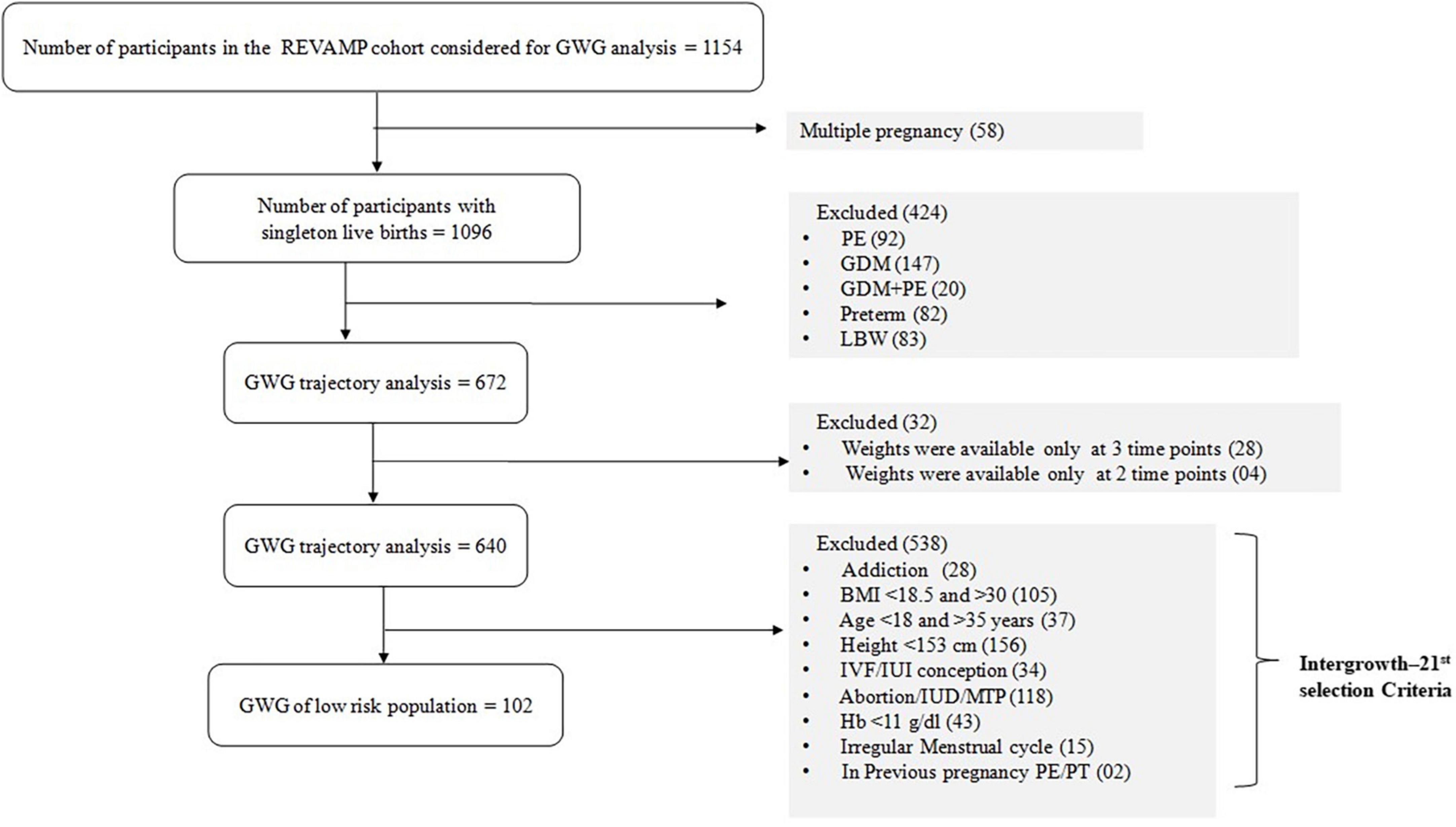
Figure 1. Flow diagram of the selection of the study population from REVAMP pregnancy cohort for gestational weight gain. PE, preeclampsia; GDM, Gestational Diabetes Mellitus; LBW, Low Birth Weight; PT, pre-term; GWG, gestational weight gain; BMI, body mass index; IVF, in vitro fertilization; IUI, intrauterine insemination; Hb, hemoglobin.
Data collection
Assessment of gestational age and anthropometric data
Gestational age was determined by last menstrual period (LMP) date, unless it differed from the gestation derived from the crown rump length at the first ultrasound scan (11–14 weeks) by > ± 7 days (18.6%), in which case the latter was used. Maternal weight (kg) at four time points (11–14, 18–22, and 26–28 weeks, and before delivery) was measured using calibrated digital weighing scales to the nearest 0.1 kg, at each antenatal visit (in both the hospitals). Height (cm) was measured once at the time of enrollment using to the nearest 0.1 cm using a stadiometer. Before the weight measurement, pregnant women were asked to take off heavy clothes, hand bags and shoes. Maternal weight and height were measured twice and the average of two reading was used for analysis.
Socio-demographic and clinical information
The socio-demographic details (socio-economic status, education) and clinical information (menstrual, obstetric data, parity, gestation, and mode of delivery), past and family history, were collected by a team of research assistants using a pre-tested questionnaire at the time of recruitment. All research assistants received on-site training prior to data collection. Socioeconomic status (SES) was recorded using the Standard of Living Index (SLI), a method developed by the International Institute for Population Sciences, Mumbai and used in India’s National Family Health Survey 2 (37). A questionnaire is used to collect data on family size, household assets, household amenities (toilets and source of drinking water), and ownership of land and livestock, from which a score is generated. At the time of recruitment hemoglobin levels were measured using venous blood sample at both hospitals on a fully automated hematology analyzer.
Assessment of gestational weight gain
We did not have pre-pregnancy weight for the participants in REVAMP, and so first trimester weight (11–14 weeks) was the baseline weight. Total weight gain was calculated by subtracting the 11–14 week weight from maternal weight on admission for labor, prior to delivery of the baby (38). Trimester specific GWG was determined by subtracting the measured weight in the first trimester from the measured weight at each subsequent prenatal visit. The recommended amount of GWG varies based on pre-pregnancy body mass index (BMI) of the women. The BMI grouping in the current study was based on IOM 2009 guidelines (15). BMI at recruitment was calculated as weight (kg)/height (m)2 and categorized was into three groups, underweight (<18.5), normal weight (18.5–24.9), and overweight/obese (≥25).
Assessment of physical activity and dietary score
Physical activity (1 month recall) was recorded at 11–14 weeks of gestation using a physical activity questionnaire that was broadly categorized by intensity (light/moderate/heavy). A daily score was calculated where higher scores indicate more activity.
Pregnant women were interviewed with a food frequency questionnaire at the same time point to estimate the frequency of consumption of foods identified using “Nutritive Values of Indian Foods” (39). All pregnant women had to indicate the frequency of each food consumed during the last 1 month for which scores were calculated.
Funding, ethical approval and informed consent
This project was funded by the Indian Council of Medical Research (ICMR), New Delhi, India (5/7/1069/13-RCH dated 31-03-2017). The study for both the hospitals was approved by the Institutional Ethics Committee, Bharati Vidyapeeth Deemed University, Pune (IEC/2015/37, dated 03.10.2015). Written informed consent was obtained from each study participant. If the participant was illiterate or could not sign, then verbal/oral consent was taken and thumb impressions were obtained in the presence of an impartial witness who signed the consent document.
Statistical analysis
Baseline characteristics (socio-demographic and clinical) of the study population were represented as median [interquartile range; IQR]. Categorical variables were expressed as number (n) and percent (%). We constructed the 3rd, 10, 25, 50, 75, 90, and 97th percentiles of GWG from 18 to 40 weeks of gestation by using the Lambda-Mu-Sigma (LMS) method via the GAMLSS model (Generalized Additive Models for Location, Scale and Shape package; version 5.1–6) in the R software (R version 4.1.2) (40).
The mean weight gain (mu), and sigma parameters of the Box-Cox Power Exponential distribution using cubic splines with five degrees of freedom were modeled against gestational age. For assessment of goodness of fit, smoothed centiles of GWG by gestational age were constructed and a visual inspection of the overall model fit was evaluated by comparing empirical centiles to the fitted centiles, using quantile–quantile plots of residuals, and plot of fitted z–scores across gestational ages.
Subsequently, we performed a comparison that was based on the 3rd, 5, 25, 50, 75, 95, and 97th GWG centiles at different gestational ages of the participants of this study with the International (Intergrowth-21st) and the National study curves (GARBH–Ini cohort). Similarly, we also compared centiles in the low-risk population group. We applied a multilevel linear regression analysis with antenatal risk factors (maternal age, BMI at the time of recruitment, height, parity, socio-economic status, family type, cooking fuel, occupation, drinking water, type of house and hemoglobin) as independent variables and GWG as the dependent variable. ANOVA was used to compare the mean GWG and BMI between the different BMI groups of first trimester BMI.
Results
Table 1 summarizes the sociodemographic and clinical details of the study cohort. The median age of women in the cohort was 28 years (IQR, 25–31 years) and gestation at the first antenatal visit was 12.3 weeks (12–13 weeks). At enrollment majority of the study participants were in the normal weight category (59%), 10% of women were underweight and 31% were overweight or obese. The vast majority (90%) belonged to ‘upper’ socio-economic status. Overall, 40% of the women were graduates and 29% of the women were postgraduates. At baseline recruitment, the median hemoglobin level was 11.7 (g/dl) (IQR, 10.8–12.4 g/dl).
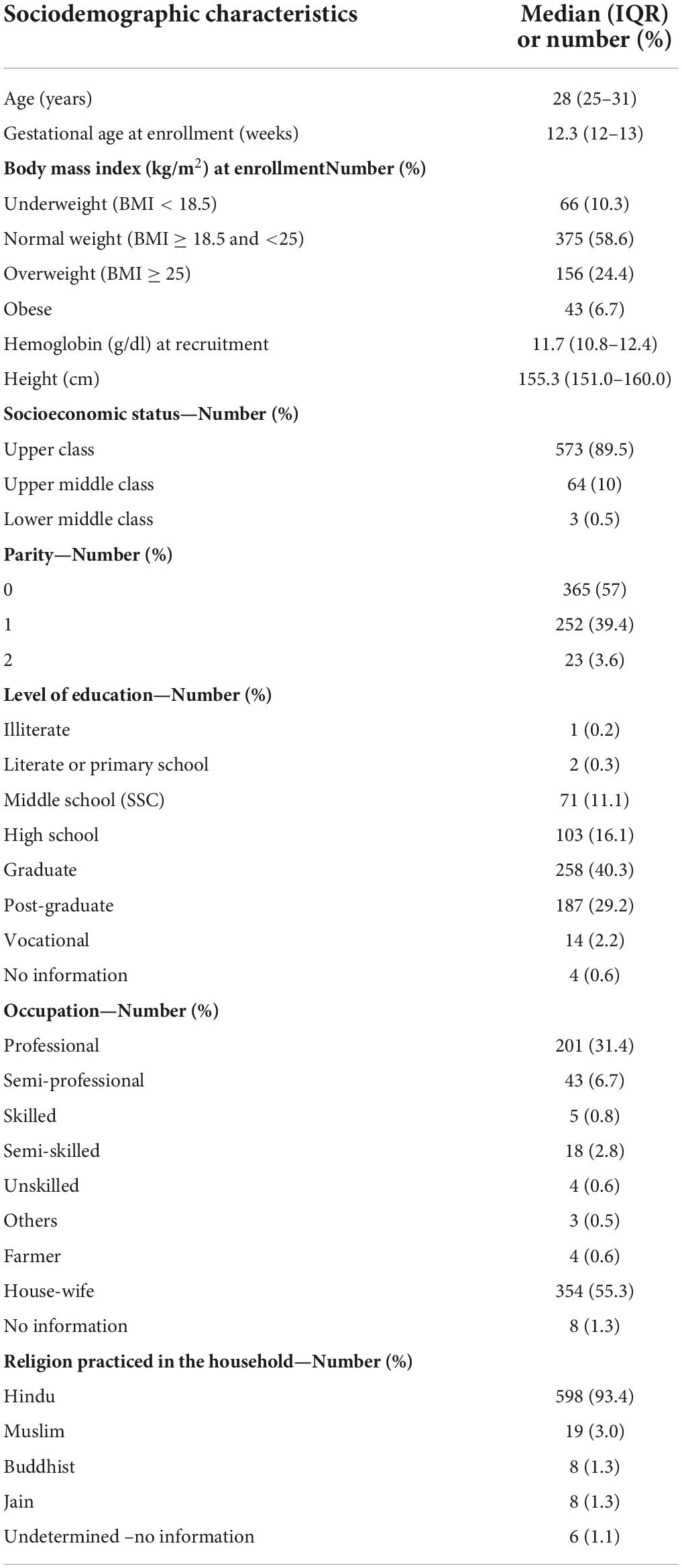
Table 1. Sociodemographic and clinical characteristics of the participants enrolled in the REVAMP cohort Pune, India (n = 640).
Gestational weight gain centile curves across pregnancy
Figure 2 depicts centile curves of gestational weight gain according to gestational age (18–40 weeks). Table 2, shows 3rd, 5, 25, 50, 75, 95, and 97th smoothed percentiles (estimated values), across pregnancy. The smoothed centile curves showed that the median GWG (kg) was 1.68, 5.80, 7.06, and 11.56 kg at gestational ages of 18, 26, 30, and 40 weeks, respectively (Table 2). Cumulative GWG between subjects was less in early pregnancy (18 weeks: IQR 0.80–2.57 kg) as compared to later gestational age (40 weeks: IQR 8.84–14.3 kg). The GWG 3rd, 5, 25, 50, 75, 95, and 97th centile at different periods of gestation for the low-risk group are tabulated in Supplementary Table 2.
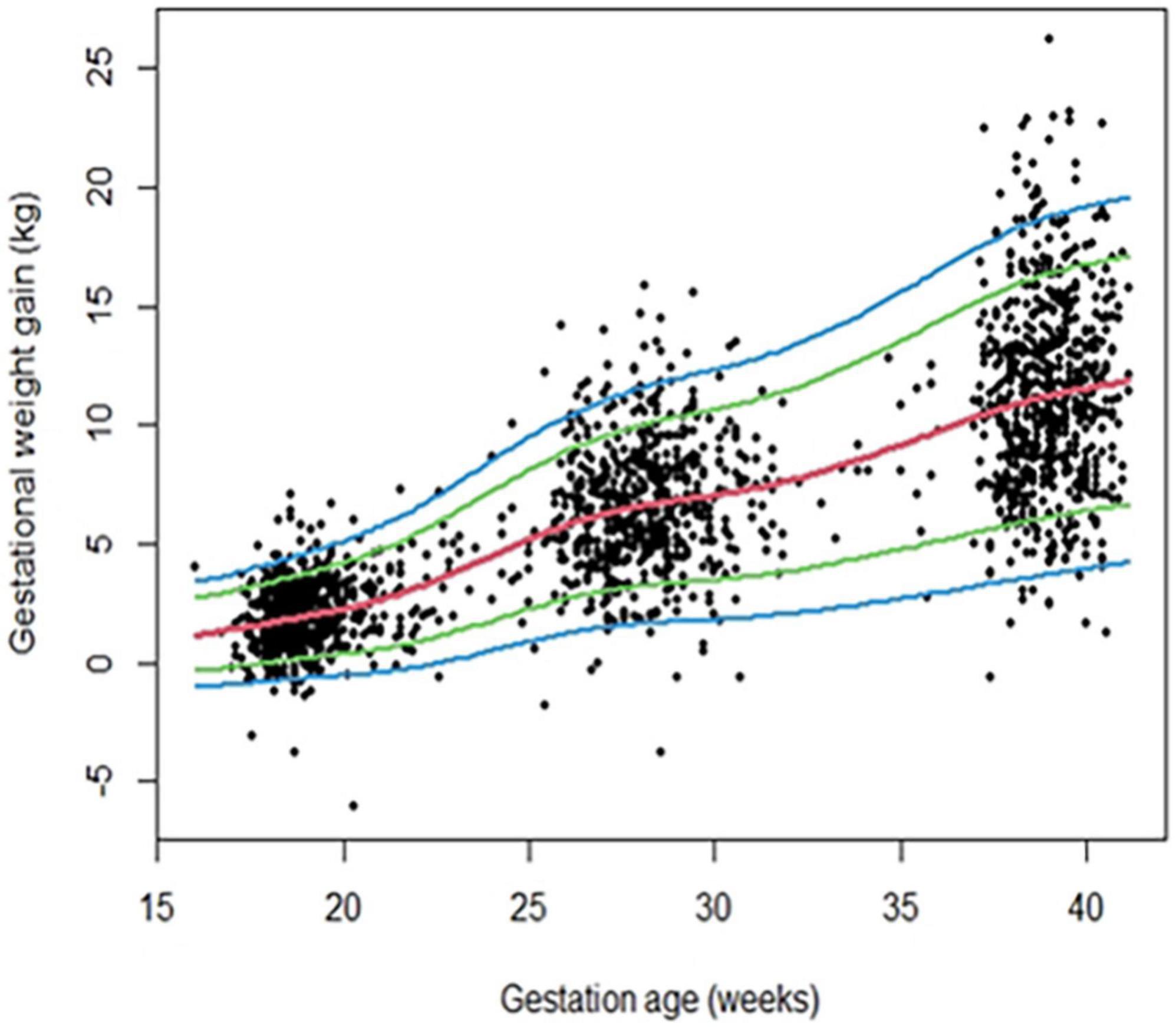
Figure 2. Gestational weight gain (GWG) pattern in the primary analytic sample (n = 640) of REVAMP cohort. Centile curves were generated for GWG using the GAMLSS.
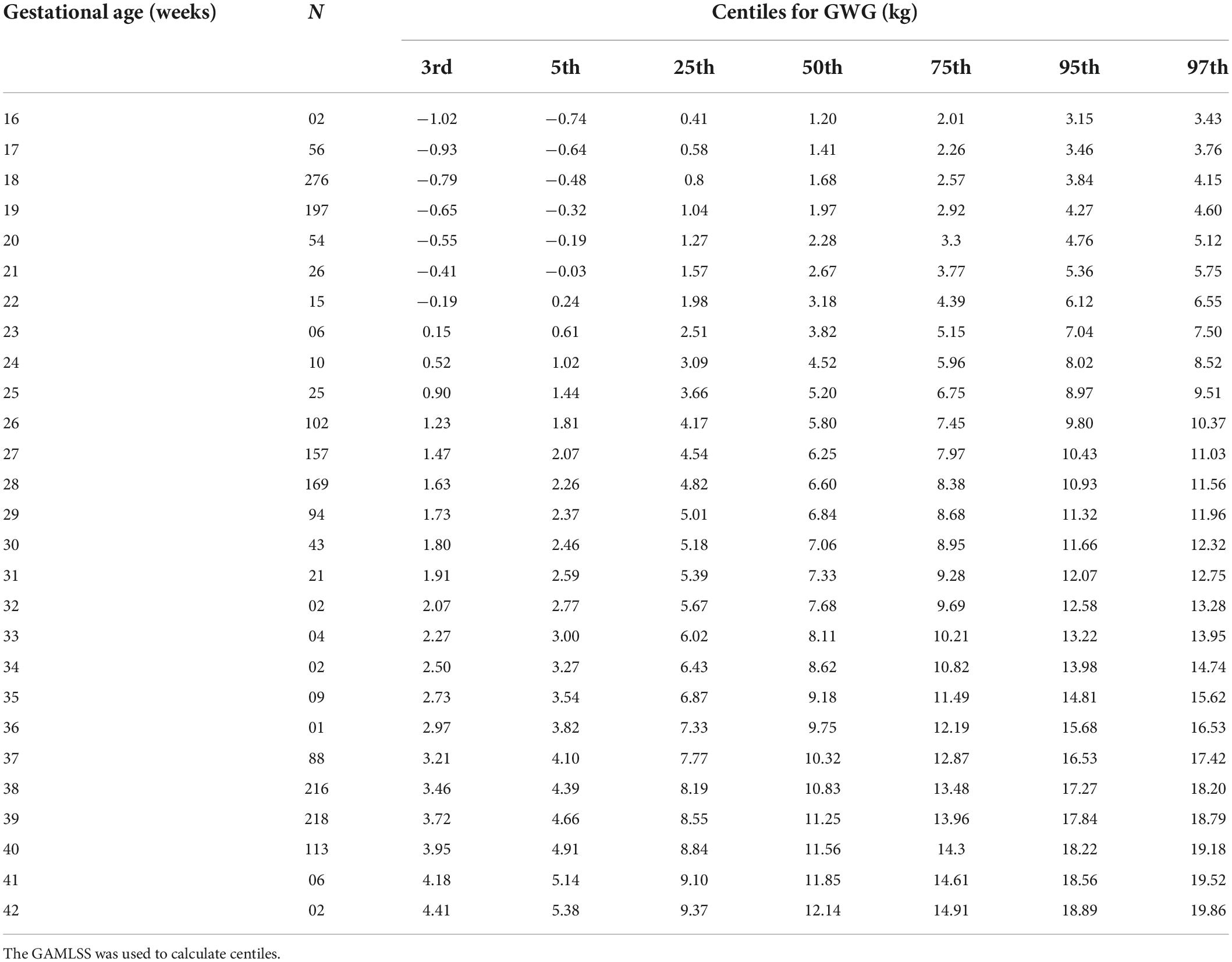
Table 2. GWG distribution (in kg) across pregnancy in the REVAMP cohort, Pune, India (primary analytic sample; n = 640).
Gestational weight gain centile curves for participants across different body mass index categories
The graphical representations of GWG centiles across pregnancy for underweight (n = 66), normal weight (n = 375), and overweight/obese (n = 199) pregnant women groups are shown in Figures 3A–C. The median values for GWG at 18 weeks were 2.62 kg in underweight women, 1.71 kg in normal weight and 1.40 kg in overweight/obese women. At 40 weeks of gestation the median value was 13.18 kg, in underweight women, 11.74 kg, in normal weight, and 10.48 kg in overweight/obese women (Table 3). GWG in overweight/obese women was significantly lower as compared to underweight or normal weight women (Supplementary Table 3).
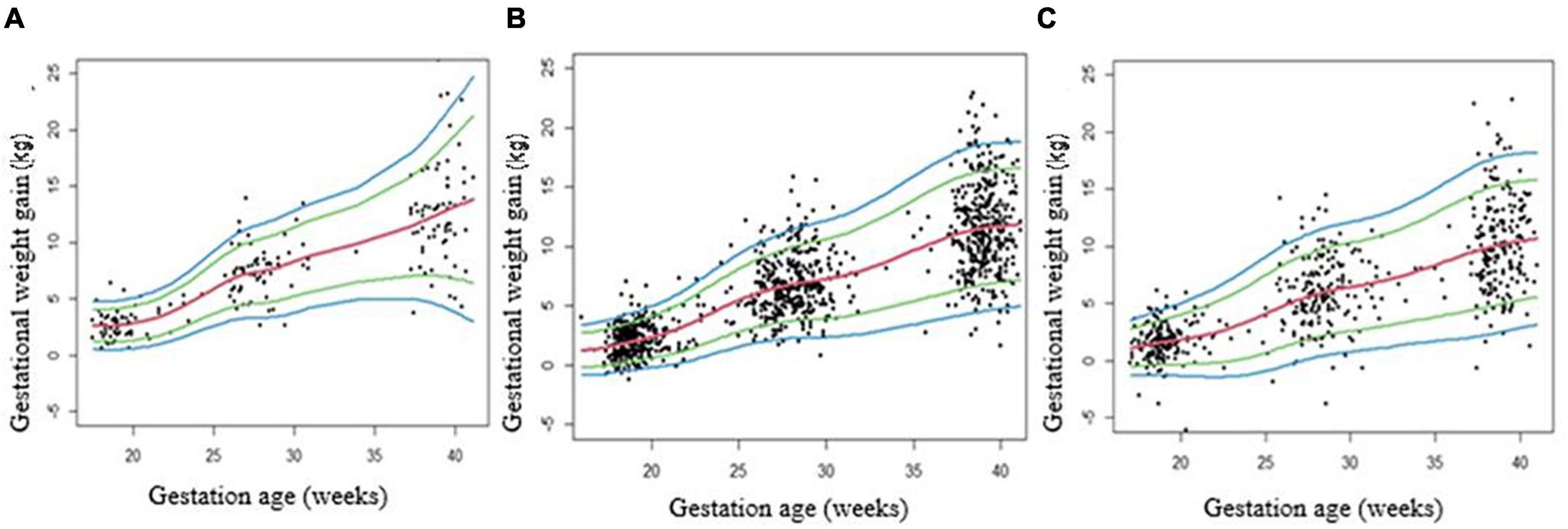
Figure 3. Gestational weight gain (GWG) centile curves for underweight (A), normal weight (B) and overweight/obese (C) pregnancies. Centile curves were generated for GWG using the GAMLSS. A: Underweight (BMI < 18.5), B: Normal (BMI ≥ 18.5 and <25), and C: Overweight/obese (BMI ≥ 25).
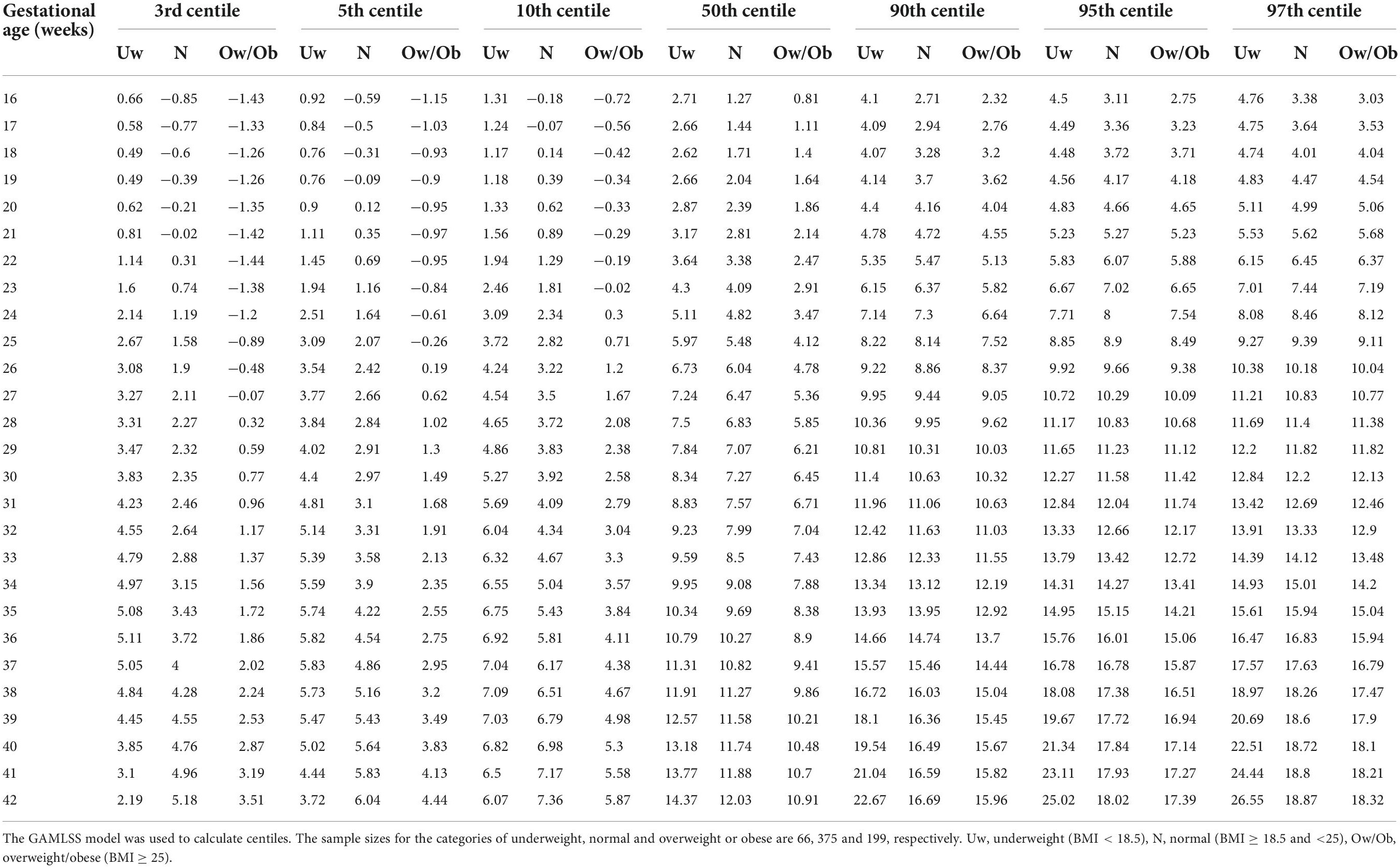
Table 3. Percentiles of cumulative GWG (in kg) at various gestational ages (in weeks) among different first trimester BMI groups.
Comparison of gestational weight gain with the Intergrowth-21st reference
The 5, 50, and 95th percentiles for GWG in the REVAMP cohort (total sample) and the low-risk group from this cohort were compared with the Intergrowth-21st reference (Figures 4A,B). The participants gained less weight throughout pregnancy compared to Intergrowth-21st and this difference was more pronounced in later pregnancy and in the higher centiles.
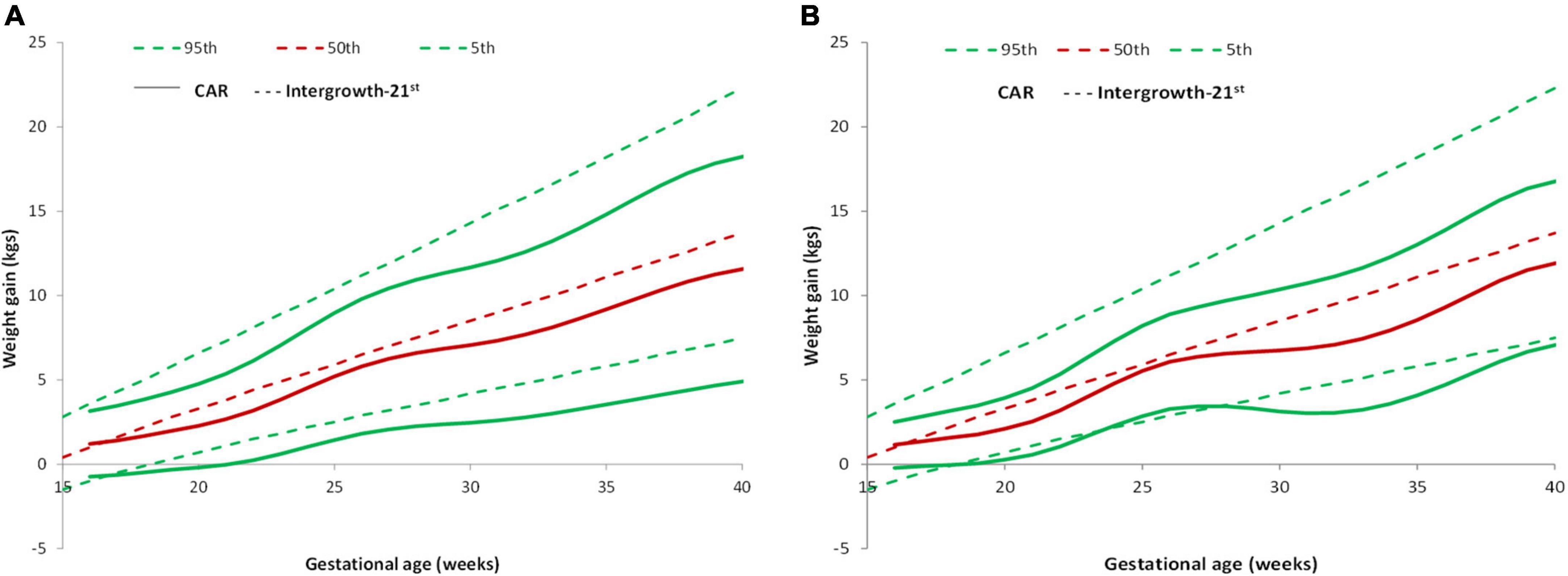
Figure 4. Pattern of GWG and their comparison with Intergrowth-21st standard. (A) Comparison of GWG curves of the REVAMP cohort (n = 640) with Intergrowth-21st by gestational age. (B) Comparison of GWG curves of the REVAMP cohort low-risk population (n = 102) with Intergrowth-21st reference.
Intergrowth–21st prescribes an average cumulative GWG of 7.47 kg at 28 weeks, 9.52 kg at 32 weeks, 11.58 kg at 36 weeks, and 13.69 kg at 40 weeks. In the REVAMP study, we observed an average cumulative GWG of 6.6 kg at 28 weeks, 7.68 kg at 32 weeks, 9.75 kg at 36 weeks, and 11.56 kg at 40 weeks (Table 2). The percentage of women with GWG < 10th centile at delivery was similar, both in the total sample (25.3%) and the low risk group (24.5%) (Table 4).

Table 4. Comparison of GWG in the REVAMP cohort and low risk population with the Intergrowth 21st reference.
Comparison of gestational weight gain curve with the GARBH-Ini cohort
The study participants in the total sample and the low-risk group gained more weight as compared to those in the GARBH-Ini cohort (Figures 5A,B). GARBH-Ini documented an average cumulative GWG of 5.07 kg (28 weeks), 6.45 kg (32 weeks), 7.86 kg (36 weeks), and 9.06 kg (40 weeks) (24). In the REVAMP study, we observed a higher average cumulative GWG; 6.6 kg (28 week), 7.68 kg (32 week), 9.75 kg (36 week), and 11.56 kg (40 week).
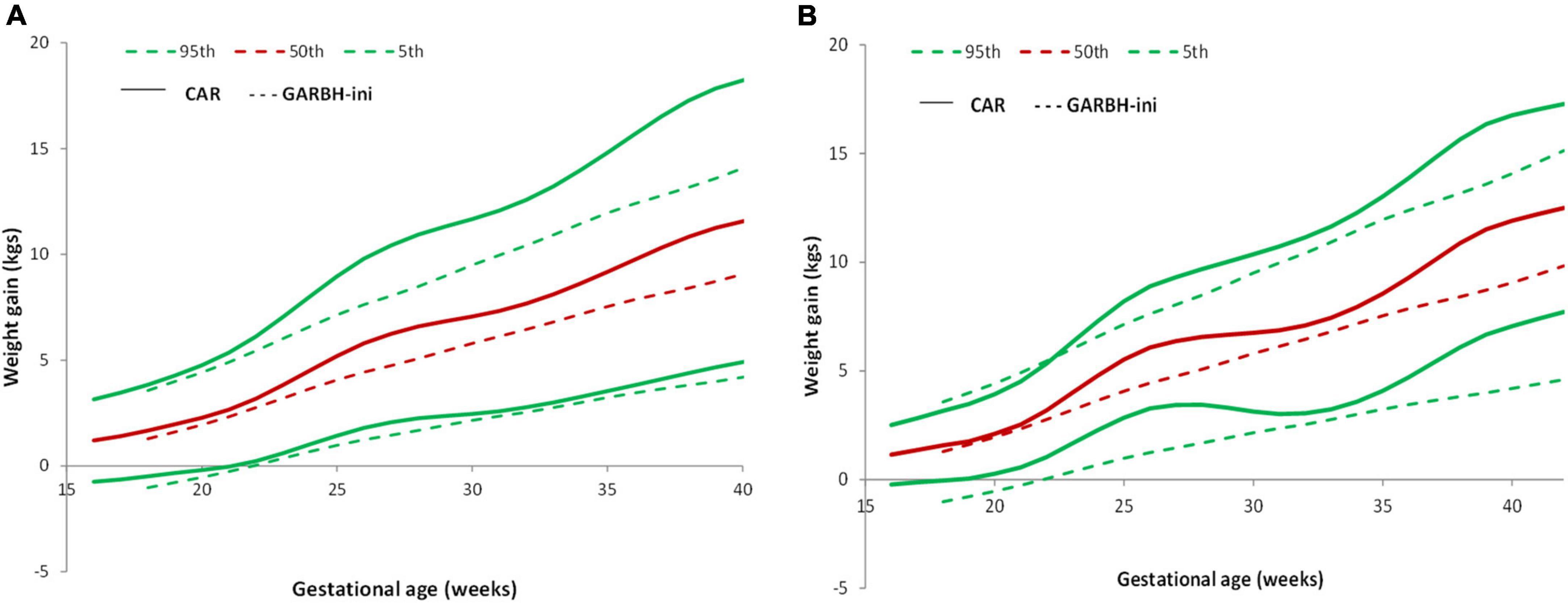
Figure 5. Pattern of GWG and their comparison with GARBH-Ini cohort. (A) Comparison of GWG curves of the REVAMP cohort (n = 640) with GARBH-Ini by gestational age. (B) Comparison of GWG curves of the REVAMP cohort low-risk population (n = 102) with GARBH-Ini reference.
In comparison to the GARBH–Ini cohort centile curves, our study participants gained more weight in all the BMI categories (24).
Maternal height and gestational weight gain
We also compared GWG data in relation to maternal height. Supplementary Table 4 presents 3rd, 5, 50, 90, and 97th centiles for gestational weight gain in short (<153 cm) and tall women (>153 cm).
Association between antenatal risk factors and total gestational weight gain
A multivariable regression analysis was undertaken to examine the association of various factors such as age, BMI, height, parity, socio-economic status, family type, cooking fuel, occupation, drinking water, type of house and hemoglobin with the total GWG at delivery (Table 5). There was a 127 g reduction in GWG for every unit (kg/m2) increase in first trimester BMI. Reports indicate that maternal socioeconomic status is an important in determining maternal health (41). Family type, cooking fuel, occupation, and drinking water, type of house, socioeconomic status and religion were not related to GWG. In addition, we found moderate physical activity (p = 0.11) and dietary score (p = 0.52) at recruitment were not associated with total GWG.
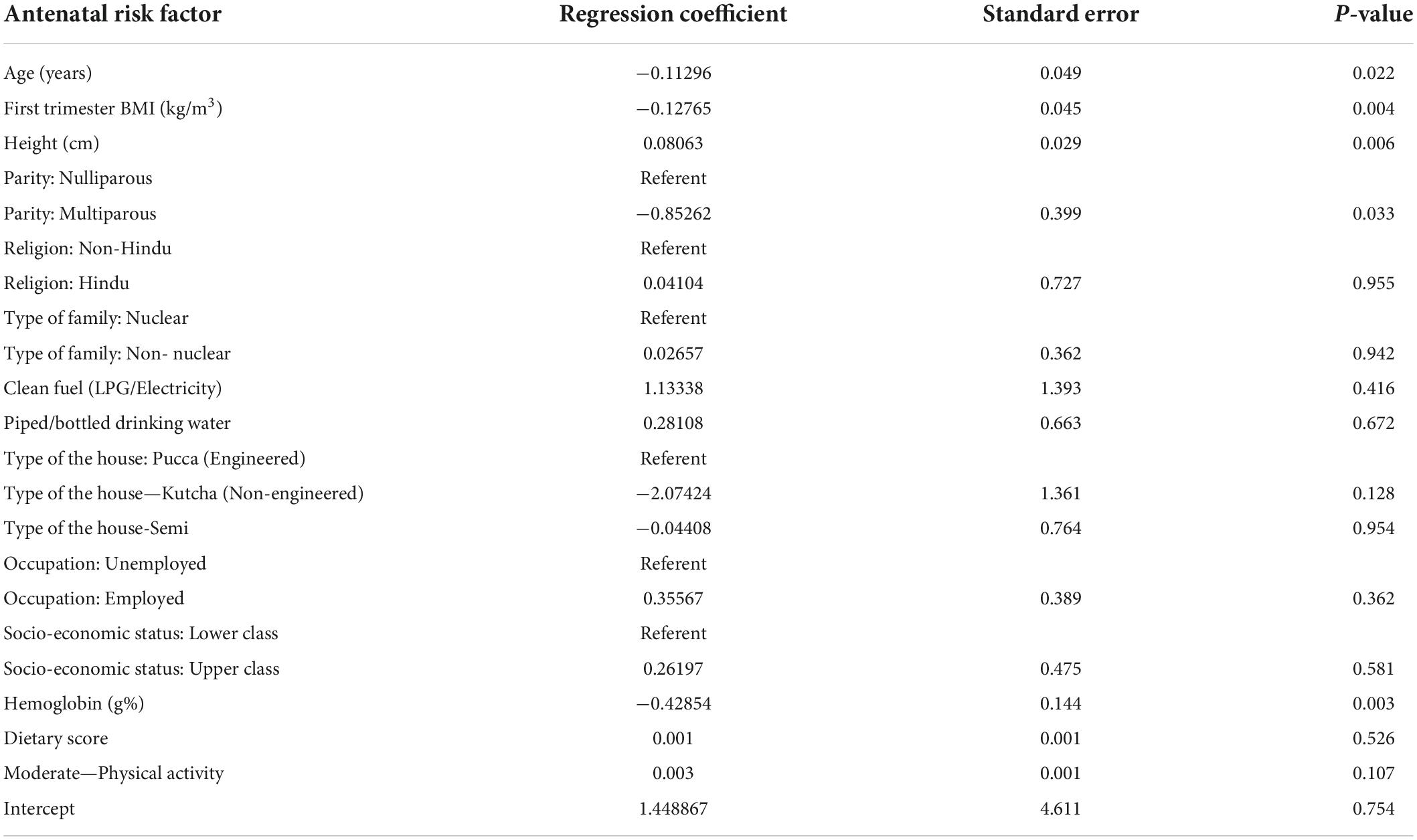
Table 5. Association between antenatal factors and total GWG evaluated in a multivariable regression analysis (n = 640).
Discussion
The current study describes the GWG among pregnant women having ante-natal care at two hospitals in Pune, Western India. In this longitudinal follow-up cohort study, we derived trimester specific reference centiles of GWG and compared them with the Intergrowth–21st reference as well as with a cohort from Northern India (GARBH-Ini). Intergrowth-21st project is the first study, to report GWG from 15–40 weeks of pregnancy in normal weight women, using data from 8 countries and it also includes three Asian countries (India, China, and Oman) (9). The Intergrowth study recruited healthy well-nourished women to arrive at recommendations for optimal weight gain. The Indian participants in Intergrowth-21st were from Nagpur, central India (N = 455). The GWG in the Nagpur women was lower as compared to women enrolled from other countries (30). The Intergrowth-21 study concluded that despite the range of cultures, behaviors, clinical practices, and traditions, patterns of gestational weight gain are similar in populations. Subsequently, GWG in Northern India women was reported to be significantly lower than the Intergrowth-21st reference (24), which raised questions on the routine using of these references to diagnose appropriate GWG in the Indian context. The current study also confirms that the GWG is lower in comparison to the Intergrowth–21st reference.
Underweight women had greater GWG than normal and overweight women. Similar findings have been reported from Italy and India (24, 42). The observed 127 g reduction in GWG per unit increase in first trimester BMI is similar to the study from Northern India (24). During pregnancy, fat is stored to secure an energy supply for fetal growth and lactation. A prospective study by Zanardo et al., reports that in obese women, no additional storage is necessary, and hence pregnancy weight gain could be restricted (43).
Earlier Asian studies on GWG were either cross-sectional or compared GWG with the IOM, 2009 guidelines. IOM guidelines are appropriate for American women, and are based on pre-pregnancy BMI, singleton pregnancies, primigravida mothers of high social status and those with no physical activity (15). Earlier Indian studies also reported inadequate GWG in comparison to the IOM reference (17–19, 42). The observed differences from IOM and Intergrowth may be due ethnicity, lifestyle, and nutritional factors.
The higher GWG in comparison to GARBH–Ini cohort may reflect the better socioeconomic status of women from our study. The majority of GARBH–Ini participants belonged to the upper lower class while our cohort is predominantly constituted by the upper class. The geographical location may also have contributed since the diet patterns vary between western and northern regions of India. International GWG curves are not classified based on BMI (9). The data from our study provides a reference chart for GWG in different BMI categories, which will help clinicians to monitor GWG during pregnancy according to BMI category.
The greater GWG in pregnant women above 153 cm in height is in conformity with earlier findings (44). The association of total GWG with maternal age, BMI, parity, and height has also been reported earlier (24, 45, 46). In addition, we observed, higher hemoglobin concentration were associated with lower GWG. Higher hemoglobin may be linked with low plasma volume expansion which may in turn lead to lower gestational weight gain.
The strength of our study includes the prospective design, accurate gestational age measurements, and multiple antenatal weight measurements which allow us to assess trimester-specific GWG. Our study has some limitations. Firstly, the initial recruitment was done in the first trimester (11–14 weeks) and the weight at this visit was approximated as the pre-conception metric. Measured weight in early pregnancy provides a reasonable quantification of pre-pregnancy weight and is used for calculating the pre-pregnancy BMI and GWG (47, 48). It has been reported that mean differences between self-reported preconception weight and measured first-trimester weights was below 2 kg, and had little impact on BMI classification and GWG calculation (48). All women included in this study are from an urban area who have better access to healthcare, and a higher educational level than rural Indian women, and did not include women from rural area. Also, for some gestational weeks, the sample size is very low.
Conclusion
In conclusion, this study provides GWG charts from “healthy” pregnant women of upper socio-economic status from an urban setting in Pune, Western, India, which confirms that the GWG of Indian women is lower than the prescriptive standards of the Intergrowth charts. These charts would be appropriate for routine obstetric use in India, particularly the Western region, to prevent misclassification errors with the available Intergrowth-21st and IOM references.
Data availability statement
The study data will be available on request subject to approval from the Institutional Ethics Committee and Health Ministry Screening Committee, Government of India.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee, Bharati Vidyapeeth Deemed University, Pune. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HS and SJ contributed to study conception and design. KD, HP, VK, and SGun contributed to data collection. HS, SM, and KR contributed to statistical analysis and interpretation of results. KD, SJ, SGup, GW, SL, NC, BK, HS, and CF contributed to draft manuscript preparation. All authors reviewed the manuscript and approved the submitted version.
Funding
This study was funded by Indian Council of Medical Research (ICMR), New Delhi, India (5/7/1069/13-RCH dated 31-03-2017).
Acknowledgments
We are grateful to the staff at both the hospitals, as well as the Mother and Child Health Unit, IRSHA, Bharati Vidyapeeth (Deemed to be University) for their constant help in this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1022990/full#supplementary-material
References
1. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25.
2. Wei X, Shen S, Huang P, Xiao X, Lin S, Zhang L, et al. Gestational weight gain rates in the first and second trimesters are associated with small for gestational age among underweight women: a prospective birth cohort study. BMC Pregnancy Childbirth. (2022) 22:106. doi: 10.1186/s12884-022-04433-4
3. Rahman M, Rahman SM, Pervin J, Aktar S, El Arifeen S, Rahman A. Body mass index in early-pregnancy and selected maternal health outcomes: findings from two cohorts in Bangladesh. J Glob Health. (2020) 10:020419. doi: 10.7189/jogh.10.020419
4. Liu X, Wang H, Yang L, Zhao M, Magnussen CG, Xi B. Associations between gestational weight gain and adverse birth outcomes: a population-based retrospective cohort study of 9 million mother-infant pairs. Front Nutr. (2022) 9:811217. doi: 10.3389/fnut.2022.811217
5. Xiao L, Ding G, Vinturache A, Xu J, Ding Y, Guo J, et al. Associations of maternal pre-pregnancy body mass index and gestational weight gain with birth outcomes in Shanghai, China. Sci Rep. (2017) 7:41073. doi: 10.1038/srep41073
6. Suliga E, Rokita W, Adamczyk-Gruszka O, Pazera G, Cieśla E, Głuszek S. Factors associated with gestational weight gain: a cross-sectional survey. BMC Pregnancy Childbirth. (2018) 18:465. doi: 10.1186/s12884-018-2112-7
7. Hasan S, Khan MA, Ahmed T. Inadequate maternal weight gain in the third trimester increases the risk of intrauterine growth restriction in rural Bangladesh. PLoS One. (2019) 14:e0212116. doi: 10.1371/journal.pone.0212116
8. Lin D, Fan D, Wu S, Chen G, Li P, Ma H, et al. The effect of gestational weight gain on perinatal outcomes among Chinese twin gestations based on Institute of Medicine guidelines. BMC Pregnancy Childbirth. (2019) 19:262. doi: 10.1186/s12884-019-2411-7
9. Ismail LC, Bishop DC, Pang R, Ohuma EO, Kac G, Abrams B, et al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ. (2016) 352:i555. doi: 10.1136/bmj.i555
10. Yaw YH, Shariff ZM, Shahdan JB. Pre-pregnancy BMI and gestational weight gain are associated with 6 months postpartum weight retention. Int J Public Health Clin Sci. (2017) 4:112–25.
11. Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. (2018) 16:153. doi: 10.1186/s12916-018-1128-1
12. Xie X, Liu J, Pujol I, López A, Martínez MJ, García-Patterson A, et al. Inadequate weight gain according to the institute of medicine 2009 guidelines in women with gestational diabetes: frequency. Clinical predictors, and the association with pregnancy outcomes. J Clin Med. (2020) 9:3343. doi: 10.3390/jcm9103343
13. Chowdhury RN, Choudhary TS, Dhabhai N, Mittal P, Dewan R, Kaur J, et al. Gestational weight gain and pregnancy outcomes: findings from North Indian pregnancy cohort. Matern Child Nutr. (2021) 18:e13238. doi: 10.1111/mcn.13238
14. Nurul-Farehah S, Rohana AJ, Hamid NA, Daud Z, Asis S. Determinants of Suboptimal Gestational Weight Gain among Antenatal Women Residing in the Highest Gross Domestic Product (GDP) Region of Malaysia. Nutrients. (2022) 14:1436. doi: 10.3390/nu14071436
15. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine Iom Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (2009).
16. Munim S, Maheen H. Association of gestational weight gain and pre-pregnancy body mass index with adverse pregnancy outcome. J Coll Physicians Surg Pak. (2012) 22:694–8.
17. Misra A, Ray S, Patrikar S. A longitudinal study to determine association of various maternal factors with neonatal birth weight at a tertiary care hospital. Med J Armed Forces India. (2015) 71:270–3. doi: 10.1016/j.mjafi.2015.03.001
18. Bhavadharini B, Anjana RM, Deepa M, Jayashree G, Nrutya S, Shobana M, et al. Gestational weight gain and pregnancy outcomes in relation to body mass index in Asian Indian Women. Indian J Endocrinol Metab. (2017) 21:588–93. doi: 10.4103/ijem.IJEM_557_16
19. Pal R, Maiti M, Roychoudhury B, Sanyal P, Chowdhury B. Association of pregestational BMI and antenatal weight gain with pregnancy outcome: a prospective observational cohort study. Int J Womens Health Reprod Sci. (2017) 5:37–40. doi: 10.15296/ijwhr.2017.07
20. Zhao R, Xu L, Wu ML, Huang SH, Cao XJ. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth. (2018) 31:e20–5. doi: 10.1016/j.wombi.2017.06.003
21. Kac G, Arnold CD, Matias SL, Mridha MK, Dewey KG. Gestational weight gain and newborn anthropometric outcomes in rural Bangladesh. Matern Child Nutr. (2019) 15:e12816. doi: 10.1111/mcn.12816
22. Morisaki N, Nagata C, Jwa SC, Sago H, Saito S, Oken E, et al. Pre-pregnancy BMI-specific optimal gestational weight gain for women in Japan. J Epidemiol. (2017) 27:492–8. doi: 10.1016/j.je.2016.09.013
23. Choi SK, Lee G, Kim YH, Park IY, Ko HS, Shin JC. Determining optimal gestational weight gain in the Korean population: a retrospective cohort study. Reprod Biol Endocrinol. (2017) 15:67.
24. Thiruvengadam R, Desiraju BK, Natchu UCM, Wadhwa N, Sachdev K, Misra S, et al. Gestational weight gain trajectories in GARBH–Ini pregnancy cohort in North India and a comparative analysis with global references. Eur J Clin Nutr. (2022) 76:855–62. doi: 10.1038/s41430-021-01040-y
25. Yang YD, Yang HX. Investigation into the clinical suitability of Institute of Medicine 2009 guidelines regarding weight gain during pregnancy for women with full term singleton fetus in China. Zhonghua Fu Chan Ke Za Zhi. (2012) 47:646-50.
26. Liu Y, Dai W, Dai X, Li Z. Prepregnancy body mass index and gestational weight gain with the outcome of pregnancy: a 13-year study of 292,568 cases in China. Arch Gynecol Obstet. (2012) 286:905-11. doi: 10.1007/s00404-012-2403-6
27. Wie JH, Park IY, Namkung J, Seo HW, Jeong MJ. Is it appropriate for Korean women to adopt the 2009 Institute of Medicine recommendations for gestational weight gain? PLoS One. (2017) 12:e0181164. doi: 10.1371/journal.pone.0181164
28. Swaminathan S, Hemalatha R, Pandey A, Kassebaum NJ, Laxmaiah A, Longvah T, et al. The burden of child and maternal malnutrition and trends in its indicators in the states of India: the Global Burden of Disease Study 1990–2017. Lancet Child Adolesc Health. (2019) 3:855–70. doi: 10.1016/S2352-4642(19)30273-1
29. Bauserman MS, Bann CM, Hambidge KM, Garces AL, Figueroa L, Westcott JL, et al. Gestational weight gain in 4 low- and middle-income countries and associations with birth outcomes: a secondary analysis of the Women First Trial. Am J Clin Nutr. (2021) 114:804–12. doi: 10.1093/ajcn/nqab086
30. Hermanussen M. Pregnant women need local references for gestational weight gain–an editorial. Eur J Clin Nutr. (2022) 76:781–2. doi: 10.1038/s41430-022-01113-6
31. Wadhwani NS, Sundrani DP, Wagh GN, Mehendale SS, Tipnis MM, et al. The REVAMP study: research exploring various aspects and mechanisms in preeclampsia: study protocol. BMC Pregnancy Childbirth. (2019) 19:308. doi: 10.1186/s12884-019-2450-0
32. World Health Organisation.WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. WHO: Geneva (2016).
33. Leung KY, Poon CF, Teotico AR, Hata T, Won HS, Chen M, et al. Recommendations on routine mid-trimester anomaly scan. J ObstetGynaecol Res. (2015) 41:653–61. doi: 10.1111/jog.12700
34. Diabetes in Pregnancy study Group India. Diagnosis & Management of Gestational Diabetes Mellitus. Chennai: Diabetes in Pregnancy study Group India (2021).
35. Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S. Gestational diabetes mellitus–guidelines. J Assoc Physicians India. (2006) 54:622–8.
36. Wadhwani N, Narang A, Mehendale S, Wagh G, Gupte S, Joshi S. Reduced maternal erythrocyte long chain polyunsaturated fatty acids exist in early pregnancy in preeclampsia. Lipids. (2016) 51:85–94. doi: 10.1007/s11745-015-4098-5
37. International Institute for Population Sciences and ORC Macro. National Family Health Survey (NFHS-2), India 1998–99. Mumbai: IIPS (2000).
38. Sato N, Miyasaka N. Stratified analysis of the correlation between gestational weight gain and birth weight for gestational age: a retrospective single-center cohort study in Japan. BMC Pregnancy Childbirth. (2019) 19:402. doi: 10.1186/s12884-019-2563-5
39. Gopalan C. Fatty acids in India - current research and government policies. Bull Nutr Found India. (2003) 24:1–5. doi: 10.1093/heapol/czu031
40. Stasinopoulos MD, Rigby RA, Heller GZ, Voudouris V, Bastiani FD. Flexible regression and smoothing: Using GAMLSS in R. Boca Raton, FL: Chapman and Hall/CRC (2017). 572 p.
41. O’Brien EC, Alberdi G, McAuliffe FM. The influence of socioeconomic status on gestational weight gain: a systematic review. J Public Health. (2018) 40:41–55. doi: 10.1093/pubmed/fdx038
42. Mahanta LB, Choudhury M, Devi A, Bhattacharya A. On the study of pre-pregnancy Body Mass Index (BMI) and weight gain as indicators of nutritional status of pregnant women belonging to low socio-economic category: a study from Assam. Indian J Community Med. (2015) 40:198. doi: 10.4103/0970-0218.158870
43. Zanardo V, Mazza A, Parotto M, Scambia G, Straface G. Gestational weight gain and fetal growth in underweight women. Ital J Pediatr. (2016) 42:74. doi: 10.1186/s13052-016-0284-1
44. Straube S, Voigt M, Briese V, Schneider KTM, Voigt M. Weight gain in pregnancy according to maternal height and weight. J Perinat Med. (2008) 36:405–12. doi: 10.1515/JPM.2008.073
45. Hill B, McPhie S, Skouteris H. The role of parity in gestational weight gain and postpartum weight retention. Womens Health Issues. (2016) 26:123–9. doi: 10.1016/j.whi.2015.09.012
46. Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. (2018) 16:201. doi: 10.1186/s12916-018-1189-1
47. Inskip H, Crozier S, Baird J, Hammond J, Robinson S, Cooper C, et al. Measured weight in early pregnancy is a valid method for estimating prepregnancy weight. J Dev Orig Health Dis. (2021) 12:561–9. doi: 10.1017/s2040174420000926
Keywords: body mass index, gestational weight gain, pregnancy, weight gain, weight gain curves
Citation: Dangat K, Gupte S, Wagh G, Lalwani S, Randhir K, Madiwale S, Pisal H, Kadam V, Gundu S, Chandhiok N, Kulkarni B, Joshi S, Fall C and Sachdev HS (2022) Gestational weight gain in the REVAMP pregnancy cohort in Western India: Comparison with international and national references. Front. Med. 9:1022990. doi: 10.3389/fmed.2022.1022990
Received: 19 August 2022; Accepted: 20 September 2022;
Published: 05 October 2022.
Edited by:
Marco La Verde, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyReviewed by:
Chao-Yu Guo, National Yang Ming Chiao Tung University, TaiwanYi Liang, Sichuan University, China
Copyright © 2022 Dangat, Gupte, Wagh, Lalwani, Randhir, Madiwale, Pisal, Kadam, Gundu, Chandhiok, Kulkarni, Joshi, Fall and Sachdev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadhana Joshi, c3Jqb3NoaTYyQGdtYWlsLmNvbQ==; Harshpal Singh Sachdev, aHBzc2FjaGRldkBnbWFpbC5jb20=
 Kamini Dangat
Kamini Dangat Sanjay Gupte
Sanjay Gupte Girija Wagh
Girija Wagh Sanjay Lalwani4
Sanjay Lalwani4 Karuna Randhir
Karuna Randhir Shweta Madiwale
Shweta Madiwale Hemlata Pisal
Hemlata Pisal Vrushali Kadam
Vrushali Kadam Shridevi Gundu
Shridevi Gundu Bharati Kulkarni
Bharati Kulkarni Sadhana Joshi
Sadhana Joshi