- 1Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 2CapitalBio Medical Laboratory, Beijing, China
- 3CapitalBio Technology Co., Ltd., Beijing, China
Introduction: This study aimed to determine the correlation between fetal fraction (FF) of cell-free DNA (cf-DNA) and pregnancy complications related to placental dysfunction in Twin Pregnancy.
Methods: This retrospective cohort study analyzed twin pregnant women who underwent non-invasive prenatal testing (NIPT) at 12+0–26+6 weeks of gestation from April 2017 to April 2021. Low fetal fraction (LFF) was defined individually as less than the 25th, 10th, 5th, and 2.5th percentile among all fetal fractions in the cohort. Primary outcomes included gestational hypertension (GH), preeclampsia (PE), gestational diabetes mellitus (GDM), and small for gestational age (SGA). Logistic regression analysis was used to assess the relationship between LFF and pregnancy complications.
Results: A total of 500 twin pregnancies (male-male twins, 245; female-female twins, 255) were included in this study. In LFF group (FF < 25th percentiles), maternal BMI was significantly higher than FF > 75th percentiles (23.6 kg/m2 vs. 21.3 kg/m2; P < 0.001). The risk of SGA increased gradually from FF < 25th percentiles [adjusted odds ratio (OR), 1.71; 95% confidence interval (CI), 1.07–2.99; P = 0.016] to FF < 2.5th percentiles (adjusted OR, 4.44; 95% CI,1.33–14.82; P < 0.015). In addition, the risks of SGA in both fetuses were higher than the risks of at least one fetus SGA in LFF group. LFF had no correlation with GH, PE, and GDM in twin pregnancy.
Conclusion: LFF has a strong association with increased risk of SGA in twin pregnancy. Moreover, FF of cf-DNA may provide a new idea for the early screening of diseases related to placental dysfunction in twin pregnancy.
Introduction
In 1997, Lo et al. (1) discovered the Y chromosome in the maternal peripheral blood of pregnant male fetuses, which proved the existence of fetal cell-free-DNA (cf-DNA) in the peripheral blood of pregnant women. In recent years, fetal cf-DNA has been widely used in fetal aneuploid screening in the early second-trimester as a biomarker for a non-invasive prenatal test (NIPT). The fetal fraction (FF) of cf-DNA is a key factor in whether NIPT can achieve effective results. In previous studies, NIPT would received no-call results when FF is <4%, which is affected by diverse maternal-fetal factors, such as gestational age, maternal weight, race, and multiple pregnancies (2–11). In addition, fetal cf-DNA is mainly derived from placenta trophoblast cell apoptosis and released into the maternal peripheral blood circulation. Therefore, FF of cf-DNA is also related to placental dysfunction, which may be a marker to the early screening for pregnancy complications with placental dysfunction, such as gestational hypertension (GH), preeclampsia (PE), small for gestational age (SGA), premature birth (PTB), and gestational diabetes mellitus (GDM).
Previous studies reported that the FF of singleton pregnant women who subsequently developed PE, GDM, PTB, and SGA were significantly different from the FF of healthy pregnant women, although the specific changes of FF were unclear. Zhong et al. suggested that the FF of PE women was higher than normal pregnant women, probably due to impaired placental function, and accelerated trophoblast apoptosis (12, 13). However, as an increasing concern of NIPT failure, several studies also found that low FF was associated with increased risks of subsequent PE, SGA, PTB, and GDM. Krishna et al. found that compared to the group with FF > 4%, the incidence of hypertension-related diseases was significantly increased in the low FF group (FF < 4%) (26.4% vs. 59.1%; P = 0.001) and that the low FF group had higher risks of adverse perinatal outcomes [adjusted odds ratio (OR), 2.5; 95% confidence interval (CI), 1.01–6.2; P = 0.049] (14). Clapp et al. determined that the proportion of low birth weight in the low FF group (FF < 5th percentile) was significantly higher than that in the FF > 5th percentile group of 2,035 singleton pregnant women (6.9% vs. 3.2%; P = 0.04) which may be related to placental dysplasia and placental volume decrease (15).
The above studies proved the relationship between FF and singleton perinatal outcomes, but were primarily limited by the lack of twin pregnancy cohorts. The frequency of twin gestations has increased over the last few decades, mainly due to the increased rates of advanced maternal age and the widespread use of assisted reproduction techniques. Twin pregnancy is associated with a higher risk of PE, GDM, and PTB than singleton pregnancy, which makes it necessary to find an appropriate marker for early screening of twin pregnancy complications. The NIPT landscape has been rapidly evolving in the prenatal screening of twin pregnancies. This study aimed to investigate the relationship between FF of cf-DNA and the pregnancy complications related to placental dysfunction in twin pregnancies and to determine whether FF of cf-DNA may also be used as a marker for early screening of placental dysfunction disorders in twin pregnancies.
Materials and methods
Study population and data collection
This retrospective cohort study analyzed all twin pregnancy women undergoing NIPT at 12–26 weeks’ gestation in Peking University Third Hospital (a university-affiliated tertiary hospital) from April 2017 to April 2021. This study was approved by the ethics committee of Peking University Third Hospital (no. M2021225) prior to the data collection. Written informed consent was obtained from each participant. The data downloaded for analysis were anonymous. We included male-male twin pregnancies and female-female twin pregnancies with the quantification of FF and pregnancy outcome information. Exclusion criteria were as follows: twin gestations with different sex; vanishing twin; fetus having chromosomal abnormalities or structural malformations; pre-gestational diseases such as chronic hypertension and diabetes; and missing or incomplete medical records. Our primary outcomes included GH, PE, GDM, and SGA. Maternal characteristics, including age, body mass index (BMI), and gestational age on NIPT, medical history, parity, method of conception, chorionicity, and FF, were obtained from our hospital’s prenatal screening database. Maternal and neonatal outcomes were obtained from our hospital’s electronic medical records, which included pregnancy complications, delivery gestations, birthweight, and sex of the two newborn babies.
Fetal fraction measurements
Two types of methods were used to calculate the fetal DNA fraction in maternal plasma. For pregnancy with male-male fetuses, reads proportion on the Y chromosome was used to estimate the FF. For pregnancy with female-female fetuses, the FF was estimated using the length distribution of cf-DNA, in which fetal DNA (130–140 bp; region A) was generally shorter than maternal DNA (155–175 bp; region B). LOESS regression was applied to calculate the FF against reads ratio in features A and B (16). However, it was difficult to accurately calculate the FF of opposite-sex twins.
Diagnostic measurements
According to the International Association of Diabetic Pregnancy Research Group criteria, diagnosis of GDM depended on a 75 g oral glucose tolerance test at 24–28 weeks’ gestation (17). Women with normal blood pressure who developed hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) after 20 weeks of gestation, were diagnosed GH. PE was identified if a woman had new onset hypertension after 20 weeks of gestation, accompanied by at least one of proteinuria, other maternal organ dysfunction (including heart, lung, liver, and kidney), hematological, digestive, neurological involvement, or uteroplacental dysfunction (18). SGA was defined as the birth weight of neonate <10th percentile for the gestational age based on the global reference for birthweight percentiles (19).
Statistical analysis
Based on the distribution of FF in the cohort, the cohort was grouped by FF quartiles to compare the baseline characteristics. The cohort was further classified into three groups to compare the relationship between FF and outcomes as follows: low FF, <2.5th, <5th, <10th, and <25th percentiles; normal FF, 2.5–97.5th, 5–95th, 10–90th, and 25–75th percentiles; and high FF, >75th, >90th, > 95th, and >97.5th percentiles.
In this study, continuous variables of baseline characteristics were non-normally distributed and expressed as median (interquartile range, IQR), whereas categorical variables of baseline characteristics and pregnancy complications were expressed as N (%). Kruskal–Wallis test and Chi–square test or Fisher’s exact test were used to analyze continuous and categorical variables, respectively. Multivariable logistic regressions were performed to determine OR and 95% CI to assess the association between low FF and pregnancy complications. Adjusted regressions were controlled for maternal age, BMI, and gestational age on NIPT, parity, method of conception, delivery gestations, which were selected a priori given their potential associations with the FF, and pregnancy complications. All data were analyzed using SPSS version 26.0. Statistical significance was set at P < 0.05.
Results
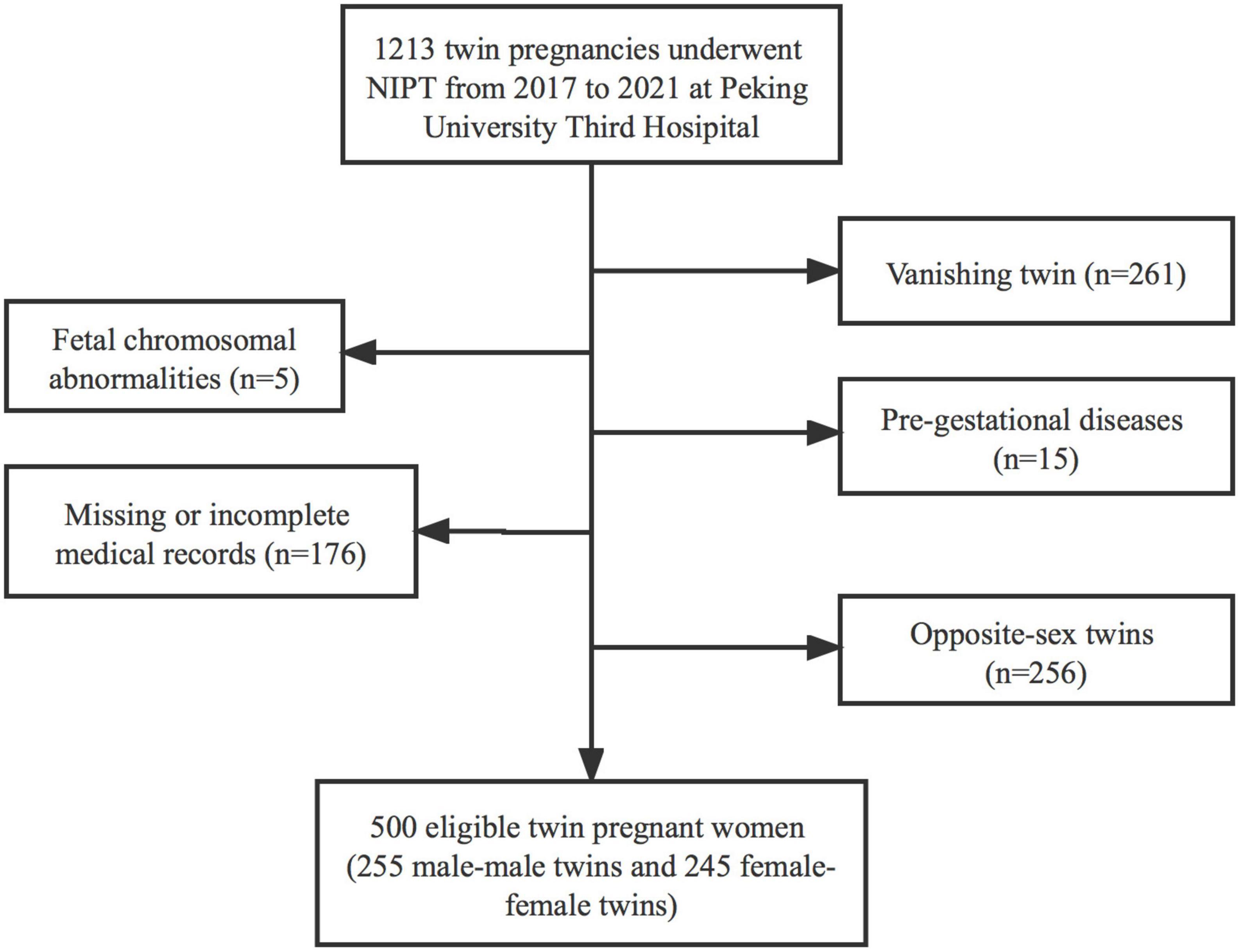
A total of 1,213 pregnant women who underwent NIPT at 12–26 weeks of gestation after obtaining their informed consent were enrolled in this study. The following were excluded from the study: 256 women due to twin pregnancies of different sex, 261 women due to vanishing twins, and 196 observational participants due to fetus having chromosomal abnormalities (n = 5), pre-gestational diseases (n = 15), missing or incomplete medical records (n = 176). Finally, 500 [male-male twin pregnancies, 255 (51.0%); female-female twin pregnancies, 245 (49.0%)] were included (Figure 1).
The baseline demographic characteristics of pregnant women and obstetrical characteristics, including maternal age, BMI, and gestational age on NIPT, parity, method of conception, chorionicity, delivery gestational age, and birthweight grouped by FF quartiles were presented in Table 1. In our study, the median FF was 18.55% (IQR, 15.00–22.80%). Maternal BMI on NIPT was significantly higher in low FF (<25th percentiles) than that in other FF groups (23.6 kg/m2 vs. 23.2 kg/m2 vs. 22.3 kg/m2 vs. 21.3 kg/m2; P < 0.001), and the proportion of obese pregnant women was also significantly increased in low FF (10.5 vs. 7.9% vs. 4.8% vs. 4.0%; P < 0.001). When compared with normal FF and high FF groups, lower birthweight of the second twin was found in FF < 25th percentiles (2,290 kg vs. 2,390 vs. 2,400 vs. 2,435 kg; P = 0.048). However, the median maternal age (31 vs. 31 vs. 32 vs. 32 years old; P = 0.750) and median gestational age of NIPT (15+0 vs. 14+0 vs. 14+0 vs. 14+1 weeks; P = 0.184) were similar among the FF quartile groups. Similarly, the proportion of parity, method of conception, chorionicity, delivery gestational age, and birthweight of the first twin were not statistically significant among different FF percentiles groups.
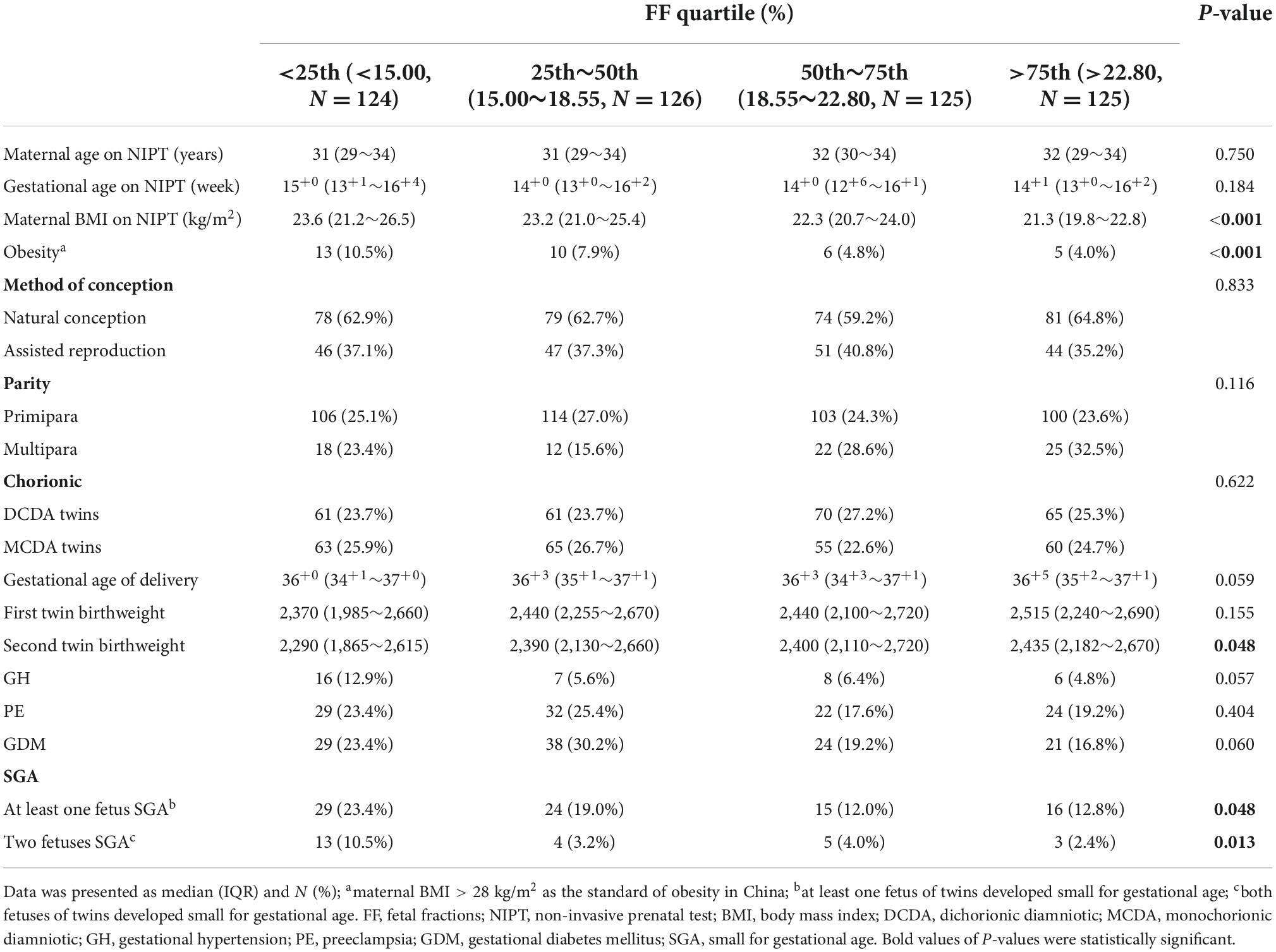
Table 1. Baseline demographic characteristics of pregnant women on NIPT in the study cohort based on the categories of FF (n = 500).
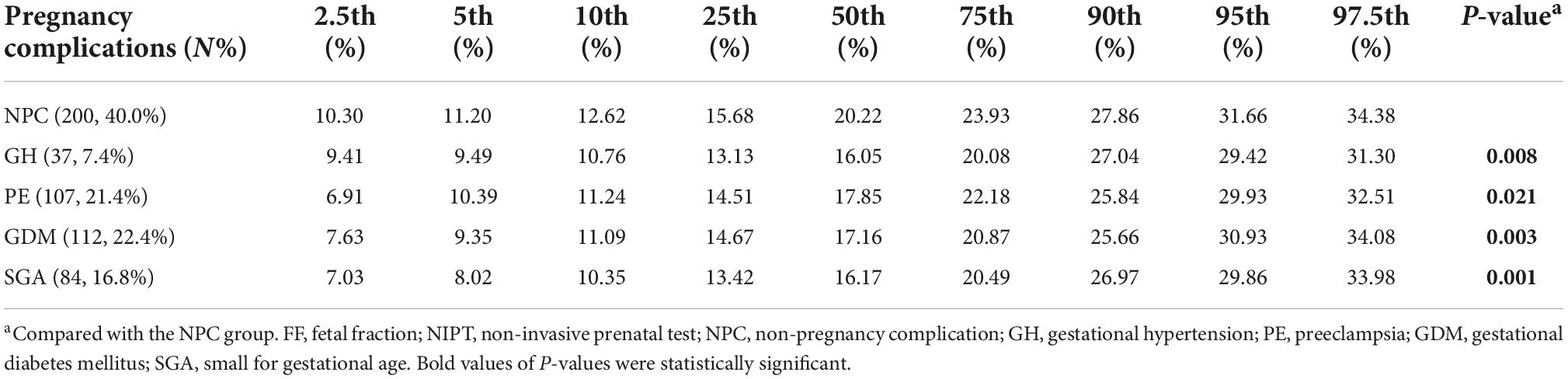
The prevalence of different complications at different percentiles groups and the distribution of plasma FF in non-pregnancy complication (NPC), GH, PE, GDM, and SGA is summarized in Tables 1, 2. Among the 500 same-sex twin pregnancies, the overall prevalence of GH, PE, GDM, and SGA was 7.4% (37), 21.4% (107), 22.4% (112), and 16.8% (84), respectively. For low FF (<25th percentiles), the incidence of SGA was significantly higher than high FF (>75th percentiles) (at least one fetus SGA: 23.4% vs. 12.8%; P = 0.048; two fetuses SGA: 10.5% vs. 2.4%; P = 0.013). The rates of GH, PE and GDM in low FF (<25th percentiles) also were higher than FF 50th–75th percentiles and FF > 75th percentiles, whereas there was no significant statistical difference (GH: 12.9% vs. 6.4% vs. 4.8%; P = 0.057; PE: 23.4% vs. 17.6% vs. 19.2%; P = 0.404; GDM: 23.4% vs. 19.2% vs. 16.8%; P = 0.060). When compared to women with NPC, the median FF was significantly lower for women who developed GH, PE, GDM, and SGA in Figure 2 and Table 2 (NPC: 20.22% vs. GH: 16.05% vs. PE: 17.85% vs. GDM: 17.16% vs. SGA: 16.17%; all P < 0.01).
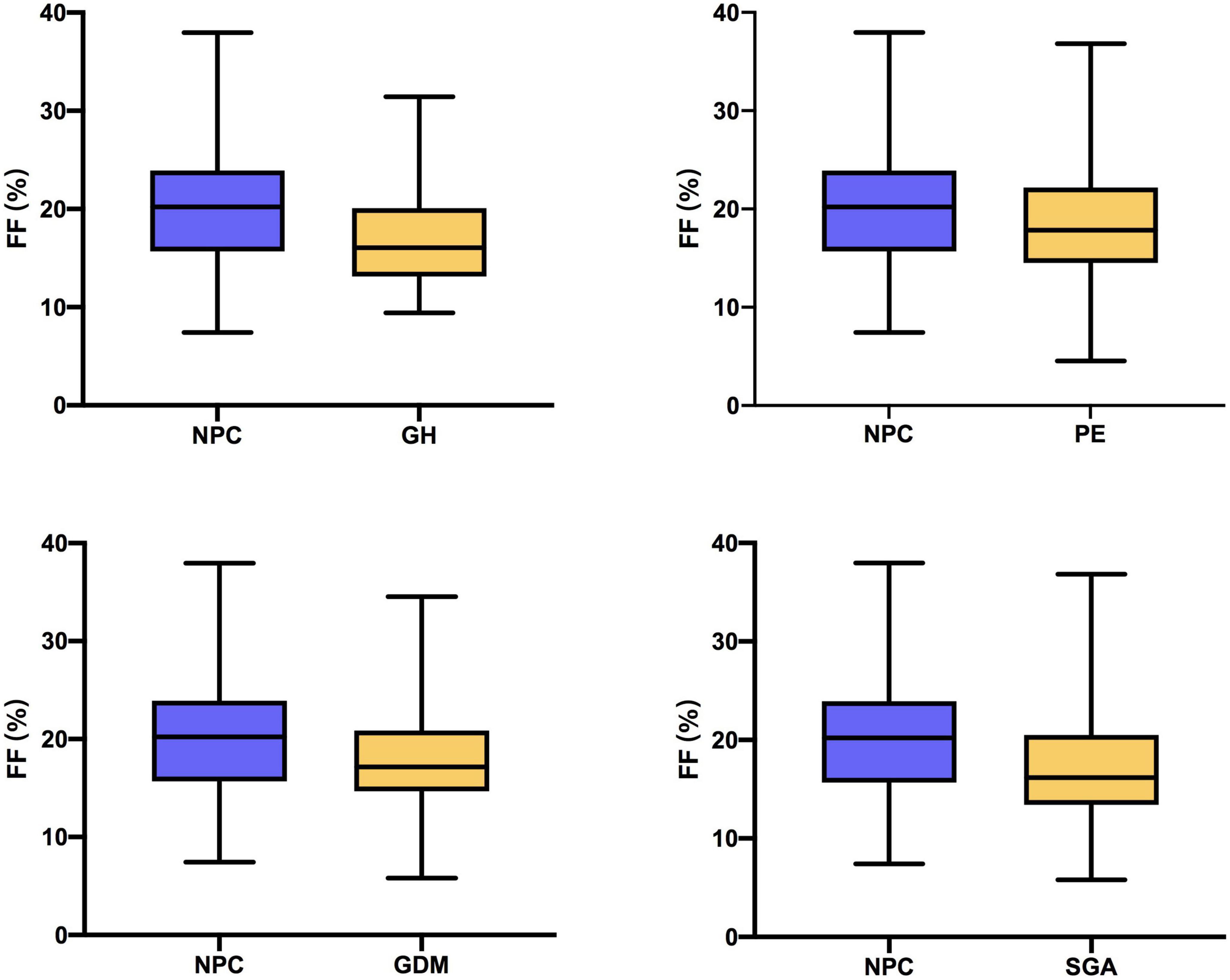
Figure 2. Fetal fraction (FF) in women with non-pregnancy complication (NPC) and women who developed gestational hypertension (GH), preeclampsia (PE), gestational diabetes mellitus (GDM), and small for gestational age (SGA).
Associations of plasma FF with risk for pregnancy complications related to placental dysfunction in adjusted models were demonstrated in Table 3. In FF quartiles groups, low FF (<25th percentiles) was associated with an increased risk of SGA (at least one fetus SGA) when compared to normal FF (adjusted OR, 1.71; 95% CI, 1.07–2.99; P = 0.016), and high FF (adjusted OR, 2.16; 95% CI, 1.05–4.42; P = 0.035). We also found that low FF below the 10th, 5th, and 2.5th percentiles were associated with gradually increased risks of SGA relative to the 10th–90th, 5th–95th, and 2.5th–97.5th percentiles, respectively (for <10th vs. 10th–90th percentiles: adjusted OR, 2.99; 95% CI, 1.08–8.28; P = 0.018; for <5th vs. 5–95th percentiles: adjusted OR, 3.43; 95% CI, 1.39–8.47; P = 0.008; for <2.5th vs. 2.5th–97.5th percentiles: adjusted OR, 4.44; 95% CI, 1.33–14.82; P = 0.015). Furthermore, the risks of both fetuses SGA were higher in low FF than the risks of at least one fetus SGA in the corresponding FF groups (for <25th vs. 25th–75th percentiles: adjusted OR, 3.00; 95% CI, 1.22–7.39; P = 0.017; for <10th vs. 10th–90th percentiles: adjusted OR, 3.19; 95% CI, 1.22–8.33; P = 0.036; for <5th vs. 5th–95th percentiles: adjusted OR, 3.84; 95% CI, 1.14–12.91; P = 0.030; for <2.5th vs. 2.5th–97.5th percentiles: adjusted OR, 6.09; 95% CI, 1.69–21.95; P = 0.006). The associations between FF and the risk of SGA were presented in the regression curve (Figure 3). However, there was no significant association between low FF and GH, PE, and GDM.
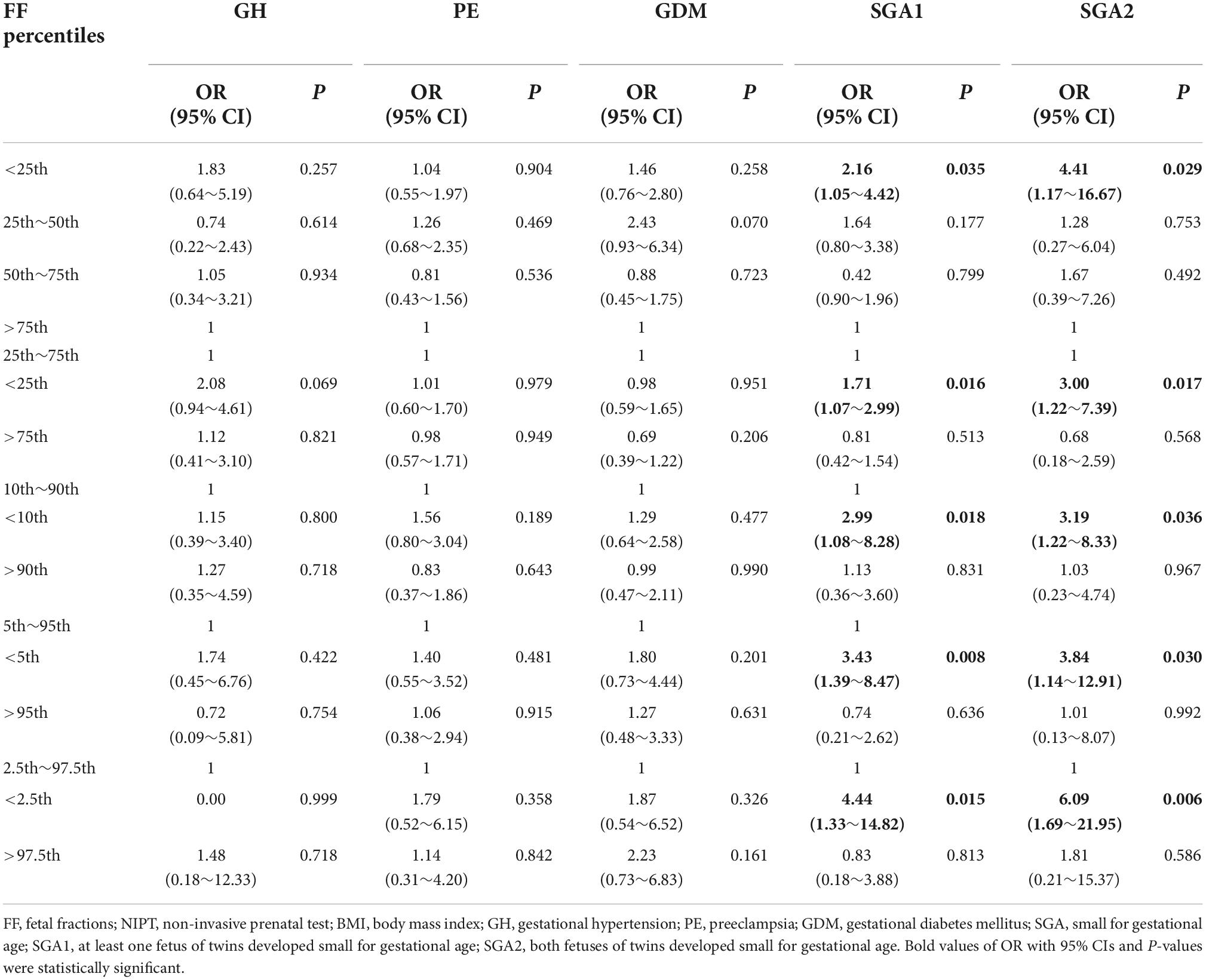
Table 3. Association between different FF percentiles on NIPT and pregnancy complications related to placental dysfunction in adjusted models.
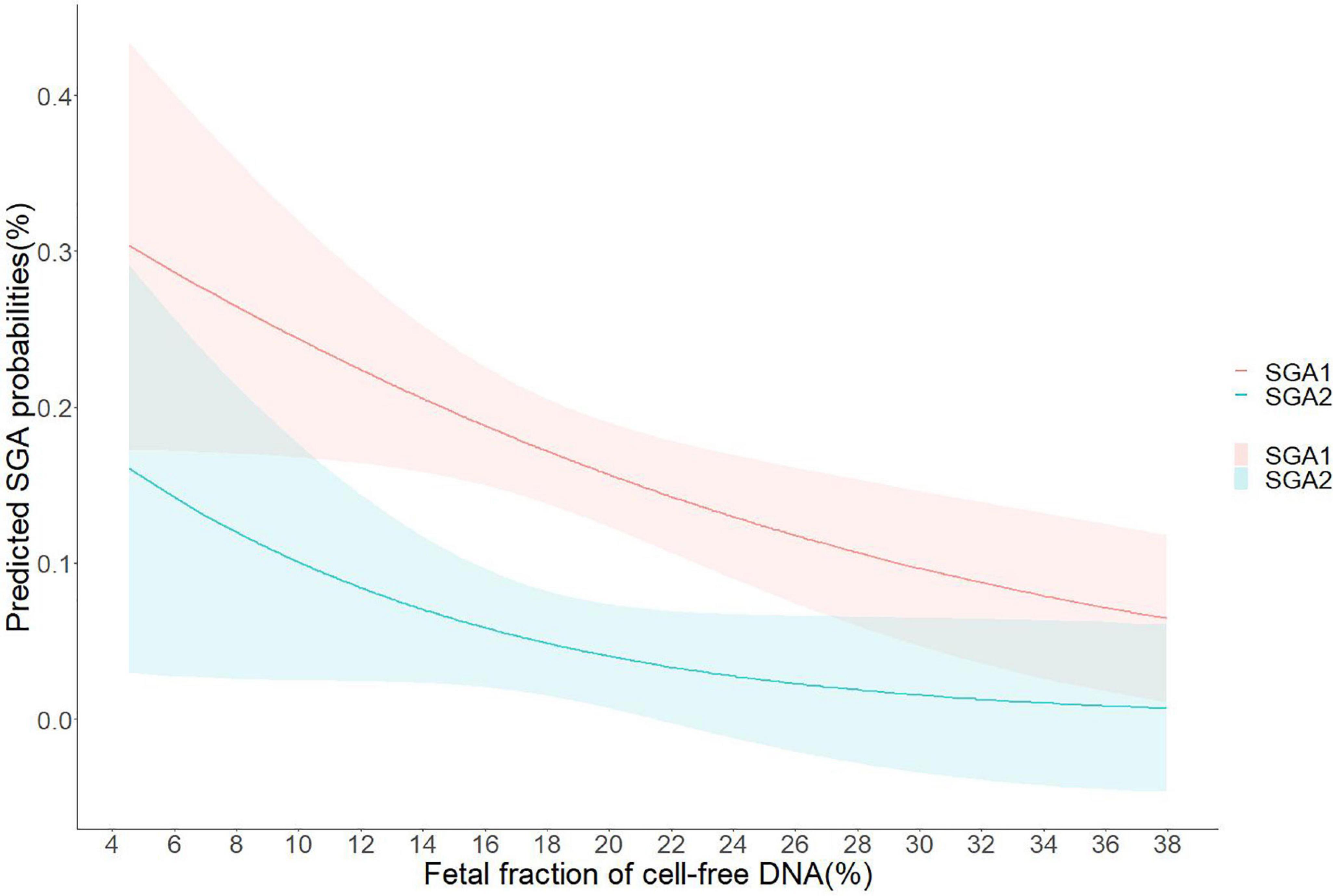
Figure 3. Predicted small for gestational age (SGA) probabilities with 95% CIs were calculated with respect to fetal fraction (FF) by performing logistic regression models; SGA1, at least one fetus of twins developed small for gestational age; SGA2, both fetuses of twins developed small for gestational age.
Discussion
This study demonstrates the association between low FF and pregnancy complications related to placental dysfunction of twin pregnancies. FF of twin pregnant maternal plasma cf-DNA in the first second-trimester may have the function as a marker of subsequent SGA. To the best of our knowledge, this is the first retrospective study that associated FF of twin pregnant maternal plasma cf-DNA at NIPT and subsequent risks of pregnancy complications in a Chinese population.
We demonstrated that low FF, defined individually as <25th, 10th, <5th, and <2.5th percentiles, at 12–26 weeks of gestation were strongly associated with the risk of SGA compared with the normal FF and high FF, especially the SGA of both fetuses. The result was consistent with previous publications using singleton women. Recently, several previous studies had investigated that low FF of singleton pregnancy was associated with pregnancy complications, such as PE, GDM, PTB, and SGA. Clapp et al. investigated that women with low FF of <5th (FF < 5.34%) percentile were associated with an increased risk of birth weights <5th and <10th percentiles in women with negative cf-DNA screening in the first trimester of a total of 7,478 singleton women (20). Similarly, Yuan et al. examined the significant relationship between FF < 5th percentile and low birth weight babies (<2,500 g) in 2,191 singleton women who underwent NIPT at 13–26 weeks of gestation (21). However, Stein et al. examined the relationship between second-trimester FF and SGA in 611 women who underwent NIPT and found no significant relationship (22). Our findings were similar to the findings that LFF was associated with SGA. Among twin pregnant women undergoing NIPT at our centers, the likelihood of at least one twin SGA with an LFF result (<25th percentile) is 1.71-fold higher, and this risk of SGA increased with decreasing FF (2.99-fold higher of FF < 10th percentile; 3.43-fold higher of FF < 5th percentile; and 4.44-fold higher of FF < 2.5th percentile). In addition, the risk of both fetuses SGA was higher than at least one twin SGA in LFF groups (FF < 25th percentile: 3.00-fold vs. 1.71-fold; FF < 10th percentile: 3.19-fold vs. 2.99-fold; FF < 5th percentile: 3.84-fold vs. 3.43-fold; and FF < 2.5th percentile: 6.09-fold vs. 4.44-fold). These findings may be related to the declined apoptosis of trophoblast cells induced by the smaller the fetal weight and the decreased placental volume.
Previous studies also examined the correlation between low FF and GH, PE, GDM, and PTB in a singleton pregnancy. Kristin et al. reported a strong association between low FF (<25th percentile) and hypertensive disease of pregnancy and preeclampsia with severe features (14). Chan et al. found that women who failed to obtain a result from NIPT were at increased risk of GDM compared with the general Australian obstetric population (23). Similarly, Yuan et al. provided evidence that low FF of <10th percentile was associated with increased risk of PE and early PTB (<34 weeks) in a singleton women retrospective cohort (21). Unlike these previous studies, we demonstrated that low FF was not significantly associated with GH, PE, and GDM, although the rates of GH and PE were higher in FF < 25th percentile. It might also be related to the sample size being too small to detect significant differences in these perinatal outcomes. Furthermore, our study did not include PTB as an adverse perinatal outcome in consideration of a wide range of infections, genetic, and maternal complex causes. More significantly, twin pregnancy also was an independent risk factor for PTB.
Fetal fraction was defined as the percentage of fetal origin DNA levels from maternal plasma total cf-DNA, which was generally in the range of 3–30% (9). The reliability of NIPT largely depended on the FF in the total cf-DNA of maternal plasma. If the FF was <4%, NIPT was largely unable to receive reliable results. Krishna et al. examined the association between low FF (<4%) and a composite of adverse pregnancy outcomes among a cohort of 370 women who underwent NIPT. However, no difference in the odds of low birthweight was observed between the FF < 4% and FF > 4% groups (14), which suggested that the incidence of NIPT failure due to FF < 4% was much less than that of the sufficient FF group. Our study considered the 25th percentile as the low FF cutoff value to increase the sample size and to ensure a similar sample size among quartile groups. We also set the 10th, 5th, and 2.5th percentile as the low FF cutoff values and adjusted for any available confounding factors in statistical analysis to determine the suitable FF threshold and predict pregnancy complications. Furthermore, FF was affected by multiple factors, including maternal BMI, gestational age, race, and twin pregnancy (24, 25). Our study also supported the association that decreased FF with increasing maternal BMI. In particular, we observed the significantly increased rates of obese women (BMI ≥ 28 kg/m2) in low FF, which suggested the increased maternal blood volume and active remodeling of adipose tissue, leading to an increased release of maternal cf-DNA into the peripheral circulation, a dilutional effect as the principal causes (26). However, we found no significant difference in the gestational age of NIPT among FF quartile groups, which may be related to the similar timing of gestational age at the obstetric examination.
Our study was novel in that we examined the relationship between low FF and pregnancy complications related to placental dysfunction among twin pregnant women who underwent NIPT in the first second-trimester. Most previous studies of the association between low FF and pregnancy complications were on singleton pregnancies. We expanded the scope of the applicable pregnancy population, including the cf-DNA test screening for pregnancy complications with placental compromise. Another strength of our study was that all twin pregnant women were admitted to and managed by our hospital, a tertiary referral care center for twin pregnancies in China, and the information regarding newborn and maternal outcomes could be recorded precisely. Moreover, the number of twin pregnancies in our center was relatively larger than in other obstetrics centers in the Beijing area, despite the fewer populations than in previous studies of singleton pregnancies.
Our study has several limitations that should be considered when interpreting our results. First, this was a retrospective study from a single institution, and FF of cf-DNA was analyzed in the same laboratory, which limited the generalizability of our findings. Multi-center prospective studies with larger twin pregnancy cohorts that would increase the generalizability are needed to further investigate FF in the first trimester using diverse laboratory technologies. In addition, longitudinal studies that analyzed the trajectory of FF over different gestational ages were also necessary to determine its predictive value for pregnancy outcomes associated with placental dysfunction. Second, although the accurate estimation of FF using length distribution of cf-DNA had been verified, we could not exclude the maternal cf-DNA that could affect FF measurements of female-female fetuses. A fairly large number of opposite-sex twins were excluded in our study, considering the difficulties in estimating and distinguishing the FF of opposite-sex twins. Finally, we use low-coverage sequencing as the routine test procedure of NIPT in this study, and zygosity information were not obtained when used low-coverage whole-genome sequencing. Analysis of single nucleotide polymorphisms (SNPs) in cf-DNA for the assessment of zygosity will be established in the follow-up study.
Conclusion
In conclusion, twin pregnant women with low FF of maternal plasma cf-DNA in the first second-trimester are more likely to subsequently develop SGA. FF of cf-DNA may serve as a biomarker for pregnancy complications related to placental dysfunction in twin pregnancies.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL and XG completed the design and writing of the manuscript. JL, BZ, YT, CP, and QH made contributions to the acquisition, analysis, and interpretation of data. YW critically revised the work. PY and TD were accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by grants from the National Key Research and Development Program [grant number: 2021YFC2700700 (YW)] and National Natural Science Foundation of China [grant number: 82171661 (YW)]. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
We thank all hospitals and health workers who contributed to the data collection and management, as well as the pregnant women who participated in our study.
Conflict of interest
Authors CP, QH, and TD were employed by CapitalBio Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. (1997) 350:485–7. doi: 10.1016/s0140-6736(97)02174-0
2. Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE, Neveux LM, Ehrich M, et al. DNA sequencing of maternal plasma to detect down syndrome: an international clinical validation study. Gene Med. (2011) 13:913–20. doi: 10.1097/GIM.0b013e3182368a0e
3. Kinnings SL, Geis JA, Almasri E, Wang H, Guan X, McCullough RM, et al. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. (2015) 35:816–22. doi: 10.1002/pd.4625
4. Galeva S, Gil MM, Konstantinidou L, Akolekar R, Nicolaides KH. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: factors affecting test failure. Ultrasound Obstet Gynecol. (2019) 53:804–9. doi: 10.1002/uog.20290
5. Lee TJ, Rolnik DL, Menezes MA, McLennan AC, da Silva Costa F. Cell-free fetal DNA testing in singleton IVF conceptions. Hum Reproduc. (2018) 33:572–8. doi: 10.1093/humrep/dey033
6. Grömminger S, Erkan S, Schöck U, Stangier K, Bonnet J, Schloo R, et al. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenat Diagn. (2015) 35:1155–7. doi: 10.1002/pd.4668
7. Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, Regan L, et al. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol. (2005) 192:23–30. doi: 10.1016/j.ajog.2004.09.029
8. Rolnik DL, da Silva Costa F, Lee TJ, Schmid M, McLennan AC. Association between fetal fraction on cell-free DNA testing and first-trimester markers for pre-eclampsia. Ultrasound Obstet Gynecol. (2018) 52:722–7. doi: 10.1002/uog.18993
9. Scott FP, Menezes M, Palma-Dias R, Nisbet D, Schluter P, da Silva Costa F, et al. Factors affecting cell-free DNA fetal fraction and the consequences for test accuracy. The journal of maternal-fetal & neonatal medicine. Int Soc Perinat Obstet. (2018) 31:1865–72. doi: 10.1080/14767058.2017.1330881
10. Hedriana H, Martin K, Saltzman D, Billings P, Demko Z, Benn P. Cell-free DNA fetal fraction in twin gestations in single-nucleotide polymorphism-based noninvasive prenatal screening. Prenat Diagn. (2020) 40:179–84. doi: 10.1002/pd.5609
11. Rava RP, Srinivasan A, Sehnert AJ, Bianchi DW. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem. (2014) 60:243–50. doi: 10.1373/clinchem.2013.207951
12. Zhong XY, Holzgreve W, Hahn S. The levels of circulatory cell free fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. (2002) 21:77–83. doi: 10.1081/prg-120002911
13. Zimmermann BG, Holzgreve W, Avent N, Hahn S. Optimized real-time quantitative PCR measurement of male fetal DNA in maternal plasma. Ann N Y Acad Sci. (2006) 1075:347–9. doi: 10.1196/annals.1368.047
14. Krishna I, Badell M, Loucks TL, Lindsay M, Samuel A. Adverse perinatal outcomes are more frequent in pregnancies with a low fetal fraction result on noninvasive prenatal testing. Prenat Diagn. (2016) 36:210–5. doi: 10.1002/pd.4779
15. Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. (2001) 158:1713–21. doi: 10.1016/s0002-9440(10)64127-2
16. Hu P, Liang D, Chen Y, Lin Y, Qiao F, Li H, et al. An enrichment method to increase cell-free fetal DNA fraction and significantly reduce false negatives and test failures for non-invasive prenatal screening: a feasibility study. J Trans Med. (2019) 17:124. doi: 10.1186/s12967-019-1871-x
17. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
18. Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology [CSOG], Chinese Medical Association [CMA]. Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China (2020). Chin J Obstet Gynecol. (2020) 55:227–38. doi: 10.3760/cma.j.cn112141-20200114-00039
19. Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. (2011) 377:1855–61. doi: 10.1016/S0140-6736(11)60364-4
20. Clapp MA, Berry M, Shook LL, Roberts PS, Goldfarb IT, Bernstein SN. Low fetal fraction and birth weight in women with negative first-trimester cell-free DNA screening. Am J Perinatol. (2020) 37:86–91. doi: 10.1055/s-0039-1700860
21. Yuan X, Zhou L, Zhang B, Wang H, Yu B, Xu J. Association between low fetal fraction of cell free DNA at the early second-trimester and adverse pregnancy outcomes. Pregnancy Hypertens. (2020) 22:101–8. doi: 10.1016/j.preghy.2020.07.015
22. Stein W, Müller S, Gutensohn K, Emons G, Legler T. Cell-free fetal DNA and adverse outcome in low risk pregnancies. Eur J Obstet Gynecol Reprod Biol. (2013) 166:10–3. doi: 10.1016/j.ejogrb.2012.09.006
23. Chan N, Smet ME, Sandow R, da Silva Costa F, McLennan A. Implications of failure to achieve a result from prenatal maternal serum cell-free DNA testing: a historical cohort study. BJOG. (2018) 125:848–55. doi: 10.1111/1471-0528.15006
24. Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. (2013) 41:26–32. doi: 10.1002/uog.12331
25. Jorgez CJ, Dang DD, Simpson JL, Lewis DE, Bischoff FZ. Quantity versus quality: optimal methods for cell-free DNA isolation from plasma of pregnant women. Genet Med. (2006) 8:615–9. doi: 10.1097/01.gim.0000241904.32039.6f
Keywords: cell-free DNA, fetal fraction, twin pregnancy, pregnancy complications, early screening
Citation: Li J, Gu X, Wei Y, Tao Y, Zhai B, Peng C, Huang Q, Deng T and Yuan P (2022) Correlation of low fetal fraction of cell-free DNA at the early second-trimester and pregnancy complications related to placental dysfunction in twin pregnancy. Front. Med. 9:1011366. doi: 10.3389/fmed.2022.1011366
Received: 04 August 2022; Accepted: 15 November 2022;
Published: 01 December 2022.
Edited by:
Raigam Jafet Martinez-Portilla, Instituto Nacional de Perinatología (INPER), MexicoReviewed by:
Ana Cecilia Jara Ettinger, Instituto Nacional de Perinatología (INPER), MexicoDavid Alejandro Martinez-Ceccopieri, Civil Hospital of Guadalajara, Mexico
Copyright © 2022 Li, Gu, Wei, Tao, Zhai, Peng, Huang, Deng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengbo Yuan, eXVhbnBlbmdibzE4NTRAZm94bWFpbC5jb20=; Tao Deng, dGRlbmdAY2FwaXRhbGJpb3RlY2guY29t
†These authors have contributed equally to this work and share first authorship
 Jiaxin Li
Jiaxin Li Xunke Gu1†
Xunke Gu1† Yuan Wei
Yuan Wei Quanfei Huang
Quanfei Huang Tao Deng
Tao Deng Pengbo Yuan
Pengbo Yuan