- Eye Center, School of Medicine, 2nd Affiliated Hospital, Zhejiang University, Hangzhou, China
Dry eye-related ocular surface examination is very important in the diagnosis and treatment of dry eye disease. With the recent advances in science and technology, dry eye examination techniques have progressed rapidly, which has greatly improved dry eye diagnoses and treatment. However, clinically, confusion remains about which examination to choose, how to ensure the repeatability of the examination, and how to accurately interpret the examination results. In this review, we systematically evaluate previous examinations of dry eye, analyze the latest views and research hotspots, and provide a reference for the diagnosis and management of dry eye.
Introduction
Dry eye is a multifactor disease defined as “the loss of tear film homeostasis accompanied by ocular symptoms, caused by tear film instability and hyperosmolar, ocular surface inflammation and damage, and neuroparesthesia” (1). The global prevalence of dry eye disease is 5–50% depending on the population and disease definition, whereas in the United States, over 16 million adults have been diagnosed with the disease (2). Traditionally, dry eye has been classified as aqueous deficiency, evaporative, or mixed mode. This classification has been extended to take into account anatomical contributions to signs and symptoms, including neurological abnormalities (1). Common diagnostic methods include consideration of the chief complaint, a questionnaire, a physical examination, a tear film rupture test, corneal punctate staining, and a tear secretion test (3–5). Others include tear osmotic pressure and the tear ferning test (6–8). However, to an extent, the pathogenic factors and disease development of dry eye are complicated, and the separation of subjective feeling and objective signs is often encountered in clinical practice, which brings certain challenges to the diagnosis and treatment of the condition (9–14). With the advent of the technological era and prolific screen reading, the incidence of dry eye has increased (15, 16). As a result, greater attention is being paid to dry eye, so clinics have in turn developed more sophisticated means of checking for the condition through more targeted point-of-care tests and imaging technologies to help clinical doctors diagnose the type and severity of dry eye more effectively (17). In view of the importance of the systematic examination and evaluation of dry eye, in this paper, we summarize the relevant examination methods currently known, evaluate the new technologies available, and explore the progress of dry eye diagnostic methods, which may be helpful in the clinical diagnosis and treatment of dry eye.
A worker must first sharpen their tools if they want to do well. The examination of dry eye is very important for the diagnosis and treatment of dry eye. With recent advances in science and technology, the examination of dry eye has progressed rapidly, which has greatly improved dry eye diagnosis and treatment. In this paper, we summarize and analyze the latest progress in dry eye examination, which may be helpful in the clinical diagnosis and treatment of dry eye.
Tear Examination
The normal tear film is made up of three layers: the lipid layer, the aqueous layer, and the mucin layer. Tears play an important role in maintaining the stability of the ocular surface microenvironment. Changes in the quality or quantity of tears will lead to the occurrence of dry eyes, and tear supplementation is an important part of dry eye treatment. The examination of the quality and quantity of tears is therefore an important indicator for the diagnosis of dry eye. In addition to the traditional tear secretion test and tear river height [tear meniscus height (TMH)] measurement, tear osmotic pressure, tear inflammatory factors, and the tear ferning test play important roles in the diagnosis of dry eye (18).
Tear Examination
Schirmer's Test
Schirmer's test has been used as a diagnostic test for dry eye disease since 1903 and can help to evaluate tear volumes (19). The test is based on the physical tendency of a fluid to travel along a strip of porous material by capillary action due to surface tension (20–22). No consensus exists on the diagnostic criteria of dry eye in Schirmer's test. A reading of <5 mm is considered to indicate dry eyes and <10 mm marginally dry eyes. Schirmer's test measures total tear secretion, including reflex, and basal tears. The test is known to measure reflex tears without anesthesia and basal tears with anesthesia (23–25). However, the concept of basal tears is uncertain. It may not be necessary to measure true basal tears with anesthesia because even light stimulation can change tear secretion (26–28).
Schirmer's test is popular for diagnosing dry eye because of its simple operation and the absence of a requirement for equipment. However, the disadvantages include poor repeatability, low sensitivity and specificity, and sharp patient discomfort (29). The change in light, room humidity, and temperature and patient's anxiety may explain the poor repeatability. Furthermore, the variability in Schirmer's test results may be caused by reflex tearing (23). To increase the reliability of Schirmer's test, many variations in Schirmer's test have been proposed. In a comparative study of Schirmer's test with and without anesthesia, Kashkouli et al. found that the value of Schirmer's test with anesthesia may be more objective and reliable than that without anesthesia (30), and they discovered that a shorter 1-min test with anesthesia not only decreased patient's discomfort but also saved time. Hamano et al. modified Schirmer's test using a cotton thread impregnated with phenol red dye instead of uncomfortable filter paper (31). In this test, which is called the phenol red thread (PRT) test, the color of the dye changes from yellow to red depending on the pH of the tears (21). However, the relationship between the PRT and Schirmer's test in the measurement of tear secretion is not clear (27). Although Schirmer's test without anesthesia may not be acceptable given its shortcomings, it can be considered a valid option for diagnosing severe dry eye because of its reproducibility (32–34).
In view of the shortcomings of the tear secretion test, nonimmersive and improved tear detection, tear composition, physical and chemical properties, and other aspects of inspection and evaluation may be new directions for exploration.
Tear Meniscus Volume
The tear menisci provide a reservoir that contributes to the formation of the preocular tear film with each blink and accommodates excess tears during reflex tearing, lacrimal obstruction, and after topical drop instillation (35). It has been reported that the tear meniscus contains 75–90% of the aqueous tear volume, which is positively correlated with the lacrimal secretory rate (36). The meniscus volume is also reported to be reduced in tear-deficient dry eye (37, 38). Thus, the quantitative assessment of tear meniscus parameters may be useful in the diagnosis of dry eye disease. Tear meniscus variables, such as height, width, cross-sectional area, and meniscus curvature, have been reported to be of value in the diagnosis of dry eye (39, 40). TMH and strip meniscometry (SM) can be used to evaluate tear meniscus volume.
Tear Meniscus Height
Tear meniscus height has been confirmed to have good reliability and accuracy in detecting tear meniscus volume (41, 42). Invasive and noninvasive techniques can be used to measure TMH (see Figure 1). TMH can be viewed under cobalt blue light using a slit lamp and fluorescein instillation (43, 44). However, invasive TMH assessments are challenging as a result of their limitations. Frequent blinking, drug stimulation, time intervals, humidity, and temperature have been reported to influence invasive TMH results (45–47). Noninvasive TMH was therefore introduced, and optical coherence tomography (OCT) measurement of the tear menisci was found to be more reliable and was validated. Noninvasive TMH can be used together with noninvasive tear breakup time (NIBUT) on the same instrument, and they have been shown to be positively correlated (48, 49).
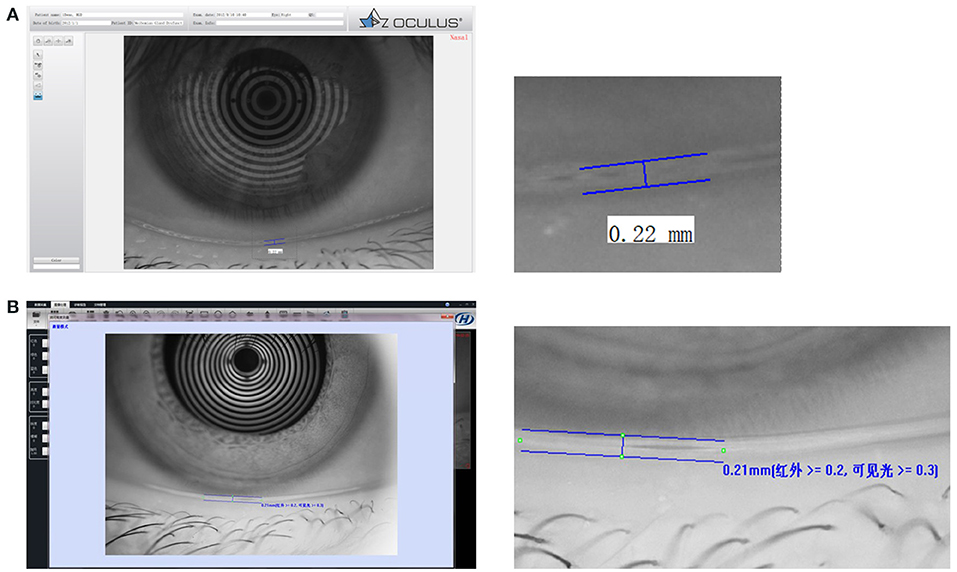
Figure 1. Tear film thickness. (A) An example of tear film thickness from OCULUS Keratograph 5M. (B) An example of tear film thickness from Kanghua dry eye analyzer.
Strip Meniscometry
Strip meniscometry (39, 40, 50–52) involves using an SMTube, which is a thin strip (length: 85 mm, width: 7 mm, and height: 0.3 mm) with a capillary absorber in the center and two columns of scale on both sides, to measure tear meniscus volume. The examiner holds the center part of the strip and immerses the tip into the tear meniscus of the lower eyelid for 5 s to absorb tears. The SMTube attached to the tear meniscus absorbs the tears into the ditch, and the strip color turns blue, indicating the volume. At the end of 5 s, the strip is taken out, and the blue-stained column length is measured. The length of the stained column for normal people is equal to or >5 mm, whereas it is <5 mm for patients with dry eye (see Figure 2). Dogru et al. (39) and Miyasaka et al. (53) demonstrated that SM results correlate with those of Schirmer's test. A good correlation between TBUT and SMTube measurements has also been reported (52). The SMTube is useful for the diagnosis of dry eye, and its validity can be evaluated effectively using the CASIA SS-1000 AS-OCT TM parameters. Lee et al. (50) compared tear meniscus measurements using SM and the Keratograph 5M between 3 patient groups with subtypes of dry eye disease and found that tear meniscus measurements using SM and the Keratograph 5M can compensate for the detection of aqueous-deficient components of dry eye (53). The SMTube is useful for evaluating tear volume and therapeutic effects in patients with lacrimal passage obstruction. Hao et al. (40) showed that the SMTube has acceptable repeatability and reproducibility and specific correlations with TMH, BUT, and Schirmer's test. SM can provide clinical staff in busy outpatient services with a swift and convenient inspection method.
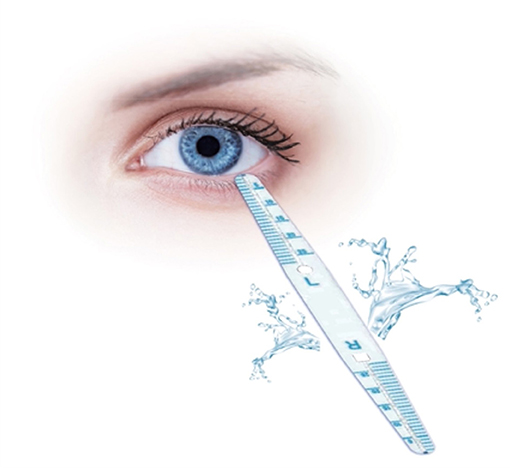
Figure 2. SMTube. The method of strip meniscometry (SM) is using SMTube which is a thin strip (length: 85 mm, width: 7 mm, and height: 0.3 mm) with a capillary absorber in the center and two columns of scale on both sides to measure the tear meniscus volume. The examiner held the center part of the strip and immersed the tip into the tear meniscus of the lower eyelid for 5 s to absorb tears. The SMTube attached to the tear meniscus absorbed the tears into the ditch and the strip color turned blue, indicating the volume. At the end of 5 s, the strip was taken out and the blue stained column length was measured. The length of the stained column for normal people was equal to or >5 mm, whereas it was <5 mm for DED patients.
Tear Ferning Test
The tear ferning test is a simple tear detection method that can reflect some biochemical characteristics of tear film relatively quickly and cheaply (see Figure 3) (44, 54). Golding et al. (55) pointed out that the salts and polymers in tears are the important factors affecting the crystallization of tear ferns in the microscopic photographic study of tear crystal patterns in the tear ferning test. In their analysis of normal tears, they found that the fern-like crystals are mainly composed of sodium chloride, potassium chloride, and trace ions, and the surface of the crystals is covered by mucins and high molecular proteins, which indirectly control the formation of the crystals. Kogbe et al. (56) found that fern-like crystal patterns and branching patterns are particularly dependent on the ratios of monovalent sodium and iron ions to divalent calcium and magnesium ions. The tear fern crystal map in the tear ferning test is a relatively inexpensive and quick test of the total biochemical level of tear samples. Traipe-Castro et al. (57) studied the dynamics of tear evaporation and found that the crystalline pattern of dry healthy tears can be divided into four structural regions, namely zone 1 (outermost), a transition zone, zone 2 (near central region crystal), and zone 3 (central main crystal). The crystal pattern of ferns produced by a single tear sample depends not only on the composition of the tear, but also on the environmental conditions of the dry tear. Reducing the pressure and humidity or increasing the temperature to speed up the drying speed of the tear results in a smaller crystal morphology and different proportions of the four structural regions in the crystal pattern. In addition to diagnosing dry eye, the tear ferning test can be used to diagnose keratoconjunctivitis and cystic fibrosis disease and for soft contact lens tolerance prediction in clinical practice (58–60). The tear ferning test has good clinical guiding significance. However, the current method has disadvantages, such as the difficulty in controlling environmental conditions and the slow speed of the experimental results. If a method for rapid crystallization could be found, this examination method may have more clinical value.
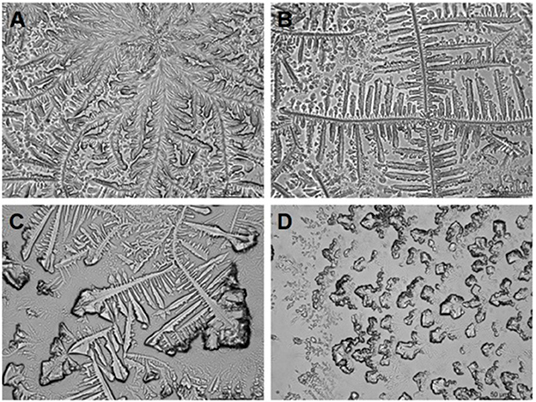
Figure 3. Tear ferning test classification descriptions (Rolando's classification). Type 1: uniform arborization in the entire field of observation without spaces between the ferns. Single ferns are big and closely branched (A). Type 2: Arborization is abundant, but the single ferns are smaller and have a lower frequency of branching than in grade 1; empty spaces appear between the ferns (B). Type 3: Single ferns are little and incompletely formed with rare or no branching (C). Type 4: No ferning is present; mucus may appear in clusters and threads (D).
Tear Osmotic Pressure
The measurement of tear osmotic pressure indicates the balance among tear secretion, evaporation, absorption, and drainage (61). Vapor pressure osmometry and freezing point depression have been used to measure tear osmotic pressure in the past (62, 63). The tear osmolarity test is considered as one of the most accurate dry eye diagnosis methods (64). Most recent techniques to measure tear osmotic pressure involve the use of the TearLab (TearLab Corporation, Escondido, CA) and I-PEN (I-MED Pharma Inc., Dollard-des-Ormeaux, Canada) osmolarity systems (65, 66).
TearLab Osmometer
The TearLab osmometer (TearLab, San Diego, CA) was approved by the US Food and Drug Administration in 2008 for the measurement of tear osmolarity in vivo by clinical practitioners. Since then, several studies have demonstrated that it is a reliable test with good performance and complements for the diagnosis of dry eye (67–69). Some studies have concluded that the TearLab osmometer is the best single marker for diagnosing and classifying dry eye levels of severity and for distinguishing between different dry eye severity levels (70, 71). On the other hand, several clinical studies have raised questions about the diagnostic ability of tear osmolarity in dry eyes measured with the TearLab osmometer (72–77).
I-PEN Tear Osmolarity Test
Tear osmolarity is performed using the I-PEN osmolarity system 5 min after a PRT test. The I-PEN osmolarity system is used a distance away from electronic devices to ensure the accuracy of the readings. Each subject is asked to close their eyelids gently for 30 s, and the disposable single-use sensor then softly makes contact with the palpebral conjunctiva from the lower eyelid at a 30° angle. By design, the I-PEN beeps after a few seconds and displays an osmolarity reading on the screen (78, 79). Tear osmolarity is measured three times in the right eye of each subject with 5-min intervals between measurements. Based on the I-PEN tear osmolarity measurements, subjects are classified as having a healthy eye (<290 mOsm/L), minor dry eye (290–310 mOsm/L), mild dry eye (310–330 mOsm/L), and moderate dry eye (330–350 mOsm/L).
Shimazaki et al. (78) studied the efficacy and safety of the handheld I-PEN in Japanese patients with dry eye disease and those without dry eye disease and found no correlations between the tear film osmolarity values obtained with the I-PEN system and any subjective or objective parameters of dry eye. Meanwhile, in the study of Fagehi et al. (79), the mean measurement of I-PEN tear osmolarity was 303.8 ± 4.8 mOsm/L, which is in agreement with the range reported for healthy subjects. The I-PEN is reliable and has the advantage of portability compared to other osmolarity systems (79). Park et al. (80) obtained similar results, indicating that the I-PEN osmometer can be considered suitable for the use in clinical settings, with good performance in the diagnosis of dry eye. König et al. (81) found that the I-PEN osmometer provided significantly higher tear film osmolarities than those measured using the TearLab osmometer. In one study, tear osmotic pressure measured using the I-PEN osmometer has shown a significant decrease after treatment with a botulinum toxin A injection in patients with intractable dry eye disease (82). Tavakoli et al. found that, in vivo, both instruments displayed poor repeatability (83). Some studies have found that after treatment, the osmotic pressure of tears in patients with dry eye is significantly reduced (74–86). However, other studies have found that the osmotic pressure of tears does not change significantly after treatment (84–88). This may be related to the duration of the treatment, the severity of the disease, and the efficacy of the different treatments. The key elements in the diagnosis of dry eye are increased osmolarity of the tear film and inflammation of the ocular surface, which are accompanied by ocular symptoms. In terms of clinical treatment, we can select different artificial tears based on the osmotic pressure of patients with dry eye.
Tear Inflammatory Factor
There is very strong and valid evidence that inflammation constitutes an important pillar in the pathophysiology of dry eye. Increased inflammatory cells, increased expression of immune activation and adhesion molecules, T-helper type 1 (Th-1) and Th-17 attracting immune pathways, cytokines, and chemokines are all evidence supporting the inflammatory pathology of dry eye (89, 90).
Currently, the only commercial options to investigate tear film biomarkers are InflammaDry® (matrix metalloproteinase 9, MMP-9) and the TearScan™ Lactoferrin Diagnostic Test Kit (91). MMP-9 is an endopeptidase that helps remodel the extracellular matrix and plays a crucial role in dry eye. The point-of-care test InflammaDry (Quidel, San Diego, CA) can detect this biomarker in tear film with a low limit of detection of 40 ng/mL (91). This test should be carried out prior to the use of anesthetics, corneal staining, or Schirmer's test. A fleece placed at the tip of a sample collector is inserted multiple times along the patient's palpebral conjunctiva. The sample collector is then placed into the supplied test cassette and secured prior to immersing it in the buffer. Once 10 min has passed, the test cassette may display a blue line, which represents a valid test. If the blue line does not appear, the test is considered as invalid and must be repeated. A positive result is indicated by the presence of pink and blue lines, thus providing a qualitative (yes or no) response. To carry out this test accurately, a sufficient sample (5 μL) needs to be collected to avoid a false-negative result. In some studies, the InflammaDry test has demonstrated high positive and negative agreement for confirming suspected dry eye disease (92, 93). These studies have further shown that tear MMP-9 positivity may serve as a reliable response predictor of topical corticosteroid treatment in dry eye. Kim et al. (94) found that the subjective 5-scale grading system in the point-of-care MMP-9 immunoassay is an easy and reliable method with acceptable accuracy. Whereas, inflammation is known to play a role in dry eye, it is not always present in those with symptoms. Lanza et al. (95) suggested that a clinical examination alone cannot identify patients with clinically significant inflammation. In their study, there was no difference in the dry eye profiles of patients when evaluating symptoms and signs and those who tested positive vs. negative for MMP-9 on the ocular surface. A randomized, double-blind, placebo-controlled dry eye study in humans reported that lactoferrin supplementation can increase tearing, whereas a randomized controlled study of cataract surgery-induced dry eye found that it can restore tearing and TBUT (96, 97).
Tear Film Examination
The most superficial layer of tear film is the lipid layer secreted by the meibomian glands. When this layer is deficient, it may result in an evaporative dry eye (18). The main methods of tear film examination are TBUT and tear film thickness, but the correlation between these two examinations needs further investigation.
Tear Breakup Time
Tear breakup time has been the most frequently adopted dry eye examination method over the past two decades (98). TBUT was first introduced by Norn (99) to assess the stability of tear film. It is traditionally defined as “the interval between the last complete blink and the first appearance of a dry spot or disruption in the tear film” (99). According to current perspectives, measurements of TBUT can be divided into two distinct patterns: invasive and noninvasive.
Invasive Tear Breakup Time
The most common clinical method of measuring invasive TBUT is sodium fluorescein (100). The general process involved in examining TBUT with fluorescein is convenient and accessible. Liquid containing light fluorescence or a sterile fluorescein paper strip infiltrated with normal saline is presented to the superior bulbar conjunctiva for 1–2 s so that the fluorescein is uniformly distributed on the precorneal ocular surface (101, 102). This study has shown that the volume of fluorescent liquid used can significantly affect TBUT results, so a micropipette can be used to control the volume (102). The use of a dry fluorescein applicator is also an effective way to minimize the influence of liquid volume (46, 103). After the patient blinks several times, the time from the last blink of the eye to the first dry spot on the tear film is measured under a cobalt blue filter. Two or three consecutive measurements are recorded. The average value provides a more reliable result (104). A recent study showed that the maximum TBUT may also be considered to diagnose dry eye (105). The sensitivity and specificity of the TBUT test are 72 and 61%, respectively. The diagnostic criteria for fluorescein TBUT in diagnosing dry eye are <10 s (105). Generally, fluorescein TBUT values can be expected to be shorter than the values of noninvasive TBUT. A fluorescein TBUT of <5 s has also been reported to contribute to the diagnosis of dry eye (106).
Noninvasive Tear Breakup Time
In recent years, due to the weak points of invasive TBUT, NIBUT, which relies on technical apparatus, has become more popular (107). Invasive TBUT may be affected by volume, the concentration and types of fluorescence used, and the experience of the examiner (108, 109). NIBUT does not involve the instillation of any substances nor is there any direct physical contact with the tear film and conjunctiva. NIBUT is measured by the automated capture of Placido disk images, which are obtained from the anterior ocular surface using a corneal topography system (110–113). Computer software is used to identify irregularities and the breakup of the precorneal tear film, and the first and average time is calculated (114, 115). These several studies have reported better sensitivity and specificity for NIBUT than invasive TBUT. NIBUT has 82–84% sensitivity and 76–94% specificity (116). In addition, 10 s has been proposed as the cutoff value, and <10 s can indicate a diagnosis of dry eye (117). The disadvantage of NIBUT using technical apparatus is that, instead of repeating the test three times, a random time is checked once, so there is room for error, and this method therefore needs to be improved in the future.
Tear Film Thickness
A stable eye tear film is a sign of good eye health, mainly because it forms the main refractor through which light enters the visual system and protects and moistens the cornea. Wolff (118) proposed a three-layer model of tear film: the mucin layer covering the eye surface reduces the hydrophobicity of epithelial cells; care of the water layer of exposed ocular epithelium by providing lubricity, some nutrients, antimicrobial proteins, and appropriate osmotic pressure; Additionally, the lipid layer prevents the water layer from being lost through overfilling and evaporation. When the eyes are open, the tears are distributed in three compartments, which are the fornical compartment, the tear menisci, and the preocular tear film (118). The preocular tear film overlies the exposed conjunctiva and cornea (119, 120).
Measurement of the lipid layer thickness (LLT) could serve as an useful examination method in clinical practice diagnosing MGD. In previous clinical routine, the LLT is usually measured indirectly by the determination of the TBUT. Nowadays, various technologies and new devices are currently being utilized to assess LLT. It is 2–5.5 μm thick in the corneal region. A suitable method for the direct quantification of the LLT is interferometry techniques (120–122). LipiView II interferometer (TearScience Inc, Morrisville, NC) could provide the clinician with the important output parameter, such as average LLT of the tear film and be capable of quantifying LLT (123). On the other hand, LipiView II could automatically detect and analyze blink rate and blinking quality through the videos recorded. It displays the number of complete blinks and incomplete blinks and blink frequency numerically to help clinician analyze blinking pattern and take images of MG for visualizing the morphology of the MGs (see Figure 4). Compelling visuals and video captures provide an opportunity to educate patients about their personal ocular health and how healthy meibomian gland function protects the ocular surface, keeping their eyes moist to ensure clear, comfortable, healthy, and stable vision (124). Willcox MDP et al. pointed that abnormal blinking habits such as partial blinking are strongly associated with MGD (125). Using meibography, the grading of dropout at baseline and subsequent examinations can be used to track long-term progression of MGD (62). A study compared the Subjective Keeler Tearscope-Plus™ with the Objective Oculus® Keratograph 5M and LipiView® and found that the results of tear stability or lipid thickness were interchangeable (126). Another study compared the LipiView ® II with IDRA® for ocular surface analysis and found no significant difference in LLT, but these devices should not be used interchangeably for the evaluation of meibomian gland dropout and partial blink rates (127).
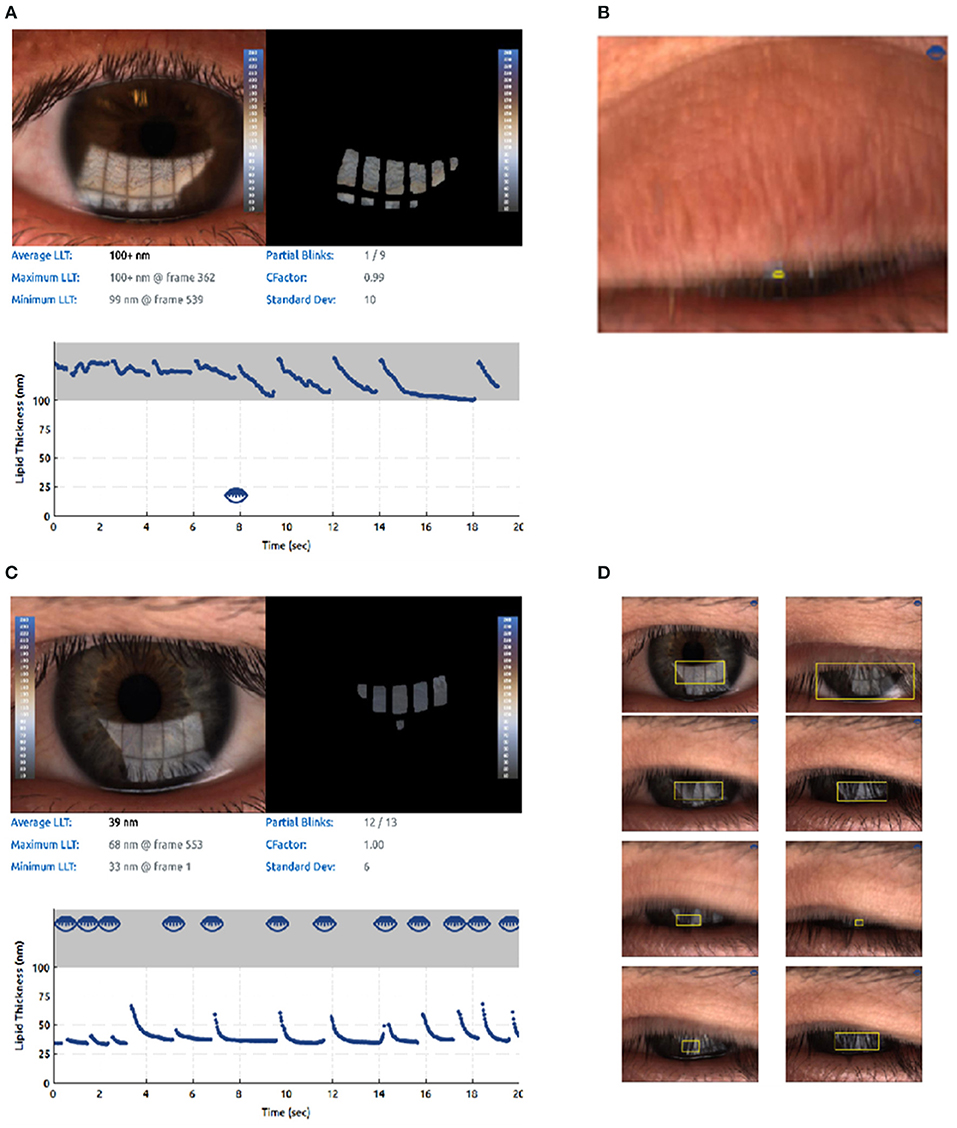
Figure 4. Lipid imagine report and incomplete blinking (an example from LipiView II ocular surface interferometer). The computer system captures a video image file that is recorded over time since the interference pattern changes as the tear film is distributed across the cornea during blinking. (A) Lipid imagine report and incomplete blinking. (B) Partial blink imagines. (C) Another for lipid imagine report and incomplete blinking. (D) Another for partial blink imagines.
Examination of the Eyelid Margin and Meibomian Glands
Meibomian gland dysfunction (MGD) is the main cause of dry eye, with 86% of dry eye cases being caused by MGD. Meibomian gland dropout is significantly correlated with the other clinical features of MGD, such as the quality of the expressed meibum, altered tear film lipid layer stability, and ocular surface damage. Meibomian gland morphology and function are routinely examined to diagnose MGD (128). Using meibography, the grading of the dropout at baseline and subsequent examinations can be used to track the long-term progression of MGD (129–131). Evaluating changes in the eyelids, eyelid margins, and meibomian glands is of great value in the diagnosis of MGD. These can be assessed by observing the opening state of the meibomian glands, squeezing the meibomian glands on the eyelid skin, and observing the difficulties and character of eyelid ester excretion. Specific scoring criteria were established following the consensus of experts on the diagnosis and treatment of MGD in China in 2017 (129).
Eyelid Margin Examination
Patients with MGD are prone to eyelid margin abnormalities. Abnormal eyelid margins can be valuable in diagnosing MGD. For example, MGD patients may have a thickened, blunt eyelid margin, an irregular shape, a Marx line (the junction of the skin and mucosa), a receded opening of the meibomian glands, hyperemia of the eyelid margin, and neovascularization. Slit lamp examination of the entire area of the upper and lower eyelids may reveal abnormal signs, including irregular eyelid margins (with notching along the eyelid margins), telangiectasis, and a shift of the mucocutaneous junction (130–132). Amano examined total MGD patients and noted that the slit lamp findings of lid margin abnormalities, a shift of the mucocutaneous junction, telangiectasia, and plugging were more frequent (133).
Meibomian Gland Infrared Meibography
Several modalities of meibomian gland imaging are used in MG examinations, including contact meibography (not currently popular), noncontact infrared meibography (e.g., IR meibography and the mobile pen-shaped meibography system), keratography (Keratograph 5M, OCULUS, Wetzlar, Germany), Kanghua Dry Eye Analyzer (DED-1L, Kanghua, Chongqing, China) and LipiView II (TearScience, Morrisville, NC) (see Figure 5), in vivo confocal microscopy (IVCM, see Section Other Examinations), and OCT meibography (134). The MG area ratio, diameter deformation, tortuosity, and signal intensity could be considered as promising biomarkers for MGD diagnosis and objective grading (131). Meibomian gland infrared meibography may provide a reference for clinical treatment of MGD, especially nondrug treatment, such as Lipiflow, IPL, and meibomian gland massage.
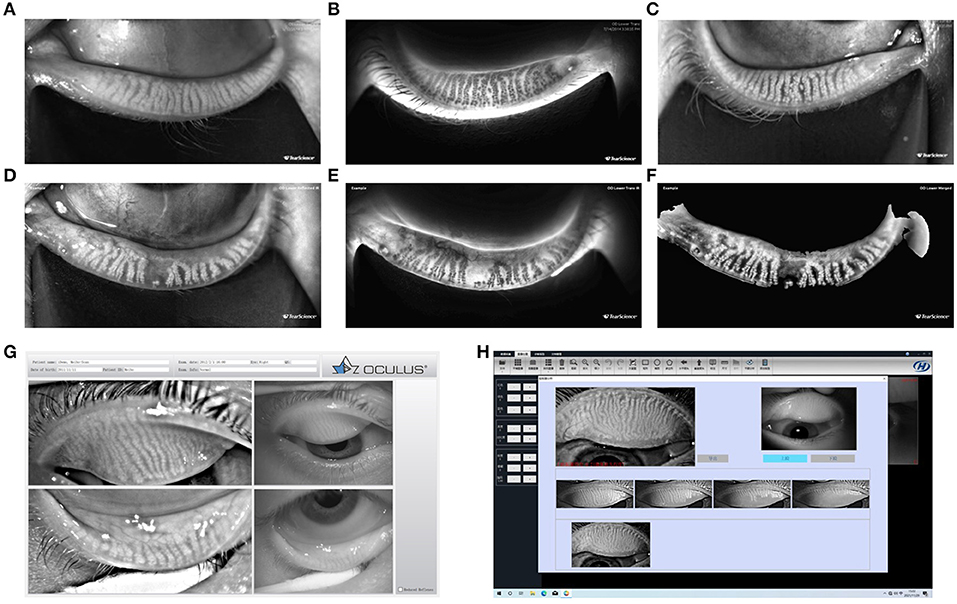
Figure 5. Meibomian gland morphology. Ocular surface interferometer—LipiView II interferometer (A–C); Dynamic Meibomian Imager—LipiScan (D–F); OCULUS Keratograph 5M (G); Kanghua Dry Eye Analyzer (H). Examples for LipiView II and LipiScan: The images of the glands may be viewed on the computer screen display and in a printed report. Three modes of gland images are provided: Reflected infrared view is shown for both upper and lower eyelid gland images. (A,D) Show only lower eyelid gland images. Transillumination infrared view is shown only for lower eyelid gland images, if images were captured using handheld near-infrared lid everter (B,E). Merged view is the combination of the reflected infrared and trans infrared views and is shown only for the lower eyelid gland images (C,F).
Lid Wiper Examination
The lid wiper is a component of the conjunctival margin of the upper and lower eyelids (135). Shiraishi et al. noted that lissamine green staining was detected in more than 10% of lid wiper epitheliopathy patients with dry eye symptoms (136). Studies have suggested that the best staining method is as follows: lissamine green or fluorescein sodium test paper is soaked and used to make contact with the conjunctiva of the lower eyelid. After more than 1 min, the conjunctiva is restained once. The degree and range of staining of the lid margin epithelial cells are then examined under a slit lamp microscope with a cobalt blue light. A staining length of ≥2 mm and/or ≥25% of the eyelid margin width are considered positive in the diagnosis of lid wiper epitheliopathy (137).
To date, studies have found that the incidence of lid wiper epitheliopathy in patients with dry eye symptoms increases, and lid margin staining can be combined with traditional dry eye diagnosis methods to improve diagnosis rates.
Inspection of the Eyelid for Demodex Mites
Ocular mite infection is significantly correlated with dry eye, MGD, and age. When Demodex mites infect the margin of the eyelid, the skin of the margin of the eyelid, the hair follicles and glands of the eyelid, and the meibomian glands will accumulate mites, and symptoms related to dry eye will occur (138). In addition, a typical clinical manifestation of Demodex blepharitis is cylindrical dandruff at the root of the eyelash, and this is considered pathognomonic for Demodex blepharitis (139). To detect Demodex mites, an optical microscope is used to examine eyelashes with typical cylindrical dandruff characteristics. These lashes are then removed and placed on a slide to observe the number and morphology of the Demodex mites. The use of IVCM (see Section Other Examinations) could be an easy way to improve this diagnosis. The advantage of IVCM is that it can be used to quickly and simultaneously detect microorganisms and eyelid structure. It is noninvasive and does not require the removal of eyelashes. It can be used repeatedly to determine a prognosis in patients, but it cannot be used to accurately to identify the type of mite.
Ocular Surface Staining
Ocular surface cell staining can evaluate the barrier function and integrity of epithelial cells as one of the evaluation indexes of the severity of dry eye (140). When the integrity of ocular surface cells has been damaged, they can be stained with specific dyeing agents to display the defects. The degree and area of staining are related to the severity of the ocular surface damage. Ocular surface cell staining can therefore be used to evaluate the barrier function and integrity of epithelial cells. Fluorescein sodium staining is commonly used in clinics although lissamine green staining and rose bengal staining are sometimes also used.
Fluorescein Staining
The mechanism of fluorescein staining involves dyeing corneal defects through the diffusion of the fluorescein between cells into the adjacent intercellular space with penetration into the lower stroma. Healthy corneal epithelial cells are therefore not stained unless they have defects. This method is mainly used to assist in the diagnosis of epithelial defect-related diseases, such as corneal abrasions, keratitis, and corneal erosion. It can also be used to evaluate the therapeutic effects of epithelial injury (140). Sodium fluorescein staining is not a specific index of dry eye but plays an auxiliary role in its diagnosis.
Sodium fluorescein is available in two forms: eye drops and impregnated paper strips. For both the eye drops and paper strip methods of instillation, the aim is to achieve highly fluorescent staining of the areas with loss of epithelial integrity (141). Studies have shown that high concentrations of sodium fluorescein are nonfluorescent and fluorescent at a concentration of <0.1% (142). It is therefore general practice for doctors to deliver 2 μL of 2% sodium fluorescein eye drops or fluorescein sodium strips soaked in physiological saline or other eye drops into the conjunctival sac using a micropipette. The whole tear film is then filled by the patient blinking their eyes. Ocular surface staining can then be observed using a blue exciter filter over the white light source and a complementary yellow or orange barrier filter over the slit lamp objective (142).
Rose Bengal Staining
A derivative of fluorescein, rose bengal is believed to not stain healthy epithelial cells. According to Roberts et al.'s study (143), the reason that the normal ocular surface does not stain with rose bengal is the obstruction of a healthy and intact ocular tear film. Accordingly, as long as the tear film protection is insufficient, there will be rose red staining. This feature can be used to assist in the diagnosis of dry eye. In a study by Korb (144), rose bengal was found to be the preferred dye for bulb conjunctival staining, whereas sodium fluorescein was the preferred dye for corneal staining, with an optimal concentration of 1%. Due to the cytotoxic effect of rose bengal, patients will feel tingling if anesthetic eye drops are not used. Furthermore, the sensitivity and specificity of rose bengal are not as good as those of sodium fluorescein, so it is not widely used and can only be used as a reference (137, 144, 145).
Lissamine Green Staining
Lissamine green staining has similar characteristics to rose bengal, but its cytotoxicity is much less than rose bengal, so lissamine green staining is commonly used as a substitute in conjunctiva staining in clinical practice (146, 147). These two dyes do not spread throughout the conjunctiva due to the blocking effect of the mucins in tear film, so their staining areas last longer than sodium fluorescein. Yoon found that a mixed solution of 1% fluorescein and 1% lissamine green can simultaneously stain the cornea and conjunctiva while maintaining the unique staining characteristics of each dye (147).
Notwithstanding, the use of mixed dyes may necessitate a long waiting time. The use of different dyes on the corneal and conjunctival epithelial cells may also have subtle effects on the damaged areas. Fluorescein sodium strips are therefore commonly used in clinical practice. Although reactive dyes have been used to stain the cornea and conjunctiva to assess ocular surface disease in dry eye for a long time, there is no uniform or clear ocular surface stain rating scale due to the different concentrations and methods of staining used in different regions. The general content is to classify the dyeing area, density, area, and type. Most studies use the Oxford scale, but the type of scale used depends on the researchers' requirements (148). Dyes are therefore an effective adjunct to the diagnosis of dry eye and to determine the type and severity of dry eye.
Dry Eye Analyzer
The dry eye analyzer is a device that integrates a variety of dry eye examinations. The dry eye analyzer can provide fast, convenient, and efficient dry eye examinations, but its relationship with traditional examinations needs to be clarified. Dry eye analyzer examinations may include tear river height (TMH) (see Section Tear Breakup Time), NIBUT (see Section Tear Breakup Time), tear film thickness (see Section Tear Film Thickness), infrared photography of the meibomian glands (see Section Eyelid Margin Examination), and conjunctival hyperemia analysis (see Figures 6, 7).
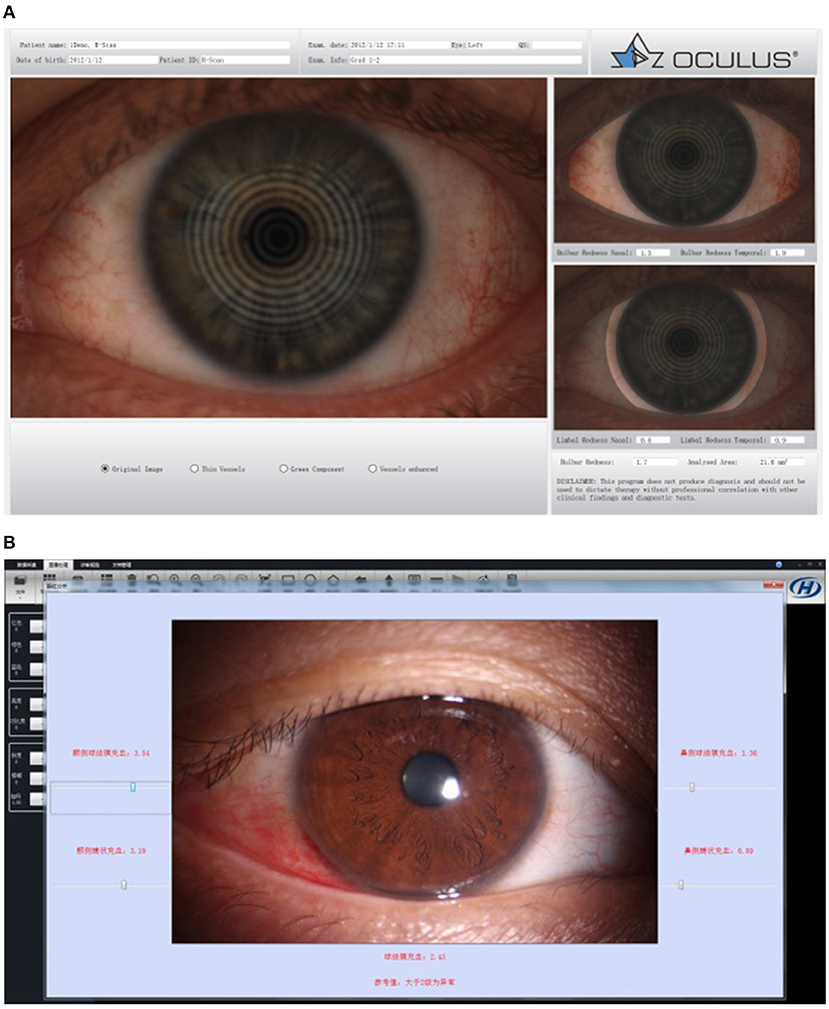
Figure 6. Bulbar redness. (A) OCULUS Keratograph 5M for bulbar redness grading scale. (B) Kanghua dry eye analyzer for bulbar redness grading scale.
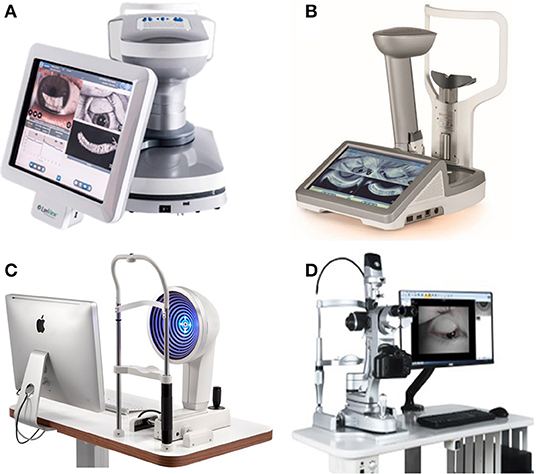
Figure 7. Dry eye analyzers. (A) LipiView II ocular surface interferometer. (B) LipiScan dynamic meibomian imager. (C) OCULUS Keratograph 5M. (D) Kanghua dry eye analyzer.
Conjunctival Hyperemia Analysis
Bulbar hyperemia is a common clinical sign and important indicator of ocular disease (149). It is one of the most common contributors of ocular complaints that prompt visits to medical centers. The translucent appearance of the conjunctiva allows immediate observation of changes in microvascular circulation. Conjunctival congestion is caused by dilation of blood vessels in microangiopathic degeneration caused by an inflammatory response. Our understanding of these neurogenic and immune-mediated pathways has progressed over time, and they are now known to play a critical role in the development of targeted novel therapies. Due to a multitude of underlying etiologies, patients must be accurately diagnosed for the efficacious management of conjunctival hyperemia. The diagnostic techniques used for the grading of conjunctival hyperemia have also evolved from descriptive and subjective grading scales to more reliable computer-based objective grading scales (150).
In 1996, Owen et al. (151) developed a novel computer software method to quantify the conjunctival plexus on the scleral background to measure its vascular surface area from photographs. Huntjens et al. employed Advanced Ophthalmic Systems software, which uses an objective approach to grading conjunctival hyperemia, and found excellent repeatability and improved agreement between experienced and novice observers (152).
Research has begun to focus on the relationship between red eyes and dry eye, but the consistency between objective and subjective tests needs to be further strengthened. Schulze et al. quantified bulbar redness using the validated bulbar redness grading scale and an automated objective method (OCULUS Keratograph 5M) in participants with dry eye disease and nondry eye disease controls, but statistically significant differences in redness between the dry eye disease and control groups were only found using the validated bulbar redness scale (153).
Other Examinations
Confocal Microscope
Confocal microscopy is a new technology that can aid in the in vivo assessment of structural changes in several ocular surface diseases on a cellular level. The application of IVCM in dry eye disease will be a powerful method to evaluate morphological changes in the ocular surface globally in the future (154). In the dry eye field, IVCM has been applied in the examination of the cornea, bulbar and palpebral conjunctiva, meibomian glands, and lacrimal glands (155–169).
First-generation confocal microscopy with an in vivo white-light through-focusing confocal microscope (Tandem Scanning Corp., Reston, VA) was developed and has advanced to an in vivo white-light slit-scanning confocal microscope (ConfoScan, Nidek Technologies, Vigonza, Veneto, Italy) (second generation). A new-generation in vivo laser-scanning confocal microscope (Heidelberg Retina Tomograph, Rostock Corneal Module; Heidelberg Engineering GmgH, Heidelberg, Baden-Württemberg, Germany) for corneal examination was recently reported to yield impressive, high-quality images in many corneal pathologies (169).
Confocal microscopy requires trained operators to acquire good quality scans but lacks built-in software to analyze nerves and inflammatory cells. As such, it is not used as often in clinical settings to diagnose dry eye. The relationship between corneal sensation and subbasal nerve morphology, as evaluated with IVCM, depends on the pathophysiological mechanism of ocular surface disease. In a recent review of the use of IVCM in dry eye, corneal subbasal nerves were implicated in the pathogenesis of the condition. There are many reports suggesting that corneal subbasal nerve density may decrease in dry eyes with Sjogren's syndrome (SS) (156, 158, 161). Ma et al. (165) found that a higher aggregated measure of tortuosity may be linked to ocular discomfort, visual function disturbance, and tear film instability. At the same time, Ma et al. (166) determined that objective visual quality is correlated with clinical symptoms and signs in patients with dry eye, which suggests that nerve changes may be a factor related to poor visual quality in these patients. Lin et al.'s study (167) showed that inflammatory dendritic cell density increases dramatically in the corneal epithelium of dry eye patients with non-SS and SS. Nerve fibers have an important influence on corneal tropism and contribute to the maintenance of healthy corneas. It has been postulated that inflammatory cells may induce a diminution of nerve fibers in the subbasal nerve plexus in dry eye patients with SS. The migration and maturation of dendritic cells are activated in response to proinflammatory stimulation. Dramatic increases in the number of dendritic cells in the corneal epithelium are thought to play a role in dry eye pathophysiology. Furthermore, some studies have determined that ocular Demodex mite infestation may be involved in ocular surface discomfort, inflammation, and meibomian gland dropout in patients with MGD (139, 168).
Incomplete Blinking
Incomplete blinking is an important parameters of dry eye disease. Spontaneous blinking can be divided into complete blinking and incomplete blinking. Incomplete blinking was associated with a higher ocular surface disease index (OSDI) score, more meibomian gland dropout (MGD), and reduced tear film stability. LipiView II automatically detects and analyzes blink rate and blinking quality through the videos recorded. It displays the number of complete blinks and incomplete blinks and blink frequency numerically to help clinician to analyze blinking pattern (124).
Outlook
The rapid progress in the development of dry eye diagnosis technologies has greatly promoted the level of diagnosis and treatment of dry eye and made dry eye a hot spot in the field of clinical ocular surfaces. In particular, the recent construction of a dry eye diagnosis and treatment center in China has received increased attention and developed rapidly (170). However, many areas worthy of exploration in the diagnosis of dry eye remained. These include the detection of the mechanism of dry eye-related neuropathy, in vivo functional examination of the meibomian glands, the correlation between tear film thickness, BUT, and noninvasive BUT, more accurate, and reliable tear and tear film examination methods. It is hoped that improvements in dry eye examination methods will promote all-round progress in the understanding, diagnosis, and treatment of dry eye and related researches (171).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XJ: conceptualization, writing—reviewing and editing, and supervision. YW: data curation, writing—original draft preparation, reviewing and editing, and visualization. CW: writing—original draft preparation. XW, KY, and YM: writing—original draft preparation. XH: writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China: [Grant Numbers: 82171013 and 81870624] and Major Science and Technology Projects of Zhejiang Province [Grant Number: 2022C03173].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
2. Tchegnon E, Liao CP, Ghotbi E, Shipman T, Wang Y, McKay RM, et al. Epithelial stem cell homeostasis in Meibomian gland development, dysfunction, and dry eye disease. JCI Insight. (2021) 6:e151078. doi: 10.1172/jci.insight.151078
3. Eom Y, Lee JS, Keun Lee H, Myung Kim H, Suk Song J. Comparison of conjunctival staining between lissamine green and yellow filtered fluorescein sodium. Can J Ophthalmol. (2015) 50:273–7. doi: 10.1016/j.jcjo.2015.05.007
4. Chen M, Miki M, Lin S, Yung Choi S. Sodium Fluorescein staining of the cornea for the diagnosis of dry eye: a comparison of three eye solutions. Med Hypothesis Discov Innov Ophthalmol. (2017) 6:105–9.
5. Ding JE, Kim YH Yi SM, Graham AD Li W, Lin MC. Ocular surface cooling rate associated with tear film characteristics and the maximum interblink period. Sci Rep. (2021) 11:15030. doi: 10.1038/s41598-021-94568-9
6. Alishahi M, Kamali R. Forced diffusion of water molecules through aquaporin-5 biomembr a molecular dynamics study. Biophys Physicobiol. (2018) 15:255–62. doi: 10.2142/biophysico.15.0_255
7. van Setten GB. Osmokinetics: A new dynamic concept in dry eye disease. J Fr Ophtalmol. (2019) 42:221–5. doi: 10.1016/j.jfo.2018.11.001
8. Fagehi R, El-Hiti GA, Alqarni BM, Alanazi MA, Masmali AM, Almubrad T. Improvement in tear ferning patterns of sheep tears after addition of various electrolyte solutions. Front Med. (2021) 8:721969. doi: 10.3389/fmed.2021.721969
9. van Setten GB. Impact of attrition, intercellular shear in dry eye disease: when cells are challenged and neurons are triggered. Int J Mol Sci. (2020) 21:4333. doi: 10.3390/ijms21124333
10. Guzmán M, Miglio M, Keitelman I, Shiromizu CM, Sabbione F, Fuentes F, et al. Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset. Immunology. (2020) 161:148–61. doi: 10.1111/imm.13243
11. Hori J, Kunishige T, Nakano Y. Immune checkpoints contribute corneal immune privilege: implications for dry eye associated with checkpoint inhibitors. Int J Mol Sci. (2020) 21:3962. doi: 10.3390/ijms21113962
12. Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E. Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: a PICASSO board review. Br J Ophthalmol. (2021) 105:446–53. doi: 10.1136/bjophthalmol-2019-315747
13. Chinese Branch of the Asian Dry Eye Soci Ocular Surface and Tear Film Diseases Group of Ophthalmology Committee of Cross-Straits Medicine Exchange Associate Ocular Surface and Dry Eye Group of Chinese Ophthalmologist Association. [Chinese expert consensus on dry eye: dry eye related to immunologic diseases (2021)]. Zhonghua Yan Ke Za Zhi. (2021) 57:898–907. doi: 10.3760/cma.j.cn112142-20210726-00350
14. Chinese Optometric Association of Chinese Ophthalmological Soci Optometry Group of Chinese Ophthalmologist Associate Refractive Surgery Group of Chinese Ophthalmologist Association. [Expert consensus on the diagnosis and treatment of dry eye during perioperative period of corneal refractive surgery in China (2021)]. Zhonghua Yan Ke Za Zhi. (2021) 57:644–50. doi: 10.3760/cma.j.cn112142-20210312-00124
15. Saldanha IJ, Petris R, Makara M, Channa P, Akpek EK. Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Ocul Surf. (2021) 22:38–46. doi: 10.1016/j.jtos.2021.06.004
16. Starr CE, Dana R, Pflugfelder SC, Holland EJ, Zhang S. Owen D, et al. Dry eye disease flares: a rapid evidence assessment. Ocul Surf. (2021) 22:51–9. doi: 10.1016/j.jtos.2021.07.001
17. Shen Lee B, Kabat AG, Bacharach J, Karpecki P, Luchs J. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol. (2020) 14:119–26. doi: 10.2147/OPTH.S228838
19. Schirmer O. Studien zur physiologie und pathologie der tranen-absonderung und tranenabfuhr. Graefes Arch Clin Exp Ophthalmol. (1903) 56:197–291. doi: 10.1007/BF01946264
20. Rodney WM, Louie J, Puffer JC. Schirmer's test of lacrimation. Am Fam Physician. (1981) 24:161–4.
21. Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E. The challenge of dry eye diagnosis. Clin Ophthalmol. (2008) 2:31–55. doi: 10.2147/OPTH.S1496
22. Shapiro A, Merin S. Schirmer test and break-up time of tear film in normal subjects. Am J Ophthalmol. (1979) 88:752–7. doi: 10.1016/0002-9394(79)90678-0
23. JordanA BaumJ. Basic tear flow. Does it exist? Ophthalmology.1980 87:920–930. doi: 10.1016/S0161-6420(80)35143-9
24. Lamberts DW, Foster CS, Perry HD. Schirmer test after topical anesthesia and the tear meniscus height in normal eyes. Arch Ophthalmol. (1979) 97:1082–5. doi: 10.1001/archopht.1979.01020010536004
25. Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. (1966) 62:47–60. doi: 10.1016/0002-9394(66)91676-X
27. Clinch TE, Benedetto DA, Felberg NT, Laibson PR. Schirmer's test. A closer look. Arch Ophthalmol. (1983) 101:1383–6. doi: 10.1001/archopht.1983.01040020385009
28. Saleh TA, McDermott B, Bates AK, Ewings P. Phenol red thread test vs Schirmer's test: a comparative study. Eye. (2006) 20:913–5. doi: 10.1038/sj.eye.6702052
29. Li N, Deng XG, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. (2012) 5:478–81. doi: 10.3980/j.issn.2222-3959.2012.04.14
30. Kashkouli MB, Pakdel F, Amani A, Asefi M, Aghai GH, Falavarjani KG, et al. modified Schirmer test in dry eye and normal subjects: open versus closed eye and 1-minute versus 5-minute tests. Cornea. (2010) 29:384–7. doi: 10.1097/ICO.0b013e3181ba6ef3
31. Hamano H, Hori M, Hamano T, Mitsunaga S, Maeshima J, Kojima S, et al. A new method for measuring tears. CLAO J. (1983) 9:281–9
32. Tsubota K, Xu KP, Fujihara T, Katagiri S, Takeuchi T. Decreased reflex tearing is associated with lymphocytic infiltration in lacrimal glands. J Rheumatol. (1996) 23:313–20.
33. Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. (2004) 23:272–85. doi: 10.1097/00003226-200404000-00010
34. Lilley J, O'Neil EC, Bunya VY, Johnson K, Ying GS, Hua P, et al. Efficacy of an Intranasal Tear Neurostimulator in Sjögren Syndrome Patients. Clin Ophthalmol. (2021) 15:4291–6. doi: 10.2147/OPTH.S312108
35. Guillon JP. Current clinical techniques to study the tear film and tear secretions. In: Korb D, Craig J, Doughty M, Guillon JP, Smith G, Tomlinson A, editors. The Tear Film. London: Butterworth Heinem (2002). p. 51–83. doi: 10.1016/B978-0-7506-4196-8.50006-7
36. Holly FJ. Physical chemistry of the normal and disordered tear film. Trans Ophthalmol Soc UK. (1985) 104:374–80.
37. Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. (1996) 15:653–61. doi: 10.3109/02713689609008906
38. Oguz H, Yokoi N, Kinoshita S. The height and radius of the tear meniscus and methods for examining these parameters. Cornea. (2000) 19:497–500. doi: 10.1097/00003226-200007000-00019
39. Dogru M, Ishida K, Matsumoto Y, Goto E, Ishioka M, Kojima T, et al. Strip meniscometry: a new and simple method of tear meniscus evaluation. Invest Ophthalmol Vis Sci. (2006) 47:1895–901. doi: 10.1167/iovs.05-0802
40. Hao Y, Tian L, Cao K, Jie Y. Repeatability and reproducibility of SMTube measurement in dry eye disease patients. J Ophthalmol. (2021) 2021:1589378. doi: 10.1155/2021/1589378
41. Vigo L, Pellegrini M, Bernabei F, Carones F, Scorcia V, Giannaccare G. Diagnostic performance of a novel noninvasive workup in the setting of dry eye disease. J Ophthalmol. (2020) 2020:5804123. doi: 10.1155/2020/5804123
42. Chang AY, Purt B. Biochemistry, tear film. In: StatPearls. Treasure Island (FL): StatPearls Publish (accessed on Jun 15, 2021).
43. Golding TR, Bruce AS, Mainstone JC. Relationship between tear-meniscus parameters and tear-film breakup. Cornea. (1997) 16:649–61. doi: 10.1097/00003226-199711000-00009
44. Shah AM, Galor A. Impact of ocular surface temperature on tear characteristics: current insights. Clin Optom. (2021) 13:51–62. doi: 10.2147/OPTO.S281601
45. Yokoi N, Bron A, Tiffany J, Brown N, Hsuan J, Fowler C. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br J Ophthalmol. (1999) 83:92–7. doi: 10.1136/bjo.83.1.92
46. Johnson ME, Murphy PJ. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Invest Ophthalmol Vis Sci. (2007) 48:4451–8. doi: 10.1167/iovs.06-1253
47. Bandlitz S, Purslow C, Murphy PJ, Pult H. The relationship between tear meniscus regularity and conjunctival folds. Optom Vis Sci. (2014) 91:1037–44. doi: 10.1097/OPX.0000000000000358
48. Li J, Ma J, Hu M, Yu J, Zhao Y. Assessment of tear film lipid layer thickness in patients with Meibomian gland dysfunction at different ages. BMC Ophthalmol. (2020) 20:394. doi: 10.1186/s12886-020-01667-8
49. Koh S, Ikeda C, Watanabe S, Oie Y, Soma T, Watanabe H, et al. Effect of non-invasive tear stability assessment on tear meniscus height. Acta Ophthalmol. (2015) 93:e135–9. doi: 10.1111/aos.12516
50. Lee KW, Kim JY, Chin HS, Seo KY, Kim TI, Jung JW. Assessment of the tear meniscus by strip meniscometry and keratograph in patients with dry eye disease according to the presence of meibomian gland dysfunction. Cornea. (2017) 36:189–95. doi: 10.1097/ICO.0000000000001033
51. Shinzawa M, Dogru M, Miyasaka K, Shimazaki J, Sekiryu T. Application of CASIA SS-1000 Optical Coherence Tomography Tear Meniscus Imaging in Testing the Efficacy of New Strip Meniscometry in Dry Eye Diagnosis. Eye Contact Lens. (2018) 44 Suppl 1:S44–9. doi: 10.1097/ICL.0000000000000312
52. Ishikawa S, Takeuchi M, Kato N. The combination of strip meniscometry and dry eye-related quality-of-life score is useful for dry eye screening during health checkup: Cross-sectional study. Medicine. (2018) 97:e12969. doi: 10.1097/MD.0000000000012969
53. Miyasaka K, Kazama Y, Iwashita H, Wakaiki S, Saito A. A novel strip meniscometry method for measuring aqueous tear volume in dogs: clinical correlations with the Schirmer tear and phenol red thread tests. Vet Ophthalmol. (2019) 22:864–71. doi: 10.1111/vop.12664
54. Rolando M. Tear mucus ferning test in normal and keratoconjunctivitis sicca eyes. Chibret Int J Ophthalmol. (1984) 2:32–41.
55. Golding TR, Baker AT, Rechberger J. Brennan NA. X-ray and scanning electron microscopic analysis of the structural composition of tear ferns. Cornea. (1994) 13:58–66. doi: 10.1097/00003226-199401000-00010
56. Kogbe O. Liotet S. An interesting use of the study of tear ferning patterns in contactology. Ophthalmologica. (1987) 194:150–3. doi: 10.1159/000309753
57. Traipe-Castro L, Salinas-Toro D, López D, Zanolli M, Srur M, Valenzuela F, et al. Dynamics of tear fluid desiccation on a glass surface: a contribution to tear quality assessment. Biol Res. (2014) 47:25. doi: 10.1186/0717-6287-47-25
58. Nebbioso M, Sacchetti M, Bianchi G, Zicari AM, Duse M, Del Regno P, et al. Tear ferning test and pathological effects on ocular surface before and after topical cyclosporine in vernal keratoconjunctivitis patients. J Ophthalmol. (2018) 2018:1061276. doi: 10.1155/2018/1061276
59. Rolando M, Baldi F, Calabria G. Tear mucus crystallization in children with cystic fibrosis. Ophthalmologica. (1988) 197:202–6. doi: 10.1159/000309944
60. Ravazzoni L, Ghini C, Macrí A. Rolando M. Forecasting of hydrophilic contact lens tolerance by means of tear ferning test. Graefes Arch Clin Exp Ophthalmol. (1998) 236:354–8. doi: 10.1007/s004170050090
61. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. (2005) 3:81–95. doi: 10.1016/S1542-0124(12)70157-X
62. Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. (2011) 52:2006–49. doi: 10.1167/iovs.10-6997f
63. Srinivasan S, Nichols KK. Collecting tear osmolarity measurements in the diagnosis of dry eye. Expert Rev Ophthalmol. (2009) 4:451–3. doi: 10.1586/eop.09.43
64. Khanal S, Tomlinson A, McFadyen A, Diaper C, Ramaesh K. Dry eye diagnosis. Invest Ophthalmol Vis Sci. (2008) 49:1407–14. doi: 10.1167/iovs.07-0635
65. Masmali AM, Murphy PJ, Purslow C. Development of a new grading scale for tear ferning. Cont Lens Anterior Eye. (2014) 37:178–84. doi: 10.1016/j.clae.2013.09.011
66. Benelli U, Nardi M, Posarelli C, Albert TG. Tear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectiveness. Cont Lens Anterior Eye. (2010) 33:61–7. doi: 10.1016/j.clae.2010.01.003
67. Versura P, Profazio V, Campos E. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. J Curr Eye Res. (2010) 35:553–64. doi: 10.3109/02713683.2010.484557
68. Jacobi C, Jacobi A, Kruse FE, Cursiefen C. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea. (2011) 30:1289–92. doi: 10.1097/ICO.0b013e31821de383
69. Masmali A, Alrabiah S, Alharbi A, El-Hiti GA, Almubrad T. Investigation of tear osmolarity using the TearLab Osmolarity System in normal adults in Saudi Arabia. J Eye contact lens. (2014) 40:74–8. doi: 10.1097/ICL.0000000000000002
70. Lemp MA, Bron AJ, Baudouin C, Benítez Del Castillo JM, Geffen D, Tauber J, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. (2011) 151:792–8.e1. doi: 10.1016/j.ajo.2010.10.032
71. Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. (2010) 51:6125–30. doi: 10.1167/iovs.10-5390
72. Messmer EM, Bulgen M, Kampik A. In Research Projects in Dry Eye Syndrome. Germany: Karger Publishers (2010). vol. 45. p. 129–138. doi: 10.1159/000315026
73. Bunya VY, Fuerst NM, Pistilli M, McCabe BE, Salvo R, Macchi I, et al. Variability of Tear Osmolarity in Patients With Dry Eye. JAMA Ophthalmol. (2015) 133:662–7. doi: 10.1001/jamaophthalmol.2015.0429
74. Szalai E, Berta A, Szekanecz Z, Szûcs G, Módis L. Evaluation of tear osmolarity in non-Sjögren and Sjögren syndrome dry eye patients with the TearLab system. J Cornea. (2012) 31:867–71. doi: 10.1097/ICO.0b013e3182532047
75. Baenninger PB, Voegeli S, Bachmann LM, Faes L, Iselin K, Kaufmann C, et al. Variability of tear osmolarity measurements with a point-of-care system in healthy subjects-systematic review. Cornea. (2018) 37:938–45. doi: 10.1097/ICO.0000000000001562
76. Tashbayev B, Utheim TP, Utheim ØA, Ræder S, Jensen JL, Yazdani M, et al. Utility of tear osmolarity measurement in diagnosis of dry eye disease. Sci Rep. (2020) 10:5542. doi: 10.1038/s41598-020-62583-x
77. Chan CC, Borovik A, Hofmann I, Gulliver E, Rocha G. Validity and reliability of a novel handheld osmolarity system for measurement of a national institute of standards traceable solution. Cornea. (2018) 37:1169–74. doi: 10.1097/ICO.0000000000001653
78. Shimazaki J, Sakata M, Den S, Iwasaki M, Toda I. Tear film osmolarity measurement in japanese dry eye patients using a handheld osmolarity system. Diagnostics. (2020) 10:789. doi: 10.3390/diagnostics10100789
79. Fagehi R, Al-Bishry AB, Alanazi MA, Abusharha A, El-Hiti GA, Masmali AM. Investigation of the repeatability of tear osmolarity using an I-PEN osmolarity device. Taiwan J Ophthalmol. (2020) 11:168–74. doi: 10.4103/tjo.tjo_65_20
80. Park J, Choi Y, Han G, Shin E, Han J, Chung TY, et al. Evaluation of tear osmolarity measured by I-Pen osmolarity system in patients with dry eye. Sci Rep. 2021 11:7726. doi: 10.1038/s41598-021-87336-2
81. König S, Priglinger S, Schaumberger M, Messmer EM. Tränenfilmosmolarität gesunder Probanden: Vergleichbarkeit zweier Osmometer [Tear Film Osmolarity in Normal Individuals: Comparison of Two Osmometers]. Klin Monbl Augenheilkd. (2020) 237:649–54. doi: 10.1055/a-1155-6468
82. Choi EW, Yeom DJ, Jang SY. Botulinum toxin a injection for the treatment of intractable dry eye disease. Medicina. (2021) 57:247. doi: 10.3390/medicina57030247
83. Tavakoli A, Markoulli M, Flanagan J, Papas E. The validity of point of care tear film osmometers in the diagnosis of dry eye. Ophthalmic Physiol Opt. (2021) doi: 10.1111/opo.12901
84. Ibrahim OM, Dogru M, Ward SK, Matsumoto Y, Wakamatsu TH, Ishida K, et al. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Invest Ophthalmol Vis Sci. (2011) 52:2194–8. doi: 10.1167/iovs.10-5986
85. Shen Z, Zhu Y, Song X, Yan J, Yao K. Dry eye after small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for Myopia: a meta-analysis. PLoS ONE. (2016) 11:e0168081. doi: 10.1371/journal.pone.0168081
86. Kim MJ, Stinnett SS, Gupta PK. Effect of thermal pulsation treatment on tear film parameters in dry eye disease patients. Clin Ophthalmol. (2017) 11:883–6. doi: 10.2147/OPTH.S136203
87. Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. (2019) 12:CD011016. doi: 10.1002/14651858.CD011016.pub2
88. García-Conca V, Abad-Collado M, Hueso-Abancens JR, Mengual-Verdú E, Piñero DP, Aguirre-Balsalobre F, et al. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. (2019) 97:e170–8. doi: 10.1111/aos.13907
89. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. (1999) 19:201–11. doi: 10.1076/ceyr.19.3.201.5309
90. Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II Tear Film Report. Ocul Surf. (2017) 15:366–403. doi: 10.1016/j.jtos.2017.03.006
91. Sambursky R, Davitt WF, Latkany R, Tauber S, Starr C, Friedberg M, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. (2013) 131:24–8. doi: 10.1001/jamaophthalmol.2013.561
92. Sambursky R, Davitt WF, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. (2014) 33:812–8. doi: 10.1097/ICO.0000000000000175
93. Ryu KJ, Kim S, Kim MK, Paik HJ, Kim DH. Short-term therapeutic effects of topical corticosteroids on refractory dry eye disease: clinical usefulness of matrix metalloproteinase 9 testing as a response prediction marker. Clin Ophthalmol. (2021) 15:759–67. doi: 10.2147/OPTH.S300047
94. Kim M, Oh JY, Bae SH, Lee SH, Lee WJ, Chun YS, et al. Assessment of reliability and validity of the 5-scale grading system of the point-of-care immunoassay for tear matrix metalloproteinase-9. Sci Rep. (2021) 11:12394. doi: 10.1038/s41598-021-92020-6
95. Lanza NL, Valenzuela F, Perez VL, Galor A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul Surf. (2016) 14:189–95. doi: 10.1016/j.jtos.2015.10.004
96. Kawashima M, Nakamura S, Izuta Y, Inoue S, Tsubota K. Dietary supplementation with a combination of Lactoferrin, Fish Oil, and Enterococcus faecium WB2000 for treating dry eye: a rat model and human clinical study. Ocul Surf. (2016) 14:255–63. doi: 10.1016/j.jtos.2015.12.005
97. Devendra J, Singh S. Effect of oral lactoferrin on cataract surgery induced dry eye: a randomised controlled trial. J Clin Diagn Res. (2015) 9:NC06–9. doi: 10.7860/JCDR/2015/15797.6670
98. Tsubota K, Pflugfelder SC, Liu Z, Baudouin C, Kim HM, Messmer EM, et al. Defining dry eye from a clinical perspective. Int J Mol Sci. (2020) 21:9271. doi: 10.3390/ijms21239271
99. Norn MS. Desiccation of the precorneal film. I corneal wetting-time. Acta Ophthalmol. (1969) 47:865–80. doi: 10.1111/j.1755-3768.1969.tb03711.x
100. Cox SM, Nichols KK, Nichols JJ. Agreement between automated and traditional measures of tear film breakup. Optom Vis Sci. (2015) 92:257–63. doi: 10.1097/OPX.0000000000000648
101. Mengher LSB A J, Tonge SR, Gilbert DJ. Effect of fluorescein instillation on the pre-corneal tear fifilm stability Curr Eye Res. (1985) 4:9–12. doi: 10.3109/02713688508999961
102. Mooi JK, Wang MTM, Lim J, Müller A, Craig JP. Minimising instilled volume reduces the impact of fluorescein on clinical measurements of tear film stability. Cont Lens Anterior Eye. (2017) 40:170–4.
103. Pult H, Riede-Pult BH. A new modified fluorescein strip: Its repeatability and usefulness in tear film break-up time analysis. Cont Lens Anterior Eye. (2012) 35:35–8. doi: 10.1016/j.clae.2011.07.005
104. Kallarackal GU, Ansari EA, Amos N, Martin JC, Lane C, Camilleri JP, et al. comparative study to assess the clinical use of Fluorescein Meniscus Time (FMT) with Tear Break up Time (TBUT) and Schirmer's tests (ST) in the diagnosis of dry eyes. Eye (Lond). (2002) 16:594–600. doi: 10.1038/sj.eye.6700177
105. Mou Y, Xiang H, Lin L, Yuan K, Wang X, Wu Y, Min J, Jin X. Reliability and efficacy of maximum fluorescein tear break-up time in diagnosing dry eye disease. Sci Rep. (2021) 11:11517. doi: 10.1038/s41598-021-91110-9
106. Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjögren's Syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren's syndrome. Annl Rheum Dis. (1994) 53:637–47. doi: 10.1136/ard.53.10.637
107. Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. (2017) 15:539–74. doi: 10.1016/j.jtos.2017.05.001
108. Tsubota K. Short tear film breakup time-type dry eye. Invest Ophthalmol Vis Sci. (2018) 59:DES64-DES70. doi: 10.1167/iovs.17-23746
109. Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye. Prog Retin Eye Res. (2020) 29:100842. doi: 10.1016/j.preteyeres.2020.100842
110. Mcmonnies CW. Tear instability importance, mechanisms, validity and reliability of assessment. J Optom. (2018) 11:203-210. doi: 10.1016/j.optom.2017.11.004
111. Braun RJ, Gewecke NR, Begley CG, King-Smith PE, Siddique JI. A model for tear film thinning with osmolarity and fluorescein. Invest Ophthalmol Vis Sci. (2014) 55:1133–42. doi: 10.1167/iovs.13-12773
112. King-Smith PE, Ramamoorthy P, Braun RJ, Nichols JJ. Tear film images and breakup analyzed using fluorescent quenching. Invest Ophthalmol Vis Sci. (2013) 54:6003–11. doi: 10.1167/iovs.13-12628
113. Wang MT, Murphy PJ, Blades KJ, Craig JP. Comparison of non-invasive tear film stability measurement techniques. Clin Exp Optom. (2018) 101:13–7. doi: 10.1111/cxo.12546
114. Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr Eye Res. (1985) 4:1–7. doi: 10.3109/02713688508999960
115. Hirji N, Patel S, Callander M. Human tear film pre-rupture phase time (TPRPT) - a non-invasive technique for evaluating the pre-corneal tear film using a novel keratometer mire. Ophthal Physiol Opt. (1989) 9:139–42. doi: 10.1111/j.1475-1313.1989.tb00833.x
116. Goto T, Zheng X, Klyce SD, Kataoka H, Uno T, Karon M, et al. A new method for tear film stability analysis using video keratography. Am J Ophthalmol. (2003) 135:607–12. doi: 10.1016/S0002-9394(02)02221-3
117. Kojima T, Ishida R, Dogru M, Goto E, Takano Y, Matsumoto Y, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci. (2004) 45:1369–74. doi: 10.1167/iovs.03-0712
118. Wolff E. The muco-cutaneous junction of the lid margin and the distribution of the tear fluid. Trans Ophthalmol Soc U K. (1946) 66:291–308.
119. Holly FJ, Lemp MA. Tear physiology and dry eyes. Surv Ophthalmol. (1977) 22:69–87. doi: 10.1016/0039-6257(77)90087-X
120. King-Smith PE, Fink BA, Hill RM, Koelling KW, Tiffany JM. The thickness of the tear film. Curr Eye Res. (2004) 29:357–68. doi: 10.1080/02713680490516099
121. King-Smith PE, Fink BA, Fogt N, Nichols KK, Hill RM, Wilson GS. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. (2000) 41:3348–59.
122. Chen Q, Wang J, Tao A, Shen M, Jiao S, Lu F. Ultrahigh-resolution measurement by optical coherence tomography of dynamic tear film changes on contact lenses. Invest Ophthalmol Vis Sci. (2010) 51:1988–93. doi: 10.1167/iovs.09-4389
123. Zhao Y, Tan CL, Tong L. Intra-observer and inter-observer repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol. (2015) 15:53. doi: 10.1186/s12886-015-0036-9
124. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf . (2019) 17:440–6. doi: 10.1016/j.jtos.2019.05.007
125. Wan T, Jin X, Lin L, Xu Y, Zhao Y. Incomplete blinking may attribute to the development of meibomian gland dysfunction. Curr Eye Res. (2016) 41:179–85. doi: 10.3109/02713683.2015.1007211
126. Markoulli M, Duong TB, Lin M, Papas E. Imaging the Tear Film: A Comparison Between the Subjective Keeler Tearscope-Plus™ and the Objective Oculus® Keratograph 5M and LipiView® Interferometer. Curr Eye Res. (2018) 43:155–62. doi: 10.1080/02713683.2017.1393092
127. Lee JM, Jeon YJ, Kim KY, Hwang KY, Kwon YA, Koh K. Ocular surface analysis: A comparison between the LipiView® II and IDRA®. Eur J Ophthalmol. (2020) 2:1120672120969035.
128. Sun Xuguang. Blepharitis and Meibomian Gland Dysfunction. Beijing: People's Health Publishing House (2015). p. 2–10.
129. Zhang Mingchang, Liu Yang. Interpretation of dry eye examination in the 2017 consensus of dry eye experts of the International Association of tear film and ocular surface[J]. Chin J Ophthalmol. (2018) 54:87–9. doi: 10.3760/cma.j.issn.0412-4081.2018.02.003
130. Asia Dry Eye Society ADES China branch Cross strait Medical Exchange Association Ophthalmology Committee Ocular surface and lacrimopathy group Ocular Ocular Surface and Dry Eye Group of Ophthalmologist Branch of Chinese Medical Doctor Association. Expert consensus on dry eye in China: Examination and diagnosis (2020). (2020) 56:741-7. doi: 10.3760/cma.j.cn112142-20200714-00477
131. Wang J, Li S, Yeh TN, Chakraborty R, Graham AD, Yu SX, et al. Quantifying meibomian gland morphology using artificial intelligence. Optom Vis Sci. (2021) 98:1094–103. doi: 10.1097/OPX.0000000000001767
132. Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom. (2018) 10:57–63. doi: 10.2147/OPTO.S142708
133. Amano S. Meibomian gland dysfunction: recent progress worldwide and in Japan. Invest Ophthalmol Vis Sci. (2018) 59:DES87-DES93. doi: 10.1167/iovs.17-23553
134. Fineide F, Arita R, Utheim TP. The role of meibography in ocular surface diagnostics: A review. Ocul Surf. (2021) 19:133–44. doi: 10.1016/j.jtos.2020.05.004
135. Efron N, Brennan NA, Morgan PB, Wilson T. Lid wiper epitheliopathy. Prog Retin Eye Res. (2016) 53:140–74. doi: 10.1016/j.preteyeres.2016.04.004
136. Shiraishi A, Yamanishi S, Yamamoto Y, Yamaguchi M, Ohashi Y. [Lid-wiper epitheliopathy in patients with dry eye symptoms]. Nippon Ganka Gakkai Zasshi. (2009) 113:596–600.
137. Korb DR, Herman JP, Blackie CA, Scaffidi RC, Greiner JV, Exford JM, et al. Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea. (2010) 29:377–83. doi: 10.1097/ICO.0b013e3181ba0cb2
138. Liang X, Li Y, Xiong K, Chen S, Li Z, Zhang Z, et al. Demodex infection changes ocular surface microbial communities, in which meibomian gland dysfunction may play a role. Ophthalmol Ther. (2021) 10:601–17. doi: 10.1007/s40123-021-00356-z
139. Huo Y, Mo Y, Wu Y, Fang F, Jin X. Therapeutic effect of intense pulsed light with optimal pulse technology on meibomian gland dysfunction with and without ocular Demodex infestation. Ann Transl Med. (2021) 9:238. doi: 10.21037/atm-20-1745
140. Kim J. The use of vital dyes in corneal disease. Curr Opin Ophthalmol. (2000) 11:241–7. doi: 10.1097/00055735-200008000-00005
141. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. (2003) 22:640–50. doi: 10.1097/00003226-200310000-00008
142. Maurice DM. The use of fluorescein in ophthalmological research. Invest Ophthalmol. (1967) 6:464–77.
143. Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. (2007) 26:805–9. doi: 10.1097/ICO.0b013e318074e460
144. Korb DR, Herman JP, Finnemore VM, Exford JM, Blackie CA. An evaluation of the efficacy of fluorescein, rose bengal, lissamine green, and a new dye mixture for ocular surface staining. Eye Contact Lens. (2008) 34:61–4. doi: 10.1097/ICL.0b013e31811ead93
145. Feenstra RP, Tseng SC. Comparison of fluorescein and rose Bengal staining. Ophthalmology. (1992) 99:605–17. doi: 10.1016/S0161-6420(92)31947-5
146. Zeev MS, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. (2014) 8:581–90. doi: 10.2147/OPTH.S45444
147. Yoon KC, Im SK, Kim HG, You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. (2011) 30:972–6. doi: 10.1097/ICO.0b013e31820687dd
148. Begley C, Caffery B, Chalmers R, Situ P, Simpson T, Nelson JD. Review and analysis of grading scales for ocular surface staining. Ocul Surf. (2019) 17:208–20. doi: 10.1016/j.jtos.2019.01.004
149. Baudouin C, Barton K, Cucherat M, Traverso C. The measurement of bulbar hyperemia: challenges and pitfalls. Eur J Ophthalmol. (2015) 25:273–9. doi: 10.5301/ejo.5000626
150. Singh RB, Liu L, Anchouche S, Yung A, Mittal SK, Blanco T, et al. Ocular redness—I: etiology, pathogenesis, and assessment of conjunctival hyperemia. Ocul Surf. (2021) 21:134–44. doi: 10.1016/j.jtos.2021.05.003
151. Owen CG, Fitzke FW, Woodward EG. A new computer assisted objective method for quantifying vascular changes of the bulbar conjunctivae. Ophthalmic Physiol Opt. (1996) 16:43. doi: 10.1046/j.1475-1313.1996.96000373.x
152. Huntjens B, Basi M, Nagra M. Evaluating a new objective grading software for conjunctival hyperaemia. Cont Lens Anterior Eye. (2020) 43:137–43. doi: 10.1016/j.clae.2019.07.003
153. Schulze MM, Ng A, Yang M, Panjwani F, Srinivasan S, Jones LW, et al. Bulbar redness and dry eye disease: comparison of a validated subjective grading scale and an objective automated method. Optom Vis Sci. (2021) 98:113–20. doi: 10.1097/OPX.0000000000001638
154. Matsumoto Y, Ibrahim OMA. Application of in vivo confocal microscopy in dry eye disease. Invest Ophthalmol Vis Sci. (2018) 59:DES41–7. doi: 10.1167/iovs.17-23602
155. Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. (2004) 45:3030–5. doi: 10.1167/iovs.04-0251
156. Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. (2007) 48:173–81. doi: 10.1167/iovs.06-0127
157. Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjögren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. (2007) 48:2017–22. doi: 10.1167/iovs.06-1129
158. Villani E, Galimberti D, Viola F, Mapelli C, Del Papa N, Ratiglia R. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Invest Ophthalmol Vis Sci. (2008) 49:560–4. doi: 10.1167/iovs.07-0893
159. Wakamatsu TH, Sato EA, Matsumoto Y, Ibrahim OM, Dogru M, Kaido M, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. (2010) 51:144–50. doi: 10.1167/iovs.08-2722
160. Matsumoto Y, Sato EA, Ibrahim OMA, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. (2008) 14:1263–71.
161. Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. (2010) 117:665–72. doi: 10.1016/j.ophtha.2009.12.029
162. Sato EA, Matsumoto Y, Dogru M, Kaido M, Wakamatsu T, Ibrahim OM, Obata H, Tsubota K. Lacrimal gland in Sjögren's syndrome. Ophthalmology. (2010) 117:1055.e3. doi: 10.1016/j.ophtha.2009.11.034
163. Villani E, Magnani F, Viola F, Santaniello A, Scorza R, Nucci P, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci. (2013) 90:576–86. doi: 10.1097/OPX.0b013e318294c184
164. Cox SM, Kheirkhah A, Aggarwal S, Abedi F, Cavalcanti BM, Cruzat A, et al. Alterations in corneal nerves in different subtypes of dry eye disease: An in vivo confocal microscopy study. Ocul Surf. (2021) 22:135–42. doi: 10.1016/j.jtos.2021.08.004
165. Ma B, Xie J, Yang T, Su P, Liu R, Sun T, Zhou Y, Wang H, Feng X, Ma S, Zhao Y, Qi H. Quantification of increased corneal subbasal nerve tortuosity in dry eye disease and its correlation with clinical parameters. Transl Vis Sci Technol. (2021) 10:26. doi: 10.1167/tvst.10.6.26
166. Ma J, Wei S, Jiang X, Chou Y, Wang Y, Hao R, et al. Evaluation of objective visual quality in dry eye disease and corneal nerve changes. Int Ophthalmol. (2020) 40:2995–3004.
167. Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. (2010) 51:122–8. doi: 10.1167/iovs.09-3629
168. Pan S, Chen Y. A clinical study on the correlation between demodex infestation and ocular surface changes in patients with meibomian gland dysfunction. Indian J Ophthalmol. (2021) 69:2389–94. doi: 10.4103/ijo.IJO_3641_20
169. Binotti WW, Bayraktutar B, Ozmen MC, Cox SM, Hamrah P. A Review of Imaging Biomarkers of the Ocular Surface. Eye Contact Lens. (2020) 46:S84–S105. doi: 10.1097/ICL.0000000000000684
170. Chinese Chinese Experts Consensus Members on Standardized Construction of Dry Eye Clinical CenDry Eye Rehabilitation Specialty Visual Visual Rehabilitation Committee of Chinese Rehabilitation Medicine Associat Jin Xiuming. Chinese experts consensus on standardized construction of dry eye clinical center. Zhonghua Shiyan Yanke Zazhi. (2021) 39:473–476. doi: 10.3760/cma.j.cn115989-20210223-00128
Keywords: dry eye, examination, tear, meibomian gland, demodex mites
Citation: Wu Y, Wang C, Wang X, Mou Y, Yuan K, Huang X and Jin X (2022) Advances in Dry Eye Disease Examination Techniques. Front. Med. 8:826530. doi: 10.3389/fmed.2021.826530
Received: 30 November 2021; Accepted: 24 December 2021;
Published: 25 January 2022.
Edited by:
Michele Lanza, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Giulia Di Benedetto, University of Catania, ItalyChuanqing Ding, SightGlass Vision, United States
Copyright © 2022 Wu, Wang, Wang, Mou, Yuan, Huang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuming Jin, bHp5anhtJiN4MDAwNDA7emp1LmVkdS5jbg==
 Yaying Wu
Yaying Wu Chunyang Wang
Chunyang Wang Xin Wang
Xin Wang Yujie Mou
Yujie Mou Kelan Yuan
Kelan Yuan Xiaodan Huang
Xiaodan Huang Xiuming Jin
Xiuming Jin