- 1Center of Gallbladder Disease, East Hospital of Tongji University, Shanghai, China
- 2Institute of Gallstone Disease, Tongji University School of Medicine, Shanghai, China
- 3Department of Radiology, Nanxiang Hospital of Jiading District, Shanghai, China
- 4Department of Hepatic Surgery and Liver Transplantation Center, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 5Organ Transplantation Institute, Sun Yat-sen University, Guangdong, China
Inflammatory pseudotumor (IPT)-like follicular dendritic cell sarcoma (FDCS) is a rare neoplasm referred to as the FDCS variant. Here we report a 66-year-old female patient suffering from hepatic IPT-like FDCS and summarize IPT-like FDCS reported in the literature. The patient presented with obvious abdominal pain without significant laboratory abnormalities and subsequently underwent surgical resection of a hepatic lesion. Postoperative pathological results demonstrated a vascular tissue-rich neoplasm (7.0-cm maximum diameter). The tumor cells expressed CD21 and CD35, and in situ hybridization detected Epstein–Barr virus-encoded RNA (EBER). Metastasis or recurrence was not detected during the 7-year follow-up.
Introduction
Follicular dendritic cells (FDCs) develop from perivascular precursors of stromal cell origin that are essential for the organization and maintenance of lymphoid architecture, induction of the germinal center reaction, production of B memory cells, and protection from autoimmune disorders (1). FDC sarcoma (FDCS) is an extremely rare neoplasm with nearly more than half of the cases occurring in lymph nodes (2). Extranodal FDCS, which mainly arises from intraabdominal organs such as liver, spleen, colon, and pancreas, may display systemic clinical symptoms (3). Inflammatory pseudotumor (IPT)-like FDCS is a recently described unique subtype of FDCS with different histological appearances and behavior compared with those of classical FDCS. The most recent World Health Organization (WHO) classification notes that IPT-like FDCS appears to be indolent; however, the data on clinical outcomes are limited (4).
The cause of IPT-like FDCS is unknown, and the diagnostic criteria are not definitive. However, Epstein–Barr virus (EBV) infection is considered one of the most important etiologies of this sarcoma (4). Distinguishing IPT-like FDCS from other tumors is very challenging, and such tumors are commonly misdiagnosed as inflammatory lesions or other malignant neoplasms. Definite diagnosis of IPT-like FDCS should rely on radiology, cellular morphology, histopathology, and immunochemistry. IPT-like FDCS exhibits indolent features, prognosis is favorable, and surgical excision is the best treatment.
Herein, we report the case of a 66-year-old female with an intraabdominal IPT-like FDCS in the hepatic right lobe. By analyzing the distinctive clinicopathologic features of this rare case combined with reviewing the related literature, here we summarize the current clinical features and diagnosis of IPT-like FDCS and discuss the treatment and prognosis of this tumor subtype.
Literature review
We systematically searched the PubMed, EMBASE, and MEDLINE databases using the search terms “inflammatory pseudotumor-like” combined with “follicular dendritic cell sarcoma,” or “follicular dendritic cell tumor,” or “fibroblastic dendritic cell sarcoma,” or “fibroblastic dendritic cell tumor” in research published from 2000 to 2022. We collated demographic, clinicopathological, and follow-up information (Table 1).
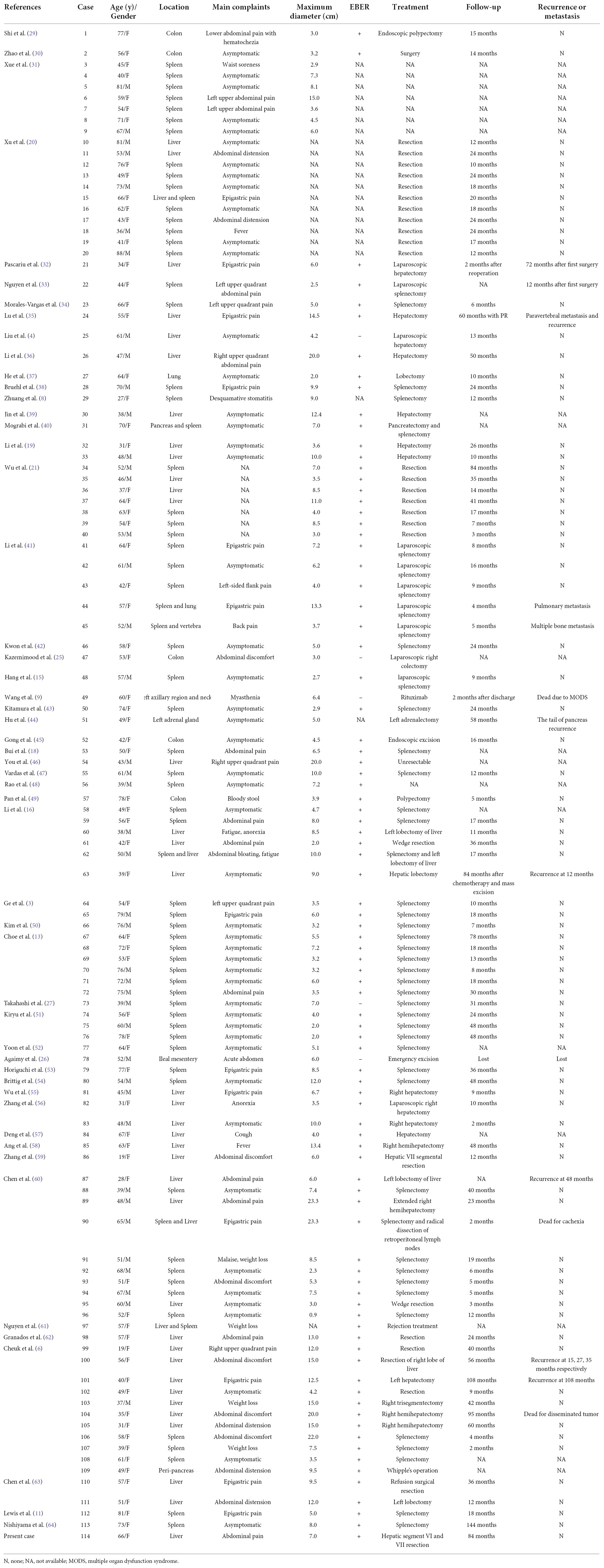
Table 1. Clinical characteristics of patients with inflammatory pseudotumor-like follicular dendritic cell sarcoma.
Case presentation
In 2015, a 66-year-old woman who suffered from right upper abdominal pain was admitted to the Department of Hepatic Surgery and Liver Transplantation Center at the Third Affiliated Hospital of Sun Yat-sen University because of a liver mass detected using abdominal ultrasound at a local hospital. Based on ultrasound examination and medical history, the preliminary diagnosis was liver-occupying lesions and diabetes mellitus type 2. The patient did not complain of vomiting, nausea, fever, or diarrhea. After admission, the values of routine tests including liver and kidney function and routine blood tests were almost within their normal limits. Liver function according to the Child–Pugh classification was class A. Serological analyses to detect hepatitis virus, syphilis, and human immunodeficiency virus were negative. Furthermore, the levels of common female-specific tumor markers, particularly α-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA)-199, and CA-125 were normal. The patient had a long history of diabetes mellitus type 2 and achieved good fasting plasma-glycemic control with acarbose combined with metformin.
Preoperative enhanced magnetic resonance imaging (MRI) showed a mass approximately 94 × 74 mm with clear borders in hepatic segments VI and VII. On enhanced phase, the images showed progressive enhancement of the lesion, while enhancement was not seen in the necrotic region. Significantly, the lesion demonstrated slightly hypointense speckled signals on in-phase and out-of-phase T1WI. Therefore, the primary diagnosis highly suggested a hepatic fat-poor angioleiomyolipoma (Figure 1).
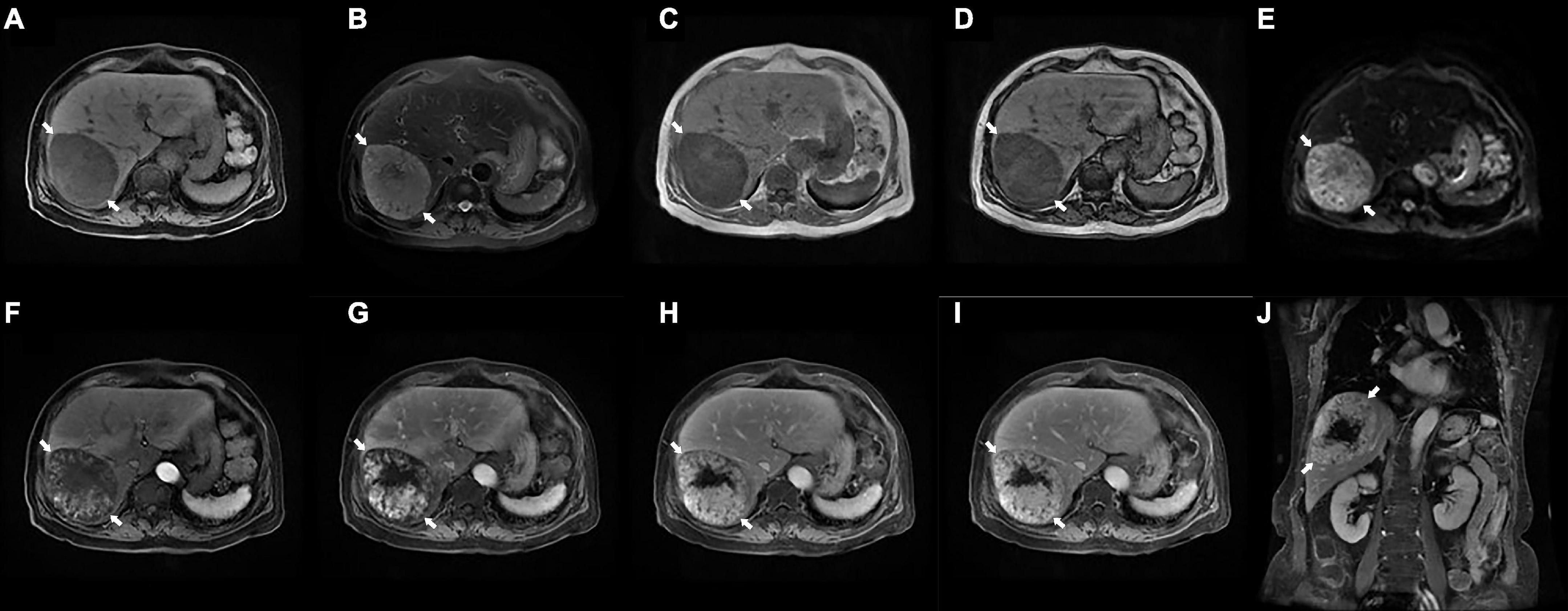
Figure 1. Preoperative enhanced magnetic resonance imaging (MRI) examination. (A) T1WI shows an ovalshaped hypointense lesion with clear border in the right lobe of the liver (arrows, 94×74 mm). (B) Fat-suppressed T2WI shows a slightly hyperintense lesions (arrows). (C,D) The in-phase (C) and out-of-phase (D) of T1WI demonstrates hypointense speckled signals within the mass (arrows). (E) DWI shows a hyperintense lesions (arrows). (F–J) Enhanced MRI scans showed progressive enhancement of the lesion (arrows) on the early (F) and late (G) arterial phase, portal venous phase (H), delayed phase (I), and coronal view (J), while no enhancement was seen in the necrotic region. MRI, magnetic resonance imaging; T1WI, T1 weighted image; T2WI, T2 weighted image; DWI, diffusion weighted imaging.
Although the diagnosis was not definitive, the large liver mass caused significant clinical symptoms (abdominal pain) in the absence of concurrent systematic disease. Our multidisciplinary hepatic surgery team therefore proposed surgical resection as the most appropriate procedure to confirm a diagnosis and further formulate the treatment strategy. Subsequently, the patient underwent resection of hepatic segments VI and VII, and minor complications occurred postoperation. During surgery, lesions were not observed in the gastrointestinal tract, spleen, mesentery, or other abdominal organs. The operation lasted 135 min, and the estimated intraoperative blood loss was approximately 150 mL.
Grossly, the size of the tumor was approximately 7.0 × 5.0 cm, presenting with an indistinct boundary and a patchy gray-red section with intratumor hemorrhage and necrosis. Postoperative pathology showed a sarcoma containing varying sizes of vessel lumens with negative surgical margins. The neoplastic tissue was extensively infiltrated by definite lymphocytes, plasma cells, and spindle cells. The tumor cells were fusiform and ovoid with a translucent cytoplasm and large vacuolated nuclei. According to the infiltration of numerous lymphocytes into the neoplastic tissues and immunohistochemical detection of CD21 and CD35 expression as well as in situ hybridization detection of Epstein–Barr virus-encoded RNA (EBER), the morphological and immunophenotypic results were consistent with a diagnosis of IPT-like FDCS (Figure 2). Hence, the final diagnosis was revised to IPT-like FDCS.
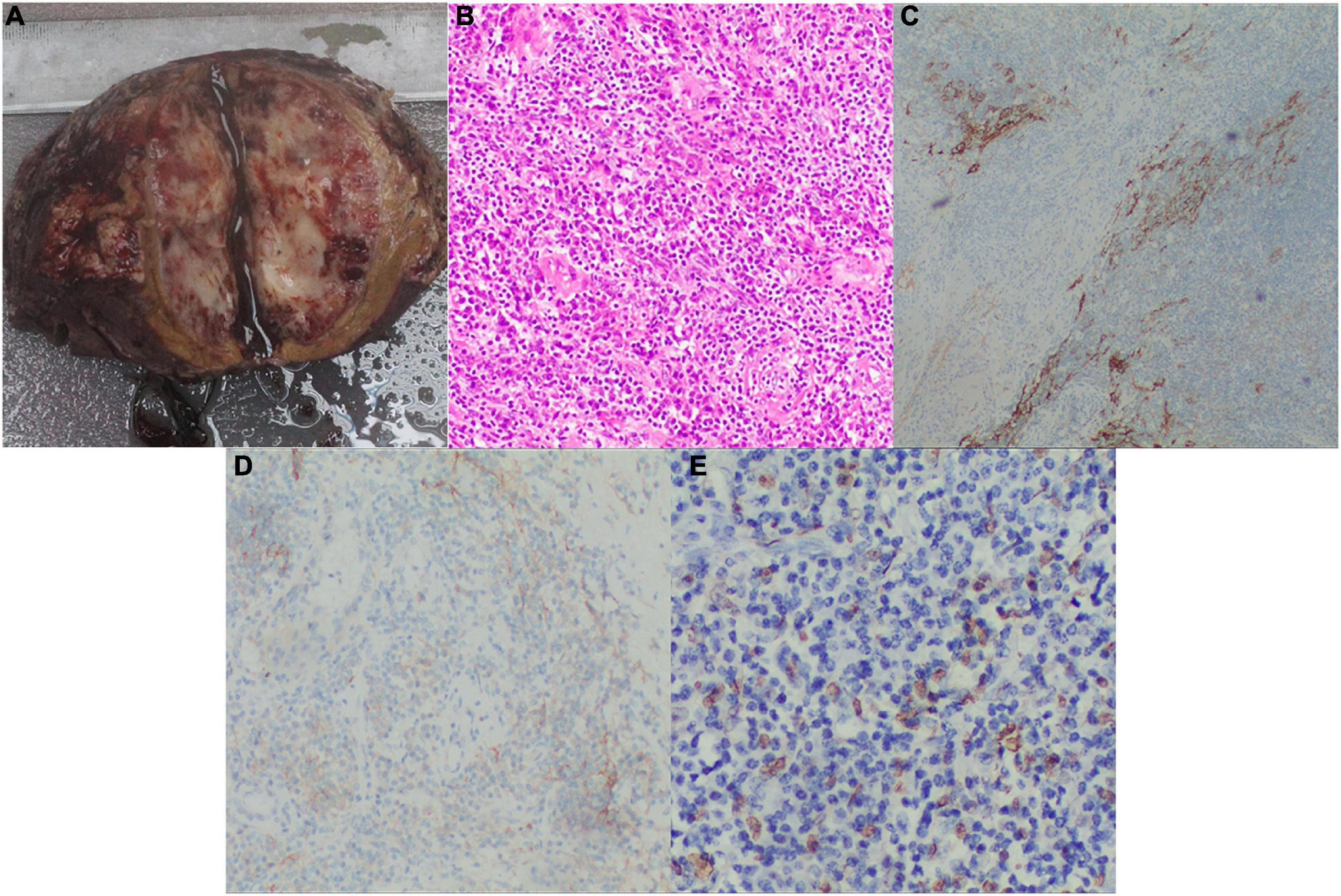
Figure 2. Postoperative pathology examinations. (A) Grossly, the cut surface of the fleshy neoplasm with necrosis and hemorrhage (tumor size: 7.0×5.0 cm); (B) H&E stained image showing that the tumor tissue had a meshwork-like architecture with abundant vascular-like proliferation, magnification: 200X; (C) The positive IHC result of CD21, magnification: 100X; (D) The positive IHC result of CD35, magnification: 100X; (E) The positive result of EBV for in situ hybridization, magnification: 200X. H&E, hematoxylin and eosin; IHC, immunohistochemical; EBV, Epstein Barr virus.
The patient was discharged without adjuvant chemotherapy or radiotherapy and has been examined at our hepatic surgery follow-up clinic every 6 months. The outcome of the 7-year follow-up was good, and metastasis or recurrence was not detected.
Discussion
Follicular dendritic cell sarcoma (FDCS) is a rare mesenchymal tumor of follicular dendritic cell origin originally identified by Monda et al. in 1986 (2). FDCS is classified into the two histopathological subtypes as follows: conventional and inflammatory pseudotumor-like (5). IPT-like FDCS is an extremely rare tumor, and only 113 cases are published (Table 1).
Inflammatory pseudotumor (IPT)-like FDCS possesses morphological and clinical features intermediate between inflammatory pseudotumors and FDC tumors and was first classified in 2001 as a distinct variant (6). Compared with conventional FDCS, IPT-like FDCS exhibits unique histopathological and clinical features that generally occur in abdominal organs, almost exclusively involving the spleen, liver, or both (104/114); and colonic (5/114), mesenteric (2/114), pancreatic (1/114), pulmonary (2/114), paranephric (1/114), and lymphatic (1/114) involvement occur as well.
Inflammatory pseudotumor (IPT)-like FDCS predominantly occurs in middle-aged adults (median age, 54.5 years; range, 19–88 years), with marked female predominance (female to male ratio = 1.71:1). Patients are mainly asymptomatic or present with abdominal distension or pain, occasionally accompanied by systemic symptoms such as back pain, waist soreness, significant weight loss, fever, and weakness. In rare cases, IPT-like FDCS exhibits paraneoplastic arthritis (7), and paraneoplastic pemphigus (8–10).
The pathogenesis and causes of IPT-like FDCS are unknown, although Epstein–Barr virus (EBV) may play an essential role in etiology. Stimulation by EBV may induce FDCs derived from mesenchymal cells to undergo neoplastic transformation and express CD21 and CD35 (11, 12). Interestingly, Choe et al. (13) found remarkable numbers of IgG4-positive plasma cell in 6 Asian patients with EBV-positive IPT-like FDCS of the spleen, suggesting that the intimate relationship between IgG4 and EBV plays a critical role in IPT-like FDCS, which may be limited to Asians. Moreover, mutations in genes encoding components of the NF-κB pathway, cell cycle regulatory genes (CDKN2A and RB1), and immune evasion genes (CD274 and PDCD1LG2) may be pathologically associated with IPT-like FDCS (14).
The morphology of IPT-like FDCS is similar to that of the conventional type. Gross examination reveals that most tumors exhibit a well-marginated, thin-walled, yellowish, soft tissue mass (maximum diameter = 7.54 ± 4.93 cm). In particular, localized hemorrhage or necrosis is observed within the tumor. The neoplastic cells, which may exhibit mild atypia, are usually spindle, ovoid, or polygonal and form storiform, fascicles, or trabecular arrays, which exhibit sparsely vesicular chromatin and distinct nucleoli (15). In particular, the inflammatory component of IPT-like FDCS, a more prominent histology, comprises mainly lymphocytes (B and T cells), plasma cells, eosinophils, and rare epithelioid histiocytes, with neoplastic cells often obscured by the inflammatory infiltration (6, 16). Owing to the lack of atypical tumor cells, IPT-like FDCS are often incorrectly identified inflammatory-reactive processes or inflammatory pseudotumor, even other various neoplasms (17). Moreover, the scarcity of cases and lack of specific clinical and imaging features present a formidable challenge to diagnosing IPT-like FDCS. Currently, the diagnosis of IPT-like FDCS requires auxiliary tests, including imaging, detecting distinctive cytological features, immunohistochemical detection of FDC markers, and in situ hybridization to detect EBER.
Although limited reports are available on the imaging features of IPT-like FDCS, they aid in making correct diagnoses before treatment when a neoplasm is detected (18). Most unenhanced computed tomography (CT) images display circular or elliptical, slightly hypodense tumors with a clear boundary. In certain cases, significant necrosis is seen within the tumor, while calcification or hemorrhage is rare. The lesions typically show heterogeneous enhancement in the enhanced phase, although the enhancement state is lower than that of the parenchyma. Therefore, the tumor area is always hypodense compared with the periparenchyma, and annular enhancement is observed in the delayed phase, sparing the central necrotic region (19). MRI and CT images are similar, and most lesions demonstrate enhancement from the center to the periphery in the arterial phase. The enhancement amplitudes of lesions in the portal, venous, and delayed phases tend to be homogeneous and diminished to varying degrees, and annular enhancements are occasionally observed (20).
The diagnosis of IPT-like FDCS is invariably supported by immunohistochemistry, and multiple FDC markers are often necessary, including CD21, CD23, CD35, CXCL-13, D2-40, Clusterin, Fascin, epidermal growth factor receptor, and CNA42 (21, 22). In particular, CD21 and CD35 are the most specific with almost universal positivity (23). Nevertheless, some EBV-related IPT-manifesting lesions do not express FDC markers (16). The immunohistochemical analysis of SSTR2a in FDCS indicates a positive rate significantly higher than CD21 and CD35 in conventional subtypes, while all IPT-like variants are negative (24). Therefore, SSTR2a shows promise as a highly sensitive and differential diagnostic marker to distinguish between FDCS and IPT-like FDCS. As mentioned previously, IPT-like FDCS is closely associated with EBV infection, while conventional types infrequently involve EBV (12). Our literature review identified only five cases of intrabdominal, EBER-negative IPT-like FDCS (4, 9, 25–27). In our present case, immunohistochemical analysis of the pathological specimen detected strongly positive expression of CD21, CD35, Ki67 (> 20%), and EBER (Figure 2).
Inflammatory pseudotumor (IPT)-like FDCS is a low-grade malignant tumor with good prognosis. Unlike FDCS, IPT-like FDCS is apparently indolent, with few instances of recurrence and metastasis (3). Disease status at the time of last follow-up is known for 92 patients, with follow-up times ranging from 2 to 144 months. Only 9.65% of patients (n = 11) experienced recurrence or metastasis during follow-up. Yet, PNP-associated IPT-like FDCS predominantly occurs in intraabdominal sites, indicating poor prognosis (8–10). Surgery is the most effective therapy for IPT-like FDCS, and only two cases (Cases 49 and 63) received chemotherapy or targeted therapy. However, chemotherapy, radiotherapy, or targeted therapy do not achieve a significant improvement in overall-or disease-free survival (28). Notably, Cases 49, 90, and 104 died because of multiple organ dysfunction syndrome, cachexia, and disseminated tumor, respectively, during treatment. The possibility of recurrence and metastasis suggests conducting long surveillance after surgery.
Conclusion
Inflammatory pseudotumor (IPT)-like FDCS is an extremely rare neoplasms that mainly occurs in the intraabdominal region. EBV probably plays an essential role in the etiology of IPT-like FDCS. The diagnosis of IPT-like FDCS is complex and usually relies on fine-needle aspiration biopsy or postoperative pathological diagnosis. Surgical resection is the most effective treatment, although the efficacy and safety of adjuvant chemotherapy, radiotherapy, or targeted therapy for postoperative management are unknown. IPT-like FDCS presents a certain risk of recurrence or metastasis after initial treatment. Thus, regular follow-up visits are strongly recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board review ([2021]12-114) at The Third Affiliated Hospital of Sun Yat-sen University in Guangzhou, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CX was the patient’s physician and responsible for the revision of the manuscript for important intellectual content. FD reviewed the literature and contributed to drafting the manuscript. CW performed the radiographic analysis. FD and HT conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors issued final approval for the version to be submitted for publication.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 82100692).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abd El-Aleem S, Saber E, Aziz N, El-Sherif H, Abdelraof A, Djouhri L. Follicular dendritic cells. J Cell Physiol. (2022) 237:2019–33. doi: 10.1002/jcp.30662
2. Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. a report of 4 cases. Am J Pathol. (1986) 122:562–72.
3. Ge R, Liu C, Yin X, Chen J, Zhou X, Huang C, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol. (2014) 7:2421–9.
4. Liu X, Cao L, Chin W, Yu J, Liu Y, Zheng S. Epstein-barr virus-negative inflammatory pseudotumor-like variant of follicular dendritic cell sarcoma of the liver: a case report and literature review. Clin Res Hepatol Gastroenterol. (2021) 45:101457. doi: 10.1016/j.clinre.2020.05.007
5. Swerdlow SH. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. editors. Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. Geneva: WHO (2017).
6. Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent epstein-barr virus association. Am J Surg Pathol. (2001) 25:721–31. doi: 10.1097/00000478-200106000-00003
7. Levi Sandri GB, Colasanti M, Vennarecci G, Ettorre GM. Paraneoplastic arthritis as first symptom of a liver inflammatory pseudotumor-like follicular dendritic cell sarcoma. Liver Int. (2016) 36:1392. doi: 10.1111/liv.13148
8. Zhuang JY, Zhang FF, Li QW, Chen YF. Intra-abdominal inflammatory pseudotumor-like follicular dendritic cell sarcoma associated with paraneoplastic pemphigus: a case report and review of the literature. World J Clin Cases. (2020) 8:3097–107. doi: 10.12998/wjcc.v8.i14.3097
9. Zhao CA. Case in which paraneoplastic pemphigus and bronchiolitis obliterans are the main manifestations of inflammatory pseudotumour-like follicular dendritic cell sarcoma. Aust J Dermatol. (2020) 61:e376–7. doi: 10.1111/ajd.13287
10. Wang L, Deng H, Mao M. Paraneoplastic pemphigus and myasthenia gravis, associated with inflammatory pseudotumor-like follicular dendritic cell sarcoma: response to rituximab. Clin Case Rep. (2016) 4:797–9. doi: 10.1002/ccr3.625
11. Lewis J, Gaffney R, Casey M, Farrell M, Morice W, Macon W. Inflammatory pseudotumor of the spleen associated with a clonal epstein-barr virus genome. Case report and review of the literature. Am J Clin Pathol. (2003) 120:56–61. doi: 10.1309/buwn-mg5r-v4d0-9yyh
12. Deyrup AT. Epstein-barr virus-associated epithelial and mesenchymal neoplasms. Hum Pathol. (2008) 39:473–83. doi: 10.1016/j.humpath.2007.10.030
13. Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased igg4-positive plasma cells. Pathol Int. (2013) 63:245–51. doi: 10.1111/pin.12057
14. Griffin G, Sholl L, Lindeman N, Fletcher C, Hornick J. Targeted genomic sequencing of follicular dendritic cell sarcoma reveals recurrent alterations in Nf-K b regulatory genes. Modern Pathol. (2016) 29:67–74. doi: 10.1038/modpathol.2015.130
15. Hang JF, Wang LC, Lai CR. Cytological features of inflammatory pseudotumor-like follicular dendritic cell sarcoma of spleen: a case report. Diagn Cytopathol. (2017) 45:230–4. doi: 10.1002/dc.23626
16. Li XQ, Cheuk W, Lam PW, Wang Z, Loong F, Yeong ML, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of liver and spleen: granulomatous and eosinophil-rich variants mimicking inflammatory or infective lesions. Am J Surg Pathol. (2014) 38:646–53. doi: 10.1097/pas.0000000000000170
17. Facchetti F, Simbeni M, Lorenzi L. Follicular dendritic cell sarcoma. Pathologica. (2021) 113:316–29. doi: 10.32074/1591-951x-331
18. Bui PL, Vicens RA, Westin JR, Jensen CT. Multimodality imaging of epstein-barr virus-associated inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: case report and literature review. Clin Imaging. (2015) 39:525–8. doi: 10.1016/j.clinimag.2014.12.021
19. Li HL, Liu HP, Guo GW, Chen ZH, Zhou FQ, Liu P, et al. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver: two case reports and literature review. World J Gastroenterol. (2019) 25:6693–703. doi: 10.3748/wjg.v25.i45.6693
20. Xu L, Ge R, Gao S. Imaging features and radiologic-pathologic correlations of inflammatory pseudotumor-like follicular dendritic cell sarcoma. BMC Med Imaging. (2021) 21:52. doi: 10.1186/s12880-021-00584-6
21. Wu YL, Wu F, Yang L, Sun H, Yan XC, Duan GJ. [Clinicopathologic features and prognosis of inflammatory pseudotumor-like follicular dendritic cell sarcomas in liver and spleen: an analysis of seven cases]. Zhonghua Bing Li Xue Za Zhi. (2018) 47:114–8. doi: 10.3760/cma.j.issn.0529-5807.2018.02.007
22. Li Z, Jin K, Yu X, Teng X, Zhou H, Wang Y, et al. Extranodal follicular dendritic cell sarcoma in mesentery: a case report. Oncol Lett. (2011) 2:649–52. doi: 10.3892/ol.2011.296
23. Shia J, Chen W, Tang LH, Carlson DL, Qin J, Guillem JG, et al. Extranodal follicular dendritic cell sarcoma: clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. (2006) 449:148–58. doi: 10.1007/s00428-006-0231-4
24. Tao LL, Huang YH, Chen YL, Yu GY, Yin WH. Sstr2a is a useful diagnostic marker for follicular dendritic cells and their related tumors. Am J Surg Pathol. (2019) 43:374–81. doi: 10.1097/pas.0000000000001205
25. Kazemimood R, Saei Hamedani F, Sharif A, Gaitonde S, Wiley E, Giulianotti PC, et al. A rare case of epstein-barr virus negative inflammatory pseudotumor-like follicular dendritic cell sarcoma presenting as a solitary colonic mass in a 53-year-old woman; case report and review of literature. Appl Immunohistochem Mol Morphol. (2017) 25:e30–3. doi: 10.1097/pai.0000000000000405
26. Agaimy A, Wünsch P. Follicular dendritic cell tumor of the gastrointestinal tract: report of a rare neoplasm and literature review. Pathol Res Pract. (2006) 202:541–8. doi: 10.1016/j.prp.2006.01.013
27. Takahashi N, Oya T, Matsumoto H, Tago K, Murotani K, Sato T, et al. A case of an inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen unrelated to epstein-barr virus. Kita Kanto Igaku. (2011) 61:207–14. doi: 10.2974/kmj.61.207
28. Facchetti F, Lorenzi L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol. (2016) 33:262–76. doi: 10.1053/j.semdp.2016.05.002
29. Shi QY, Zheng Z, Wu HY, Chen JY, Fan XS. [Colonic inflammatory pseudotumor-like follicular dendritic cell sarcoma: report of a case]. Zhonghua Bing Li Xue Za Zhi. (2022) 51:138–40. doi: 10.3760/cma.j.cn112151-20210727-00531
30. Zhao M, Du X, OuYang B, Li M, Yang H. Inflammatory pseudotumor-like follicular dendritic cell sarcoma mimicking a colonic polyp. J Gastrointest Surg. (2021) 25:2429–30. doi: 10.1007/s11605-021-04961-y
31. Xue N, Xue X, Sheng C, Lu M, Wang Y, Zhang S, et al. Imaging features of inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen. Ann Palliat Med. (2021) 10:12140–8. doi: 10.21037/apm-21-2776
32. Pascariu AD, Neagu AI, Neagu AV, Bãjenaru A, Beţianu CI. Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor: a case report. J Med Case Rep. (2021) 15:410. doi: 10.1186/s13256-021-02957-5
33. Nguyen A, Negrete LM, Bulterys PL, Shen L. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: a case report and approach to differential diagnosis. Radiol Case Rep. (2021) 16:3213–6. doi: 10.1016/j.radcr.2021.07.078
34. Morales-Vargas B, Deeb K, Peker D. Clinicopathologic and molecular analysis of inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: a case report and review of literature. Turk Patoloji Derg. (2021) 37:266–72. doi: 10.5146/tjpath.2021.01523
35. Lu SS, Wang Z, Zhao S, Liu WP. [Hepatic inflammatory pseudotumor-like follicular dendritic cell sarcoma with paravertebral metastasis and recurrence: report of a case]. Zhonghua Bing Li Xue Za Zhi. (2021) 50:958–60. doi: 10.3760/cma.j.cn112151-20210325-00234
36. Li J, Tao HS, Chen D, Huang ZY, Zhang EL. Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor with hepatic lymphoma history: a case report and literature review. Medicine. (2021) 100:e27392. doi: 10.1097/md.0000000000027392
37. He H, Xue Q, Tan F, Yang L, Wang X, Gao Y, et al. A rare case of primary pulmonary inflammatory pseudotumor-like follicular dendritic cell sarcoma successfully treated by lobectomy. Ann Transl Med. (2021) 9:77. doi: 10.21037/atm-20-4965
38. Bruehl FK, Azzato E, Durkin L, Farkas DH, Hsi ED, Ondrejka SL. Inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcomas of the spleen are EBV-associated and lack other commonly identifiable molecular alterations. Int J Surg Pathol. (2021) 29:443–6. doi: 10.1177/1066896920949675
39. Jin K, Li MN, Li S, Li J, Chen N. [Hepatic inflammatory pseudotumor-like follicular dendritic cell sarcoma]. Zhonghua Gan Zang Bing Za Zhi. (2020) 28:172–4. doi: 10.3760/cma.j.issn.1007-3418.2020.02.015
40. Mograbi M, Stump MS, Luyimbazi DT, Shakhatreh MH, Grider DJ. Pancreatic inflammatory pseudotumor-like follicular dendritic cell tumor. Case Rep Pathol. (2019) 2019:2648123. doi: 10.1155/2019/2648123
41. Li X, Shi Z, You R, Li Y, Cao D, Lin R, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: computed tomography imaging characteristics in 5 patients. J Comput Assist Tomogr. (2018) 42:399–404. doi: 10.1097/rct.0000000000000700
42. Kwon H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Turk J Gastroenterol. (2018) 29:128–30. doi: 10.5152/tjg.2018.17220
43. Kitamura Y, Takayama Y, Nishie A, Asayama Y, Ushijima Y, Fujita N, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen: case report and review of the literature. Magn Reson Med Sci. (2015) 14:347–54. doi: 10.2463/mrms.2014-0052
44. Hu J, Chen LL, Ding BW, Jin DY, Xu XF. Resection is an effective treatment for recurrent follicular dendritic cell sarcoma from retroperitoneum: unusual presentation of a rare tumor. Int J Clin Exp Med. (2015) 8:8218–21.
45. Gong S, Auer I, Duggal R, Pittaluga S, Raffeld M, Jaffe ES. Epstein-barr virus-associated inflammatory pseudotumor presenting as a colonic mass. Hum Pathol. (2015) 46:1956–61. doi: 10.1016/j.humpath.2015.08.011
46. You Y, Shao H, Bui K, Bui M, Klapman J, Cui Q, et al. Epstein-barr virus positive inflammatory pseudotumor of the liver: report of a challenging case and review of the literature. Ann Clin Lab Sci. (2014) 44:489–98.
47. Vardas K, Manganas D, Papadimitriou G, Kalatzis V, Kyriakopoulos G, Chantziara M, et al. Splenic inflammatory pseudotumor-like follicular dendritic cell tumor. Case Rep Oncol. (2014) 7:410–6. doi: 10.1159/000365000
48. Rao L, Yang Z, Wang X, Zhang X, Shen B. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumor of spleen. Clin Nucl Med. (2014) 39:e286–9. doi: 10.1097/RLU.0b013e3182952bfe
49. Pan ST, Cheng CY, Lee NS, Liang PI, Chuang SS. Follicular dendritic cell sarcoma of the inflammatory pseudotumor-like variant presenting as a colonic polyp. Korean J Pathol. (2014) 48:140–5. doi: 10.4132/KoreanJPathol.2014.48.2.140
50. Kim HJ, Kim JE, Kang GH, Kim JY, Park K. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen with extensive histiocytic granulomas and necrosis: a case report and literature review. Korean J Pathol. (2013) 47:599–602. doi: 10.4132/KoreanJPathol.2013.47.6.599
51. Kiryu S, Takeuchi K, Shibahara J, Uozaki H, Fukayama M, Tanaka H, et al. Epstein-barr virus-positive inflammatory pseudotumour and inflammatory pseudotumour-like follicular dendritic cell tumour. Br J Radiol. (2009) 82:e67–71. doi: 10.1259/bjr/66918927
52. Yoon S, Ko H, Kim B-H, Kwon G, Jeon Y, Kim C-G. Epstein-barr virus-associated inflammatory pseudotumor-like follicular dendritic cell tumor in the spleen of a patient with diffuse large B cell lymphoma: a case report and review of the literature. Korean J Pathol. (2007) 41:198–202.
53. Horiguchi H, Matsui-Horiguchi M, Sakata H, Ichinose M, Yamamoto T, Fujiwara M, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int. (2004) 54:124–31. doi: 10.1111/j.1440-1827.2004.01589.x
54. Brittig F, Ajtay E, Jaksó P, Kelényi G. Follicular dendritic reticulum cell tumor mimicking inflammatory pseudotumor of the spleen. Pathol Oncol Res. (2004) 10:57–60. doi: 10.1007/bf02893411
55. Wu CY, Wang RC, Chen BJ, Chen WY, Jhuang JY, Chang MC, et al. Granuloma with an underlying lymphoma: a diagnostic challenge and a wider histologic spectrum including adult T-cell leukemia/lymphoma. Appl Immunohistochem Mol Morphol. (2020) 28:316–24. doi: 10.1097/pai.0000000000000731
56. Zhang BX, Chen ZH, Liu Y, Zeng YJ, Li YC. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: a brief report of two cases. World J Gastrointest Oncol. (2019) 11:1231–9. doi: 10.4251/wjgo.v11.i12.1231
57. Deng S, Gao J. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: a rare presentation of a hepatic mass. Int J Clin Exp Pathol. (2019) 12:3149–55.
58. Ang W, Bundele M, Shelat V. Follicular dendritic cell sarcoma: rare presentation of incidental large hepatic mass. Ann Hepatobiliary Pancreat Surg. (2019) 23:74–6. doi: 10.14701/ahbps.2019.23.1.74
59. Zhang X, Zhu C, Hu Y, Qin X. Hepatic inflammatory pseudotumour-like follicular dendritic cell tumor: a case report. Mol Clin Oncol. (2017) 6:547–9. doi: 10.3892/mco.2017.1188
60. Chen Y, Shi H, Li H, Zhen T, Han A. Clinicopathological features of inflammatory pseudotumour-like follicular dendritic cell tumour of the abdomen. Histopathology. (2016) 68:858–65. doi: 10.1111/his.12851
61. Nguyen B, Roarke M, Yang M. Synchronous hepatic and splenic inflammatory pseudotumour-like follicular dendritic cell sarcomas. Liver Int. (2015) 35:1917. doi: 10.1111/liv.12738
62. Granados R, Aramburu JA, Rodríguez JM, Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. (2008) 36:42–6. doi: 10.1002/dc.20744
63. Chen T, Kuo T, Ng K. Follicular dendritic cell tumor of the liver: a clinicopathologic and epstein-barr virus study of two cases. Modern Pathol. (2001) 14:354–60. doi: 10.1038/modpathol.3880315
Keywords: inflammatory pseudotumor, follicular dendritic cell, sarcoma, case report, hepatic
Citation: Ding F, Wang C, Xu C and Tang H (2022) Case report: Hepatic inflammatory pseudotumor-like follicular dendritic cell sarcoma: A rare case and minireview of the literature. Front. Med. 9:1002324. doi: 10.3389/fmed.2022.1002324
Received: 25 July 2022; Accepted: 25 October 2022;
Published: 08 November 2022.
Edited by:
Liam Chen, University of Minnesota, United StatesReviewed by:
Hui Li, Chongqing University, ChinaShunyou Gong, Northwestern University, United States
Copyright © 2022 Ding, Wang, Xu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tang, Y2hlbnV4QGFsaXl1bi5jb20=; Chi Xu, Y2hpeHV6b2VAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Fan Ding
Fan Ding Chao Wang
Chao Wang Chi Xu4,5*
Chi Xu4,5*