- 1Department of Anesthesiology, Stomatology Hospital Affiliated Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China
- 3Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing, China
Background: High-flow nasal oxygenation (HFNO) has been suggested as an alternative oxygenation method during procedural sedation. This randomized, non-inferiority trial evaluated the safety and efficacy of HFNO compared with laryngeal mask airway (LMA) in pediatric ambulatory oral surgery under deep sedation.
Methods: In total, 120 children aged 2–7 years (weight: 10–30 kg) were equally assigned into two groups, namely, HFNO with propofol total intravenous anesthesia infusion (HFNO-IV) or LMA with propofol total intravenous anesthesia infusion (LMA-IV). The primary objective was to monitor carbon dioxide (CO2) accumulation during perioperative surgery. Secondary objectives included monitoring transcutaneous oxygen saturation, grade exposure to the surgical field, perioperative adverse events, or other events. The predefined non-inferiority margin was 7 mmHg. During the COVID-19 pandemic, a novel WeChat applet was implemented to gather follow-up data after discharge.
Results: Non-inferiority could be declared for HFNO relative to LMA (mean difference in transcutaneous CO2 (TcCO2) = −1.4 mmHg, 95% CI: −2.9, 0.1 mmHg; P > 0.05). The pre-surgical TcCO2 of the HFNO-IV group (45.4 ± 4.5 mmHg) was similar to that of the LMA-IV group (44.0 ± 3.5 mmHg), within the clinically acceptable normal range. All the children maintained SpO2 levels of >97%. The surgical field exposure score of the HFNO group was significantly better than that of the LMA group. There was no significant difference between the two groups regarding risk or adverse events.
Conclusion: HFNO was not inferior to LMA for maintaining oxygenation and ventilation in patients undergoing pediatric ambulatory oral surgery under deep sedation under strict isolation from the oral cavity to the upper airway.
Introduction
Preschool children (aged 3–6 years) in need of dental care may require general anesthesia or deep sedation because of anxiety, fear, having special needs, or an inability to cooperate with oral procedures. As a method of respiratory support, the laryngeal mask airway (LMA) combined with sevoflurane inhalation has been preferred in our department since 2010 for the greater comfort and efficiency of pediatric patients who undergo ambulatory oral surgeries, compared with nasal or oral endotracheal intubation (1).
General anesthesia with LMA or oral/nasal endotracheal intubation in ambulatory pediatric oral surgeries is disadvantaged by obstruction of the surgical field or the risks of laryngeal trauma and epistaxis. High-flow nasal oxygenation (HFNO) has been applied in the anesthetic management of tubeless laryngeal surgeries, gastrointestinal endoscopy, and difficult airway management (2–6). It significantly improves the oxygenation safety for tubeless laryngeal surgery and gastrointestinal endoscopy, with less interference to the surgical field. HFNO can not only provide a good oxygen supply but also relieve the pain of patients caused by difficult airway tracheal intubation. HFNO was found to prolong apnoeic time safely and facilitate tubeless laryngeal surgery, especially in children (7).
The application of HFNO in spontaneously breathing patients under general anesthesia was first described by Booth et al. in adult microlaryngoscopic surgery (8). Unfortunately, previous studies of HFNO in pediatric surgeries showed inconsistent results. A retrospective study showed that HFNO in spontaneously breathing patients under intravenous (IV) anesthesia was an effective and feasible option in pediatric airway surgery (9). However, it did not increase respiratory stability in sedated children undergoing upper gastrointestinal tract endoscopy, compared with low flow nasal cannula (10). Whether the application of HFNO can maintain good oxygenation under general anesthesia or even facilitate the work of the surgeon is yet unknown.
We hypothesized that HFNO would not be inferior to LMA for maintaining adequate and efficient respiratory support during deep sedation for pediatric dental procedures or oral surgeries. Therefore, this randomized controlled study evaluated the efficacy and safety of HFNO in pediatric outpatient oral surgery under deep sedation in comparison with LMA.
Methods
Patients and center
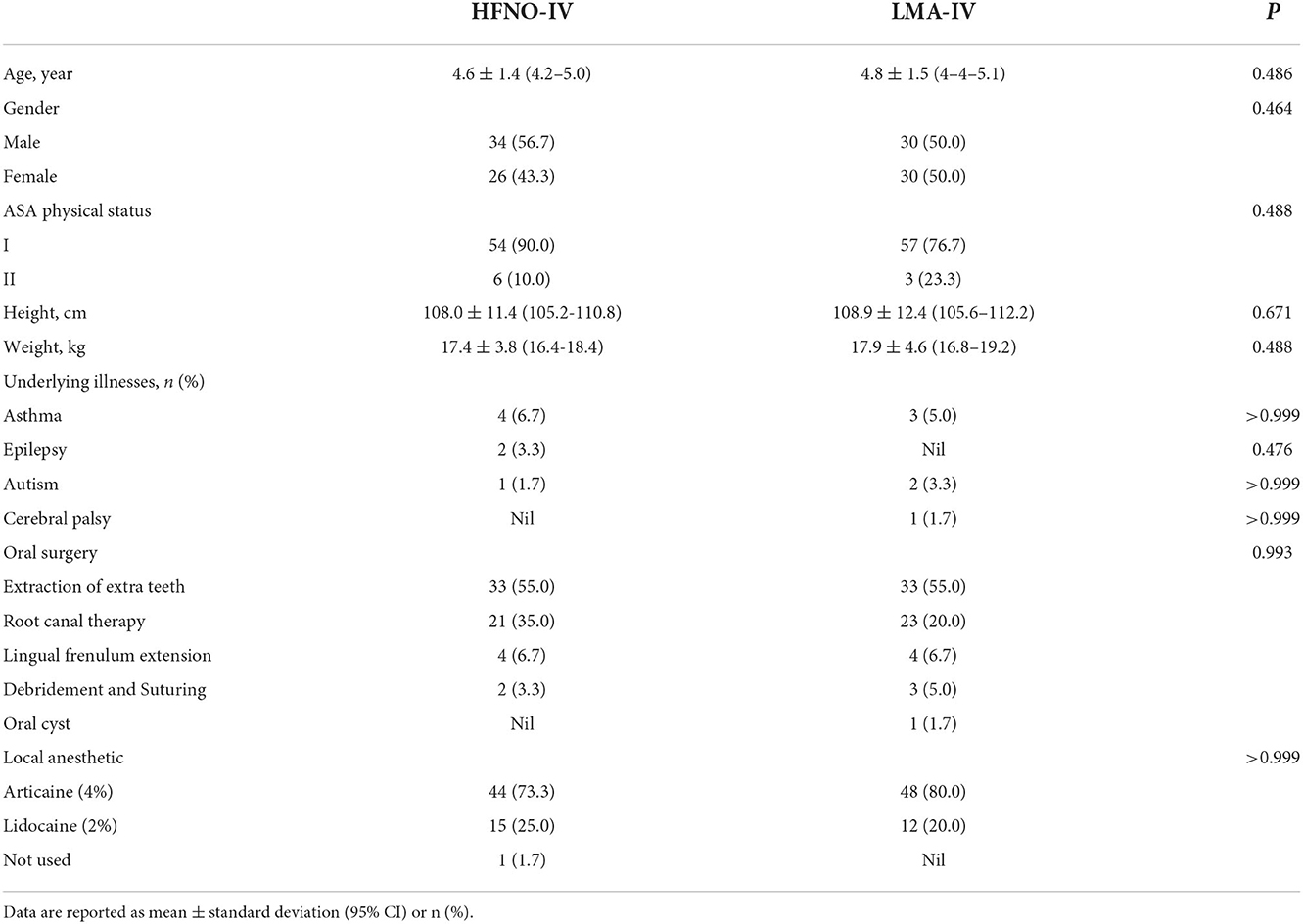
This single-center, prospective, open-label, randomized, non-inferiority trial recruited preschoolers aged 2–7 years who were treated in the Comfort Oral Center in Stomatology Hospital affiliated with Chongqing Medical University, China. Potential participants scheduled to undergo extraction of extra teeth, root canal therapy, lingual frenulum extension and debridement, and suturing were identified from surgery lists. The inclusion criteria were as follows: weight, 10–30 kg; American Society of Anesthesiologists (ASA) status, I or II; scheduled for elective outpatient oral surgery under general anesthesia or deep sedation; and having an expected surgery duration of <1 h. Patients with any of the following conditions were excluded: upper respiratory tract infection; known or anticipated difficult airway; heart or lung disease; history of upper airway obstruction; or with obesity (body mass index >30 kg/m2). Patients were invited to enroll in the study during the preoperative examination and randomly allocated by randomization software (STATA version 15.1) to the study arms.
Trial procedures
After obtaining the guardian's written informed consent, children were randomly assigned to one of two groups (study arms), namely, HFNO with propofol total intravenous anesthesia infusion (HFNO-IV) and LMA with propofol total intravenous anesthesia infusion (LMA-IV). The anesthesia nurse announced the group allocation after inhalational induction. Due to the nature of the intervention, blinding the clinicians to the treatment was not feasible, but eligible children's parents or caregivers were blinded to group allocation. The operations were performed by one of five surgeons who had experience performing more than 100 operations before this study.
Vital signs were recorded before laryngeal mask insertion or HFNO (timepoint T1, baseline), at the start of the operation (T2) and immediately after the operation (T3). Vital signs included peripheral oxygen saturation (SpO2), heart rate (HR), body temperature (T), and mean arterial pressure (MAP), where MAP = (systolic pressure + 2 × diastolic pressure)/3. Because it is an open system, measuring continuous end-tidal CO2 and blood CO2 levels by blood gas analysis was not feasible during HFNO anesthesia and in an outpatient setting. For these reasons, transcutaneous CO2 (i.e., TcCO2) was used to monitor arterial CO2 attached to the skin of the forearm (6). TcCO2 is more reliable than end-tidal PCO2 for children undergoing invasive mechanical ventilation (11).
In addition, transcutaneous oximetry (TcO2) and TcCO2 were recorded at timepoints T2 and T3. Transcutaneous CO2 was measured with a TCM4 system (Radiometer Medical ApS, Denmark). TcO2 and bispectral index (BIS; Covidien, St. Louis, Missouri, USA) were monitored continuously throughout the procedure. The BIS value was maintained at 40–50.
Each child was prepared before general anesthesia by fasting, water restriction, and blood analysis, and was given a physical examination. Anesthesia induction was 5 to 8% sevoflurane with 5 L/min oxygen inhalation via a face mask. After intravenous access, the treatment area in the mouth was isolated with a rubber dam, and a sterile gauze was placed at the base of the tongue to avoid leakage of high-flow oxygen and upper airway isolation; 2% lidocaine hydrochloride or 4% articaine hydrochloride injection was used for local anesthesia during the operation. Spontaneous breathing was maintained in all the study arms.
In the HFNO-IV group, after inhalational induction, the face mask was replaced with age-appropriate nasal prongs (10–20 kg: EM05-503, Excellentcare, Huizhou, Guangdong, China; 20–30 kg: EM05-502) and weight-specific high flow delivered using an HFNO system (OH-70C, Micomme, Shenzhen, China). The system is composed of a humidifier base, a heater wire, a temperature probe, an oxygen/air blender, and a circuit that included a humidifier chamber, a tubing system, and a pressure relief valve for the pediatric circuit. The fraction of inspired oxygen (FiO2) was set at 100%, temperature controlled to 1–2°C below the baseline body temperature, and the oxygen flow rate set at 2 L/kg/min that could be increased to 70 L/min if needed.
Deep sedation was maintained with continuous infusion of alfentanil hydrochloride (2ml:1mg, SFDA No. 13S03021, Yichang, Humanwell, Yichang, Hubei, CHN) 0.2 μg/kg/min and plasma target-controlled infusion of propofol titrate (injectable emulsion, 10ml:0.1g, SFDA No. 2104062, Sichuan Guorui Pharmaceutical, LeShan, Sichuan, CHN) with an initial target concentration of 3–5 μg/ml (Kataria model; Infusion Workstation Cabinet Body, HP-80, Medcaptain, Shenzhen, Guangdong, China) (12). HFNO was terminated for any of the following: TcCO2 ≥ 65 mmHg; apnea time ≥ 10 min; or SpO2 ≤ 95%.
In the LMA-IV group, the LMA size 2.0 was used in children weighing 10–20 kg and size 2.5 was used in children weighing 20–30 kg. After insertion, the LMA was connected to a semi-closed circle breathing system (Fabius GS; Drager Medical, Lübeck, Germany). Deep sedation was maintained with continuous infusion of alfentanil hydrochloride at 0.2 μg/kg/min and TCI-propofol titrate with concentration of plasma (Cp) at 3–5 μg/ml based on the vital signs and BIS value; spontaneous breathing was maintained throughout the procedure. At the end of the operation, the LMA was removed after oral secretion suction.
Outcomes
The primary outcome was carbon dioxide (CO2) accumulation of the HFNO-IV group, relative to that of the LMA-IV group. Secondary outcomes included transcutaneous oxygen saturation, surgical field exposure, perioperative adverse events, or other reactions related to the airway management techniques being investigated. At the end of the operation (T3), all anesthesia drugs were stopped, and patients were given 100% oxygen at 5 L/min by face mask. Vital signs and TcO2 and TcCO2 were recorded. Patients were then transferred to the recovery room. The duration of anesthesia, recovery time (from the end of anesthesia until the child could answer questions from the guardian), and length of oral surgery (from the beginning of the operation until the end of anesthesia) were recorded from an electronic anesthesia recording system. All intraoperative or postoperative events were recorded, including the number of operation interruptions, cardiac arrhythmia, laryngospasm, aspiration, emergence delirium (ED) (Pediatric Anesthesia Emergence Delirium Scale, PAEDS) (13), and sore throat. Dental surgeons were asked to grade exposure to the surgical field as excellent, obstructed view but able to operate, or poor.
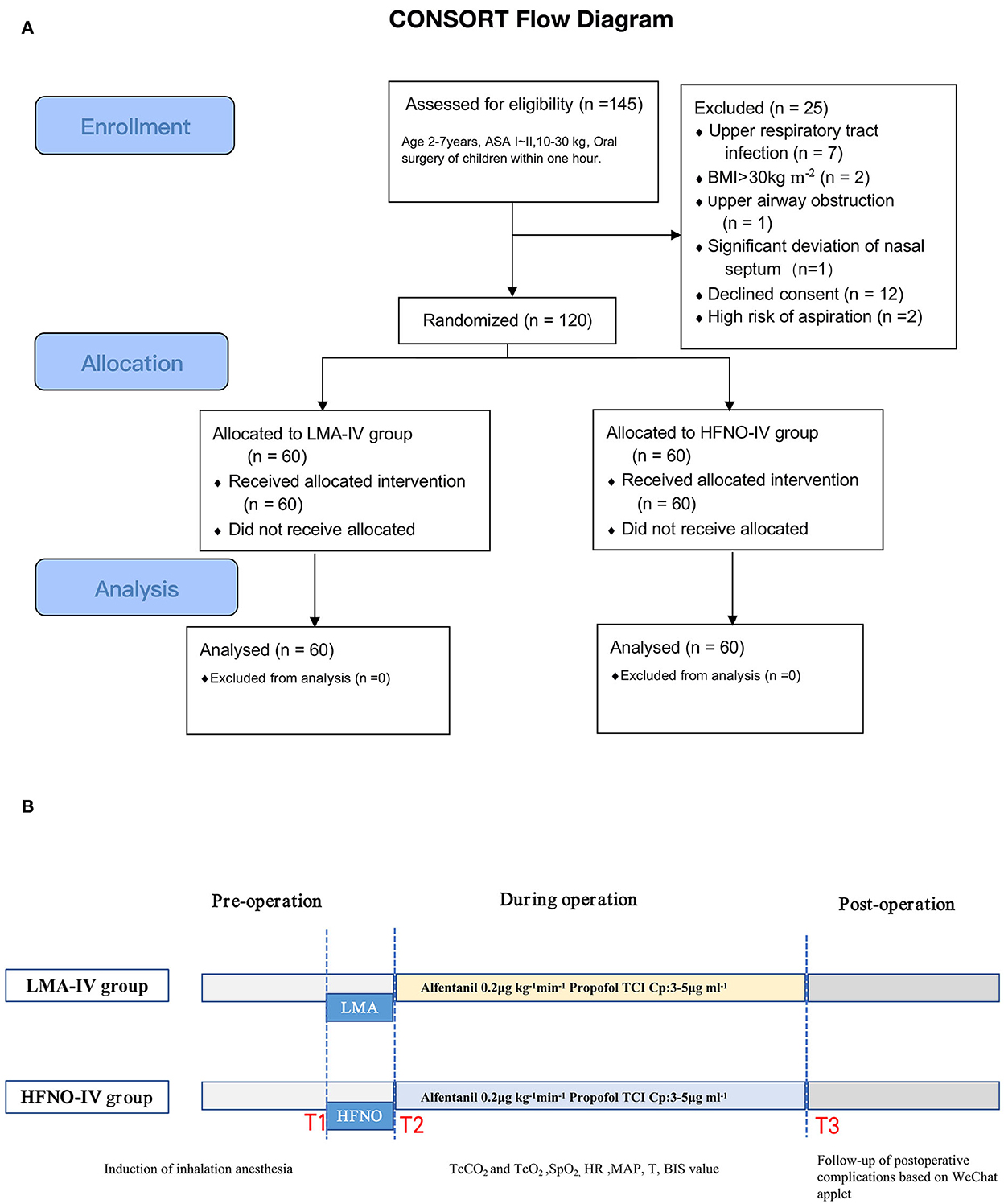
Children were discharged from the hospital after they passed a modified Aldrete score for discharge (14) (Figure 1). After discharge, the patients were followed for postoperative adverse events (e.g., nausea, vomiting, pain, bleeding, or itching) using a novel smartphone-based WeChat applet (Four I) 24 h, 48 h, and 72 h after surgery (Supplementary Figure S1). In addition to its use as a follow-up tool, Four I was used to provide treatment suggestions to improve service quality for children and their families, record adverse events, obtain immediate feedback, maintain medical services, and reduce physical contact during the COVID-19 pandemic.
Statistical analysis
To calculate the sample size, we conducted a prior pilot study with 15 patients who underwent oral surgeries under LMA with propofol total intravenous deep sedation infusion in our institution. In the pilot study, the TcCO2 in LMA was 41.4 ± 7.2 mmHg at the start of the operation. To test the non-inferiority of HFNO compared with LMA regarding the TcCO2 during surgery, 60 subjects per group (a total of 120 children) were required for a non-inferiority margin of 7 mmHg, which was chosen by clinical consensus, with a one-sided level of significance of 0.025 and power of 80%, including a dropout rate of 10% (PASS 15.0, NCSS, USA).
Qualitative variables were expressed as numbers and percentages. Quantitative variables were shown as mean ± standard deviation (95% CI) or median (interquartile range) as appropriate. The normality of distribution was tested with the Kolmogorov-Smirnov test. The non-parametric Mann-Whitney U test or independent t-test was applied for comparisons of continuous outcomes, as appropriate. Categorical outcomes were compared using the chi-squared test. Intention-to-treat analysis was applied. Statistical analyses were performed using SPSS22.0. A 2-tailed P-value of < 0.05 was considered statistically significant.
Ethics approval
We ensure that this work conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki, IR.SUMS.REC.1397.759) for experiments involving humans, and informed consent was obtained from all subjects. This study was approved by the Ethics Committee of the Stomatology Hospital affiliated with Chongqing Medical University (registration no. CQHS-NT10-2020) and registered at http://www.chictr.org.cn/ (registration no. ChiCTR2100043269) before enrollment.
Results
From October 2020 to August 2021, 120 pediatric patients were randomly assigned to the HFNO-IV group (34 boys) or the LMA-IV group (30 boys), P > 0.05 (Figure 1). The two groups were statistically similar in age, gender, and other baseline characteristics (Table 1, P > 0.05). No patients were lost to follow-up.
Primary outcomes
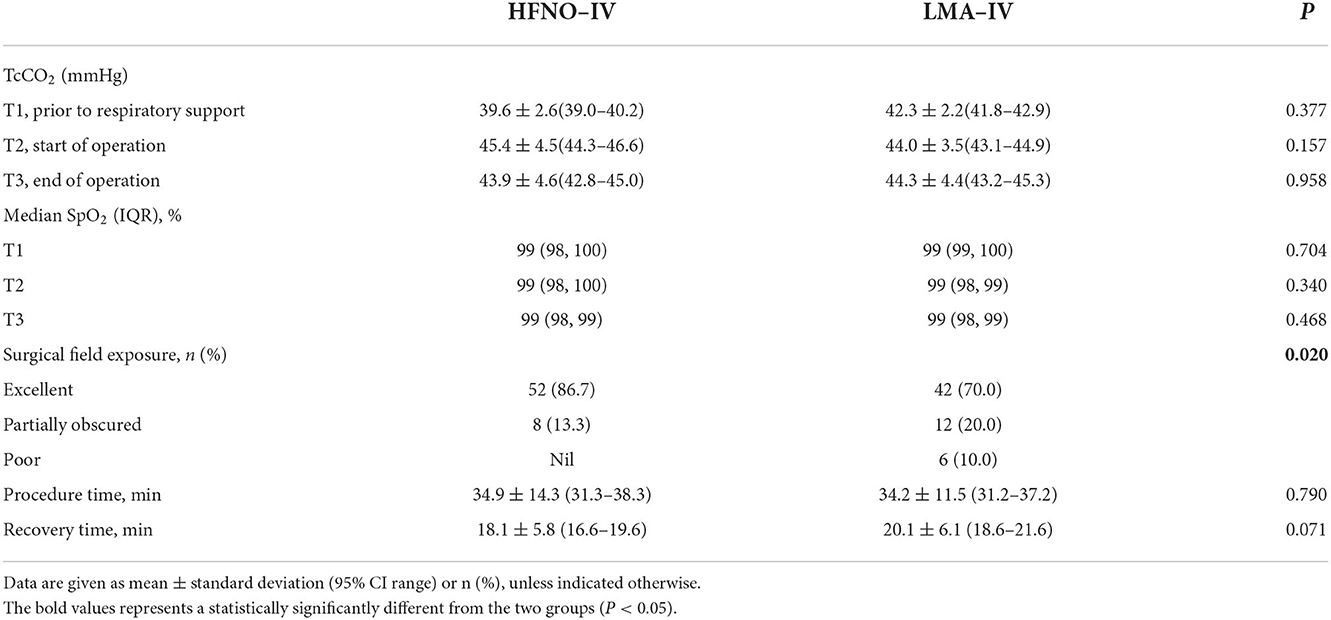
At the baseline (T1), the TcCO2 of the HFNO-IV and LMA-IV groups was 39.6 ± 2.6 (39.0–40.2) mmHg and 42.3 ± 2.2 (41.8–42.9) mmHg, respectively (Figure 2, P > 0.05). Just before surgery (T2), the respective recordings for TcCO2 were not different at 45.4 ± 4.5 (44.3–46.6) mmHg and 44.0 ± 3.5(43.1–44.9) mmHg, with a mean difference of −1.4 mmHg (95% CI: −2.9 and 0.1 mmHg, P > 0.05). At the end of the procedure (T3), the TcCO2 of the HFNO-IV group (43.9 ± 4.6[42.8–45.0] mmHg) was similar to that of the LMA-IV [44.3 ± 4.4(43.2–45.3) mmHg] (Figure 2, Table 2, P > 0.05).
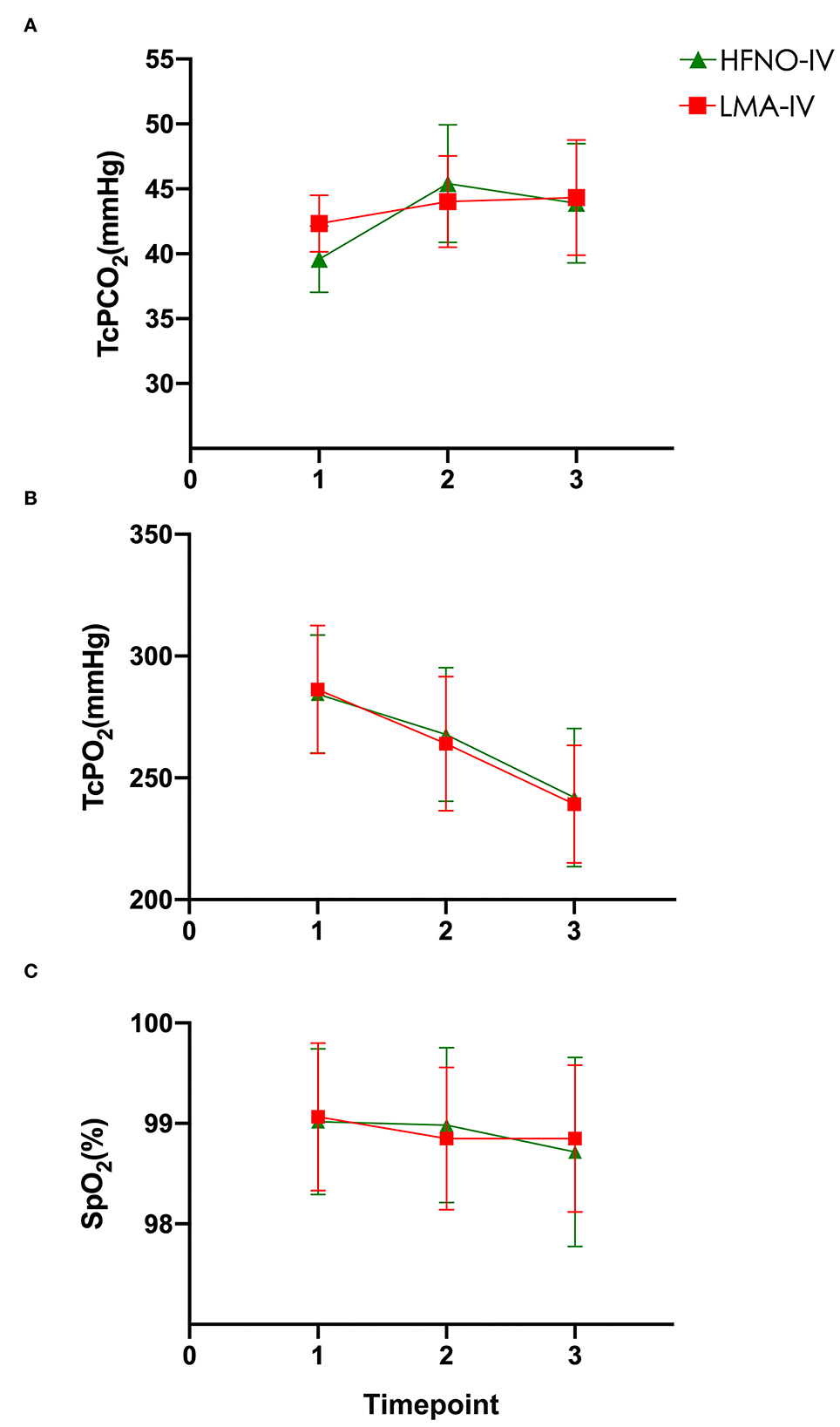
Figure 2. (A–C) Carbon dioxide, oxygen, and SpO2 levels were measured at baseline, at the beginning of the operation, and at the end of the operation.
Secondary outcomes
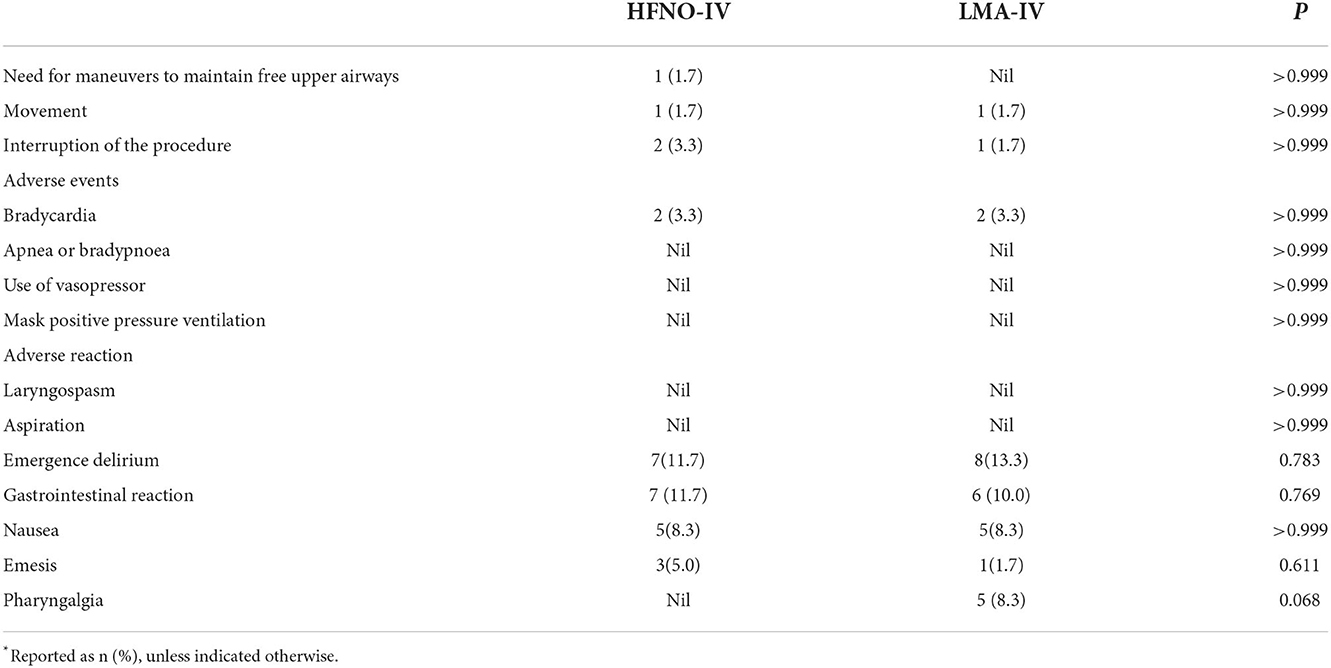
From T1 to T3, TcO2 decreased gradually from the peak value recorded at T1. The SpO2 of each child was above 97% at each timepoint (Figure 2, Table 2, P > 0.05). There was one child in each of the two groups with minor movements during the operation. For both children, the treatment was completed after adjusting the depth of anesthesia. There was no significant difference between the two groups regarding movement, interruption of procedure, the incidence of bradycardia or apnea/bradypnoea, or use of vasopressor, or mask positive pressure ventilation (Table 3, P > 0.05).
Agitation after awakening from anesthesia was common. The difference in the incidence of agitation as measured by the PAEDS score was insignificant: 13.3 and 11.7%, respectively, for the LMA-IV and HFNO-IV groups. Fewer children in the HFNO-IV group (nil) experienced pharyngalgia through the postoperative follow-up compared with the LMA-IV (5, 8.3%; P > 0.05).
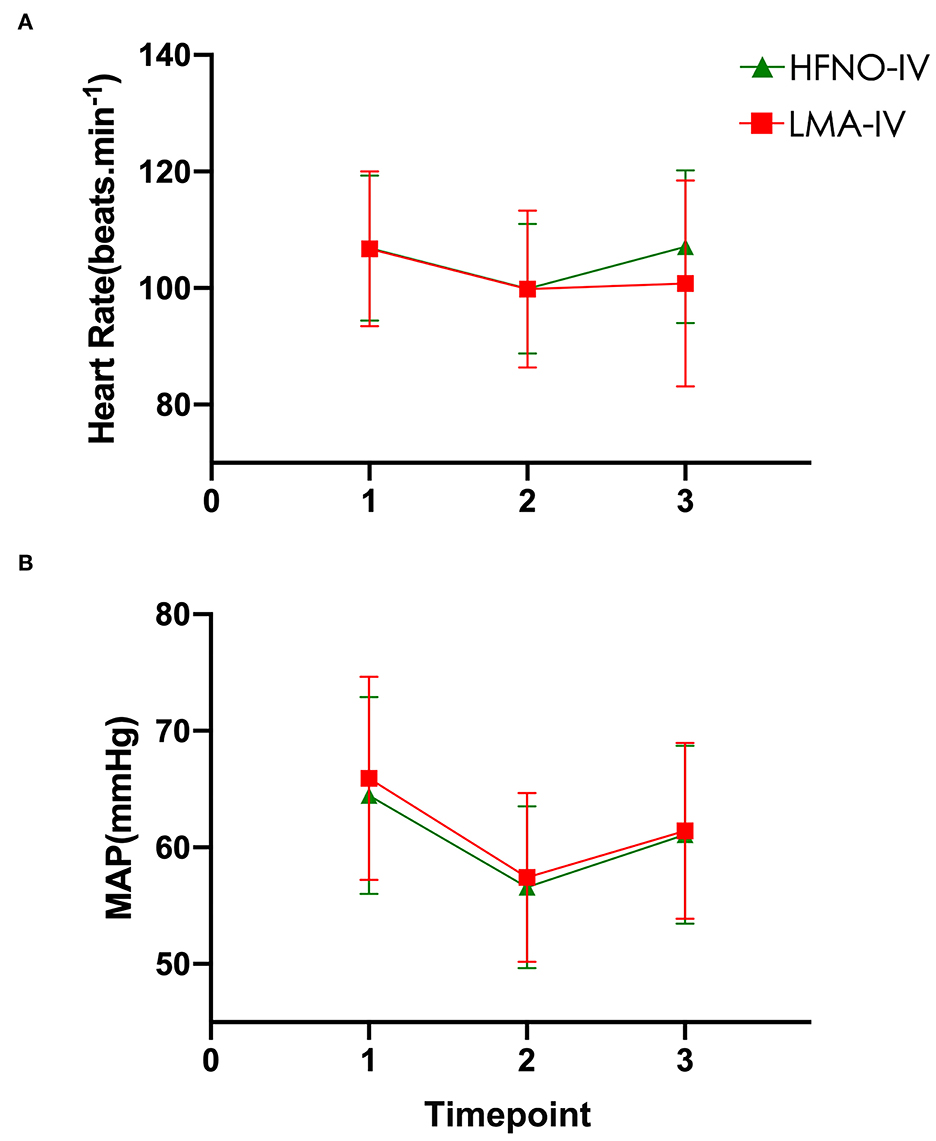
At timepoint T2, the heart rates and the MAPs of the HFNO-IV and LMA-IV groups were lower than that at the baseline and postoperative surgery (Figure 3). Specifically, at T2, the MAPs of HFNO-IV and LMA-IV were 56.6 ± 6.9 (54.8–58.4) mmHg and 57.4 ± 7.3 (55.6–59.3) mmHg, respectively, P > 0.05. The times to awaken in HFNO-IV and LMA-IV groups were 18.1 ± 5.8 (16.6–19.6) and 20.1 ± 6.1 (18.6–21.6) min, P > 0.05.
The surgeons scored the visual field for each surgery as excellent, partially obscured, or poor. Between the groups, the percentage of excellent scores for HFNO (86.7%) was significantly higher than that for LMA (70%; Table 2, P < 0.05)
Discussion
This study found that HFNO is effective for the maintenance of oxygenation and CO2 clearance in pediatric ambulatory oral surgery under deep sedation and allows better operative field exposure for the surgeon. It also found that there was no difference between the two groups regarding SpO2 levels or duration of SpO2 ≤ 95%.
The high-flow nasal oxygenation minimizes nasopharyngeal inspiratory resistance by providing gas flow that matches or exceeds peak inspiratory flow. It also prevents nasopharyngeal collapse by reducing inspiratory effort (15–17). HFNO has been shown to provide a degree of continuous positive airway pressure (18), which could prevent upper airway obstruction caused by deep sedation (19). It provides CO2 washout in spontaneously breathing patients, but in apneic patients it only provides oxygenation support. Riva et al. (20) reported that high-flow 100% oxygen (2 L/kg/min) did not extend the safe apnea time for children weighing 10–20 kg, compared with low-flow nasal cannula oxygen (0.2 L/kg/min). Unlike in adults, HFNO failed to show a CO2 clearance effect in children (21, 22). That is, HFNO failed to ventilate pediatric patients with apnea. Although the TcCO2 levels were higher in both the HFNO and LMA groups at some timepoint, the TcCO2 levels in both groups remained in the normal range throughout the procedures. When anesthesia was too deep, spontaneous breathing would reduce or stop altogether and blood CO2 would quickly accumulate. After spontaneous breathing was restored, CO2 was rapidly cleared if the airway was unobstructed (15).
This study showed that TcO2 decreased from baseline in both groups. We consider that propofol is a greater suppressant of external respiration compared with sevoflurane. The most likely reasons are atelectasis and intrapulmonary shunts, which are common during anesthesia. Of note, Booth et al. (23) found that the partial pressure of oxygen (PaO2) decreased gradually during tubeless anesthesia using HFNO. The benefits of HFNO under spontaneous breathing are that the respiratory support and anesthesia maintenance are delivered concurrently, yet independently, allowing titration according to the patient's vital signs and responses (24).
Perioperative adverse events were recorded in this study. We previously showed that ED was the most common complication after general anesthesia for dental treatment and was related to the duration of surgery (25). A recent meta-analysis determined that ED in children was less likely to occur after propofol compared with sevoflurane anesthesia (26). Prophylactic administration of the μ-opioid agonist significantly decreased the incidence of ED associated with sevoflurane anesthesia in children (27). In this study, local anesthetics and μ-opioid agonists were used, and the duration of surgery was <1 h, resulting in a low incidence of ED. We concluded that HFNO-IV may not promote ED when the duration of anesthesia is relatively brief. Because no tubes were placed in the larynx, throat discomfort was avoided in the HFNO group in this study. There was no significant difference between the groups regarding perioperative adverse events, suggesting that HFNO is safe for providing anesthesia during pediatric outpatient oral procedures. No aspiration occurred in either group.
Previous studies showed that if the mouth was closed, the positive end-expiratory pressure (PEEP) of the HFNO device increased non-linearly with increases in the flow rate. When the mouth was opened, the PEEP levels dropped rapidly to close to nil (28). In our experience, maintaining an open oral cavity and airway has proved important for adequate oxygenation, and we place moist gauze on the base of the tongue. A rubber dam is installed to prevent foreign body aspiration and ensure adequate HFNO use. The most popular sedation agents used during ambulatory gastrointestinal endoscopy in China are propofol and fentanyl (29). The application of alfentanil has only occurred more recently, and co-administration of alfentanil and propofol has proved more useful in ambulatory anesthesia compared with combined fentanyl and propofol (30).
The results of this study showed that the procedural time when using HFNO was similar to that of LMA. There was no significant difference in perioperative adverse events between the two groups. This study suggests that total intravenous anesthesia with propofol and alfentanil is safe in pediatric oral surgery and can be used without tracheal intubation. HFNO in ambulatory pediatric oral surgeries when sharing the same airway (oral cavity) with the surgeon is advantaged by guaranteed oxygenation, broadened surgical field, and decreased risks of laryngeal trauma and epistaxis.
This trial had several limitations. First, the data are from a single center, potentially compromising the generalizability of the findings, and by its nature, it was impossible to blind all the participants to the allocation of the two oxygen delivery modalities. The patient population was relatively narrow, i.e., included only healthy children aged 2–7 years. It was reported that FiO2 > 0.8 in anesthetized children with normal lungs decreases lung volume in the immediate postoperative period, accompanied by persistent ventilation inhomogeneity (31). In this study, the FiO2 in HFNO was set at 100%, and the oxygen flow rate was 2 L/kg/min. The actual FiO2 may be lower than 100%.
Spontaneous breathing was maintained throughout the trial, and our results may not apply to deeply sedated children who experienced prolonged periods of apnea. In future studies, specific FiO2 monitoring during pediatric HFNO administration is needed to determine the optimal FiO2 and flow rate that will ensure safe oxygenation.
Of note, this study involved only elective procedures, which precludes extrapolation to emergency settings, younger children, or those with difficult airways, heart or lung disease, a history of upper airway obstruction, or obesity.
Conclusion
The high-flow nasal oxygenation was not inferior compared with LMA in pediatric outpatient oral surgery in spontaneously breathing patients under deep sedation. It can be considered an alternative to LMA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Stomatology Hospital affiliated Chongqing Medical University (Registration No. CQHS-NT10-2020). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
CY, LR, GH, YY, and CZ contributed to the study design, performance, analysis, and manuscript preparation. YWu, YWa, and CZ contributed to patient recruitment, the conduct of the study, and the interpretation of data. CY, LR, and GH contributed to the study design and finalizing the manuscript. All authors contributed to the manuscript revision.
Funding
This trial was supported by the Intelligent Medicine Project of Chongqing Medical University, China (Grant No. ZHYX202116) and the CSA Clinical Research Fund (CSA-A2021-05).
Acknowledgments
The authors thank the oral and maxillofacial surgeons of the Stomatology Hospital of Chongqing Medical University for their generous assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1001213/full#supplementary-material
References
1. Zhao N, Deng F, Yu C. Anesthesia for pediatric day-case dental surgery: a study comparing the classic laryngeal mask airway with nasal trachea intubation. J Craniofac Surg. (2014) 25:e245–e8. doi: 10.1097/SCS.0000000000000547
2. Maupeu L, Raguin T, Hengen M, Diemunsch P, Schultz P. Indications of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in laryngoscopy, a prospective study of 19 cases. Clin Otolaryngol. (2019) 44:182–6. doi: 10.1111/coa.13252
3. Yang SH, Wu CY, Tseng WH, Cherng WY, Hsiao TY, Cheng YJ, et al. Nonintubated laryngomicrosurgery with Transnasal Humidified Rapid-Insufflation Ventilatory Exchange: A case series. J Formos Med Assoc. (2019) 118:1138–43. doi: 10.1016/j.jfma.2018.11.009
4. Nay MA, Fromont L, Eugene A, Marcueyz JL, Mfam WS, Baert O, et al. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). Br J Anaesth. (2021) 127:133–42. doi: 10.1016/j.bja.2021.03.020
5. Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. (2015) 70:323–9. doi: 10.1111/anae.12923
6. Gustafsson IM, Lodenius, Tunelli J, Ullman J, Jonsson FM. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE)–a physiological study. Br J Anaesth. (2017) 4:610–7. doi: 10.1093/bja/aex036
7. Riva T, Seiler S, Stucki F, Greif R, Theiler L. High-flow nasal cannula therapy and apnea time in laryngeal surgery. Paediatr Anaesth. (2016) 26:1206–8. doi: 10.1111/pan.12992
8. Booth A, Vidhani K, Lee PK, Thomsett CM. SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen (STRIVE Hi) maintains oxygenation and airway patency during management of the obstructed airway: an observational study. Br J Anaesth. (2017) 118:444–51. doi: 10.1093/bja/aew468
9. Jyj A, Ehk B, Jhl B, Yej B, Hsk B, Skk A. Pediatric airway surgery under spontaneous respiration using high-flow nasal oxygen–science direct. Int J Pediatr Otorhinolaryngol. (2020) 134:110042. doi: 10.1016/j.ijporl.2020.110042
10. Klotz D, Seifert V, Baumgartner J, Teufel U, Fuchs H. High-flow nasal cannula versus standard respiratory care in pediatric procedural sedation: a randomized controlled pilot trial. Pediatr Pulmonol. (2020) 55:2706–12. doi: 10.1002/ppul.24975
11. Duyu M, Mocan Çaglar Y, Karakaya Z, Usta Aslan M, Yilmaz S, Ören Leblebici AN, et al. Comparison of arterial CO(2) estimation by end-tidal and transcutaneous CO(2) measurements in intubated children and variability with subject related factors. J Clin Monit Comput. (2021) 35:101–11. doi: 10.1007/s10877-020-00569-w
12. Engelhardt T, Mccheyne AJ, Morton N, Karsli C, Bissonnette B. Clinical adaptation of a pharmacokinetic model of Propofol plasma concentrations in children. Paediatr Anaesth. (2010) 18:235–9. doi: 10.1111/j.1460-9592.2007.02407.x
13. Nancy Si. Jerrold, Lerman. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. (2004) 100:1138–45. doi: 10.1097/00000542-200405000-00015
14. Patel SS, Goa KL. Sevoflurane. A review of its pharmacodynamic and pharmacokinetic properties and its clinical use in general anaesthesia. Drugs. (1996) 51:658–700. doi: 10.2165/00003495-199651040-00009
15. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. (2009) 103:1400–5. doi: 10.1016/j.rmed.2009.04.007
16. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. (2014) 15:1–6. doi: 10.1097/PCC.0000000000000011
17. Pham TM, O'Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. (2015) 50:713–20. doi: 10.1002/ppul.23060
18. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. (2011) 39:1103–10. doi: 10.1177/0310057X1103900620
19. Sago T, Watanabe K, Kawabata K, Shiiba S, Watanabe S. A Nasal High-flow system prevents upper airway obstruction and hypoxia in pediatric dental patients under intravenous sedation. J Oral Maxillofac Surg. (2020) 79(Suppl. 1):18. doi: 10.1016/j.joms.2020.10.018
20. Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. (2018) 120:592–9. doi: 10.1016/j.bja.2017.12.017
21. Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. (2017) 2:232–8. doi: 10.1093/bja/aew401
22. Riva T, Préel N, Theiler L, Greif R, Bütikofer L, Ulmer F, et al. Evaluating the ventilatory effect of transnasal humidified rapid insufflation ventilatory exchange in apnoeic small children with two different oxygen flow rates: a randomised controlled trial*. Anaesthesia. (2021) 76:15335. doi: 10.1111/anae.15335
23. Booth AWG, Vidhani K, Lee PK, Coman SH, Pelecanos AM, Dimeski G, et al. The Effect of high-flow nasal oxygen on carbon dioxide accumulation in apneic or spontaneously breathing adults during airway surgery: a randomized-controlled trial. Anesth Analg. (2021) 133:133–41. doi: 10.1213/ANE.0000000000005002
24. McCormack JG, Krosnar S, Baxter A. Reply to Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. (2017) 119:172. doi: 10.1093/bja/aex160
25. Chao Z, Gui Jin H, Cong Y. The effect of general anesthesia for ambulatory dental treatment on children in Chongqing, Southwest China. Paediatr Anaesth. (2017) 27:98–105. doi: 10.1111/pan.12983
26. Kanaya A, Kuratani N, Satoh D, Kurosawa S. Lower incidence of emergence agitation in children after propofol anesthesia compared with sevoflurane: a meta-analysis of randomized controlled trials. J Anesth. (2014) 28:4–11. doi: 10.1007/s00540-013-1656-y
27. Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. (2016) 26:139–50. doi: 10.1111/pan.12815
28. Luo JC, Lu MS, Zhao ZH, Jiang W, Xu B, Weng L, et al. Positive end-expiratory pressure effect of 3 high-flow nasal cannula devices. Respir Care. (2017) 62:888–95. doi: 10.4187/respcare.05337
29. Zhou S, Zhu Z, Dai W, Qi S, Tian W, Zhang Y, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. (2021) 127:56–64. doi: 10.1016/j.bja.2021.01.028
30. Hui JK, Critchley LA, Karmakar MK, Lam PK. Co-administration of alfentanil-propofol improves laryngeal mask airway insertion compared to fentanyl-propofol. Can J Anaesth. (2002) 49:508–12. doi: 10.1007/BF03017932
Keywords: high-flow nasal oxygenation, pediatric anesthesia, child ambulatory oral surgery, deep sedation, non-invasive ventilation
Citation: Ran L, Huang G, Yao Y, Wu Y, Zhang C, Wang Y and Yu C (2022) Efficacy of high-flow nasal oxygenation compared with laryngeal mask airway in children undergoing ambulatory oral surgery under deep sedation: A randomized controlled non-inferiority trial. Front. Med. 9:1001213. doi: 10.3389/fmed.2022.1001213
Received: 23 July 2022; Accepted: 04 November 2022;
Published: 02 December 2022.
Edited by:
Ivan Pavlov, Hôpital de Verdun, CanadaReviewed by:
Diansan Su, Shanghai Jiao Tong University, ChinaYonglei Huang, Shanghai Jiao Tong University, China
Copyright © 2022 Ran, Huang, Yao, Wu, Zhang, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Yu, NTAwMTU4QGhvc3BpdGFsLmNxbXUuZWR1LmNu
 Longkuan Ran1,2,3
Longkuan Ran1,2,3 Cong Yu
Cong Yu