- 1Department of Internal Medicine, University of Maryland Medical Center, Baltimore, MD, United States
- 2Institute for Genome Sciences, University of Maryland School of Medicine, Baltimore, MD, United States
- 3Department of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, United States
- 4Department of Gastroenterology and Hepatology, Veterans Affairs Maryland Health Care System, Baltimore, MD, United States
Despite the impact of the Coronavirus Disease 2019 (COVID-19) pandemic, vaccine hesitancy remains common in the general public and patients with Inflammatory Bowel Diseases (IBD). We sought to examine the reasons for vaccine hesitancy in patients with IBD. In this case-control study, we performed a retrospective chart review of 1,349 IBD patients and 215 non-IBD patients seen at University of Maryland Medical Center, a tertiary referral medical center, between March 2020 and October 2021. Data obtained included demographics, vaccination records, disease history, number of IBD-related surgeries, and IBD medications. 813/1,349 (60.3%) IBD patients received at least one dose of either the Pfizer/BioNTech, Moderna, or Johnson & Johnson vaccines. In a multivariate logistic regression, COVID vaccination was found to be positively associated with older age (p-value = 1.65e-5), female sex (p = 0.00194), Asian and White races (p = 0.02330, 0.00169), number of clinic visits (p = 1.11e-08), and biologic use (p = 7.82e-5). There was no association between vaccination and other types of vaccination nor with the use of other IBD medications. There was a negative association between vaccination status and the total number of IBD related surgeries (p = 0.02857). In non-IBD patients, only the number of clinic visits was positively associated with COVID-19 vaccination. Although the majority of IBD patients are immunosuppressed, COVID-19 vaccination rate was only 60.3%. Younger adults, males, African Americans, and those requiring IBD-related surgeries were less likely to receive COVID-19 vaccine. Healthcare providers need to recognize these potential risk factors for COVID-19 vaccine hesitancy.
Introduction
When Coronavirus disease 2019 (COVID-19) vaccines became available, adults who were on immunosuppressive medications were among the earlier groups recommended by the Centers for Disease Control and Prevention to receive the vaccines. Among these groups were patients diagnosed with Inflammatory Bowel Diseases (IBD), which includes Crohn's Disease and Ulcerative Colitis. These are diseases of innate and adaptive immune system dysregulation leading to chronic intestinal inflammation. An estimated 3.1 million adults (1) in the United States live with IBD and many patients require immunosuppressive medications such as corticosteroids, immunomodulators, anti-Tumor Necrosis Factors, and other biologic agents. These medications have been associated with increased susceptibility to infections (2–5). Consequently, the fear of COVID-19 infection in patients with IBD is more pronounced, especially in those taking immunosuppressants (6). Despite the global impact of the COVID-19 pandemic, vaccine hesitancy remains in both the general public (7, 8) and the IBD population (9).
Vaccine hesitancy in IBD patients is not a novel concern to gastroenterologists. Prior studies have shown that IBD patients are less likely to receive vaccinations made necessary from their immunocompromised state (10). Some concerns from the past have transferred to COVID-19 vaccines. Studies in the United States (U.S.) and Europe reported COVID-19 vaccination intent among IBD patients as rates ranging from 54 to 96.4% (9, 11–14). Many IBD patients voiced concerns that the nature of their disease may trigger worse adverse side-effects from the COVID-19 vaccine and/or the vaccine will cause an IBD flare. Others were concerned about the overall efficacy and validity of the vaccines (15, 16). However, consensus among physicians is in support (17) of vaccination of all IBD patients as data shows COVID-19 vaccines available in the U.S. are safe (18–21) and effective (22–25) for these patients.
Recent studies have shown that the rate of COVID-19 infection in IBD patients is similar to the rate of infection in the general population (26–28). They have also shown that risk factors for adverse COVID-19 outcomes in IBD patients are also similar to the risk factors of the general population which include age and comorbidities (29–33). There have been varying reports of the effects of IBD therapies on COVID-19 infection outcomes. Some studies have found that IBD medications are not associated with more adverse COVID-19 infections (34). However, several other studies have shown 5-ASA/Sulfasalazine (31, 35) systemic corticosteroids (31, 32, 35, 36) and thiopurines (35) may be associated with worse clinical outcomes. Interestingly, anti-TNF drugs and biological therapies have not been shown to be associated with worse clinical outcomes (32, 34, 36).
Therefore, given the vulnerable nature of IBD patients in the setting of the ongoing COVID-19 pandemic, it is important to address any vaccine hesitancy seen in patients with inflammatory bowel disease and use this information to then increase rates of vaccination. We sought to investigate the barriers to vaccination by examining rate of vaccine hesitancy in patients with IBD as well as associated demographic and socioeconomic risk factors.
Materials and methods
This study was a single-center, retrospective analysis of 1,349 patients with IBD who were seen at the University of Maryland Medical Center between March 2020 and October 2021. 215 non-IBD patients, also seen in the same clinic, were used as the control group. The period between January 2020 and October 2021 was selected to account for the disruption of the pandemic on patients' clinic appointments. This period maximizes patient capture and ensures inclusion of those who presented to clinics infrequently; patients on therapies like mesalamine are seen yearly. Furthermore, as this was a retrospective chart review on an electronic medical record system, we were able to obtain current vaccine records even in patients only seen prior to when vaccines were first made available in January 2021. The diverse control group encompasses every patient seen at the clinic without IBD. They range from patients presenting for common and general gastroenterological needs to those with cancer and/or end stage diseases. Their medication use also vary, and can range from no medications to immunosuppressants/chemotherapy.
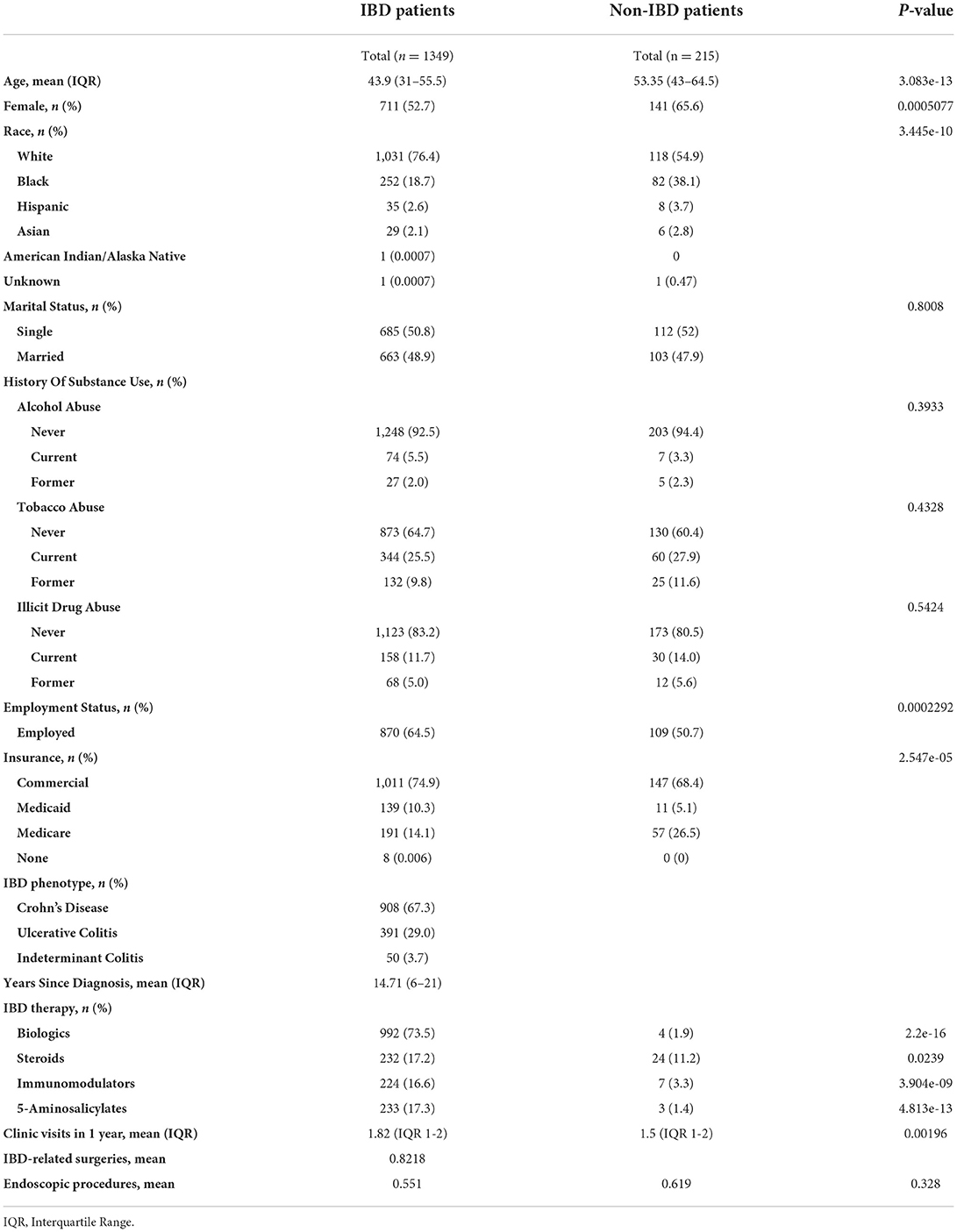
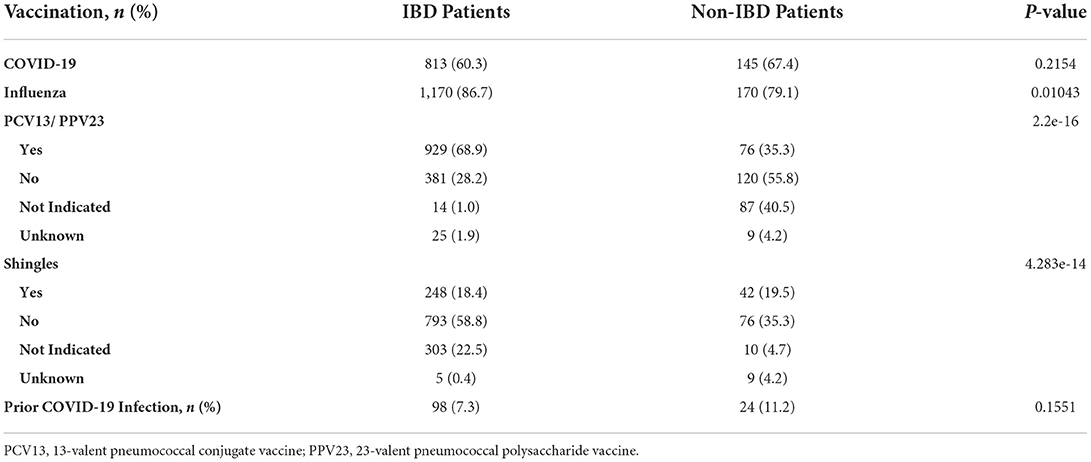
Data was obtained by performing a chart review of the electronic health record, Epic®. The data that was collected included demographics (age, sex, race, marital status, employment, insurance type), substance use (tobacco, alcohol, illicit drugs), IBD diagnosis, year of diagnosis, number of years since diagnosis, number of IBD-related surgeries, and IBD therapy received between October 2020 and 2021 including biologics, steroids, mesalamine, thiopurines or methotrexate. The number of IBD clinic visits and IBD-related gastroenterology procedures between October 2020 and 2021 were also reported (Table 1). Information was obtained on prior COVID-19 infection and recommended vaccines including the influenza vaccine, 23-valent pneumococcal polysaccharide vaccine, 13-valent pneumococcal conjugate vaccine, recombinant zoster vaccine, and COVID-19 vaccines. Patients were recorded as having received a COVID-19 vaccine if they received at least one dose (the first dose of the two-dose series for the Pfizer/BioNTech or Moderna COVID-19 vaccines, or the single dose Johnson & Johnson COVID-19 vaccine) (Table 2).
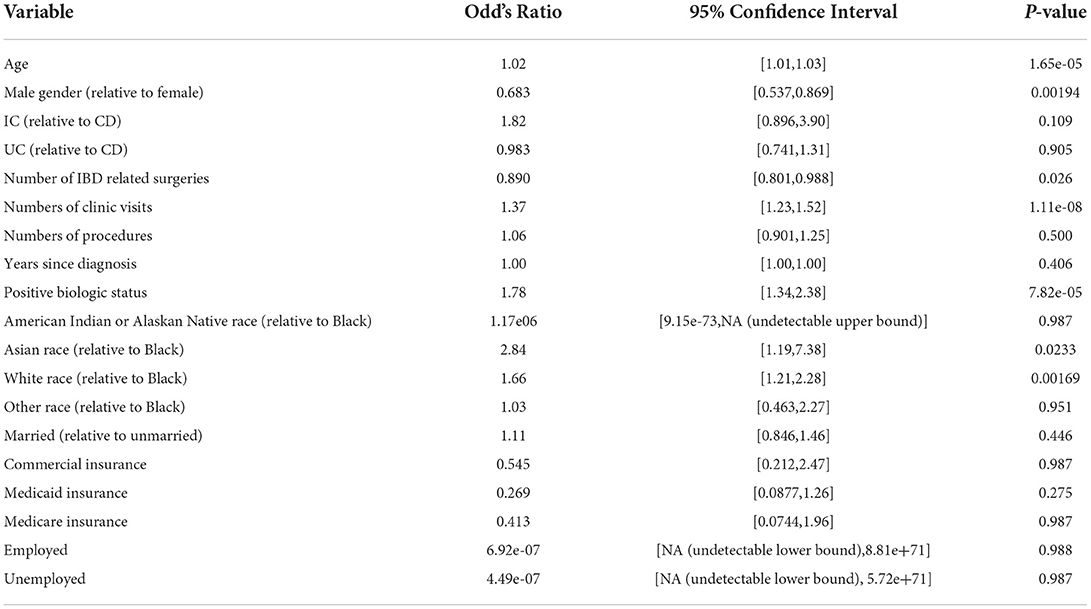
Multiple regression analysis was used to assess the relationships between several clinical and demographic factors, and likelihood of receiving a COVID vaccine, and both models and odd's ratios were calculated using a GLM (generalized linear model). All variables compared were considered as factors except for age, number of clinic visits and procedures, and years since diagnosis. Two group differences for various factors were compared using Fisher's exact test. All tests were performed using the R “stats” package, version 4.0.4.
Results
60.3% (813/1349) of IBD patients received at least one dose of either the Pfizer/BioNTech (BNT162b2), Moderna (mRNA-1273), or Johnson & Johnson (JNJ-78436735) vaccines (Table 2). In a multivariate regression, COVID vaccination was found to be positively associated with a number of factors including older age (OR 1.022, p-value = 1.65e-5), female sex (OR 1.46, p = 0.00194), Asian and White races (OR 2.84, 1.66, p = 0.02330, 0.00169), number of clinic visits in the past 12 months (OR 1.37, p = 1.11e-08), and biologic use (OR 1.78, p = 7.82e-5; Table 3). This was true while controlling for IBD type; marital status; insurance (Commercial vs. Medicaid vs. Medicare); employment status; years since diagnosis; and tobacco, alcohol, and substance use history. Years since diagnosis and age were not found to have a significant interaction suggesting older age independently predicts likelihood of vaccination. There was a negative association between vaccination status and the total number of IBD related surgeries a patient had undergone (OR 0.890, p = 0.02857). There was no association between COVID vaccination and the number of endoscopic procedures in the past 12 months, employment status, other types of vaccination (influenza vaccine, 23-valent pneumococcal polysaccharide vaccine, 13-valent pneumococcal conjugate vaccine, recombinant zoster vaccine), or with the use of other IBD medications. 992 patients with IBD received a biologic agent, but only 232, 224, and 233 received steroids, thiopurines or methotrexate, or 5-ASA agents, respectively, suggesting the difference in use may be responsible for the lack of significant relationship between vaccination status and non-biologic treatments. In contrast, age, race, sex, marital status, use of biologic, insurance type, and employment status had no relationship with likelihood of vaccination in those patients without IBD. Only the number of clinic visits a patient had was positively associated with likelihood of receiving a COVID vaccine (OR 1.54, p = 0.00383).
Discussion
Our study examined COVID-19 vaccination rates in a diverse, adult IBD and non-IBD population from a single institution in the state of Maryland. 60.3% (813/1349) of our IBD population received the vaccine, which is lower than the 88.4% of the general, adult U.S population and 95% of the Maryland population (as of April 04, 2022) (37). In our study, 67.4% (145/215) of non-IBD patients were vaccinated.
In prior studies of IBD patients, factors such as female gender, younger age, minority race, lack of prior vaccinations, shorter duration of IBD diagnosis, and current steroid therapy, appear to be negative determinants of COVID-19 vaccination in IBD patients (15, 38, 39). Whereas older age (13, 40), male gender (9, 13), White race (13), prior COVID-19 infection (13), prior routine vaccinations (12, 40, 41), current biologics (9, 13), and immunomodulators use (38), and higher education levels (13, 40) were associated with greater incidences of COVID-19 vaccination.
In prior studies examining the relationship between race in IBD patients and vaccination rates, the patient populations investigated were predominantly White. We included a more diverse patient population. Our study also showed that White race was associated with increased vaccination for COVID-19 in IBD patients. But we also demonstrated that Asian race was associated with increased vaccine acceptance, which had not been previously reported. Interestingly, African American race was not a negative determinant of COVID-19 vaccination, as previously demonstrated. However, African American IBD patients were less likely to be vaccinated relative to White and Asian patients. When compared to African American non-IBD patients, African American IBD patients were equally as likely to receive the COVID-19 vaccine. Concentrated efforts must continue to address the many health disparities which have become more accentuated during the pandemic.
Interestingly, in contrast to the other study, our study showed that female IBD patients were more likely to be vaccinated for COVID-19. Our study had 711 (52.7%) female IBD patients and 141 (65.6%) female non-IBD patients. Women in both groups were more likely to be vaccinated against COVID-19 than men. It is possible that non-IBD women were found to be more likely than non-IBD men to be vaccinated for COVID as women comprised a significantly larger portion of the non-IBD population in our study (65.6%).
We found that IBD-related surgeries were negatively associated with COVID vaccination suggesting that patients with history of severe IBD disease may be more hesitant about getting vaccinated. One possible explanation is that the patients fear the vaccine may exacerbate their disease and therefore lead to more traumatic surgeries. Another potential explanation is that patients with more severe disease and more IBD related surgeries may be more likely to be non-compliant with medication (42, 43). Those less compliant with medications are also likely to be less compliant with recommended vaccinations, such as the COVID-19 vaccine.
Interestingly, we did not find an association between prior vaccinations and COVID vaccination in IBD patients as other studies have shown. It is unlikely that IBD patients have an aversion to vaccinations in general as they are significantly more likely than non-IBD patients to receive other types of vaccinations (influenza, pneumococcal, shingles). And, oppositely, non-IBD patients are more likely to be vaccinated for COVID-19 than the routinely recommended vaccines. The IBD patients' reluctance may be due to the relative novelty of the COVID-19 vaccine versus the other vaccinations that have been available for a significantly longer period. Therefore, assumptions should not be made regarding patients' willingness to be vaccinated for COVID based on their vaccination history.
Our study also showed that biologic use was positively associated with COVID-19 vaccine. However, other immunosuppressive medications did not show any relationship as other studies have. This may be because a higher proportion of patients were on biologics (992, 73.5%) at this institution given it is a large tertiary care referring center, and only 232 (17.2%), 224 (16.6%), and 233 (17.3%) received either steroids, thiopurines or methotrexate, or 5-ASA agents, respectively.
We did not find an association between steroid use and COVID-19 vaccination. This may represent a dichotomy of perception of the patients: some may perceive that a COVID-19 vaccination might exacerbate their acute flare, and some may perceive that because they are in an acute flare, they wish to prevent a worse COVID-19 infection outcome.
In congruence with prior studies, older age appears to be associated with more likelihood of being vaccinated for COVID-19 in IBD patients. Age was not a predictor in non-IBD patients. This is likely due to the IBD patients' perception of higher risk for severe outcomes of COVID-19 infection given the evidence that the disease has a higher likelihood of negatively impacting the older population. Another reason why older IBD patients have higher rates of vaccination may be because they have had longer exposure and interaction to the healthcare system and this has required them to have regular contact with their health care providers.
This study also highlights the importance of regular visits with IBD patients, especially those who are immunosuppressed. We demonstrated that both IBD and non-IBD patients with more clinic visits over the 12-month period were more likely to be vaccinated against COVID-19. Clinic visits present opportunities for patients to ask questions regarding the vaccine and how COVID-19 infection can impact their disease. It is possible that patients who have higher numbers of clinic visits represent patients that are in an acute phase of their disease. Therefore, these patients may perceive a heightened risk and vulnerability to COVID-19, leading them to receive vaccinations for COVID-19. In addition, number of clinic visits was the only factor that was positively associated with vaccination in non-IBD patients, which further demonstrates its importance across both populations. Therefore, communication between physician and patient is one of the best contributing factors to getting vaccinated. Unfortunately, it has become easy for patients to be lost to follow-up during the pandemic. As providers, we must continue to educate our patients during clinic or telemedicine visits on the importance of obtaining a COVID-19 vaccine. Often, health maintenance conversations surrounding vaccines can be pushed to the end of the appointment or never spoken about given time restraints. We must continue to make this a priority during appointments given the ongoing global pandemic.
One limitation of this study is relying on the accuracy of the electronic medical record (EMR) for variables including vaccination status; marital status; employment status; and history of tobacco, alcohol, and illicit substance use. Data recorded are based solely on information disclosed by the patient.
In conclusion, greater vaccination efforts should be made for IBD patients, specifically targeting patients that are male, younger in age, African American, and have history of multiple IBD-related surgeries. In addition, efforts should be made to continue regular visits with patients when indicated to improve communication, educational opportunities, and thus increase COVID-19 vaccination uptake.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
As this was a retrospective chart review study, individual informed consent was waived and oversight of the protocol was governed by the University of Maryland, Baltimore Human Research Protections Office. All patient data collected were de-identified of any protected health information.
Author contributions
HK and KP are co-first authors and both planned the study, collected the data, and wrote the manuscript. KP submitted the study. MA performed data analysis and also wrote the manuscript. MBe, EZ, MBo, PP, and LS collected the data. UW was the Principal Investigator who planned and organized the study. All authors contributed to the article and approved the submitted version.
Funding
MA was supported by T32 DK067872 from the NIDDK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohn's Colitis. (2014) 8:443–68. doi: 10.1016/j.crohns.2013.12.013
2. Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years - United States, 2015. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/mmwr/volumes/65/wr/mm6542a3.htm. Published August 17, 2017 (accessed April 2, 2022).
3. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. (2018) 155:337–46. doi: 10.1053/j.gastro.2018.04.012
4. Long MD, Farraye FA, Okafor PN, Martin C, Sandler RS, Kappelman MD. Increased risk of Pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis. (2013) 19:1018–24. doi: 10.1097/MIB.0b013e3182802a9b
5. Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. (2018) 25:369–76. doi: 10.1093/ibd/izy243
6. Grunert PC, Reuken PA, Stallhofer J, Teich N, Stallmach A. Inflammatory bowel disease in the COVID-19 pandemic: The patients' perspective. J Crohn's Colitis. (2020) 14:1702–8. doi: 10.1093/ecco-jcc/jjaa126
7. Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. (2021) 194:245–51. doi: 10.1016/j.puhe.2021.02.025
8. Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. (2021) 39:2024–34. doi: 10.1016/j.vaccine.2021.02.005
9. Walldorf J, von Arnim U, Schmelz R, Riesner-Wehner A, Michl P, Grunert PC, et al. SARS-CoV-2 vaccination in patients with inflammatory bowel disease—fear and desire. Inflamm Bowel Dis. (2021) 27:1858–61. doi: 10.1093/ibd/izab150
10. Chan W, Salazar E, Lim TG, Ong WC, Shim HH. Vaccinations and inflammatory bowel disease – a systematic review. Digest Liver Dis. (2021) 53:1079–88. doi: 10.1016/j.dld.2021.04.015
11. Caron B, Neuville E, Peyrin-Biroulet L. Inflammatory bowel disease and covid-19 vaccination: A patients' survey. Digest Dis Sci. (2021) 1–7. doi: 10.1007/s10620-021-07040-z
12. Crispino F, Brinch D, Carrozza L, Cappello M. Acceptance of SARS-CoV-2 vaccination among a cohort of IBD patients from Southern Italy: a cross-sectional survey. Inflamm Bowel Dis. (2021) 27:134–5. doi: 10.1093/ibd/izab133
13. Dalal RS, McClure E, Marcus J, Winter RW, Hamilton MJ, Allegretti JR. Covid-19 vaccination intent and perceptions among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2021) 19:1730–2. doi: 10.1016/j.cgh.2021.02.004
14. Giannini EG, Demarzo MG, Bodini G. Elevated adherence to vaccination against SARS-CoV-2 among patients with inflammatory bowel disease. J Crohn's Colitis. (2021) 15:2142–3. doi: 10.1093/ecco-jcc/jjab104
15. Wu X, Lin J, Buch H, Ding Q, Zhang F, Cui B, et al. The COVID-19 vaccination hesitancy among the people with inflammatory bowel disease in China: A questionnaire study. Front Public Health. (2021) 9. doi: 10.3389/fpubh.2021.731578
16. Ellul P, Revés J, Abreu B, Gisbert JP, Mariangela A, Fiorino G, et al. Implementation and short-term adverse events of anti-SARS-CoV-2 vaccines in inflammatory bowel disease patients: An international web-based survey. J Crohn's Colitis. (2022). doi: 10.1093/ecco-jcc/jjac010
17. Siegel CA, Melmed GY, McGovern DPB, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut. (2021) 70:635–40. doi: 10.1136/gutjnl-2020-324000
18. Botwin GJ Li DFigueiredo J, et al. Adverse events after SARS-CoV-2 mrna vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. (2021) 116:1746–51. doi: 10.14309/ajg.0000000000001342
19. Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: Results from prevent-covid. Inflamm Bowel Dis. (2021) 20:1497–1505. doi: 10.1093/ibd/izab302
20. Lev-Tzion R, Focht G, Lujan R, Mendelovici A, Friss C, Greenfeld S, et al. Covid-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. (2021) 20:e1263–82.doi: 10.1016/j.cgh.2021.12.026
21. Hadi YB, Thakkar S, Shah-Khan SM, Hutson W, Sarwari A, Singh S. Covid-19 vaccination is safe and effective in patients with inflammatory bowel disease: Analysis of a large multi-institutional research network in the United States. Gastroenterology. (2021) 161:1336–9. doi: 10.1053/j.gastro.2021.06.014
22. Caldera F, Knutson KL, Saha S, Wald A, Phan HS, Chun K, et al. Humoral immunogenicity of mrna COVID-19 vaccines among patients with inflammatory bowel disease and healthy controls. Am J Gastroenterol. (2021) 117:176–9. doi: 10.14309/ajg.0000000000001570
23. Shehab M, Abu-Farha M, Alrashed F, Alfadhli A, Alotaibi K, Alsahli A, et al. Immunogenicity of BNT162B2 vaccine in patients with inflammatory bowel disease on infliximab combination therapy: a multicenter prospective study. J Clin Med. (2021) 10:5362. doi: 10.3390/jcm10225362
24. Kappelman MD, Weaver KN, Boccieri M, Firestine A, Zhang X, Long MD, et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology. (2021) 161:1340–3. doi: 10.1053/j.gastro.2021.06.016
25. Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. (2021) 161:827–36. doi: 10.1053/j.gastro.2021.05.044
26. Taxonera C, Alba C, Olivares D. What is the incidence of COVID-19 in patients with IBD in western countries? Gastroenterology. (2021) 160:1901–2. doi: 10.1053/j.gastro.2020.05.099
27. Allocca M, Chaparro M, Gonzalez HA, et al. Patients with inflammatory bowel disease are not at increased risk of COVID-19: A large multinational cohort study. J Clin Med. (2020) 9:3533. doi: 10.3390/jcm9113533
28. Singh AK, Jena A. Kumar-M P, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. United Eur Gastroenterol J. (2021) 9:159–76. doi: 10.1177/2050640620972602
29. Derikx LAAP, Lantinga MA, de Jong DJ, van Dop WA, Creemers RH, Römkens TEH, et al. Clinical outcomes of covid-19 in patients with inflammatory bowel disease: A nationwide cohort study. J Crohn's Colitis. (2020) 15:529–39. doi: 10.1093/ecco-jcc/jjaa215
30. Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS, et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. (2020) 159:1541–4. doi: 10.1053/j.gastro.2020.05.066
31. Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology. (2020) 159:481–91. doi: 10.1053/j.gastro.2020.05.032
32. Wetwittayakhlang P, Albader F, Golovics PA, Hahn GD, Bessissow T, Bitton A, et al. Clinical outcomes of COVID-19 and impact on disease course in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. (2021) 2021:1–9. doi: 10.1155/2021/7591141
33. Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An Ig-IBD Study. Gut. (2020) 69:1213–7. doi: 10.1136/gutjnl-2020-321411
34. Bezzio C, Armuzzi A, Furfaro F, Ardizzone S, Milla M, Carparelli S, et al. Therapies for inflammatory bowel disease do not pose additional risks for adverse outcomes of SARS-CoV-2 infection: An ig-ibd study. Aliment Pharmacol Ther. (2021) 54:1432–41. doi: 10.1111/apt.16663
35. Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut. (2020) 70:725–32. doi: 10.1136/gutjnl-2020-322539
36. Khan N, Mahmud N, Trivedi C, Reinisch W, Lewis JD. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. (2021) 70:1657–64. doi: 10.1136/gutjnl-2021-324356
37. CDC, Covid Data tracker,. Centers for Disease Control and Prevention. Available online at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-onedose-pop-5yr (accessed April 4, 2022).
38. Nishida Y, Hosomi S, Kobayashi Y, Nakata R, Ominami M, Nadatani Y, et al. Acceptance of covid-19 vaccines among patients with inflammatory bowel disease in Japan. Healthcare. (2021) 10:6. doi: 10.3390/healthcare10010006
39. Schell TL, Richard LJ, Tippins K, Russ RK, Hayney MS, Caldera F. High but inequitable COVID-19 vaccine uptake among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2021) 20:1606–8. doi: 10.1016/j.cgh.2021.12.013
40. Viola A, Muscianisi M, Voti RL, Costantino G, Alibrandi A, Fries W. Predictors of covid-19 vaccination acceptance in IBD patients: a prospective study. Eur J Gastroenterol Hepatol. (2021) 33:1042–5. doi: 10.1097/MEG.0000000000002320
41. Costantino A, Noviello D, Conforti FS, Aloi M, Armuzzi A, Bossa F. Covid-19 vaccination willingness and hesitancy in patients with inflammatory bowel diseases: Analysis of determinants in a national survey of the Italian IBD patients' association. Inflamm Bowel Dis. (2021) 28:474–8. doi: 10.1093/ibd/izab172
42. Kane S, Chao J, Mulani P. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn's disease patients. Adv Ther. (2009) 26:936–46. doi: 10.1007/s12325-009-0069-7
Keywords: vaccine hesitancy, immunosuppression, COVID-19, inflammatory bowel disease, vaccines
Citation: Kwon HJ, Panagos K, Alizadeh M, Bell M, Bourmaf M, Zisman E, Paul P, Sibel L and Wong U (2022) Patients with inflammatory bowel disease are more hesitant about Coronavirus disease 2019 vaccination. Front. Med. 9:1005121. doi: 10.3389/fmed.2022.1005121
Received: 27 July 2022; Accepted: 12 October 2022;
Published: 15 November 2022.
Edited by:
Fuqiang Cui, Peking University, ChinaReviewed by:
Elizabeth J. Ryan, University of Limerick, IrelandJonathan Braun, Cedars-Sinai Medical Center, United States
Copyright © 2022 Kwon, Panagos, Alizadeh, Bell, Bourmaf, Zisman, Paul, Sibel and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine Panagos, a2F0aGVyaW5lLnBhbmFnb3NAc29tLnVtYXJ5bGFuZC5lZHU=
†These authors share first authorship
 Hyuk Joon Kwon
Hyuk Joon Kwon Katherine Panagos
Katherine Panagos Madeline Alizadeh
Madeline Alizadeh Mack Bell1
Mack Bell1