
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 September 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1000084
This article is part of the Research Topic New Advances in Bedside Assessment and Monitoring of Acute Respiratory Failure Patients View all 5 articles
 Rolf Erlebach1†
Rolf Erlebach1† Lennart C. Wild2†
Lennart C. Wild2† Benjamin Seeliger3†
Benjamin Seeliger3† Ann-Kathrin Rath4
Ann-Kathrin Rath4 Rea Andermatt1
Rea Andermatt1 Daniel A. Hofmaenner1
Daniel A. Hofmaenner1 Jens-Christian Schewe2
Jens-Christian Schewe2 Christoph C. Ganter1
Christoph C. Ganter1 Mattia Müller1
Mattia Müller1 Christian Putensen2
Christian Putensen2 Ruslan Natanov5
Ruslan Natanov5 Christian Kühn5,6
Christian Kühn5,6 Johann Bauersachs6,7
Johann Bauersachs6,7 Tobias Welte3,6
Tobias Welte3,6 Marius M. Hoeper3,6
Marius M. Hoeper3,6 Pedro D. Wendel-Garcia1
Pedro D. Wendel-Garcia1 Sascha David1*‡
Sascha David1*‡ Christian Bode2‡
Christian Bode2‡ Klaus Stahl4‡ for the BonHanZA (Bonn-Hannover-Zurich-ARDS) study group
Klaus Stahl4‡ for the BonHanZA (Bonn-Hannover-Zurich-ARDS) study groupObjective: Veno-venous (V-V) extracorporeal membrane oxygenation (ECMO) is increasingly used to support patients with severe acute respiratory distress syndrome (ARDS). In case of additional cardio-circulatory failure, some experienced centers upgrade the V-V ECMO with an additional arterial return cannula (termed V-VA ECMO). Here we analyzed short- and long-term outcome together with potential predictors of mortality.
Design: Multicenter, retrospective analysis between January 2008 and September 2021.
Setting: Three tertiary care ECMO centers in Germany (Hannover, Bonn) and Switzerland (Zurich).
Patients: Seventy-three V-V ECMO patients with ARDS and additional acute cardio-circulatory deterioration required an upgrade to V-VA ECMO were included in this study.
Measurements and main results: Fifty-three patients required an upgrade from V-V to V-VA and 20 patients were directly triple cannulated. Median (Interquartile Range) age was 49 (28–57) years and SOFA score was 14 (12–17) at V-VA ECMO upgrade. Vasoactive-inotropic score decreased from 53 (12–123) at V-VA ECMO upgrade to 9 (3–37) after 24 h of V-VA ECMO support. Weaning from V-VA and V-V ECMO was successful in 47 (64%) and 40 (55%) patients, respectively. Duration of ECMO support was 12 (6–22) days and ICU length of stay was 32 (16–46) days. Overall ICU mortality was 48% and hospital mortality 51%. Two additional patients died after hospital discharge while the remaining patients survived up to two years (with six patients being lost to follow-up). The vast majority of patients was free from higher degree persistent organ dysfunction at follow-up. A SOFA score > 14 and higher lactate concentrations at the day of V-VA upgrade were independent predictors of mortality in the multivariate regression analysis.
Conclusion: In this analysis, the use of V-VA ECMO in patients with ARDS and concomitant cardiocirculatory failure was associated with a hospital survival of about 50%, and most of these patients survived up to 2 years. A SOFA score > 14 and elevated lactate levels at the day of V-VA upgrade predict unfavorable outcome.
Extracorporeal membrane oxygenation (ECMO) has become an integral part in supporting patients with severe acute respiratory distress syndrome (ARDS) at specialized referral centers, due to the results of the CESAR trial and affected by the pandemics of H1N1 in 2009 and SARS-CoV-2 in 2019–2022 (1–3), despite controversial results from the randomized EOLIA trial (4). A veno-venous (V-V) cannulation technique is primarily employed to correct life-threatening hypoxemia and/or hypercapnia and to enable lung protective ventilation strategies (5). In cases of additive refractory cardio-circulatory deterioration, an upgrade of the V-V-system using an additional arterial return cannula (termed V-VA ECMO) to retain sufficient organ perfusion has been used by experienced centers (6). In such a triple cannulation set-up, V-VA ECMO provides both respiratory and hemodynamic support potentially representing a therapeutic option for patients with ARDS who develop secondary severe hemodynamic impairment (or heart failure). However, the literature of ARDS patients with secondary shock supported by V-VA ECMO is scarce and confined to case reports (7–10) and small series (11–13). Moreover, patient populations were heterogeneous, including both primary cardiogenic shock patients (starting with V-A ECMO) who were later upgraded with an additional venous cannula for treatment of respiratory failure (8, 12), as well as patients with primary ARDS (starting on V-V ECMO) who were later upgraded to V-VA for treatment of secondary cardio-circulatory failure (10, 13). Heterogeneity in cannulation sequences makes conclusions about the outcomes of patients with ARDS that subsequently require an arterial cannulation, upgrade difficult. Additionally, no data exist concerning long-term survival of these patients beyond the period of critical care or hospital stay and the extent of chronic organ failure in survivors is unknown.
This retrospective study from three ECMO referral centers aimed at describing the short and long-term outcomes of a cohort of patients with predominant ARDS receiving V-V ECMO support who required an upgrade to V-VA ECMO because of additional cardio-circulatory failure. Additionally, factors associated with poor outcome of V-VA ECMO support strategy were analyzed.
In this retrospective observational cohort study, we aimed to describe characteristics and outcome of patients with ARDS and additional acute cardio-circulatory failure under V-VA ECMO support. Data were collected from the clinical information system by the local study team of two centers in Germany (Hannover Medical School, University Hospital Bonn) and one center in Switzerland (University Hospital Zurich). Inclusion criteria were ARDS with V-V ECMO support and upgrade to V-VA ECMO or direct V-VA ECMO implantation to simultaneously treat primary respiratory failure and secondary cardio-circulatory deterioration during the period from January 2008 to September 2021. In the contributing centers an escalation from V-V to V-VA ECMO is considered in refractory shock after optimization of conventional respiratory and hemodynamic support. Patients with primary cardiac failure requiring V-A ECMO therapy that later developed respiratory failure and required additional venous cannulation (e.g., upgrade from V-A ECMO to V-AV ECMO) were excluded from this analyses. The study was approved by the institutional review boards at all sites (Ethikkommission Hannover Medical School: #9720 BO K 2021, 2021/04/21; Kantonale Ethikkommission Zürich: ZH 2021-01804, 2021/10/08; Ethikkommission University Hospital Bonn: #488/21, 2021/05/07). Informed consent was waived by the regulatory body for all patients at both sites in Germany and for patient in Zurich before 2016 and later if death occurred before consent could be obtained. Consent has been obtained for all patients not falling under above conditions. All analyses performed involving human data were in accordance with the ethical standards of the institutional and national research committee of Switzerland and Germany and with the 1964 Helsinki Declaration and its latest amendments.
Extracorporeal membrane oxygenation (ECMO) nomenclature based on the ELSO Maastricht Treaty for ECLS Nomenclature (14), where V-VA ECMO stands for an upgrade of V-V ECMO in patients with predominant ARDS with an additional arterial return cannula. Differential return blood flow of V-VA ECMO was regulated with gate clamps and additional flow monitors at the venous return cannula.
We collected demographic data, current illness leading to ECMO support and relevant comorbidities. Respiratory and hemodynamic parameters and the extent of organ support were analyzed at two time points – before V-V ECMO implantation and before V-VA ECMO upgrade. ECMO configuration and initial settings for V-V ECMO and V-VA ECMO were collected. The following outcome parameters were included: ECMO runtime, ICU and hospital length of stay, organ-specific outcomes (lung transplantation, long-term oxygen therapy, chronic kidney disease, congestive heart failure), mortality during ICU- and hospital stay and after one and two years. Additional, Vasoactive-inotropic score and serum lactate 24 h after V-VA ECMO upgrade was collected. If patients deceased in the first 24 h, the latest value before discontinuation of life-sustaining therapies was documented.
ARDS was defined according to the Berlin definition (15). ARDS was further classified as primary, when a direct lung insult was the most likely cause, or as secondary in case of an extra-pulmonary origin of ARDS. Primary ARDS was further divided into identified lung insults according to the RESP-score (16). The PRESERVE mortality risk score comprises pre-ECMO parameters that were shown to be correlated with mortality as a lower PRESERVE score is associated with a lower risk of death 6 months after ICU discharge (17). The Sequential Organ Failure Assessment (SOFA) score was used to assess the severity of organ dysfunction and to determine the predicted mortality risk (18). The Vasoactive-inotropic score (VIS) was used to quantify pharmacologic hemodynamic support by different inotropes and vasopressors and to compare it between groups (19).
We used a clinical definition of acute cardio-circulatory deterioration based on evidence of cardiac impairment on bed-side echocardiography or extended hemodynamic monitoring including cardiac output measurements, the degree of hemodynamic support, signs of impaired organ perfusion on clinical examination and laboratory parameters such as lactate levels and urine output. Left ventricular ejection fraction (LVEF) and right ventricular ejection fraction (RVEF) were semi-quantitatively categorized as good/sustained and reduced, respectively, because exact measurements were limited due to time-critical ECMO upgrade.
Comorbidities were extracted from the clinical information system. For immunosuppression we used the definition of the APACHE II Score (20, 21) and defined high-dose steroid therapy as prednisone-equivalent doses of ≥ 7.5 mg/day. Obesity was defined as body-mass index (BMI) of ≥ 30 kg/m2.
Intracranial hemorrhages were classified as minor when occasionally identified on routine cerebral imaging or as major when requiring neurosurgical intervention or resulting in any neurological deficit. Anemia requiring four or more red blood cell concentrates within 24 h after V-VA ECMO upgrade was chosen as a clinically relevant cut-off for bleeding complications.
Comparison of variables between two time-points was performed using the Wilcoxon Signed Rank and Chi-Squared Test, as appropriate. A two-sided p < 0.05 was considered statistically significant. Comparison between variables at V-VA ECMO upgrade and 24 h follow-up was performed using the paired Wilcoxon Signed Rank Test. Clinically relevant population characteristics and characteristics at the time of V-VA ECMO implantation were stratified according to ICU-mortality and compared using Cox proportional-hazards model for 60-day ICU-mortality. Variables with a signification association in the univariate Cox-model were entered into the multivariate Cox-model. Ordinal variables (SOFA score) were further categorized into two groups with the cut-off chosen according to the receiver operating characteristic (ROC) curve and Youden Index. Proportional-hazards assumptions were checked visually and with Schoenfeld Individual Test. After model reduction method and input of interaction terms, variables were only retained if they were found to contribute to the model. Survival plots were generated for overall survival and 60-day survival stratified by variables in the multivariate Cox-model using the best cut-off chosen with ROC curve and Youden Index. Missing data are indicated in Supplementary Tables 1–4 of the Supplementary material.
In the three study centers, 73 patients met the inclusion criteria and were analyzed. In 53 (73%) patients V-V ECMO was upgraded to V-VA ECMO after a median of 48 (Interquartile Range, 8-120) hours. In 20 (27%) patients, primary V-VA ECMO support was applied due to simultaneous presence of respiratory and cardio-circulatory failure. Most common reason for respiratory failure was primary ARDS (n = 65, 89%), particularly bacterial pneumonia (n = 33, 47%). Table 1 summarizes the patient characteristics.
In those patients where echocardiographic data were available, reduced right ventricular systolic function was observed in 64% of patients before V-VA ECMO upgrade (25 out of 39 patients with available data). A trend toward higher vasopressor and inotropic doses was observed before V-VA ECMO upgrade, represented by a numerically higher median VIS of 27 (0–77) at V-V ECMO implantation and 53 (12–123) at V-VA ECMO upgrade (p = 0.054). Epinephrine was used significantly more frequently before V-VA ECMO (n = 18, 25%) than before V-V ECMO (n = 3, 6%) (p = 0.014). Eleven (15%) patients had undergone cardiopulmonary resuscitation before V-VA ECMO implantation. Clinical condition and organ support before V-V and V-VA ECMO are summarized and further stratified by initial V-VA ECMO implantation or later V-VA ECMO upgrade in Table 2.
The femoral site for venous drainage (n = 66, 90%) and the jugular site for venous return (n = 63, 86%) was the most frequent configuration of V-V ECMO. Cannulation of both the femoral artery (n = 41, 56%) and the subclavian artery (n = 32, 44%) where used in V-VA ECMO upgrade. The most frequent complication following ECMO insertion was anemia requiring four or more red blood cell concentrates in 24 h (n = 38, 52%). ECMO configurations and complications are summarized in Table 3.
VIS decreased significantly from 53 (12–123) at V-VA upgrade to 9 (3–37) after 24 h (p < 0.001). During the same time interval lactate levels decreased from 2.5 (1.6–5.9) to 1.8 (1.2–3.2) (p = 0.053). Both comparisons are visualized in Figure 1. V-VA ECMO and V-V ECMO was successful weaned in 47 (64%) and 40 (55%) patients, respectively. Thirty-five (48%) V-VA ECMO patients died during their ICU stay. Two patients (3%) died during the later hospital course. Of those patients, one died of pericardial tamponade and another patient died of recurrent respiratory failure due to progressive lung allograft dysfunction. After hospital discharge further two patients (3%) died during a two-year follow-up. Given that in six (8%) patients follow-up time was less than two years, an overall two year-mortality of 58% (39 of 67) was observed. Follow-up data and organ-specific outcomes are summarized in Table 4.
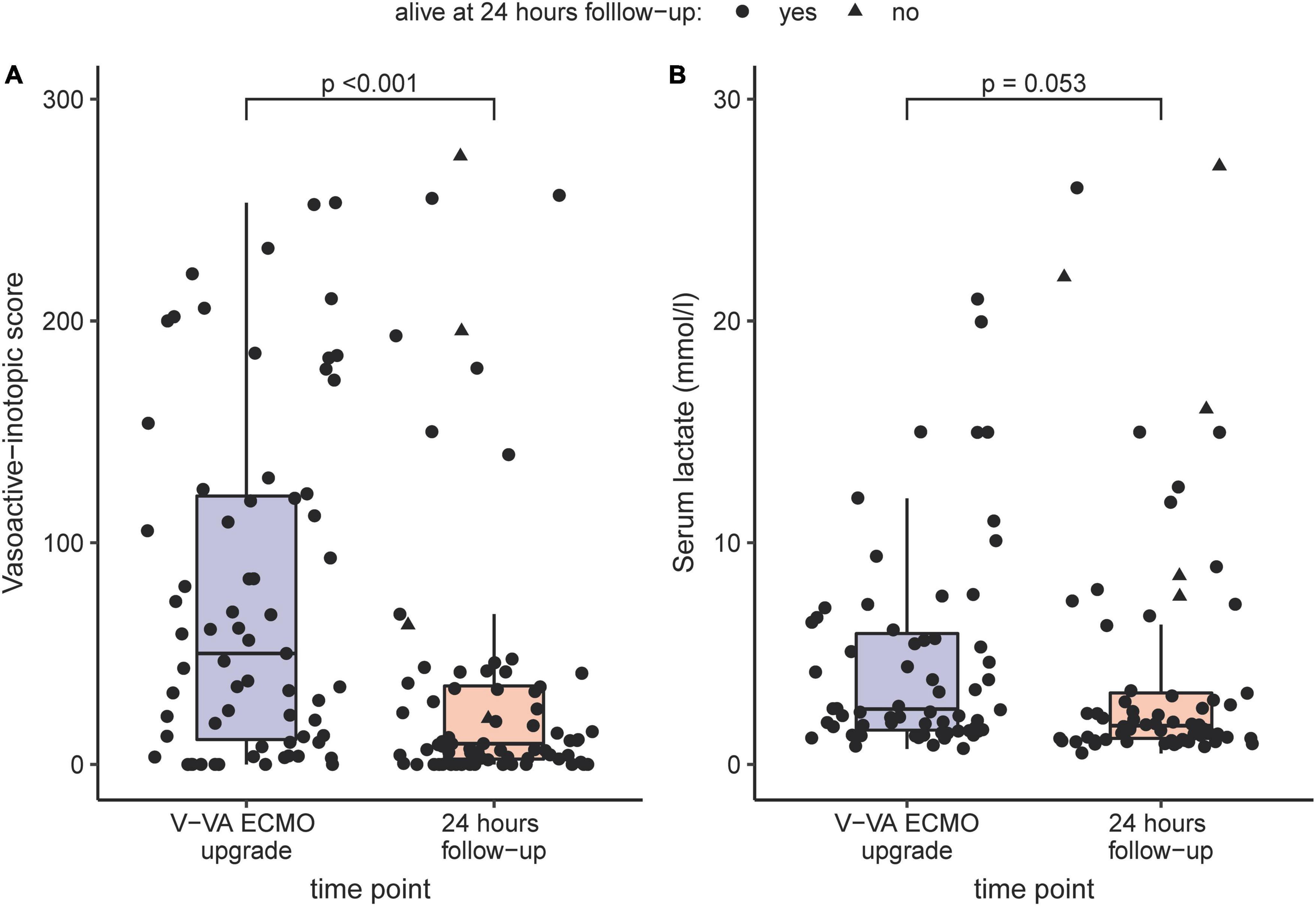
Figure 1. Comparison of Vasoactive-inotropic score (A) and serum lactate (B) before V-VA ECMO upgrade and after 24 h under V-VA ECMO support visualized as boxplots and scatterplots. If patients deceased in the first 24 h, the latest value before discontinuation of life-sustaining therapies was chosen.
Stratification of predictive variables at the time of V-VA ECMO implantation and results from Cox regression for 60-day ICU-mortality are shown in Figure 2. Of the variables that showed a significant association with 60-day ICU-mortality, five variables (SOFA score, lactate, VIS, renal replacement therapy and pH) were entered into the multivariate analysis. The PaO2/FIO2 ratio was excluded because it is not a reliable parameter for oxygen requirements under ECMO support. In the final multivariable model, SOFA score > 14 (Hazard ratio 4.28; 95% CI: 1.55–11.80, p = 0.005) and lactate level [(Hazard ratio 1.004; 95% CI: 1.000–1.008), p = 0.049] were significantly associated with 60-day ICU-mortality. Neither in-hospital nor 60-day nor 2-year survival was different between patients receiving initial V-VA cannulation and those receiving initial V-V cannulation with later V-VA upgrade (Supplementary Table 5). The results of the Cox proportional-hazards model are provided in Table 5. Survival plots stratified for these predictors are shown in Figure 3 and Supplementary Figure 1 (Supplementary material). Reduction in VIS 24 h after V-VA ECMO upgrade was significantly associated with improved survival (Supplementary Figure 2 and Supplementary Table 6).
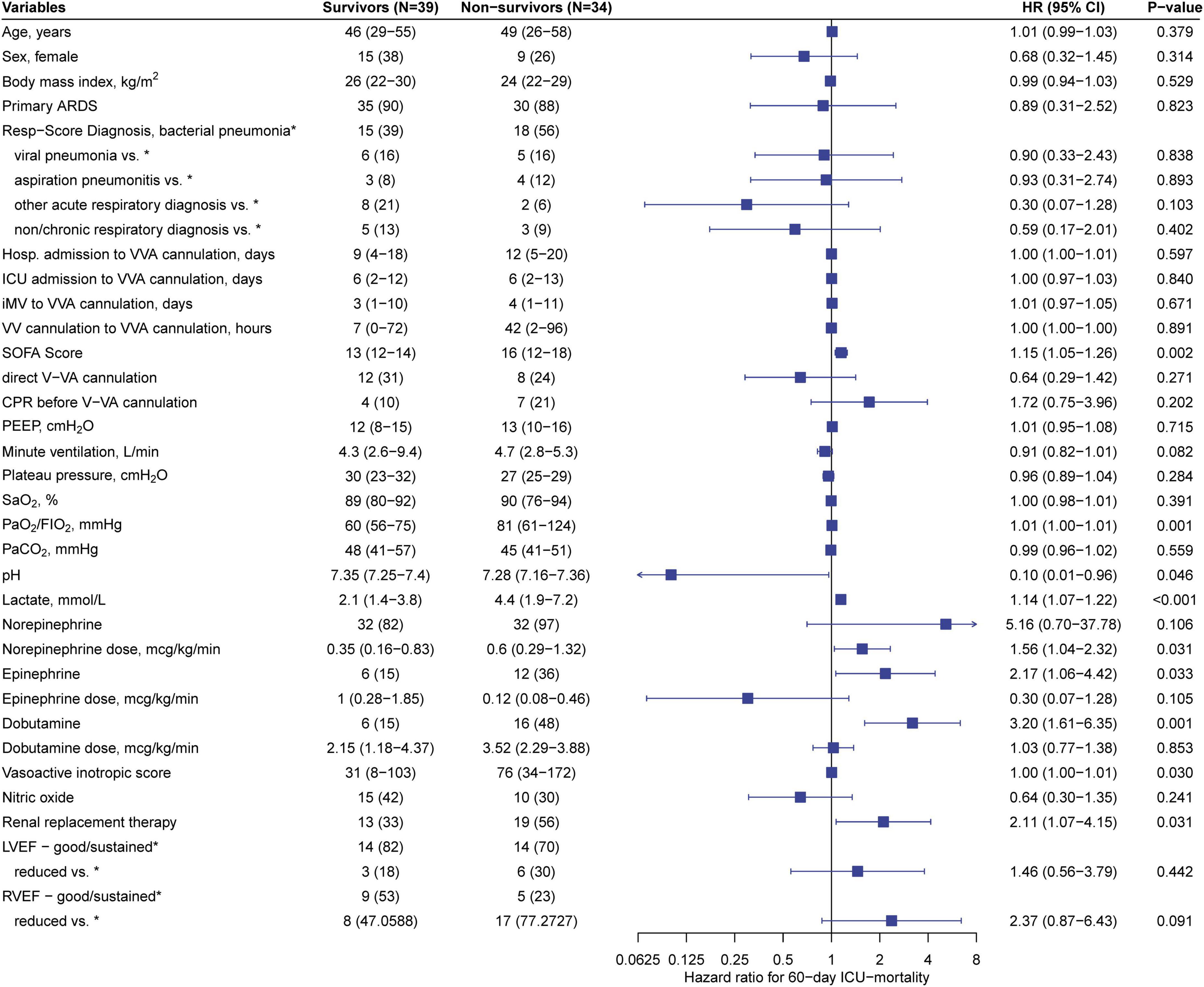
Figure 2. Stratification of predictive variables at veno-veno-arterial ECMO cannulation. Left: Predictive variables at the time of V-VA ECMO cannulation stratified in survivors and non-survivors according to 60-day ICU mortality. Values are expressed as n (%) or median (interquartile range). Right: Forest-plot and univariate cox regression for 60-day ICU-mortality. Values are expressed as Hazard ratio (HR) with 95% confidence interval (CI) and p-value. ARDS, acute respiratory distress syndrome; CPR, cardiopulmonary resuscitation; FIO2, fraction of inspired oxygen; Hosp., Hospital; ICU, intensive care unit; iMV, invasive mechanical ventilation; LVEF, left ventricular ejection fraction; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
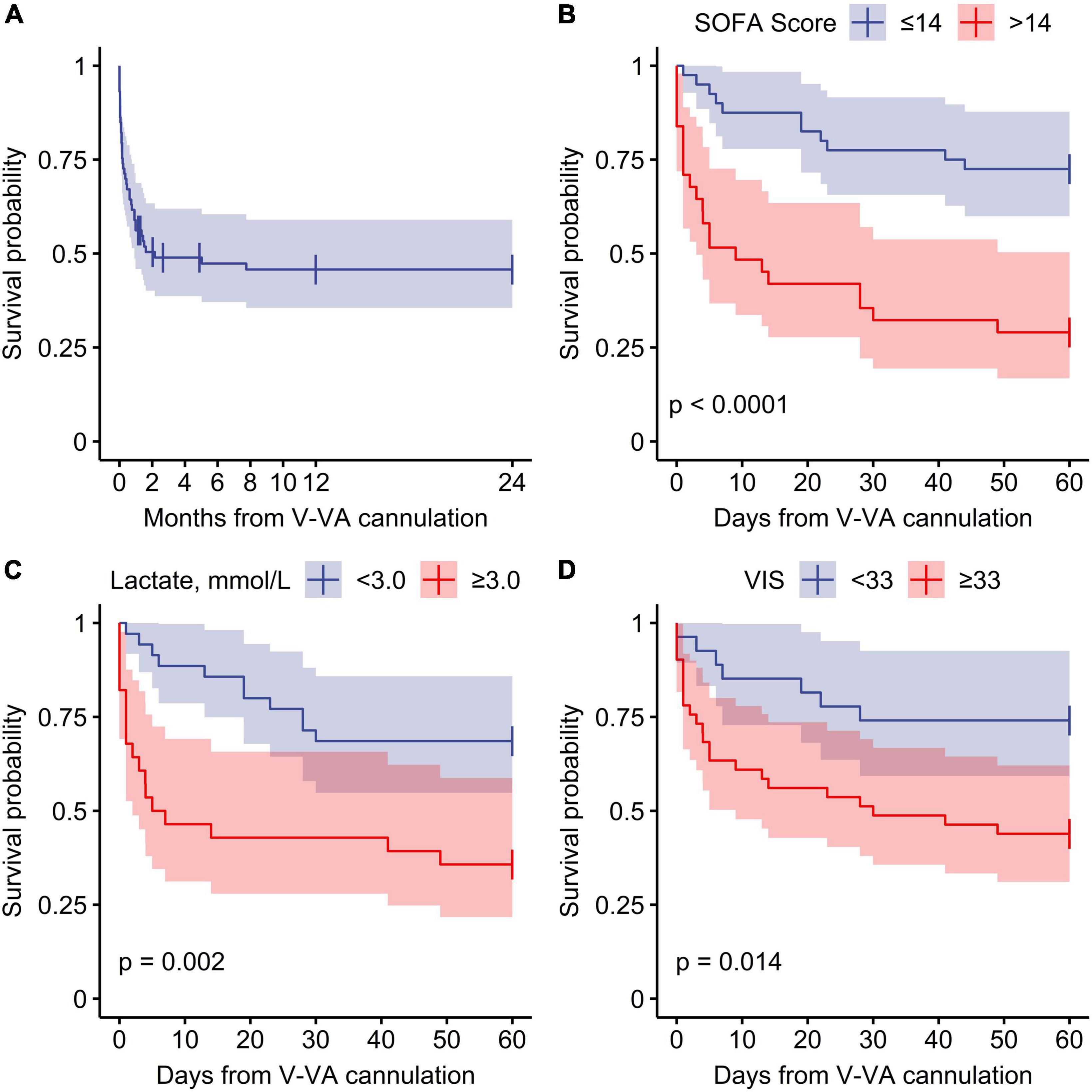
Figure 3. Survival function from veno-venoarterial ECMO cannulation. (A) Overall survival, (B–D) stratified survival function for 60-day ICU mortality. SOFA, Sequential Organ Failure Assessment; VIS, Vasoactive inotropic score.
In the present retrospective study, patients with predominant ARDS on V-V ECMO support who required additional V-VA ECMO support due to acute cardio-circulatory failure had an encouraging ICU survival rate of 52%. Two patients died during the later hospital course thereafter and only an additional two died in the two-years follow-up. Besides this unexpected high long-term survival only a minority of survivors suffered from relevant persistent organ dysfunction. A SOFA-score of more than 14 at the day of V-VA ECMO upgrade independently predicted an unfavorable outcome in these critically ill patients.
Previous studies and case series have found survival rates of patients with V-VA ECMO support ranging from 39 to 75% (11–13, 22–27). This wide range might be attributable to heterogeneity of the patient cohorts, including those with cardiogenic shock requiring initial V-A ECMO and later venous ECMO upgrade grouped together with ARDS patients on initial V-V ECMO support with a later arterial upgrade. Furthermore, the number of investigated patients in these studies (11, 13, 24–26) was small (1–21 patients), with high risk of bias, which might contribute to the wide range of survival outcomes. The registry of the Extracorporeal Life Support Organization (ELSO) showed a survival rate of 38% in patients requiring V-VA ECMO support (28). The reason for the more favorable outcome of patients in the current study might be explained by a more stringent selection of patients and by a homogenization focusing on a group with severe acute respiratory failure and a subsequent or concomitant cardio-circulatory deficit.
After hospital discharge, only 2 patients died during the 2-year follow-up, and survivors showed a surprisingly good organ function. Consistently, previous studies demonstrated good long-term outcomes after classical ECMO support (i.e., V-V- or V-A-cannulation) with most patients’ health almost restored to their previous level (1, 29, 30). V-A ECMO patients seem to have a worse long-term health status, what might be explained by a more serious initial clinical condition [e.g., acute (on chronic) heart failure, eCPR] (29). The fact that long-term outcome presented in this study is comparable with outcome of individuals after classical V-A or V-V EMCO support (1, 29, 30) shows that V-VA ECMO upgrading is feasible and should be considered for appropriate patients.
Identifying patients who will benefit from V-VA ECMO upgrade remains a challenge and poor selection is associated with unfavorable outcomes on ECMO (31, 32). We showed that the outcome of V-VA patients significantly worsened when their SOFA score exceeded 14 at the time of V-VA consideration. While the SOFA score was initially developed to describe the degree of organ function (18), it is increasingly used to predict mortality for patients with various conditions in the ICU (33, 34). While reliable data for triple cannulated V-VA ECMO patients are missing, a recent study found a higher SOFA score in non-survivors compared to survivors before classical V-V ECMO implantation, but with only moderate prognostic performance (35). In contrast, there was no difference in V-A ECMO patients in terms of survival reported in the same study (35). Besides the prognostic value of the SOFA-score as a global marker of organ dysfunction, we found that parameters of hemodynamic compromise, e.g., high serum lactate levels and an increased need for vasopressors, were associated with ICU-mortality. The high doses of vasopressors probably represent refractory circulatory failure with subsequent right ventricular dysfunction in a substantial proportion of patients (64%). After 24 h under V-VA ECMO support, vasopressor requirements were significantly lower indicating that V-VA ECMO could be effective in restoring hemodynamic stability in these patients.
While the significance of elevated serum lactate on outcome of patients who are commenced on either V-A- or V-V ECMO support is widely appreciated (36–38), this is to our knowledge the first study that extents the value of these clinical parameters to prognosis prediction before V-VA-cannulation. We found that elevated lactate levels independently predicted ICU-mortality. Therefore, the clinical decision for V-VA ECMO implementation should not rely solely on a risk score such as SOFA but be incorporated in the complex interaction of clinical status including lactate levels, need for vasopressor support and assessment of renal function in addition to clinical experience.
When patients develop cardio-circulatory failure while under V-V ECMO support, an alternative to V-VA upgrade might be converting from V-V to V-A cannulation. Falk et al. have shown that patients who required a conversion from V-V to V-A ECMO had a higher mortality than patients with initial V-A cannulation (39). Similarly, another study showed that initial V-A cannulation in ARDS patients is an independent predictor for increased mortality (40). In pronounced RV failure, adding a second venous drainage cannula (VV-A) to improve RV preload reduction and intracardiac shunt flow may be beneficial, but larger clinical studies have not been conducted to verify a clinical benefit.
In the current work, patients were approximately half of their overall ECMO runtime on V-VA configuration, suggesting that the need for respiratory support outlives the requirement for cardio-circulatory support. Since V-A ECMO support increases the risk for bleeding (41), renal failure, vascular complications and the Harlequin syndrome (42), downgrading V-VA to V-V cannulation, when hemodynamic stability has reached, might improve outcome compared to continued V-A ECMO support. In line with this approach, Stöhr et al. showed a lower 30-day-mortality for ARDS patients with V-VA cannulation when compared to V-A or V-V ECMO support (11).
Limitations of the present study are the retrospective design including missing data on follow-up and of hemodynamic variables. Since echocardiography data were not available in about half of the patients, the exact cause of acute cardiocirculatory failure could not be exactly differentiated in those patients. Due to similar reasons, extended hemodynamic monitoring was mostly not installed at the time of V-VA ECMO upgrade and retrospective interpretation is difficult under V-V ECMO support. However, insertion of the third arterial cannula was often carried out in an absolute emergency setting therefore not allowing performance of in depth echocardiography imaging. On the other hand, in only six patients the follow-up time was less than two years, allowing a reasonable interpretation of long-term outcome. Hemodynamic parameters other than vasopressor support and lactate after V-VA ECMO upgrade were not analyzed, hence limiting conclusions about the direct effect of V-VA ECMO. Because physiologic parameters are difficult to interpret retrospectively, mortality was chosen as a more robust endpoint. The analysis of three high-volume centers data might provide real-world clinical experience to ECMO providers. The design and missing data, however, limits the possibility of objectifying the individual clinical decisions that led to V-VA ECMO upgrade/cannulation. In addition, mechanisms leading to cardiocirculatory deterioration may differ between patients with initial triple cannulation and patients on V-V ECMO that were upgraded later to V-VA. Furthermore, patients were recruited over a long time span of 13 years in which the therapy of ARDS and handling of ECMO support has evolved (43). Therefore, the population is likely highly heterogeneous covering patients over a long time span and with no prespecified ethiological/physiological inclusion criteria but only clinical. Regarding the organ specific outcomes, patients with worse outcomes might have been more likely to drop out of the follow up. A prospective evaluation or matched cohort of patients with and without later V-VA ECMO upgrade would overcome most of the limitations above, but is unlikely to be conducted in the near future because of the time-critical setting and relatively few affected patients.
In summary, this work demonstrated in the currently largest cohort of V-VA ECMO patients coming from V-V ECMO due to initial ARDS that approximately every second patient survived until hospital discharge. This encouraging survival rate was preserved over a two-year period where only a minority suffered from relevant organ dysfunction. Thus, an arterial upgrade of V-V ECMO patients suffering from ARDS to V-VA ECMO should not be rendered as futile per se. In our cohort, a SOFA score > 14 and elevated lactate levels at the time of V-VA upgrade evaluation predicted unfavorable outcome.
The data analyzed in this study was subject to the following licenses/restrictions: Authors can confirm that all relevant data are included in the article and/or its Supplementary material. The corresponding author may provide specified analyses or fully de-identified parts of the dataset upon reasonable request. Requests to access these datasets should be directed to SD, c2FzY2hhLmRhdmlkQHVzei5jaA==.
The study was approved by the institutional review boards at all sites (Ethikkommission Hannover Medical School: #9720 BO K 2021, 2021/04/21; Kantonale Ethikkommission Zürich: ZH 2021-01804, 2021/10/08; and Ethikkommission University Hospital Bonn: #488/21, 2021/05/07). Informed consent was waived by the regulatory body for all patients at both sites in Germany and for patient in Zurich before 2016 and later if death occurred before consent could be obtained. Consent has been obtained for all patients not falling under above conditions.
RE, LW, BS, A-KR, PW-G, SD, CB, and KS conceived and designed the research project. RE, BS, LW, A-KR, RA, MM, and PW-G handled the data acquisition. RE, BS, DH, PW-G, SD, CB, and KS analyzed the data. RE, LW, A-KR, and RA wrote the first draft of the manuscript. All authors substantially contributed to the interpretation of the data, critically revised the draft, read and approved the final manuscript, has agreed both to be personally accountable for the author’s own contributions, and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding was solely provisioned from internal resources of the trial centers: Hannover Medical School (Germany), University Hospital Bonn (Germany), and University Hospital Zurich (Switzerland).
Members of the BonHanZA (Bonn-Hannover-Zurich-ARDS) study group are: RE, LW, BS, A-KR, RA, DH, CG, MM, CP, TW, MH, PW-G, SD, CB, and KS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1000084/full#supplementary-material
1. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. (2009) 374:1351–63. doi: 10.1016/S0140-6736(09)61069-2
2. Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. (2011) 37:1447. doi: 10.1007/s00134-011-2301-6
3. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. (2021) 398:1230–8. doi: 10.1016/S0140-6736(21)01960-7
4. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. (2018) 378:1965–75. doi: 10.1056/NEJMoa1800385
5. Serpa Neto A, Schmidt M, Azevedo LCP, Bein T, Brochard L, Beutel G, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis : Mechanical ventilation during ECMO. Intensive Care Med. (2016) 42:1672–84. doi: 10.1007/s00134-016-4507-0
6. Napp LC, Kühn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. (2016) 105:283–96. doi: 10.1007/s00392-015-0941-1
7. Madershahian N, Wittwer T, Strauch J, Franke UFW, Wippermann J, Kaluza M, et al. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. (2007) 22:180–4. doi: 10.1111/j.1540-8191.2007.00381.x
8. Choi JH, Kim SW, Kim YU, Kim SY, Kim KS, Joo SJ, et al. Application of veno-arterial-venous extracorporeal membrane oxygenation in differential hypoxia. Multidiscip Respir Med. (2014) 9:55. doi: 10.1186/2049-6958-9-55
9. Lee JH, Park JH, Min HK, Seo GW, Song PS, Her C, et al. Veno-veno-arterial ECMO support for acute myocarditis combined with ARDS: a case report. Int J Artif Organs. (2015) 38:667–70. doi: 10.5301/ijao.5000460
10. Jeon YJ, Byun JH, Hwang SW, Park JH, Lee JH. Successful application of veno-venoarterial extracorporeal membrane oxygenation for acute exacerbation of asthma followed by stress cardiomyopathy. Yonsei Med J. (2016) 57:536–7. doi: 10.3349/ymj.2016.57.2.536
11. Stöhr F, Emmert MY, Lachat ML, Stocker R, Maggiorini M, Falk V, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: is the configuration mode an important predictor for the outcome? Interact Cardiovasc Thorac Surg. (2011) 12:676–80. doi: 10.1510/icvts.2010.258384
12. Biscotti M, Lee A, Basner RC, Agerstrand C, Abrams D, Brodie D, et al. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. ASAIO J. (2014) 60:635–42. doi: 10.1097/MAT.0000000000000139
13. Ius F, Sommer W, Tudorache I, Avsar M, Siemeni T, Salman J, et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg. (2015) 20:761–7. doi: 10.1093/icvts/ivv035
14. Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, Di Nardo M, et al. The ELSO Maastricht Treaty for ECLS Nomenclature: abbreviations for cannulation configuration in extracorporeal life support - a position paper of the Extracorporeal Life Support Organization. Crit Care. (2019) 23:36. doi: 10.1186/s13054-019-2334-8
15. Ards Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33.
16. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. (2014) 189:1374–82. doi: 10.1164/rccm.201311-2023OC
17. Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. (2013) 39:1704–13. doi: 10.1007/s00134-013-3037-2
18. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
19. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass*. Pediatr Crit Care Med. (2010) 11:234–8. doi: 10.1097/PCC.0b013e3181b806fc
20. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
21. Headley J, Theriault R, Smith TL. Independent validation of APACHE II severity of illness score for predicting mortality in patients with breast cancer admitted to the intensive care unit. Cancer. (1992) 70:497–503. doi: 10.1002/1097-0142(19920715)70:2<497::AID-CNCR2820700220>3.0.CO;2-H
22. Werner NL, Coughlin M, Cooley E, Haft JW, Hirschl RB, Bartlett RH, et al. The University of Michigan Experience with Veno-Venoarterial Hybrid Mode of Extracorporeal Membrane Oxygenation. ASAIO J. (2016) 62:578–83. doi: 10.1097/MAT.0000000000000405
23. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. (2014) 97:610–6. doi: 10.1016/j.athoracsur.2013.09.008
24. Vogel DJ, Murray J, Czapran AZ, Camporota L, Ioannou N, Meadows CIS, et al. Veno-arterio-venous ECMO for septic cardiomyopathy: a single-centre experience. Perfusion. (2018) 33(suppl 1):57–64. doi: 10.1177/0267659118766833
25. Yeo HJ, Jeon D, Kim YS, Cho WH, Kim D. Veno–veno–arterial extracorporeal membrane oxygenation treatment in patients with severe acute respiratory distress syndrome and septic shock. Crit Care. (2015) 20:28. doi: 10.1186/s13054-016-1205-9
26. Cakici M, Gumus F, Ozcinar E, Baran C, Bermede O, Inan MB, et al. Controlled flow diversion in hybrid venoarterial–venous extracorporeal membrane oxygenation. Interact CardioVasc Thoracic Surg. (2018) 26:112–8. doi: 10.1093/icvts/ivx259
27. Blandino Ortiz A, Belliato M, Broman LM, Lheureux O, Malfertheiner MV, Xini A, et al. Early findings after implementation of veno-arteriovenous ecmo: a multicenter european experience. Membranes (Basel). (2021) 11:81. doi: 10.3390/membranes11020081
28. Extracorporeal Life Support Organization. ECLS Registry Report. International Summary April, 2022 [Internet]. (2022). Available online at https://www.elso.org/Portals/0/Files/Reports/2022_April/International%20Report%20April%202022.pdf
29. Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock*. Crit Care Med. (2008) 36:1404–11. doi: 10.1097/CCM.0b013e31816f7cf7
30. Lindén VB, Lidegran MK, Frisén G, Dahlgren P, Frenckner BP, Larsen F. ECMO in ARDS: a long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand. (2009) 53:489–95. doi: 10.1111/j.1399-6576.2008.01808.x
31. Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, et al. High In-Hospital Mortality Rate in Patients with COVID-19 Receiving Extracorporeal Membrane Oxygenation in Germany: A Critical Analysis. Am J Respir Crit Care Med. (2021) 204:991–4. doi: 10.1164/rccm.202105-1145LE
32. Karagiannidis C, Bein T, Welte T. ECMO during the COVID-19 pandemic: moving from rescue therapy to more reasonable indications. Eur Respir J. (2022) 59:2103262. doi: 10.1183/13993003.03262-2021
33. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. (2001) 286:1754–8. doi: 10.1001/jama.286.14.1754
34. Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care. (2008) 12:R161. doi: 10.1186/cc7160
35. Fisser C, Rincon-Gutierrez LA, Enger TB, Taccone FS, Broman LM, Belliato M, et al. Validation of prognostic scores in extracorporeal life support: a multi-centric retrospective study. Membranes (Basel). (2021) 11:84. doi: 10.3390/membranes11020084
36. Chen WC, Huang KY, Yao CW, Wu CF, Liang SJ, Li CH, et al. The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care. (2016) 20:336. doi: 10.1186/s13054-016-1520-1
37. Bonizzoli M, Lazzeri C, Cianchi G, Boddi M, Cozzolino M, Di Valvasone S, et al. Serial lactate measurements as a prognostic tool in venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg. (2017) 103:812–8. doi: 10.1016/j.athoracsur.2016.06.087
38. Shah N, Said AS. Extracorporeal support prognostication-time to move the goal posts? Membranes (Basel). (2021) 11:537. doi: 10.3390/membranes11070537
39. Falk L, Fletcher-Sandersjöö A, Hultman J, Broman LM. Conversion from venovenous to venoarterial extracorporeal membrane oxygenation in adults. Membranes. (2021) 11:188. doi: 10.3390/membranes11030188
40. Kon ZN, Bittle GJ, Pasrija C, Pham SM, Mazzeffi MA, Herr DL, et al. Venovenous versus venoarterial extracorporeal membrane oxygenation for adult patients with acute respiratory distress syndrome requiring precannulation hemodynamic support: a review of the ELSO registry. Ann Thorac Surg. (2017) 104:645–9. doi: 10.1016/j.athoracsur.2016.11.006
41. Delius R, Anderson H, Schumacher R, Shapiro M, Otsu T, Toft K, et al. Venovenous compares favorably with venoarterial access for extracorporeal membrane oxygenation in neonatal respiratory failure. J Thorac Cardiovasc Surg. (1993) 106:329–38. doi: 10.1016/S0022-5223(19)34132-7
42. Rupprecht L, Lunz D, Philipp A, Lubnow M, Schmid C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. (2015) 7:320–6.
Keywords: extracorporeal life support (ECLS), triple cannulation, acute respiratory distress syndrome, sequential organ failure assessment (SOFA) score, vasoactive inotropic score, shock, survival analysis
Citation: Erlebach R, Wild LC, Seeliger B, Rath A-K, Andermatt R, Hofmaenner DA, Schewe J-C, Ganter CC, Müller M, Putensen C, Natanov R, Kühn C, Bauersachs J, Welte T, Hoeper MM, Wendel-Garcia PD, David S, Bode C and Stahl K (2022) Outcomes of patients with acute respiratory failure on veno-venous extracorporeal membrane oxygenation requiring additional circulatory support by veno-venoarterial extracorporeal membrane oxygenation. Front. Med. 9:1000084. doi: 10.3389/fmed.2022.1000084
Received: 21 July 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Eizo Watanabe, Aichi Medical University, JapanCopyright © 2022 Erlebach, Wild, Seeliger, Rath, Andermatt, Hofmaenner, Schewe, Ganter, Müller, Putensen, Natanov, Kühn, Bauersachs, Welte, Hoeper, Wendel-Garcia, David, Bode and Stahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sascha David, c2FzY2hhLmRhdmlkQHVzei5jaA==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.