
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 December 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1019752
Background: The guidelines of the Surviving Sepsis Campaign suggest using invasive blood pressure (IBP) measurement in septic shock patients, without specifying for a preferred arterial site for accuracy in relation to the severity of septic shock. The objective of this study was to determine the mean arterial pressure (MAP) gradient between the femoral and radial artery sites in septic shock patients.
Method: This prospective study was carried out at a 20-bed ICU in a university hospital. Simultaneous MAP measurements at femoral and radial arterial sites were obtained in septic shock patients receiving norepinephrine (≥0.1 μg/kg/min), with a pre-planned subgroup analysis for those receiving a high dose of norepinephrine (≥0.3 μg/kg/min).
Results: The median norepinephrine dose across all 80 patients studied, including 59 patients on a high dose, was 0.4 (0.28–0.7) μg/kg/min. Overall, simultaneous measurement of MAP (mmHg) at the femoral and radial arterial sites produced mean (95% CI) MAP values of 81 (79–83) and 78 (76–80), respectively, with a mean difference of 3.3 (2.67–3.93), p < 0.001. In Bland–Altman analysis of MAP measurements, the detected effect sizes were 1.14 and 1.04 for the overall and high-dose cohorts, respectively, which indicates a significant difference between the measurements taken at each of the two arterial sites. The Pearson correlation coefficient indicated a weak but statistically significant correlation between MAP gradient and norepinephrine dose among patients receiving a high dose of norepinephrine (r = 0.289; p = 0.026; 95% CI 0.036–0.508).
Conclusion: In septic shock patients, MAP readings were higher at the femoral site than at the radial site, particularly in those receiving a high dose of norepinephrine.
Clinical trial registration: [ClinicalTrials.gov], identifier [NCT03475667].
Invasive blood pressure (IBP) monitoring is a common procedure carried out in critically ill patients admitted to the intensive care unit (ICU) to optimize their hemodynamics. The international guidelines of the Surviving Sepsis Campaign (SSC) for the management of sepsis also suggest the placement of an arterial catheter as soon as practical in all patients requiring vasopressors (1). The accurate measurement of systolic blood pressure (SBP) and mean arterial pressure (MAP) is essential both for identification of circulatory shock and for maintenance of hemodynamic targets for better clinical outcomes while minimizing the untoward side effects of vasopressor agents (1–4). The artery most commonly used for catheter placement is the radial artery, and this site is considered to be both easily accessible and safe, with fewer complications arising in comparison to other sites, such as the femoral, dorsalis pedis, posterior tibial, ulnar, or brachial artery (5–8).
Generally, in physiological conditions, systolic blood pressure (SBP) is higher and diastolic blood pressure (DBP) is lower, while mean arterial pressure (MAP) is unchanged, when measurements are taken at a peripheral arterial site (radial) in comparison to a central arterial site (femoral) (9). However, in various clinical conditions, it has been reported that simultaneous MAP measurements taken at the most commonly used sites in each case (the radial artery and the femoral artery) differ significantly. During deep hypothermic cardiac arrest and during high-risk surgeries, including cardiopulmonary bypass and liver transplantation, MAP measurements have been found to be significantly different, with femoral site readings being higher than radial site readings (10–16). There is a possibility that this site-specific difference in IBP measurements might be explained by poorly understood pathophysiological changes, such as regional auto-regulation, atherosclerotic conditions, and tunica media area, and their relationships with the effects of vasopressors (norepinephrine alone or with other vasoactive agents) (17, 18). These studies suggest giving preference to central artery site measurement in high-risk peri-operative and medical patients experiencing circulatory shock requiring vasopressor therapy in order to deliver optimal care through accurate hemodynamic monitoring.
Among extant studies in the septic population, many have reported observing higher MAP values at the femoral artery site (19–23), while others have not (24, 25). However, across all the studies available, MAP measurements have also been included either from patients with non-septic conditions or from those not receiving vasopressors, which might have influenced their results. The available findings of these studies suggest the need for further evidence in the septic shock population, including separate subgroup analysis for patients receiving a high dose of norepinephrine, as this treatment is not uncommon (26), and underestimation of MAP measurements at the radial artery site could lead to unnecessarily high-dose vasopressor therapy and its related complications, including arrhythmia and digital or limb necrosis (27–29). This is especially important in septic shock patients, to whom vasopressors are frequently administered for an extended period of time.
Our study aimed to determine the MAP gradient between the femoral and radial artery sites in septic shock patients receiving norepinephrine (≥0.1 μg/kg/min), with pre-planned subgroup analysis for those receiving a high dose of norepinephrine (≥0.3 μg/kg/min).
This prospective observational study was conducted at a tertiary care center, specifically the 20-bed ICU of a university hospital in India, from Apr 2018–Jan 2020. The primary objective of this study was to compare femoral and radial arterial invasive blood pressure measurements in patients receiving norepinephrine (≥0.1 μg/kg/min) for septic shock and to correlate the pressure gradient with the dose of norepinephrine. The study protocol was approved by the Institutional Ethics Committee (IEC code: 2018-27-DM-EXP). A waiver of consent was granted by the Ethics Committee. The study was recorded on the ClinicalTrials.gov website (ClinicalTrials.gov identifier: NCT03475667).
Critically ill adult septic shock patients (as per Sepsis-3 definition) requiring norepinephrine infusion (≥0.1 μg/kg/min) and in whom the site of arterial invasive blood pressure monitoring was changed from radial to femoral, due to the current practice in our ICU as per the existing literature, were considered for inclusion in this study. All consecutively presenting eligible patients were included if they received a static dose of norepinephrine for at least 30 min with a functional radial artery monitoring system in place. Patients below the age of 18 years, pregnant women, patients with abdominal compartment syndrome, patients in whom a supine position was not feasible due to their clinical condition, and patients with a history of peripheral artery disease were all excluded from the study.
All relevant demographic details, clinical characteristics, all vasopressor doses, and each patient’s need for mechanical ventilation were recorded, along with scores on two measures of ICU severity, namely the Sequential Organ Failure Assessment (SOFA) and the Acute Physiology and Chronic Health Evaluation II (APACHE II). In addition to IBP (SBP, DBP, and MAP) readings, we also noted the number of patients in whom an absolute pressure gradient of ≥5 and ≥10 mm Hg was observed.
In each participating patient, a 16-gauge single-lumen arterial catheter (SLR 16 GA 8’ Arrow international, C.R.a.s. Jamska 2359/47) was used for femoral artery cannulation, while a 20-gauge catheter (BD Venflon™ Pro IV Cannula) was used for the radial artery. The arterial lines (pressure monitoring lines) used at both sites were similar. Blood pressure measurements were recorded in a supine position in cases in which both procedures were carried out on the same side (left or right), with the radial arterial line transducer at the level of the 5th rib in the mid-axillary line (phlebostatic axis), and the femoral line transducer placed at the same level as the radial line transducer. The equality of the levels of both the transducers was confirmed via the spirit level technique (30). The adequacy of damping was assessed by the fast-flush test.
All pressure values (SBP, DBP, and MAP) were recorded simultaneously three times within a 5-minute period, by freezing or taking a snapshot of the monitor, after placement of the femoral arterial catheter and before removal of the radial arterial line. The three readings for each value were averaged for analysis purposes.
In a previous observational study, the minimum average paired difference in MAP (mean ± SD) between radial (86.7 ± 10.7) and femoral (91.1 ± 11.5) site blood pressure recordings was 4.4 ± 11.1 mmHg (effect size = 0.396) (22). Based on this effect size, with a two-sided 95% confidence interval and 80% power for the study, the estimated sample size required with paired groups was 53. In our study, we also planned to carry out a subgroup analysis in patients receiving a high dose of norepinephrine (≥0.3 μg/kg/min). Therefore, we included 80 patients. Sample size was estimated using the G*Power software package, version 3.1.9.2 (Düsseldorf University, Germany).
The normality of the continuous variables were assessed; variable was considered to be normally distributed when the Z score of the skewness was within a range of ±3.29 (31). Normally distributed data are reported in the form of means (95% confidence interval, CI); other data are presented in the form of medians (interquartile range, IQR). Categorical variables are reported as numbers (percentage). A paired sample t-test was used to test the significance level of the mean differences observed. A Bland–Altman analysis was conducted, in which the bias (mean difference between two paired measurements) and corresponding 95% limits of agreement (reported in the form of mean ± 1.96 SD for the paired differences) were calculated for both the overall group and the high-dose group. To evaluate the absolute change in mean values in paired observations with respect to the corresponding pooled standard deviation, the effect size of the mean difference was calculated. The Pearson correlation coefficient (r) was used as an index of the relationship between norepinephrine dose and blood pressure gradient. All statistical analyses were performed using SPSS for Windows, version 23.0 (SPSS Inc., Chicago, IL, USA), and a two-tailed P-value < 0.05 was considered to represent statistical significance.
During the study period, a total of 520 patients were admitted to the ICU, and 422 of these received vasopressor therapy for septic shock. Among septic shock patients, 314 (74%) received a norepinephrine dose of at least 0.1 μg/kg/min at any point during their ICU stay (Figure 1). In accordance with the inclusion and exclusion criteria, 80 patients were included in this study, among whom the median norepinephrine dose was 0.4 (0.28–0.7) μg/kg/min. Of these 80 patients, 21 were receiving a norepinephrine dose of 0.1–0.29 μg/kg/min, while 59 were receiving ≥0.3 μg/kg/min. Across all included patients, the median age was 46.5 (31–56) years and the median APACHE II score at ICU admission was 23 (18–29). On the day of study inclusion, the median SOFA score was 15.5 (12–18), 66 patients (82.5%) were on mechanical ventilation, and none of the patients were receiving inotropic medication, including dobutamine (Table 1).
Across all included patients (n = 80), invasive blood pressure readings (mmHg) taken simultaneously at the femoral and radial arterial sites revealed that the mean (95% CI) SBP values were 122 (118–126) mmHg and 119 (115–123) mmHg, respectively, with a statistically significant difference between the means of 2.9 (1.1–4.7), p = 0.002; additionally, the mean (95% CI) MAP values were 81 (79–83) mmHg and 78 (76–80) mmHg, respectively, with a statistically significant difference between the means of 3.3 (2.7–3.9), p < 0.001. In the subgroup of patients receiving a high dose of norepinephrine (n = 59), the mean (95% CI) MAP values (mmHg) at the femoral and radial arterial sites were 80 (78–83) and 78 (75–80), with a statistically significant difference between the means of 2.9 (0.6–5.2), p < 0.001 (Table 2).
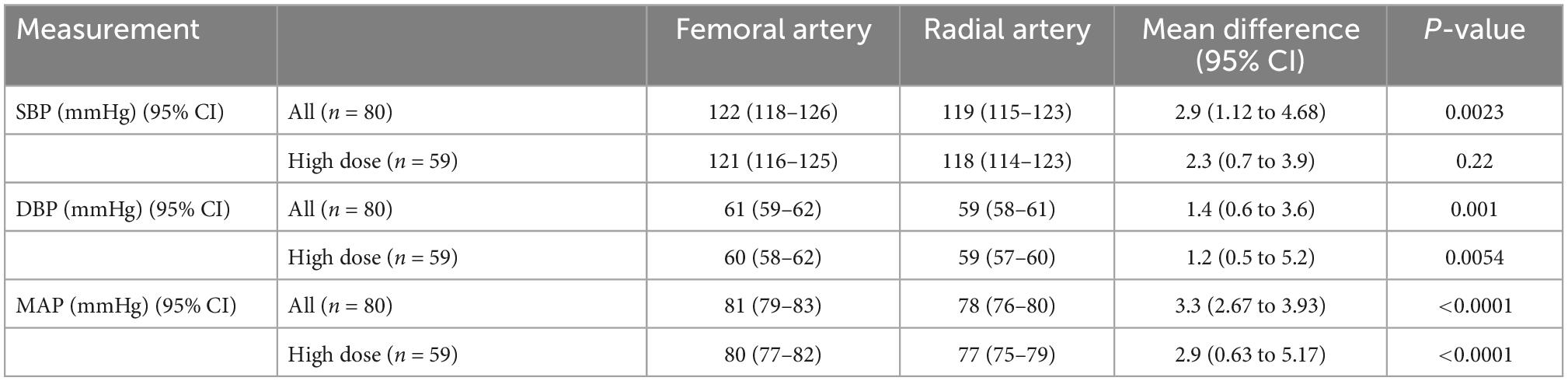
Table 2. Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) at femoral and radial arterial sites in study patient cohort.
Overall, an absolute pressure gradient of ≥5 mmHg (femoral > radial) was observed for SBP in 21 patients (26%) and for MAP in 17 patients (21%). Among the subgroup of patients receiving high-dose norepinephrine, an SBP gradient of ≥5 mmHg was observed in 13 patients (22%), and a MAP gradient of ≥5 mmHg was observed in 11 patients (19%), with the radial site frequently underestimating the central blood pressure (Table 3).
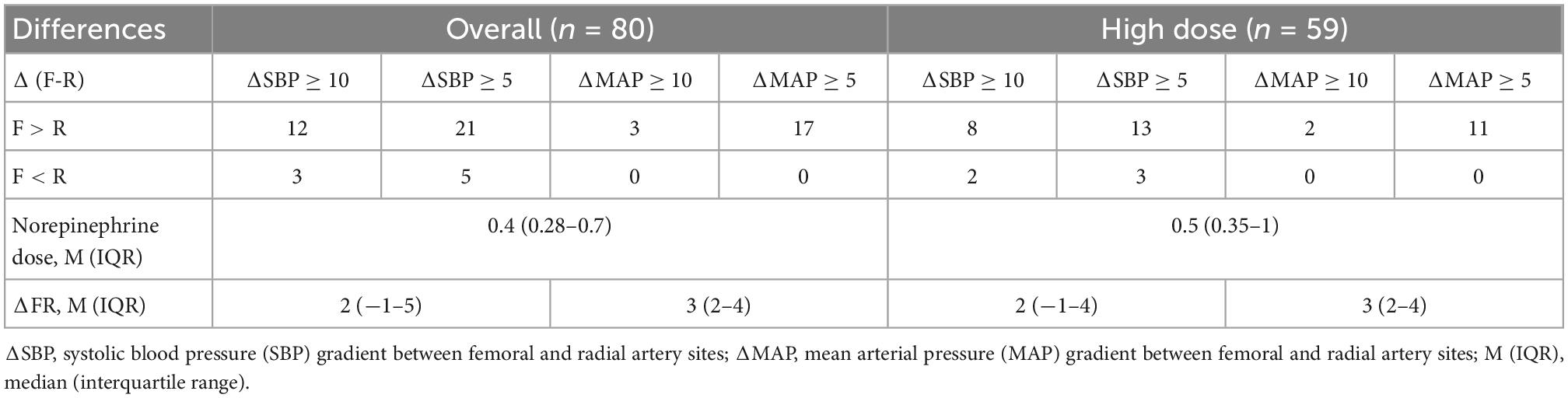
Table 3. Number of patients having systolic blood pressure (SBP) and mean arterial pressure (MAP) differences between femoral (F) and radial (R) sites, with cut-off values 5 and 10 mm of Hg.
Bland–Altman analysis indicated the presence of marked discrepancies or uncertainty in the measurement between the two methods, as the 95% limits of agreement indicated no specific direction for the change in mean difference (Figure 2 and Table 4). To quantify the absolute change in mean values in paired observations with respect to the corresponding pooled standard deviation, the effect size of the mean difference was also calculated. This showed that the effect sizes for the overall and high-dose cohorts, respectively, were 0.35 and 0.32 for SBP (effect sizes ranging from 0.2 to 0.49 are considered small) and 1.14 and 1.04 for MAP (effect sizes ≥ 0.8 are considered large) (Figure 2 and Table 4).
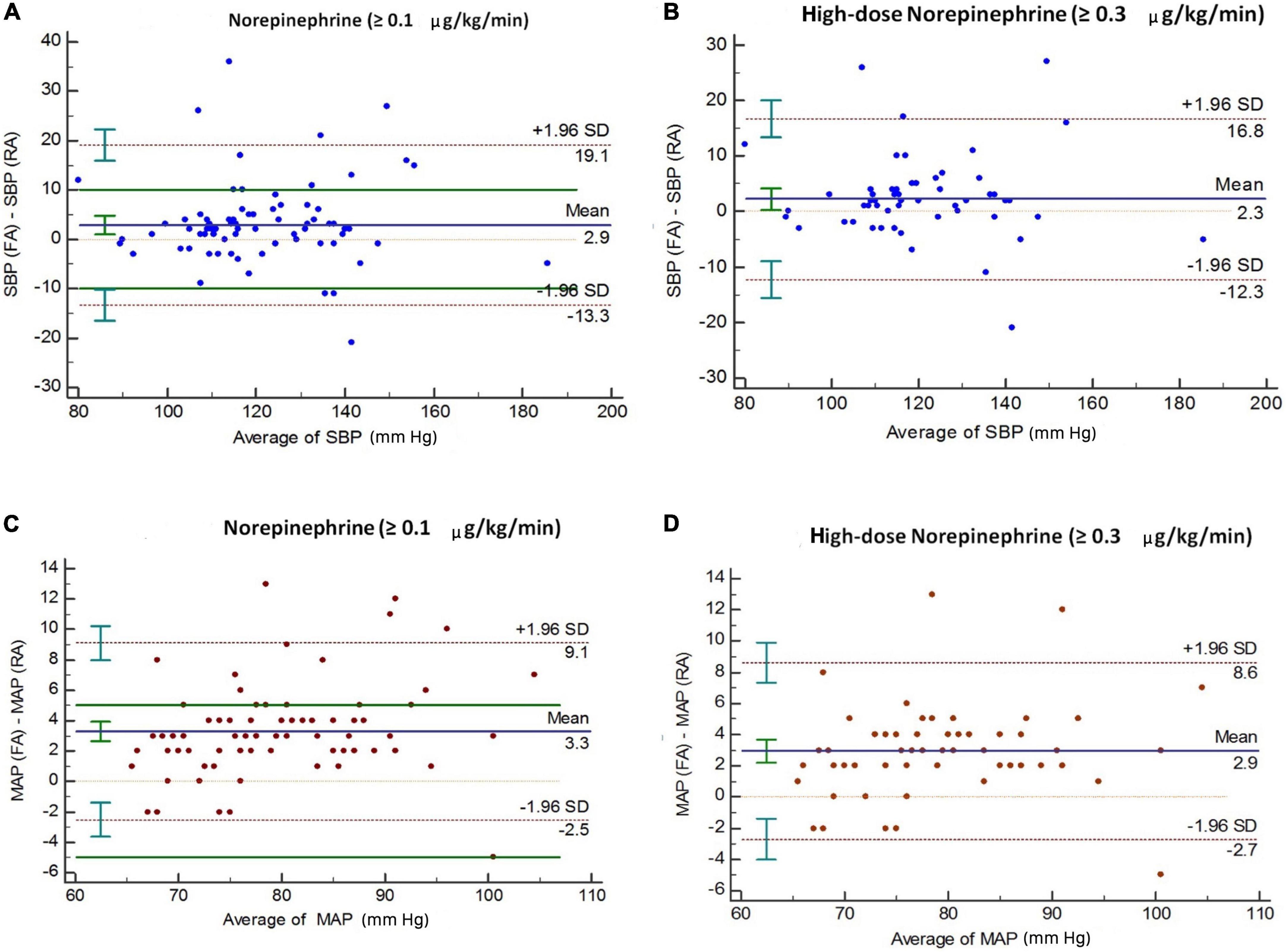
Figure 2. Bland–Altman analysis of differences between femoral and radial arterial pressure among all patients (n = 80), and among those on high-dose norepinephrine (n = 59). Panels (A,B) represent SBP; panels (C,D) represent MAP. The solid line represents bias (the mean difference between simultaneous measurements). Dotted lines show 95% limits of agreement (bias ± 1.96 SD). SBP, systolic blood pressure; MAP, mean arterial pressure.
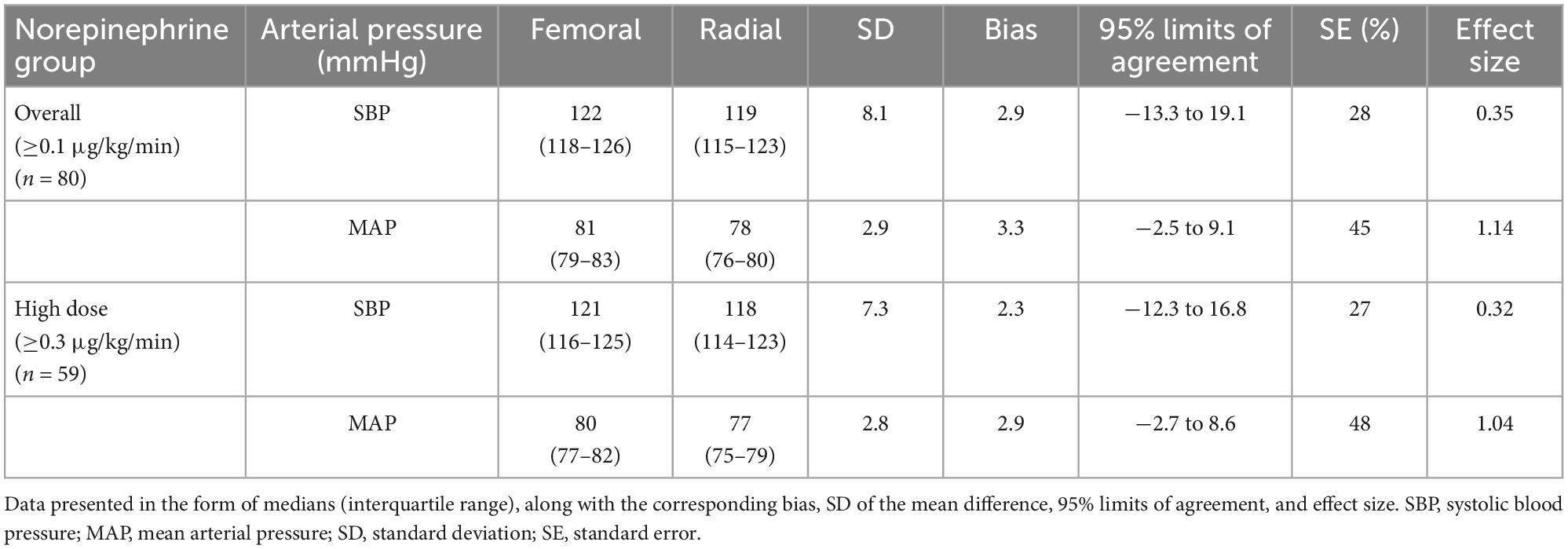
Table 4. Bias (mean of the difference) and 95% limits of agreement between arterial pressure measurements at the femoral and radial arterial sites, calculated via Bland–Altman analysis accounting for repeated simultaneous measurements.
The Pearson coefficient representing the correlation between MAP gradient (femoral > radial) and norepinephrine dose was not found to be significant across the entire sample, i.e., among patients receiving a norepinephrine dose ≥0.1 μg/kg/min (r = 0.105; p = 0.805; 95% CI: −0.118 to 0.317). However, there was a statistically significant weak positive correlation between MAP gradient and norepinephrine dose among patients receiving a high dose of norepinephrine (r = 0.289; p = 0.026; 95% CI: 0.036 to 0.508) (Figures 3A, B).
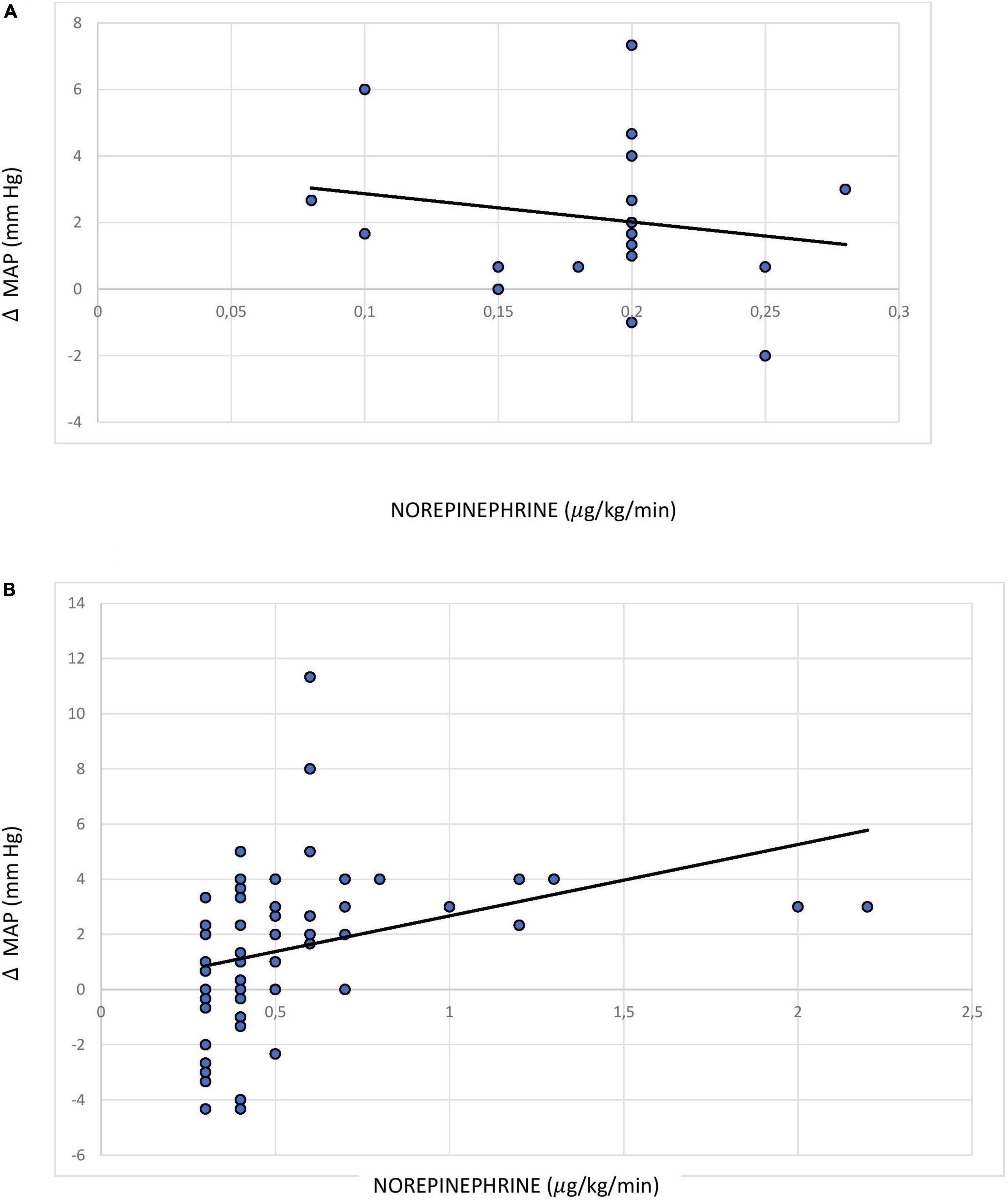
Figure 3. Pearson correlation between norepinephrine dose and mean arterial pressure difference between the femoral and radial sites among patients receiving (A) norepinephrine dose <0.3 (0.1–0.29) μg/kg/min; (B) norepinephrine dose ≥0.3 μg/kg/min.
Our study found that MAP measurements taken at the radial artery site are frequently underestimates in comparison to those taken at the femoral artery site in septic shock patients requiring norepinephrine (≥0.1 μg/kg/min). In previous studies comparing MAP measurements between the femoral and radial arterial sites in septic shock patients, findings have been inconsistent (Table 5). Studies by Dorman, Compton, Galluccio, Kim, and Wisanusattra have reported significantly higher MAP at the femoral arterial site when measured simultaneously with the radial site (19–23), while those by Mignini and Antal have not observed this (24, 25). Among these, only the studies conducted by Dorman and Kim recruited a cohort of only septic shock patients.

Table 5. Summary of studies examining differences in mean arterial pressure (MAP) between the femoral and radial sites in critically ill septic shock patients.
The population of the study by Dorman (19) included a small number of septic shock patients (n = 14) receiving a mean norepinephrine dose of 86 mcg/min, and the mean femoral site MAP observed was 15 mmHg higher than that observed at the radial artery site. Kim (22) included 37 septic shock patients who were receiving a mean norepinephrine dose of 0.1 μg/kg/min, with multiple measurements taken after inclusion, and found that radial MAP was on average 5 mmHg lower (95% CI: −17 to +7). In a recently published study by Wisanusattra (23), 32 patients (28 with septic shock) who were receiving a mean norepinephrine dose of 0.85 mcg/kg/min showed a MAP gradient between the femoral and radial sites of 7.6 mmHg over multiple hourly serial readings over the course of 24 h. In contrast, the results of the studies conducted by Compton, Galluccio, Mignini, and Antal did not exclusively represent a septic shock cohort, and their findings might have been influenced by the inclusion of non-septic shock patients (Table 5).
In our study, across all septic shock patients included, the median norepinephrine dose was 0.4 μg/kg/min and the mean radial site MAP was 3.3 mmHg lower than the femoral arterial site MAP. More than 20% of patients had a higher MAP (gradient ≥ 5 mmHg) at the femoral site, while no patients had a higher MAP (gradient ≥ 5 mmHg) at the radial artery site. This higher MAP (gradient ≥ 5 mmHg) at the femoral site was also observed in 58% of patients in the Kim study (22) and in 59% of patients receiving a high dose of norepinephrine in the study by Wisanusattra (23). Across all studies, there were either fewer (2–4%) or no patients with MAP readings ≥5 mmHg higher at the radial artery site compared to the femoral site (19–25).
According to the pre-planned analysis conducted in our study in the subgroup of patients receiving high-dose norepinephrine [median (IQR) dose: 0.5 (0.35–1) μg/kg/min], the Pearson correlation coefficient indicated a weak correlation (r = 0.289; p = 0.026) between MAP gradient and vasopressor dose, which is a similar finding to that of the study by Kim (r = 0.33, p < 0.001) (22). In another recent study conducted by Wisanusattra, a strong correlation was observed between MAP gradient and vasopressor dose (r = 0.89; p < 0.0001) (23). This correlation might be relevant for clinical outcomes, as prolonged unnecessary exposure to a higher dose of norepinephrine during septic shock leads to its own complications, such as arrhythmia and digital or limb necrosis. However, we acknowledge that the risk–benefit ratio of giving preference to the femoral arterial site for MAP measurement in patients with septic shock is still not clear and needs to be investigated further. Indeed, a recent study has found that the difference in non-invasive blood pressure readings between the brachial and radial artery correlates with the MAP difference between the femoral and radial artery (32); this could be considered as a means of identifying patients who are candidates for preferential use of the femoral artery site for invasive pressure measurement.
Our study has certain limitations. First, we did not include patients who were receiving a norepinephrine dose <0.1 μg/kg/min. Second, we did not investigate the effects of age, radial artery diameter, intra-thoracic pressure, or intra-abdominal pressure on the MAP gradient between the femoral and radial arterial sites. Additionally, we did not analyze the effect of concurrent use of vasopressin on the MAP gradient observed in patients who were receiving this. The long-term adverse effects of femoral artery site cannulation, such as digital ischemia, thrombosis, or other complications, were not followed up in our study. On the other hand, the primary strength of our study is that we included only septic shock patients receiving a norepinephrine dose of at least 0.1 μg/kg/min, including a pre-planned analysis with an adequate sample size of patients receiving a norepinephrine dose of at least 0.3 μg/kg/min.
In comparison to the femoral artery site, invasive pressure monitoring at the radial artery site frequently underestimates SBP and MAP in septic shock patients receiving norepinephrine therapy. Hence, the findings of our study suggest that the femoral artery could be considered over the radial artery for accurate MAP measurement in septic shock patients, particularly in those who are receiving a high dose of norepinephrine, in order to minimize possible side effects arising from unnecessary exposure to higher doses of vasopressor agents for a prolonged period of time. However, the risk–benefit ratio of giving preference to the femoral arterial site in these patients is still not clear and needs to be investigated further.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee, SGPGIMS, Lucknow, India. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BB: data curation, investigation, and original draft preparation. MG: conceptualization, methodology, project administration, supervision, validation, and writing – review and editing. PM: methodology, formal analysis, and software. AA: supervision and writing – review and editing. BP: methodology, and writing – review and editing. AB: project administration and writing – review and editing. All authors contributed to the article and approved the submitted version.
Shreyas H. Gutte, Mallikarjun Dube, Mohd. Chand, and Saumitra Misra for their support during data collection. Presented, in part, as an abstract at the 6th SG ANZICS, Intensive Care Forum, Apr 18–22, 2019, Singapore (also received Education Grant Award), and at the 26th Annual Conference of the Indian Society of Critical Care Medicine (ISCCM), Feb 28–Mar 1, 2020, at Hyderabad, India.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith C, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
2. Anantasit N, Boyd J, Walley K, Russell J. Serious adverse events associated with vasopressin and norepinephrine infusion in septic shock. Crit Care Med. (2014) 42:1812–20.
3. Patidar K, Peng J, Pike F, Orman E, Glick M, Kettler C, et al. Associations between mean arterial pressure and poor ICU outcomes in critically ill patients with cirrhosis: is 65 the sweet spot?. Crit Care Med. (2020) 48:e753–60. doi: 10.1097/CCM.0000000000004442
4. Daroca-Pérez R, Carrascosa M. Digital necrosis: a potential risk of high-dose norepinephrine. Ther Adv Drug Saf. (2017) 8:259–61. doi: 10.1177/2042098617712669
5. Lodato R, Schlichting R. Arterial pressure monitoring. Arterial catheterization: complications. In: Tobin M editor. Principles and practice of intensive care monitoring volume part III. 2nd ed. New York, NY: McGraw Hill (1998). p. 733–56.
6. Frezza E, Mezghebe H. Indications and complications of arterial catheter use in surgical or medical intensive care units: analysis of 4932 patients. Am Surg. (1998) 64:127–31.
7. Gurman G, Kriemerman S. Cannulation of big arteries in critically ill patients. Crit Care Med. (1985) 13:217–20.
8. Russell J, Joel M, Hudson R, Mangano D, Schlobohm R. Prospective evaluation of radial and femoral artery catheterization sites in critically ill adults. Crit Care Med. (1983) 11:936–9. doi: 10.1097/00003246-198312000-00007
9. O’Rourke M, Blazek J, Morreels C Jr, Krovetz L. Pressure wave transmission along the human aorta. changes with age and in arterial degenerative disease. Circ Res. (1968) 23:567–79. doi: 10.1161/01.res.23.4.567
10. Gravlee G, Brauer S, O’Rourke M, Avolio A. A comparison of brachial, femoral, and aortic intra-arterial pressures before and after cardiopulmonary bypass. Anaesth Intensive Care. (1989) 17:305–11. doi: 10.1177/0310057X8901700311
11. Baba T, Goto T, Yoshitake A, Shibata Y. Radial artery diameter decreases with increased femoral to radial arterial pressure gradient during cardiopulmonary bypass. Anesth Analg. (1997) 85:252–8.
12. Kanazawa M, Fukuyama H, Kinefuchi Y, Takiguchi M, Suzuki T. Relationship between aortic-to-radial arterial pressure gradient after cardiopulmonary bypass and changes in arterial elasticity. Anesthesiology. (2003) 99:48–53. doi: 10.1097/00000542-200307000-00011
13. Chauhan S, Saxena N, Mehrotra S, Rao B, Sahu M. Femoral artery pressures are more reliable than radial artery pressures on initiation of cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (2000) 14:274–6.
14. Manecke G Jr, Parimucha M, Stratmann G, Wilson W, Roth D, Auger W, et al. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth. (2004) 18:175–9. doi: 10.1053/j.jvca.2004.01.023
15. Acosta F, Sansano T, Beltran R, Palenciano C, Reche M, Roques V, et al. Is femoral and radial artery pressure different during reperfusion in liver transplantation? Transplant Proc. (2000) 32:2647.
16. Arnal D, Garutti I, Perez-Peña J, Olmedilla L, Tzenkov I. Radial to femoral arterial blood pressure differences during liver transplantation. Anaesthesia. (2005) 60:766–71.
17. Magder S. The meaning of blood pressure. Crit Care. (2018) 22:257. doi: 10.1186/s13054-018-2171-1
18. Ohta H, Ohki T, Kanaoka Y, Koizumi M, Okano H. Pitfalls of invasive blood pressure monitoring using the caudal ventral artery in rats. Sci Rep. (2017) 7:41907. doi: 10.1038/srep41907
19. Dorman T, Breslow M, Lipsett P, Rosenberg J, Balser J, Almog Y, et al. Radial artery pressure monitoring underestimates central arterial pressure during vasopressor therapy in critically ill surgical patients. Crit Care Med. (1998) 26:1646–9. doi: 10.1097/00003246-199810000-00014
20. Compton F, Zukunft B, Hoffmann C, Zidek W, Schaefer J. Performance of a minimally invasive uncalibrated cardiac output monitoring system (flotrac/vigileo) in haemodynamically unstable patients. Br J Anaesth. (2008) 100:451–6. doi: 10.1093/bja/aem409
21. Galluccio S, Chapman M, Finnis M. Femoral-radial arterial pressure gradients in critically ill patients. Crit Care Resusc. (2009) 11:34–8.
22. Kim W, Jun J, Huh J, Hong S, Lim C, Koh Y, et al. Radial to femoral arterial blood pressure differences in septic shock patients receiving high-dose norepinephrine therapy. Shock. (2013) 40:527–31.
23. Wisanusattra H, Khwannimit B. Agreement between mean arterial pressure from radial and femoralartery measurments in refractory shock patients. Sci Rep. (2022) 12:8825. doi: 10.1038/s41598-022-12975-y
24. Mignini M, Piacentini E, Dubin A. Peripheral arterial blood pressure monitoring adequately tracks central arterial blood pressure in critically ill patients: an observational study. Crit Care. (2006) 10:R43. doi: 10.1186/cc4852
25. Antal O, Ştefãnescu E, Hagãu N. Does norepinephrine infusion dose influence the femoral-to-radial mean arterial blood pressure gradient in patients with sepsis and septic shock?. Blood Press Monit. (2019) 24:74–7. doi: 10.1097/MBP.0000000000000363
26. Jentzer J, Vallabhajosyula S, Khanna A, Chawla L, Busse L, Kashani K, et al. Management of refractory vasodilatory shock. Chest. (2018) 154:416–26.
27. Asfar P, Meziani F, Hamel J, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. (2014) 370:1583–93.
28. Auchet T, Regnier M, Girerd N, Levy B. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. (2017) 7:43.
29. Martin C, Medam S, Antonini F, Alingrin J, Haddam M, Hammad E, et al. Norepinephrine: not too much. too long. Shock. (2015) 44:305–9.
30. Sahoo J. Transducer and pressure monitoring. In: Gurjar M editor. Manual of ICU procedures. New Delhi: Jaypee Brothers Medical Publishers (2016). p. 322–35.
31. Mishra P, Pandey C, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. (2019) 22:67–72.
Keywords: septic shock, invasive blood pressure, mean arterial pressure, hemodynamic monitoring, arteries, femoral-radial arterial pressure gradient, vasoconstrictor, norepinephrine
Citation: Bhaskar B, Gurjar M, Mishra P, Azim A, Poddar B and Baronia AK (2022) Arterial site selection for measurement of mean arterial pressure in septic shock patients on high-dose norepinephrine. Front. Med. 9:1019752. doi: 10.3389/fmed.2022.1019752
Received: 15 August 2022; Accepted: 01 December 2022;
Published: 23 December 2022.
Edited by:
Mathieu Jozwiak, Centre Hospitalier Universitaire de Nice, FranceReviewed by:
Pascal Laferriere-Langlois, Hôpital Maisonneuve-Rosemont, CanadaCopyright © 2022 Bhaskar, Gurjar, Mishra, Azim, Poddar and Baronia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohan Gurjar, ✉ bS5ndXJqYXJAcmVkaWZmbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.