- 1General, Visceral and Transplant Surgery, Department of Surgery, Medical University of Graz, Graz, Austria
- 2Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Kidney transplantation remains the gold standard treatment for patients suffering from end-stage kidney disease. To meet the constantly growing organ demands grafts donated after circulatory death (DCD) or retrieved from extended criteria donors (ECD) are increasingly utilized. Not surprisingly, usage of those organs is challenging due to their susceptibility to ischemia-reperfusion injury, high immunogenicity, and demanding immune regulation after implantation. Lately, a lot of effort has been put into improvement of kidney preservation strategies. After demonstrating a definite advantage over static cold storage in reduction of delayed graft function rates in randomized-controlled clinical trials, hypothermic machine perfusion has already found its place in clinical practice of kidney transplantation. Nevertheless, an active investigation of perfusion variables, such as temperature (normothermic or subnormothermic), oxygen supply and perfusate composition, is already bringing evidence that ex-vivo machine perfusion has a potential not only to maintain kidney viability, but also serve as a platform for organ conditioning, targeted treatment and even improve its quality. Many different therapies, including pharmacological agents, gene therapy, mesenchymal stromal cells, or nanoparticles (NPs), have been successfully delivered directly to the kidney during ex-vivo machine perfusion in experimental models, making a big step toward achievement of two main goals in transplant surgery: minimization of graft ischemia-reperfusion injury and reduction of immunogenicity (or even reaching tolerance). In this comprehensive review current state of evidence regarding ex-vivo kidney machine perfusion and its capacity in kidney graft treatment is presented. Moreover, challenges in application of these novel techniques in clinical practice are discussed.
Introduction
Kidney transplantation (Tx) remains the gold-standard treatment for end-stage kidney disease. Due to advanced kidney replacement therapies, improved care, and increased survival of such patients, the number of people on the waiting list for a deceased donor kidney is stably high or growing. In 2020, in the EuroTransplant region, <3,000 deceased donor kidneys and <1,000 living donor kidneys were transplanted while ~11,000 patients were on the active kidney waiting list (1). To meet growing organ demands, utilization of grafts donated after circulatory death (DCD), or retrieved from extended criteria donors (ECD) is unavoidable. The main challenge preventing the expansion of the donor pool is the susceptibility of ECD kidneys for ischemia-reperfusion injury (IRI). Moreover, DCD and ECD grafts are more immunogenic, which leads to difficulties in immune regulation after implantation and a higher risk of acute and chronic rejection (2–4).
The development of organ preservation techniques has the potential to overcome these challenges. Therefore, it has been the main focus of research in the solid organ Tx field for at least 30 years (5, 6). Kidney graft preservation relied on static cold storage (SCS) for a long time (7–9) but increasing donor age and comorbidities urgently require more reliable preservation techniques. Since publication of the largest randomized clinical trial (RCT) comparing hypothermic dynamic kidney machine perfusion (HMP) and SCS (10), HMP took root in routine clinical practice (11–14). HMP improves at least short-term outcomes of all types of kidney grafts, especially of DCD and ECD organs, by achieving the first goal of organ preservation—maintain organ quality until implantation. However, to increase the number of transplantable organs, the main focus now is on the improvement of graft quality, graft conditioning, and repair (6). Extensive research on different ex-vivo dynamic preservation temperatures [normothermic (NMP) and subnormothermic (SNMP)], perfusate composition, oxygenation, and perfusion duration revealed that machine perfusion could be used as a tool to treat kidneys prior to implantation by creating nearly-physiological conditions (15). Moreover, machine perfusion seems to be a perfect platform for the various biological and pharmacological agents' delivery directly to the kidney graft. Ex-vivo treatment is attractive because it would allow avoiding toxicity caused by systemic recipient treatment and logistic difficulties of the donor therapy (16–18). In addition, some novel promising agents just failed to reach the graft when applied systemically (19–21). This issue could be solved by targeted therapy delivery during ex-vivo machine perfusion. Moreover, perfusion parameters and perfusate markers would allow constant evaluation of the kidney state during treatment (22). Multiple experimental and clinical works investigated various ex-vivo kidney perfusion therapies for three main goals: (i) to reduce ischemia-reperfusion injury, (ii) reduce the immunogenicity of the kidney graft and (iii) promote healing and regeneration of already injured organ.
In this review, we discuss current evidence of kidney ex-vivo perfusion techniques and graft treatment during ex-vivo perfusion. Of note, in the overview of ex-vivo therapies, we did not include usual perfusate components, such as oxygen, nutrition supplement, heparin, vasodilators, and antibiotics, as this is out of our scope. We aim to outline the newest experimental and clinical studies of kidney ex-vivo cell therapy, gene therapy, application of nanotechnologies, delivery of different gasses, biological, and pharmacological agents for kidney treatment and pre-conditioning.
Overview of Kidney EX-VIVO Machine Perfusion Protocols
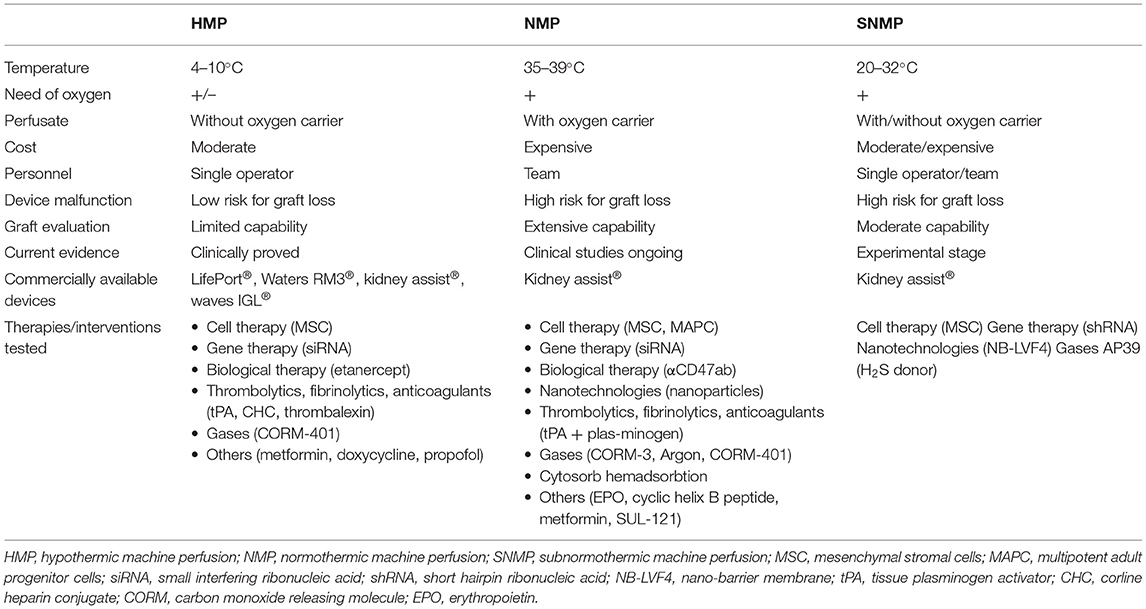
Ex-vivo organ perfusion techniques are usually classified according to the perfusate temperature. Below, we briefly present the concepts and the current protocols of hypothermic and (sub)normothermic kidney machine perfusion. The main differences of perfusion techniques are summarized in Table 1.
Hypothermic Machine Perfusion
During HMP, cold (4–10°C) acellular preservation solution is pumped through the kidney vasculature (5). Several kidney HMP devices are currently commercially available, including LifePort® (Organ Recovery Systems; Itasca, Illinois), Kidney Assist® (OrganAssist; Groningen, Netherlands), Waters RM3® kidney perfusion system (Rochester, Minnesota) and Waves IGL® (Lyon, France). In the current ex-vivo perfusion machines, roller or centrifugal pumps are used to generate pressure-controlled pulsatile flow, avoiding perfusion-related graft injury. For kidney HMP, low pressure (25–30 mmHg) is preferable to prevent kidney edema and endothelial damage (5, 23). The perfusion solutions used are qualitatively different from SCS solutions. The only clinically proven fluid for kidney HMP is Kidney Perfusion Solution-1 (KPS-1®), which has the same composition as Belzer MPS® UW (Machine Perfusion Solution University of Wisconsin) (14). Unlike the conventional UW solution and other SCS solutions, KPS-1 has an extracellular-like Na+/K+ balance. Moreover, it contains other impermeants (gluconate and mannitol instead of lactobionate and raffinose) and glucose. A variant of commonly used HTK (histidine-tryptophan-ketoglutarate, Custodiol®) solution Custodiol-N® supplemented with dextran 40 was used for kidney HMP in several porcine models (24). Moreover, a novel Custodiol-MP® solution has been recently developed exclusively for aerobic machine perfusion (25). Despite promising results of experimental models, further studies are necessary to prove the superiority of Custodiol-N® or Custodiol-MP® over KPS-1® in terms of kidney graft function and transplant outcomes (24, 25).
MP was widely used at the beginning of the solid organ Tx era when the first kidney transplant programs were developed. This method was considered the only safe and reliable way to preserve grafts, especially for long periods of organ storage. These machines were large and difficult to transport (26, 27). Later, Collins et al. presented a method to preserve and transport kidneys on ice in a preservation solution (7). More elaborated preservation solutions granted safe, easy, and much cheaper SCS with satisfactory Tx outcomes decreasing HMP's popularity, inaugurating the era of SCS (7–9). SCS served well as a preservation method of healthy kidney grafts. However, with the growing demand for organs and the aging donor population, the usage of kidneys from ECD lately became unavoidable. The limited applicability of SCS for higher-risk organs encouraged extensive research on alternative and more sophisticated preservation methods. The HMP landmark study in 2009 by Moers et al. brought the machine perfusion back to clinics. This EuroTransplant study, which included 672 kidneys retrieved in 60 European Tx centers, revealed a significantly improved graft survival at 1- and 3-years as well as reduced DGF rates in HMP kidneys compared to SCS (10). Even though this study included mainly donation after brain-death (DBD) grafts, Zhong et al. also found significant benefits in 1- and 3-years graft survival of HMP in the DCD kidneys cohort (28). An independently powered extension of Jochmans et al. initial RCT showed reduced delayed graft function (DGF) rates in DCD kidneys that underwent HMP (29). Similarly, another RCT reported that HMP significantly diminished DGF rates and improved 1-year death-censored graft survival in ECD kidneys in comparison with SCS (30). The superiority of kidney HMP vs. SCS was also confirmed by many systematic reviews and meta-analyses: reduction of DGF rates was observed in both DCD (31, 32) and ECD kidneys (33), and across all donor types (11–14, 34). Similarly, meta-analyses reported improved ECD kidney graft survival at 1-year (33) and among all donor types at 3-years (13). A Cochrane Review, including 16 studies published during the previous 10-years including 2,266 patients, revealed that HMP significantly reduced DGF rates in both DBD and DCD kidney Tx. However, the number needed to treat to prevent one episode of DGF was lower for DCD kidneys (14). Several systematic reviews and meta-analyses demonstrated significant reduction rates in kidney primary non-function (PNF) following HMP compared to SCS (12, 35).Nevertheless, the HMP effect on short- and long-term kidney graft function remains inconclusive (14).
In depth, knowledge about the exact mechanism, how pulsatile hypothermic perfusion improves kidney outcome, is scarce. However, several experimental studies brought some clarity into the field. Similar to SCS, during HMP, the rate of metabolism is reduced to ~10% of physiological temperature. Although HMP does not prevent cold-related depletion of adenosine triphosphate (ATP) and accumulation of metabolic products in the graft (5), the dynamic manner of preservation gives its beneficial effects. Firstly, HMP allows the elimination of debris, toxic metabolites, and free radicals produced during hypothermia (36, 37). Hemodynamic stimulation of the graft vasculature helps prevent endothelial damage, leading to anti-inflammatory effects and may even reduce the graft's immunogenicity. Pulsatile flow generates vascular shear stress, which influences endothelial gene expression and function (5, 38). Moreover, HMP enhances endothelial nitric oxide (NO) synthase (eNOS) phosphorylation, preventing vasospasm and promoting NO-depended vasodilatation at reperfusion. Good microcirculation at the time of implantation vastly increases the chances of immediate graft function (39). On the other hand, even though cellular metabolism during HMP is reduced, it is not entirely ceased. Therefore, the problem of oxygen deficiency remains: a decrease in ATP levels results in inhibition of Na+/K+ pumps and leads to acidosis. Mitochondrial dysfunction and ROS production due to oxygen deficiency promote graft lesions during reperfusion (40). In a porcine DCD model, Kaminski et al. reported a rapid decrease in perfusate oxygen pressure from 150 mmHg at 10 min of perfusion to 6.8 mmHg at 200 min and kidney cortical oxygen pressure dropped from 10.2 to 0 within the first 60 min of perfusion (41). While healthy kidney grafts endure anoxia at low temperature for some time, such conditions may be harmful to ECD or DCD kidneys significantly diminishing the optimal preservation time. Not surprisingly, despite reported superiority of HMP when compared to SCS, RCT and meta-analyses reveal a lack of evidence that HMP improves long-term graft function or long-term survival of DCD kidneys (14, 29). Moreover, even with HMP, the kidney graft outcome depends on cold ischemia time (CIT). Therefore, preservation time cannot be extended (30, 42). HMP itself does not have a conditioning effect and does not improve kidney quality but rather helps maintaining kidney quality as it was at the time of retrieval.
These limitations of HMP led to a growing interest in oxygenated HMP (HMPO2). During HMP higher levels of ATP are produced than during SCS, and oxygen is further able to support ATP synthesis (43). Rodent models revealed that oxygenation in HMP reduced nuclear injury, tubular damage, macrophage activation, increased kidney function and even suppressed T-cell response after implantation (37, 44). In pre-clinical porcine models, HMPO2 improved early kidney graft function and reduced fibrosis (43, 45–48). The COPE-COMPARE study (49, 50) included 106 paired kidneys retrieved from DCD donors aged ≥50 years and compared HMPO2 to standard HMP. It demonstrated that HMPO2 is safe and feasible resulting in significantly less severe complications after Tx in the HMPO2 group. HMPO2-perfused grafts did not show significant improvement in estimated glomerular filtration rate (eGFR) at 12 months post-transplant when comparing functioning kidneys from both groups. However, sensitivity analysis, considering all-cause graft failure, showed significantly improved 1-year kidney graft function in the HMPO2 group. Interestingly, HMPO2-preserved kidneys had a significant relative risk reduction of acute rejection compared to HMP. No statistically significant difference was seen in terms of DGF and PNF (49). Another clinical trial (COPE-POMP) randomized ECD kidneys to end-HMPO2 after SCS vs. SCS alone with graft survival at 12 months post-transplant as a primary endpoint. The minimum HMPO2 time was 120 min before implantation. Both groups had comparable 1-year graft survival. Moreover, no significant differences in terms of DGF, PNF, eGFR, and acute rejection between the groups were observed. The overall graft survival rate was high in this study leading to the assumption that the analysis might be underpowered (50). All in all, it seems that oxygen is the key factor when considering HMP not only as a preservation technique but also as an organ conditioning tool. However, additional clinical studies are necessary to confirm this hypothesis.
Normothermic Machine Perfusion
Despite the benefits of HMP, the detrimental effect of the cold remains, and the kidney graft outcome is still very dependent on CIT. Even after adoption of HMP into clinical practice, the utilization of marginal kidneys, especially DCD, remains limited. The emerging technology of NMP that reduces or even eliminates CIT and harm caused by low temperatures has a great potential in expanding the donor pool (5, 6). During NMP, the organ is perfused with oxygenated blood or other oxygen carrier containing perfusate, nutrients, and medications at body temperature (35–37°C) (5, 6, 15). The preclinical trials by Hosgood and Nicholson found that the optimal arterial pressure for kidney NMP is the lower end of the physiological range for kidney autoregulation [mean arterial pressure (MAP) ~95 mmHg] (51). The main difference between HMP and NMP perfusion solutions is that NMP requires an oxygen carrier. Leukocytes and thrombocytes depleted autologous whole blood has been used for kidney NMP in experimental studies (52–54). Elimination of white cells and platelets allows restoration of organ function without induction of inflammatory reactions and thrombosis, occurring during normal in-vivo reperfusion. The remaining plasma contains albumin and globulins, which maintain stable osmotic pressure and electrolytes that regulate pH. However, it can be logistically demanding to harvest autologous blood and prepare perfusate on the spot in clinical situations. Moreover, the remaining fibrinogen may promote micro thrombus formation (15). Therefore, red blood cells (RBC)-based solution is used much more commonly. RBCs efficiently carry oxygen, as well as, their flow reduces shear stress in kidney vessels maintaining normal endothelial function (15) but the increased risk of hemolysis due to RBC contact with artificial surfaces in the perfusion circuit should not be under estimated. Longer-banked RBCs may not be suitable because of higher levels of non-transferrin-bound iron and time-dependent metabolic alterations (15, 55–57). As an alternative, artificial oxygen carriers, including polymerized bovine hemoglobin-based oxygen carriers (HBOC) and pyridoxylated bovine hemoglobin, as well as manufactured, acellular oxygen-carrying media, including Lifor, Aqix RS-I, STEEN solution, have been proposed. The properties of different oxygen carriers are discussed in detail elsewhere (15). In addition, in most of the protocols NMP perfusates contain heparin (if it is blood- or RBC-based perfusate), vasodilators, mannitol, corticosteroids, antibiotics, nutrient preparations with glucose, amino acids and insulin (5, 6, 15).
Such graft preservation in almost physiological conditions enables normal cellular metabolism and recovery of ATP production (58). Some experimental works revealed that 1 h NMP promotes IL-6, IL-8 (59), and heat shock proteins (HSP) expression in kidney grafts (59, 60). Moreover, induction of protective stress responses, down-regulation of cell death, and enhanced proliferation have been observed (61). It has been demonstrated that long periods of NMP can support de novo protein synthesis and promote recovery of cytoskeletal integrity in ischemically-damaged kidneys (62). Interestingly, the release of inflammatory cytokines and chemokines into the perfusate and recirculation during NMP has been observed by several investigators (63–65). Transcriptional profiling of NMP perfused kidneys revealed that not only oxidative phosphorylation genes but also many immune and pro-inflammatory pathway genes are significantly up-regulated after 4 h of NMP. Most likely, circulating cytokines promote inflammatory gene expression; therefore, their removal could be beneficial (discussed below) (64, 65). On the other hand, NMP initiates donor-derived T- and B- lymphocytes, natural killer (NK)-cells, macrophages, and granulocytes diapedesis and removal (63).
Current evidence shows, that NMP itself has a therapeutic effect on kidney grafts, though the full mechanism remains to be revealed. In addition, NMP should be an ideal platform for the delivery of various ex-vivo therapies as pharmacokinetics and pharmacodynamics of drugs should not be altered by low temperature (5, 6). Another advantage is the possibility of kidney evaluation in physiological conditions before implantation, which could both alleviate decision-making in graft utilization and allow graft assessment during ex-vivo therapy (22). Hosgood et al. established a kidney quality score based on renal blood flow (RBF), urine output (UO) during NMP, and macroscopic graft appearance (66). Extensive research is ongoing to find an ideal perfusate or urine biomarker (or a combination of biomarkers) for graft quality and function assessment (22).
So far, most of the experimental and clinical experience has been acquired with short-term pre-implantation kidney NMP. One of the pioneering groups, established the NMP technology using modified pediatric cardiopulmonary bypass equipment and demonstrated its feasibility and superiority over SCS in multiple animal experiments (52, 58, 59). In 2013 they published the first pilot clinical series, in which 18 ECD kidneys underwent 1 h pre-implantation NMP revealing a significantly lower incidence of DGF when compared to the consecutive historical control group (ECD kidneys implanted after SCS) (67). Later they reported successful implantation of a pair of previously considered non-transplantable human kidneys after 1 h NMP at the end of SCS (68). Currently, the same group is conducting the first RCT (ISRCTN15821205), comparing pre-implantation NMP vs. SCS for DCD kidneys. Three hundred thirty-eight patients have been recruited, and results of this trial are expected in 2021 (69). On the other hand, just a few studies have directly compared NMP with HMP or HMPO2 so far. Several experimental models demonstrated the superiority of a short period of NMP after HMP (52) or prolonged SCS (58) vs. HMP-only. Contrary to those findings, in the recent study by Vallant et al. porcine kidneys after 4 h of end-ischemic HMP had higher urine output, oxygen consumption, perfusate flow rates, and lower number of apoptotic cells than paired grafts that underwent 4 h of end-ischemic NMP (70). This is in consistence with the results of Darius et al. (46) and Blum et al. (71) who also did not find a beneficial effect of kidney NMP compared to HMP or HMPO2 in their experiments. One of the reasons for this inconsistency with previously mentioned results could be different protocols and compositions of preservation solutions. Importantly, another RCT has recently started in the Netherlands, aiming to compare 2 h NMP after HMP vs. HMP-only for DCD or ECD kidneys (NCT04882254).
Currently, Kidney Assist® (OrganAssist; Groningen, Netherlands) is the only commercially available device for kidney NMP. As this machine is not portable, kidney NMP remains a static method and can be used only in combination with SCS or HMP, which are the only commercially available options for organ transportation. Moreover, the optimal duration of NMP is still to be determined. Even though clinically, short-term pre-implantation kidney NMP has been demonstrated as sustainable, the maintenance of NMP for long periods is challenging. One of the main issues is the toxic products unavoidably deriving from the cells' activity that need to be eliminated (70). Moreover, the NMP procedure itself is expensive and requires a lot of human resources; not surprisingly, its cost-effectiveness remains questionable. The risk of the device malfunction, which would lead to organ loss, should also be taken into account. Nevertheless, there is growing evidence demonstrating the benefits of long-term NMP. Recently, a Dutch group registered a new clinical trial PROPER (NCT04693325), aiming to prolong NMP up to 6 h. One of the leading groups in kidney graft preservation at the University of Toronto explored prolonged NMP up to 16 h in porcine experiments. They revealed that long-term normothermic preservation significantly reduces tubular injury and improves kidney function, compared to SCS, HMP or end-ischemia short-term NMP (72–78). The most recent works already demonstrated the safety and feasibility of 24 h kidney NMP, though these grafts were not implanted (79–82). Importantly, Weissenbacher et al. in porcine and non-transplantable human kidneys experiments revealed, that one key factor allowing prolongation of NMP is urine recirculation (80–82). Perfusate homeostasis and stable kidney arterial flow could be achieved only by urine recirculation (80, 82). Moreover, proteomics analysis showed decreased damage-associated molecular patterns, angiotensinogen levels, and enhanced levels of enzymes involved in kidney metabolism, perfused with urine recirculation (81). Pool et al. recently demonstrated that perfusate composition also significantly impact kidney injury and perfusion parameters during prolonged NMP (83). Basile et al. observed stable perfusion parameters and no evidence of damage during 72 h of NMP, revealing that even several days of perfusion could be feasible (79). However, further investigation of principal perfusate components is necessary to establish optimal NMP conditions, allowing long-term NMP. In the studies by Weissenbacher et al. a pre-clinical automatic portable NMP machine was used, confirming that it is only a matter of time until NMP becomes feasible even for organ transportation (80–82).
Although some challenges remain to be overcome, the results of the previously mentioned studies suggest that long periods of NMP may be necessary for re-conditioning of the more severely damaged kidneys. Moreover, the ability to extend ex-vivo preservation time could allow applying regenerative techniques and other treatments regimen. It is most likely, that different NMP protocols and perfusate components may be necessary considering different donation situations and kidney quality. Finally, kidney Tx surgery could become an elective daytime procedure.
Subnormothermic Machine Perfusion
A less investigated but also promising alternative for kidney graft preservation is SNMP. Organ perfusion at 20–32°C aims to avoid cold-induced graft injury but does not increase metabolism to a level requiring oxygen carriers for adequate oxygenation (5). Currently, this technique is still at an experimental stage. One study showed that 1 h end-ischemia kidney perfusion at 37°C preserves tubular and kidney functions better than the same duration perfusion at 32°C, which raised a concern that lower than physiological temperatures may reduce RBC oxygen-carrying capacity (84). However, a study by Hoyer et al. revealed that SNMP is feasible and beneficial even without oxygen carrier: 7 h perfusion of porcine DCD kidneys at 21°C with Custodiol-N, supplemented with dextran 40, resulted in a better-preserved organ structure when compared to oxygenated HMP or SCS. Moreover, a 2-fold increase in creatinine clearance (CrCl) compared to oxygenated HMP, and a 10-fold increase in CrCl, compared to SCS was demonstrated (85). Another group demonstrated that 4 h porcine kidney perfusion with autologous blood at 22°C significantly reduced IRI-related structural injury, hemorrhage and clotting, as well as increased UO and RBF in comparison to kidneys, perfused at 15 and 37°C. Moreover, SNMP diminished the expression of Toll-like receptor signaling molecules [high mobility group box 1 (HMGB1), myeloid differentiation factor 88 (MyD88), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB)] and reduced the levels of the kidney injury marker neutrophil gelatinase lipocalin (NGAL) and IL-6 (86). In another study, the same authors showed the feasibility of a hemoglobin-based oxygen carrier (HBOC-201) for 22°C kidney SNMP (87). STEEN solution has also been used for prolonged (24 h) SNMP at 21°C and demonstrated the advantage over 37°C blood-based solutions in terms of vascular resistance during perfusion (88). Brasile et al. used 32°C for kidney perfusion with EMS medium (89).
Moreover, SNMP has been successfully used for delivery of pharmacological agents (90). For example, the cytoprotective property of the H2S donor AP29 in University of Wisconsin (UW) solution seemed more pronounced at 21 °C than in hypothermic or normothermic conditions, suggesting that SNMP might be an optimal platform for certain agents delivery (90, 91).
Lastly, a controlled oxygenated rewarming strategy has been proposed as a safer gradual transition from cold to warm before reperfusion, avoiding a sudden heat shock (92–94). According to the protocol of Minor et al. (93) and von Horn and Minor (94) following SCS the kidney is perfused ex-vivo starting at 8°C and 30 mmHg and gradually elevating temperature and pressure up to 35°C and 75 mmHg during the first 90 min of 2 h perfusion. Pre-clinical studies demonstrated improved mitochondrial recovery (94) and kidney function following auto-Tx (95) after controlled oxygenated rewarming compared to SCS alone (93). The same group recently demonstrated that 2 h of controlled-oxygenated rewarming after 6 h SCS improved kidney function of ischemically damaged porcine kidneys at a similar level as 8 h NMP (96). One successful Tx of a human ECD kidney after gradual oxygenated rewarming has been reported (93). Despite promising pre-clinical results, further investigation is necessary to prove the applicability of these techniques and establish optimal protocols in terms of timing and perfusate composition.
Kidney Graft Therapy During EX-VIVO Machine Perfusion
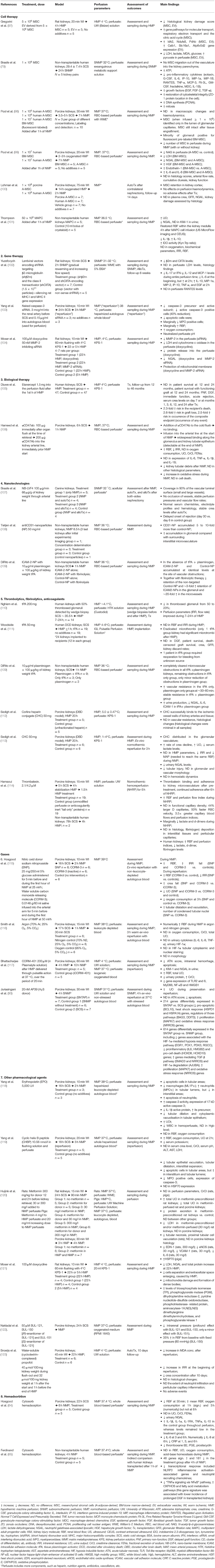
Many different pharmacological and biological therapies, nanotechnologies, and hemadsorbtion techniques have been applied for kidney grafts during ex-vivo machine perfusion. Experimental and clinical studies investigating kidney ex-vivo machine perfusion therapies are summarized in Table 2 and discussed in detail below.
Cell Therapy
One of the emerging strategies in ex-vivo machine perfusion therapeutics is cell therapy. Mesenchymal stromal cells (MSC) are multipotent cells mainly derived from bone marrow and adipose tissue, but also present in the umbilical cord, placenta, peripheral blood, and other tissues. Due to their stem-cell-like properties, after the engraftment into organs, they can differentiate into various functional cells and interfere with detrimental pathophysiological processes (124). International Society for Cellular Therapy established criteria defining MSC, including plastic-adherence when maintained in standard culture conditions, expression of cluster of differentiation (CD)73, CD90, and CD105 surface markers, lack of expression of endothelial and blood cell markers CD45, CD34, CD14 or CD11b, CD79α or CD19, and human leukocyte antigen (HLA)-DR, and capacity to differentiate into osteoblasts, chondroblasts, and adipocytes in vitro (125). Low immunogenicity (the lack of HLA class II expression, low HLA class I and costimulatory molecules expression), immunomodulatory and regenerative properties, as well as unelaborate growth in vitro (126) make MSC an attractive therapeutic agent in the field of solid organ Tx (124, 127). Like stem cells, they are able to differentiate into specified cells or deliver organelles to injured cells and consequently restore the function of the damaged organ (128). However, the secretion of growth factors, cytokines, and extracellular vesicles (EV) containing lipids, proteins, mRNAs, miRNAs, non-coding RNAs, and sometimes genomic DNA as well as the modulation of the graft's microenvironment are probably even more crucial mechanisms of action (129–132). Specifically, MSCs may not directly replace damaged kidney epithelial cells, but rather MSCs secretome may promote kidney regeneration from residual mature kidney cells, which become capable, dedifferentiate, and replicate (133). Nevertheless, it has been revealed that not only paracrine activity but also direct cell-to-cell interactions between MSC and host cells play a crucial role in acquiring regenerative, anti-inflammatory, and pro-tolerogenic effects in the target organ (127).
Several clinical kidney Tx trials already showed that systemic recipient treatment with autologous or allogenic MSC modulates the immune response and allows reducing doses of immunosuppressive drugs (134–136).Those studies focused on systemic immune modulation but not on the reparative or regenerative capacities of MSC therapy. Despite promising results, including safety and feasibility, it has become clear that intravenous infusion of MSC might not be ideal for several reasons. Firstly, it has been observed that the majority of intravenously delivered MSC are trapped in the microcapillary system of the lungs and liver hence not reaching the target organ (19–21). Another major problem is the short life span of intravenously infused MSC, meaning that multiple infusions may be necessary (19, 21). On the other hand, intra-arterial delivery of MSC directly to the kidney graft is feasible, efficient, prolongs MSC survival and cell-to-cell contact in situ, as well as, off-target migration of infused cells is minimal (137, 138), supporting the idea that cell therapy could be successfully applied for organ preconditioning and repair ex-vivo prior to implantation.
Five experimental studies in which MSCs were delivered into the kidney via ex-vivo machine perfusion have been published (79, 97–100). Gregorini et al. perfused rat kidneys, harvested after 20 min of warm ischemia, for 4 h with hypothermic UW solution containing MSC or extracellular vesicles derived from MSC. Both treatments significantly reduced histological lesions such as bleb formation, tubular necrosis, tubular lumen obstruction, and overall diminished global kidney damage score after HMP. Moreover, gene sets and individual genes responsible for molecular transport, citric acid cycle, respiratory electron transport, and antioxidant activity were significantly up-regulated. Correspondingly, perfusate biochemical analysis showed significantly lower levels of ischemia markers lactate and lactate dehydrogenase (LDH), oxidative stress marker malondialdehyde (MDA), increased pyruvate, and decreased glucose levels, demonstrating more active kidney metabolism during HMP. Importantly the positive effect was even more considerable in the EV group, compared to MSC-perfused kidneys. It suggests that prompt and direct delivery of free soluble EV mediators could be beneficial during short-term ex-vivo perfusion (97). Brasile et al. used 24 h NMP (so-called exsanguinous metabolic support) to deliver MSC to non-transplantable human kidneys focussing on the regenerative potentiality of this therapy. Infusion of 108 MSC (higher doses led to higher MAP, lower vascular flow, and diminished oxygen consumption) resulted in significantly increased ATP concentration in both kidney cortex and medulla, reduced synthesis of pro-inflammatory cytokines, and increased synthesis of growth factors. Moreover, up-regulated DNA synthesis and increased incidence of mitosis associated with MSC therapy were observed. Interestingly, 24 h NMP alone normalized the cytoskeletal integrity of injured kidneys, and MSC therapy further improved this restoration by 4.81%. During 24 h of perfusion, MSC remained in the vascular compartment of the kidney and did not migrate to parenchyma, as proved by histological evaluation and perfusate investigation (79). Similarly, in the study by Pool et al. human MSC stayed in glomerular capillaries of porcine DCD kidneys and remained undamaged throughout 7 h of NMP. Nevertheless, MSC accumulated only in the minority of glomeruli rather than distributed uniformly, even in well-perfused kidneys. Moreover, in this study, a gradual decrease of MSC counts in the perfusate was observed in experiments with kidney as well as without kidney connected to the perfusion machine. That suggests that MSC might be susceptible to perfusion conditions, such as flow and pressure, suggesting to be a limitation of long-term machine perfusion (98). In contrast, Brasile et al. could recover more than 95% of initially infused MSC from the perfusate at the end of 24 h perfusion (79). However, it should be taken into account that Pool et al. used human-derived MSC in porcine kidneys, so there could be a possibility of a xeno-effect, even for leukocyte-depleted perfusate lacking some immune components (98). In a later study the same group demonstrated that porcine DCD kidneys, 7 h perfused with MSC in 37°C, presented with lower levels of kidney injury markers (NGAL and LDH) and increased levels of hepatocyte growth factor (HGF), as well as immunomodulatory cytokines IL-6 and IL-8 in the perfusate. No differences in kidney function or diuresis were observed, which could also be explained by the relatively short perfusion time. Importantly, no apparent differences between adipose and bone marrow-derived MSC were found (99), even though several studies suggested that MSC of different sources may differ in activity and treatment effects (139, 140). The main drawback of the before-mentioned experiments is the lack of data from kidney assessment after reperfusion in vivo. Recently, Lohman et al. published their results of porcine DCD kidney auto-Tx after 14 h oxygenated HMP and subsequent 4 h NMP with human or porcine adipose-derived MSC. After 14 days of follow-up, no beneficial effects on kidney function or kidney injury markers were observed. Nevertheless, the treatment neither negatively affected perfusion parameters nor caused adverse events after auto-Tx, thus encouraging further machine perfusion studies with subsequent Tx in vivo to investigate MSC impact on ischemia-reperfusion injury, organ regeneration capacity, and immune modulation (100).
Another type of cells, potentially useful for kidney treatment during ex-vivo machine perfusion, are multipotent adult progenitor cells (MAPC). Genetically MAPCs are similar to MSCs, reside in the bone marrow, and even have comparable function and mechanism of action. Particular growth and expansion characteristics lead to phenotypically different features of those two cells' populations (141). MAPC immunomodulatory capacity has already been demonstrated in a couple of Tx studies. Treatment with allogenic MAPC in a rat heterotopic heart transplant model allowed to withdraw pharmacological immunosuppressive therapy and successfully achieve long-term survival (142). MAPCs have also been successfully used in a human liver Tx case, which resulted in a pro-tolerogenic profile of the recipient‘s leucocytes and reduced immunogenicity (143). Thompson et al., in their very recent study, used ex-vivo NMP to deliver MAPC to non-transplantable human kidneys. MAPC treatment resulted in higher UO, lower NGAL concentration in perfusate, but not other kidney injury biomarkers kidney injury molecule-1 (KIM-1) and flavine mononucleotide (FMN). MAPC was also associated with the changes in cytokine profile—decreased IL-1β and increased IL-10 levels, as well as up-regulated indoleamine-2,3-dioxygenase activity, which is known for its role in pro-tolerant mechanisms and suppression of inflammatory processes (101).
Although the results of machine perfusion cell-therapy studies are promising, several questions remain to be answered. The fate of ex-vivo delivered MSC or MAPC is still not determined. It is known that their lifespan in the target organ is limited; however, there is evidence that once immunomodulatory processes have been promoted, these beneficial effects are maintained even after inactivation or death of MSC (144, 145). Therefore, it is necessary to investigate if supportive cell therapy would be beneficial after implantation and at which time points it would be the most efficient. Additionally, it is crucial to thoroughly check into possible immunogenicity of allogenic MSC and MAPC in the machine perfusion setting, as extraction and preparation of autologous cells in case of Tx is usually logistically demanding (146). The perfect duration and conditions of machine perfusion also need to be determined. As discussed previously, several hours of ex-vivo cell therapy could be sufficient to promote immunomodulatory and anti-inflammatory processes; however, apparently a much longer time is needed for kidney regeneration and repair (79, 99, 101). Recent study revealed that machine perfusion conditions also affect MSC viability, metabolism, and function, which may not allow reaching the maximum effect of cell therapy (147). Therefore, further work on machine perfusion prolongation, ex-vivo organ viability maintenance, and optimal conditions for therapeutic cells is needed.
Gene Therapy
Ex-vivo machine perfusion is a promising platform for organ-specific gene therapy or even genetic engineering. One of the emerging approaches is posttranscriptional gene silencing with small interfering double-stranded RNA (siRNA), which induces degradation of homologous mRNA transcripts and blocks the desired gene expression (148, 149). They can be transported by viral vectors or just injected as a synthetic “naked” form. The main advantage of siRNA, as a tool for gene therapy, is its simple delivery—they are small and do not need to cross the nucleus membrane to become active. siRNA, silencing the expression of RelB, Caspase 3, IKKb, Fas or complement genes, delivery by simple intravenous, or hydrodynamic injection or via renal artery, has been already successfully applied in multiple rodent ischemia-reperfusion models (148–152).
However, even though gene therapy techniques, including siRNA, have been developing for the past 20 years, they remain in the experimental stage, not proceeding to clinics. The main problem is low efficiency and insufficient organ specificity of non-viral or viral gene therapy in vivo (153). Short lasting effects due to rapid degradation and excretion of the agent, delivered via the systemic route, is challenging too. Therefore, ex-vivo machine perfusion of the graft could help overcome those drawbacks by specific and efficient application of the gene therapy hence avoiding off-target effects (154).
Yang et al. published the first ex-vivo kidney perfusion study with siRNA—naked synthetic caspase-3 siRNA was infused directly into the renal arteries of ischemic porcine kidneys prior to 24 h of SCS followed by addition to autologous blood perfusate used for 3 h NMP. As expected, this treatment significantly reduced caspase-3 precursor and active subunit expression in perfused kidneys. Moreover, treated grafts had 40% lower number of apoptotic cells, shown by histology and immunohistochemistry (IHC). The caspase-3 siRNA group also demonstrated marginally improved RBF, significantly increased oxygen consumption, and improved perfusate pH regulation at 3 h of perfusion. However, no difference in CrCl and UO was observed (103). In another study, siRNA inhibiting matrix metalloproteinase-2 (MMP-2) gene expression was added to HMP perfusate. Ischemic rat kidneys were perfused for 22 h, which diminished MMP-2 and NGAL levels in perfusate to a level similar to that observed at 5 h of perfusion without treatment. Moreover, protection of mitochondrial membranes was observed (104). It suggests that gene therapy could help prolong organ preservation time until implantation and protect from additional injury. Recently, Yuzefovych et al. (102) adopted a similar technique to reduce the immunogenicity of the rat kidney allografts by silencing rat MHC I and MHC II expression. Rat kidneys underwent 2 h of SNMP with short hairpin RNA (shRNA), designed to target rat β2-microglobulin (β2m) and rat class II transactivator genes (CIITA) and carried by a lentiviral vector. Kidneys were implanted, and recipients were followed up for 6 weeks. As a result, transcript levels of β2m and CIITA were reduced by 71 and 70%, respectively. As the vector contained the sequence for nanoluciferase, the bioluminescence activity in plasma and urine was detectable during the whole 6 weeks after Tx, confirming a stable transferred gene expression. Moreover, a cytokine shift toward a pro-tolerogenic milieu was detected in perfusate during perfusion [increased secretion of IL-10, macrophage inflammatory protein (MIP)-1a, MIP-2, interferon γ-induced protein (IP)-10, epidermal growth factor (EGF), and decreased IL-12, IL-17, monocyte chemoattractant protein (MCP-1), interferon γ (IFN-γ)]. Importantly, no vector-related damage of the kidney allograft, as confirmed by LDH levels and histological investigation became evident. Even more, as no off-target distribution of the vector was observed, this diminishes the burden of risks associated with a lentiviral vector, such as tumorigenesis and other systemic side effects (102, 155).
Undoubtedly, these results are a step forward toward the goal to make a kidney graft immunologically invisible and reduce or even eliminate the need for systemic immune suppression for the recipient. Nevertheless, the long-term immunological state of the recipient, the incidence of acute and chronic rejection, the need for pharmacological immune suppression, graft function, and other clinical questions were not investigated in this study and remain to be answered.
Biological Therapy
The idea to use ex-vivo kidney machine perfusion to modulate the biological response of the graft before Tx by administering biological agents into perfusion solution has recently emerged. Inhibition of pro-inflammatory molecules, or their precursors' secretion and action by using specific monoclonal antibodies, blocking target sites, or even changing gene expression at the organ level before implantation gives a rationale that IRI, immunogenicity, and, therefore, need for systemic therapies could be reduced (105, 106). In a clinical study by Diuwe et al. (105) the tumor necrosis factor (TNF)-α inhibitor Etanercept was added to HMP of human kidneys, which were subsequently implanted. Although no negative impact on perfusion parameters was observed, no difference in patient survival at 12 and 24 months, PNF, DGF, an immediate graft function, acute rejection, or serum creatinine levels could be found. Unexpectedly, the proportional hazard Cox model showed that etanercept caused a 2.3-fold increase in the risk of recipient's death and a 2.6-fold increased risk of graft loss. It should be taken into account that all recipients still received a standard immunosuppression regimen of three drugs: corticosteroid, tacrolimus, and mycophenolic acid ester or sodium salt, therefore this study was not able to determine if the need or at least dosage of systemic immune suppression could be reduced by local biological therapy during machine perfusion. Moreover, it is not clear if, in the hypothermic environment, the highest effectiveness of the TNF-α inhibitor could be reached. It is more likely that in higher temperatures, the bioavailability and activity of the drug may be different. Therefore, adoption of (S)NMP into clinical practice would allow more efficient testing of drugs and determining optimal conditions for ex-vivo application of biological agents (105). Recently, an experimental study using αCD47Ab, an inhibitor of thrombospondin mediated IRI signaling, was conducted. One dose of αCD47Ab was infused via the renal artery immediately following cold perfusion of porcine kidneys at the time of retrieval, while another dose was added to NMP via the arterial line. Interestingly, the addition of αCD47Ab into the cold solution did not result in antibody binding to the kidney structures, whereas after 1 h of NMP containing the agent, αCD47Ab was detectable widely spread along the glomerulus and kidney tubular epithelium. Moreover, increased RBF and lower intrarenal resistance (IRR) during NMP could be observed in treated kidneys. However, oxygen consumption, UO, CrCl, and fractional sodium excretion (FENa) did not differ from control organs. Histologically, a significant increase in tubular dilatation and vacuolation in 1 h of NMP was detected in all kidneys whereas αCD47Ab-treated organs had reduced kidney tubular debris. NMP-induced oxidative stress was reduced in treated kidneys but the extent of cell death remained similar in comparison to control kidneys. Although no reperfusion was performed and the beneficial effects of this treatment on IRI remain questionable, the finding of successful delivery and binding of the antibody to graft structures during only 1 h of NMP encourages further investigation of machine perfusion as a platform for organ-targeted biological therapy (106).
Nanotechnologies
Ex-vivo machine perfusion is an attractive platform to apply novel nanotechnology in the solid organ Tx field. The main limitation of its use in vivo is some difficulties with systemic administration. NPs are not able to escape from the bloodstream and reach extravascular targets (156, 157). Moreover, they tend to be trapped in liver and spleen phagocytes (158) or be absorbed by serum proteins and form “protein corona “which also disturbs specific targeting (159). Therefore, delivery of NPs directly to the graft in leukocyte-depleted, serum-free perfusate is promising.
So far, endothelial cells of kidney graft vasculature have been chosen as the main target for nanomedicine. Firstly, because the endothelium is the primary point, where ischemia-reperfusion or immune response-caused graft injury starts and secondly because it is directly accessible to the perfusate (107, 108). Brasile et al. used SNMP of 32 °C as a platform to deliver a receptor-mediated bioengineered nano-barrier membrane (NB-LVF4), made of laminin, vitrogen, fibronectin, and type IV collagen, to canine kidney grafts, to “immunocloak” the vasculature and reduce the antigenicity of the endothelium. The main idea was to provide a physical nano-barrier between recipient's immune cells and graft endothelium without interfering with the transport of nutrients and oxygen and thus making the vascular surface non-immunogenic and non-thrombogenic. Three hours of perfusion with the agent allowed a coverage of 90% of small and large kidney vessels without any occlusion or disturbances in perfusion parameters. Autotransplanted kidneys revealed a good function, proving that NB-LVF4 local treatment ex-vivo did not additionally damage the kidney. AlloTx experiments demonstrated a significant delay in the onset of rejection in treated kidneys in the absence of any systemic immunosuppression (107).
NPs are also attractive due to their ability to release the specific agents gradually. It has been found that pharmacological agents encapsulated within NPs can be incorporated into the endothelium, thereafter slowly releasing drugs by hydrolysis (160). This slow-release is especially interesting in handling the host's immune response against allografts, as it only evolves within several weeks after implantation. The work by Tietjen et al. focused on the improvement of NPs targeting kidney graft endothelium during NMP. Anti-human CD31 antibodies conjugated NPs were used to facilitate the binding and internalization of NPs by endothelial cells by delivery to non-transplantable human kidney grafts via NMP for 8.5 h. CD31-conjugated NPs accumulation was 5 to 10-fold higher than for non-conjugated NPs with no extravascular accumulation being observed. However, this increase was much less pronounced than observed in vitro where CD31-NPs accumulation was 80-fold higher than for control-NPs. Interestingly, non-specific NPs accumulated within the interstitial microvessels, where RBC-enriched vascular plugs were found. The extent of non-specific binding accordingly correlated to worse kidney perfusion. Nevertheless, despite the limitation of specific targeting of NPs in poor-perfused kidneys, the feature of non-specific accumulation of NPs could serve as a diagnostic/prognostic tool indicating vascular obstruction (108). The same group recently pre-treated non-transplantable human kidneys with tissue plasminogen activator (tPA) and plasminogen via NMP aiming ameliorate cold storage-caused microvascular obstructions to be able to deliver intercellular adhesion molecule (ICAM)-2-NPs. They observed a 3-fold increased retention of ICAM-2-targeted NPs in the glomeruli as well as ~20-fold increase in the microvessels meaning that more effective delivery of NPs and graft modification could be achieved by application of thrombolytics as an essential first step during NMP (109).
Thrombolytics, Fibrinolytics, Anticoagulants
Formation of microthrombi is one of the most common problems in DCD kidneys or grafts retrieved from donors with disseminated intravascular coagulation, which often accompanies head injuries or multiorgan failure. Microvascular thrombosis can lead to poor graft perfusion and subsequent DGF or even PNF. Therefore, the discard rate of such kidneys remains high. Moreover, interactions among endothelial damage, inflammation, and activation of the coagulation system are well-known resulting in microthrombi, subsequent microcirculatory failure, and the “no-reflow” phenomenon as usual manifestations of IRI and acute antibody-mediated rejection (161, 162). As conventional systemic anticoagulation after kidney graft implantation does not eradicate pre-existing thrombi, does not guarantee the prevention of thrombosis, and carries its risk of bleeding (163, 164), the idea of anticoagulants or thrombolytics introduction directly to the graft has gained interest (165, 166). Several attempts of local thrombolysis or kidney pre-treatment with anticoagulants using ex-vivo machine perfusion have been published. Nghiem et al., used 14 human kidneys with biopsy-proven 50% thrombosed glomeruli that underwent 12–16 h of HMP with 200 mg tPA and were subsequently implanted. Reduction in glomerular thrombosis from 50 to 23% was observed at the end of perfusion, and 10 out of 14 recipients had immediate graft function after implantation (110). In the later RCT, after HMP with tPA, microthrombi were eradicated in all but one DCD kidney. No significant difference in kidney function, recipient survival, and death-censored graft survival could be observed which could also be due to the small study population (111). Despite promising results, it should be considered that tPA activity is reduced at lower temperatures suggesting that HMP, used in both studies, might not be optimal (167). DiRito et al. recently revealed that microvascular plugging in the kidney graft is induced by prolonged cold storage, which promotes fibrinogen production in proximal tubular cells and accumulation within the tubular epithelium. Upon restoration of physiological temperatures during NMP or implantation, rapid fibrinogen secretion to urine and microvasculature as well as RBC aggregation causes microvascular obstructions, occurring within 15 min after normothermic temperature restoration impairing adequate graft perfusion. Interestingly, those cold-induced obstructions seem to be different from traditional microthrombi. The investigators delivered tPA with plasminogen at the beginning of 1 h NMP of non-transplantable human kidneys and managed to clear microvascular obstructions completely. It subsequentially resulted in more stable perfusion parameters, decreased levels of NGAL, IL-6, and ICAM-1, and increased urine production. On the other hand, differently from the above-mentioned studies, neither tPA nor plasminogen alone had such an effect (109).
Another kidney ex-vivo perfusion study used thrombalexin, an endothelial localizing, cell membrane binding synthetic thrombin inhibitor. Nineteen ischemically damaged porcine kidneys and 2 non-transplantable human kidneys underwent 1.5 h of HMP with thrombalexin and were subsequentially hemoperfused for 6 h at physiologic temperature to mimic reperfusion. Perfect thrombalexin binding and adherence to graft microvasculature were confirmed after HMP remaining stable during 6 h of reperfusion. In comparison to the control group, treated kidneys demonstrated superior blood flow, 44% larger D capillaries, 50% faster RBC velocity, 3.5 times improved capillary blood flows and perfusion indices in orthogonal polarization spectral imaging, as well as lower d-dimer levels, confirming the anticoagulant activity of thrombalexin. Accordingly, cortical lactate levels were lower in treated kidneys, showing reduced ischemic damage and giving evidence that HMP could be successfully used to deliver cytotopic anticoagulant to the graft thus improving macro-and microvascular perfusion (114).
Sedigh et al. used HMP to deliver Corline heparin conjugate (CHC), a macromolecular heparin, consisting of >20 heparin molecules, to porcine DBD kidney grafts. CHC not only inhibits coagulation, platelet adhesion, and complement activity but also, differently from conventional heparin, irreversibly binds to collagen structures and therefore may be able to restore damaged endothelial glycocalyx and also locally express functional heparin (112, 113). In their first study, the group confirmed the safety and feasibility of CHC pre-treatment during HMP. It successfully bound to vessel walls of the ischemic kidneys, did not cause excess histological damage, or changed perfusion parameters (112). The later work on 3 h ex-vivo reperfusion showed that pre-coating vessels with CHC during HMP reduces preservation injury and improves organ function at least in the acute period. Treated kidneys had higher OU, faster decline in creatinine levels, lower urine NGAL levels, and less tubular damage in histological specimens (113).
Although none of those studies investigated the fate of delivered anticoagulant agents in longer periods after reperfusion and the actual need for systemic anticoagulation therapy afterwards, cytotopic delivery, and coating graft with anticoagulants during machine perfusion seems to be promising and provides a field for further studies before implementing this strategy into clinical practice.
Gases
Several investigators used ex-vivo machine perfusion to deliver various gases (other than pure oxygen or 95% oxygen and 5% carbon dioxide mix) for kidney graft treatment. Multiple animal models revealed that such gaseous molecules, like carbon monoxide (CO) or NO, have cytoprotective, anti-inflammatory, antiapoptotic, or vasoregulatory effects and may be beneficial in the reduction of IRI or immune responses in the Tx setting (168–170). However, despite the long history of experiments, systemic donor or recipient treatment with gases never set foot in clinical practice due to side effects, difficulties to deliver gases in a safe and controlled manner, and logistic issues, which all might be solved by ex-vivo organ-specific treatment.
Hosgood et al. after 10 min of WI and 16 h of SCS, applied 2 h of NMP with NO donor sodium nitroprusside (SNP) or CO-releasing molecule (CORM-3) infused during the first hour of perfusion to porcine kidneys. Despite the short-acting time of these agents, the treatment not only increased RBF at the time of preservation but also improved hemodynamic parameters during 3 h of ex-vivo reperfusion. However, in this study, the renoprotective effects of CORM-3 were more apparent than for SNP in terms of RBF, oxygen consumption, UO, and CrCl. Moreover, the histological evaluation showed an increase in ischemic structural changes, such as tubular dilatation, vacuolation, and the number of condensed tubular nuclei in SNP-treated kidneys (115). In the later work, Bhattacharjee et al. used the fourth generation of CO releasing molecules CORM-401, which is more potent and allows more controlled CO release than previously synthesized molecules (117, 171). It has been demonstrated that at 37°C, CORM-401 releases 15 times more CO than at 5°C during the same period, suggesting that NMP could be superior to HMP in case of gas delivery. Another important finding of the study was that 200 μM of CORM-401 in plasmaLyte solution, delivered via the renal artery, over 10 h results only in a minimal and non-toxic level of carboxyhemoglobin (COHb) hence considering it safe. CORM-401 was delivered to kidneys originating from a porcine DCD model in a pulsatile manner at 37°C for 20 min followed by immediate reperfusion with autologous blood. This treatment increased RBF and total OU during reperfusion, reduced graft injury (lower histological acute tubular necrosis score, less necrosis, intrarenal hemorrhage, and apoptosis, diminished KIM-1 and NGAL levels in urine), urinary protein levels, and increased CrCl to compare with control kidneys. Interestingly, this treatment had an anti-inflammatory effect by significant downregulation of toll-like receptor (TLR)-2, 4, and 6, as well as MyD88, NF-κB, and HMGB1 genes expression (117).
A very recent study investigated the third gasotransmitter's (H2S) donor AP39 use during SNMP (21°C) (90). Its beneficial cytoprotective effects have already been demonstrated by supplementing preservation solutions of SCS in murine kidney IRI and Tx models (172, 173). Juriasingani et al. stored porcine DCD kidneys for 24 h in UW supplemented with AP39 at 21°C. Surprisingly, this strategy preserved kidneys better than SCS in UW without additives, giving rationale that delivery of H2S at higher temperatures could be even more beneficial (91). Indeed, SNMP with AP39 increased UO both during preservation and 4 h of normothermic reperfusion with stressed autologous blood. It also lowered the extent of apoptosis after reperfusion. RNA sequencing detected 214 genes, differentially expressed in the treatment group compared to the SCS group, including downregulated pro-apoptotic, heat shock response genes including regulators of those pathways as well as increased proliferation and oxidative stress response genes. When compared to SNMP without additives, the treatment group differentially expressed 614 genes with reduced expression of genes associated with the hypoxia inducible factor (HIF)-1α-mediated hypoxia response pathway, pro-inflammatory, and cell death-attenuating genes. On the contrary, the genes mediating proliferation, oxidative stress response, transforming growth factor (TGF)-β pathway, and HIF-1α degradation were upregulated (90).
Among other gases, 70% of argon has also been investigated as potential gasotransmitter for ischemically damaged porcine kidneys during 1 h of NMP. However, subsequent 3 h of ex-vivo reperfusion showed no measurable beneficial effect in graft histology, functional parameters, or inflammatory markers (116).
Nevertheless, current evidence suggests that ex-vivo delivery of gases, especially using gas-releasing molecules, is likely feasible and safe. Development of machine perfusion strategies and increasing experience of S(NMP) perfusion may be especially beneficial in targeted kidney graft treatment with gas not only due to more efficient gas release from gas-donor molecules to compare with hypothermic conditions but also due to easier control of such treatment via perfusion parameters. The efficacy still needs to be determined in auto- or alloTx models and subsequent clinical trials.
Other Pharmacological Agents
EPO
Several studies investigated erythropoietin's (EPO) role in cytoprotection and preconditioning of kidney graft in the course of ex-vivo machine perfusion. Endogenous EPO is mainly produced by kidney cortical fibroblasts and not only participates in erythropoiesis but also acts locally through autocrine and paracrine axis via receptors in kidney tubular epithelium, endothelial cells, and mesangium. As a protective agent, it coordinates the response to injury of kidney cells by modulating pathways of apoptosis, necrosis, and inflammation (174). Nevertheless, to achieve a therapeutic effect, high doses of EPO are required. It vastly increases the risk of complications, such as hypertension and thrombosis, when administered systemically (17, 18). EPO contributes to tissue remodeling when added to NMP perfusate of ischemically damaged porcine kidneys. Two hours of NMP with EPO increased caspase-3 activity and the number of neutrophils, free cells, and cellular debris in tubular lumen but reduced the number of apoptotic cells and macrophages in tubulointerstitial areas compared to NMP alone. Reduced activity of pro-inflammatory cytokine IL-1β was also observed. Moreover, tubular apoptosis, dilatation, and cytoplasmic vacuolization in the tubular epithelium were significantly diminished, and UO increased in EPO-treated kidneys. The augmentation of apoptotic cells in tubular lumen but not in interstitial areas shows that EPO likely alleviates the clearance of dead inflammatory material, which could be beneficial for tissue conditioning before implantation and confrontation with the host's immune system (118). In another porcine study, cyclic helix B peptide, derived from the 3-dimensional structure of EPO using the cyclization method, was investigated. Such a structure improves cytoprotection but avoids induction of erythropoiesis and its related negative effects. Therefore, such peptide would be potent for systemic use. In this work, ex-vivo machine with whole blood as perfusate was used to simulate reperfusion and cyclic helix B peptide effect during the kidney reperfusion phase. Similar to the previous work, such treatment significantly decreased the number of apoptotic cells in the tubular areas, but increased in the lumen, again confirming EPO contribution in apoptotic cells clearance. Downregulation of caspase-3 expression (both precursor and active subunits) and upregulation of heat shock protein (HSP)70 in the treatment group was observed. Furthermore, cyclic helix B peptide improved the hemodynamic parameters RBF and oxygen consumption and increased UO. Even though drug administration during reperfusion is outside of the subject of this review, the results of this study suggest that cyclic helix B peptide could be used as an alternative to conventional EPO during the preservation phase for improved organ conditioning and protection (119).
Metformin
Huijink et al. investigated the potential beneficial effect of metformin on kidney graft injury by the donor pre-treating and/or adding it to NMP solution in rats and pigs experiments (120). Metformin is not only known as an antihyperglycemic agent but also has some pleiotropic effects, due to its ability to inhibit the complex 1 of the mitochondrial respiratory chain, coordinate cellular energy state, inhibit apoptosis, and regulate endothelial function via NO production (175, 176). Organo-protective action of metformin has also been demonstrated in an ischemia-reperfusion setting (177, 178). Nevertheless, the results of the Huijink et al. study were inconclusive as the beneficial effect on kidney integrity was observed mainly in rodent experiments when metformin was used for preconditioning (donor treatment). However, perfusate supplementation during NMP was not as effective. Nevertheless, downregulation of eosinophil-derived neurotoxin (EDN)-1, eNOS, vascular cell adhesion molecule (VCAM)-1, IL-6 genes in rat kidneys and upregulation of HSP-70 in porcine kidneys was observed. Moreover, metformin-perfused kidneys revealed lower kidney injury in histological specimens, encouraging further investigation of metformin-treatment using an ex-vivo perfusion platform (120).
Doxycycline
Another kidney graft pharmacological treatment strategy is inhibition of matrix metalloproteinases, which play a role in the pathogenesis of ischemia-reperfusion (179), as well as acute and chronic immune injury (180, 181). Activation of MMPs leads to acute tubular injury, necrosis, apoptosis, tubular atrophy, fibrosis, and damage of the basal membrane in the kidney (179, 182). Moser et al. observed increased levels of MMP-9 and MMP-2 during HMP both in human and rat kidneys, along with elevated levels of total protein, NGAL, and LDH in perfusate, indicating preservation injury. Twenty-two hours of rat kidney HMP resulted not only in higher levels of MMPs and kidney injury markers but also in structural impairment of mitochondrial integrity compare to kidneys perfused for only 5 h. Upon supplementing HMP perfusate with doxycycline, which is not only an antibiotic but also a clinically approved MMP inhibitor, 22 h HMP levels of MMP-2 and MMP-9, LDH, cytochrome c oxidase, NGAL, and total protein dropped to the levels of 5 h perfusion without treatment. Moreover, the doxycycline effect on mitochondrial membrane protection was demonstrated by electronic microscopy (104). Extensive proteomic analysis showed a significant increase in 8 proteins in doxycycline perfused rat kidneys, including glycolysis enzymes triosephosphate isomerase (TPI), phosphoglycerate kinase 1 (PK-1), phosphoglycerate mutase (PGM), urea cycle enzyme aminoacyclase-1A, NO synthesis regulator N(G),N(G), dimethylarginine dimethylaminohydrolase, as well as, other enzymes, such as dihydropteridine reductase-2, pyridine nucleotide-disulfide oxidoreductase and phosphotriesterase-related protein. Interestingly, TPI, PK-1, and N(G),N(G), dimethylarginine dimethylaminohydrolase levels were reduced by HMP, while treatment with doxycycline allowed correction of this reduction, again proving mitochondrial preservation (121).
SUL Compounds
Another class of novel agents that protect cells from hypothermia-associated damage by preserving mitochondrial structure and function and reactive oxygen species (ROS) scavenging are 6-chromanol derivates (SUL compounds) (183, 184). Nakladal et al. added SUL-121 and its enantiomers SUL-150 and SUL-151 into the NMP solution of porcine kidneys after 24 h of SCS. An apparent and immediate increase in RBF and decrease in intrarenal pressure, mainly through SUL-150 enantiomer, was observed. In vitro experiments with isolated intraarterial arteries using various agonists showed that the beneficial vascular effect of SUL-121/SUL-150 is mediated by specific competitive inhibition of α1-adrenoreceptors on the vascular smooth muscle. This study did not investigate SUL-121 impact on ROS production and mitochondrial function when it is administered specifically during machine perfusion. Therefore, although ex-vivo kidney graft treatment with 6-chromanol derivates after prolonged cold ischemia seems feasible and promising, further investigation is necessary (122).
Propofol
Besides the anesthetic properties, the well-known agent propofol has common structural elements like α-tocopherol and acts as antioxidant by preventing lipid peroxidation in cell membranes (185, 186). Snoeijs et al. investigated renoprotective features of propofol by delivering it to ischemically damaged porcine kidney grafts via 22 h HMP. To make it water-soluble, they prepared a cyclodextrin inclusion complex. The treatment prevented lipid peroxidation, reduced the increase in renovascular resistance at reperfusion after autologous kidney implantation. Moreover, treatment with propofol slightly improved kidney function in the early period after Tx. However, leucocyte infiltration in kidneys was not diminished by propofol treatment. The main advantage of HMP delivery was that it allowed high tissue concentrations of the agent without any adverse effects after graft implantation. Propofol concentrations were undetectable in recipients' plasma in the early reperfusion periods (123).
Hemadsorbtion
It has been recently found that, despite leukocytes- and complement-free perfusate, kidney tubular epithelial cells and circulating cells locally produce cytokines and chemokines, hence up-regulating inflammatory pathways within the graft already during ex-vivo machine perfusion (59, 65). As it is well-known that pro-inflammatory mediators aggravate the severity of IRI, the removal of cytokines and chemokines from the kidney using machine perfusion seems to be a logical strategy of organ preparation before implantation. This question was analyzed in two recent experimental studies (64, 65). Cytosorb hemadsorbtion filter, which is currently widely used in intensive care to treat severe inflammatory states, such as systemic inflammatory response syndrome and sepsis (187, 188), was connected to the perfusion machine and used to remove cytokines from perfusate during kidney NMP (64, 65). In the first study on porcine kidneys, a significant increase of IL-1β, IL-1α, IL-1RA, TNFα, IL-10 was observed after 6 h of NMP in control kidneys, whereas levels of these cytokines remained low in the hemadsorbtion group. Decreased levels of IL-6, IL-8, c reactive protein (CRP), and thromboxane B2 were also observed in treated kidneys. No effect on UO, CrCl, and FENa was observed, but kidney injury marker NGAL levels in urine were significantly lower in the Cytosorb group (probably due to direct filtering of the NGAL molecule). Interestingly, even though overall blood flow throughout 6 h of NMP was increased in the treatment group a rapid decrease in RBF was observed at 30 min of perfusion, following a decline of vasodilators prostaglandin E (PGE) and prostacyclin. Cytosorb advantage is the ability to filter a wide range of mediators according to their size (10–50 kDa). However, it is not specific and also removes anti-inflammatory agents, such as IL-10 or IL-1RA, and vasodilators PGE and prostacyclin. This problem could be partly solved by adding vasodilators to the perfusate when hemadsorbtion is used (64). Ferdinand et al., used the same technique to perfuse non-transplantable human kidney pairs, focused on changes in graft gene expression after cytokine removal. They showed that NMP has a double effect on gene regulation. It promotes oxidative phosphorylation pathway genes, which are crucial for energy generation. However, at the same time, several pro-inflammatory and immune pathway genes are induced. Using the samples from the clinical study of kidney NMP, the investigators found that this “gene signature” is associated with prolonged DGF after implantation. In non-transplantable human kidneys, NMP with Cytosorb hemadsorbtion not only diminished levels of cytokines but also significantly reduced inflammatory gene expression and up-regulated genes of the oxidative phosphorylation pathway. These surprising results suggest that locally produced and during NMP recirculating cytokines likely play a role in kidney gene regulation and its shift toward a pro-inflammatory state, culminating in aggravated graft injury after implantation. Hemadsorption during ex-vivo kidney perfusion seems to be a promising approach to cease this inflammation exacerbating loop however, more experimental studies followed by kidney implantation and clinical studies are necessary to strengthen this hypothesis (65).
Challenges and Considerations
In the last decade, significant progress has been made in the kidney preservation field. Many ex-vivo machine perfusion therapeutic strategies have been developed and proved feasible in multiple experimental and a few clinical studies. Nevertheless, the kidney machine perfusion therapeutics is still in its infancy therefore, several challenges remain and need to be considered.
Currently, among other ex-vivo kidney perfusion strategies, only HMP is fully established and routinely used in clinical practice. Therefore, it could be the fastest and easiest way to implement kidney machine perfusion therapeutics into clinical practice. Indeed, several studies demonstrated a successful delivery of MSC and EV (97), siRNA (104), fibrinolytics and anticoagulants (110–114), doxycycline (104, 121), and propofol (123). However, the pharmacokinetics and pharmacodynamics of different agents at 4°C is not well-known (105). The possibility that low temperature might reduce the effectiveness of the therapy (167) or even enhance the accumulation of the agent, which can be damaging after reperfusion, should be considered. Moreover, the kidney outcomes after HMP still depend on CIT (30, 42). Therefore, it could be used only for several hours of therapy, which might not be sufficient for tissue regeneration strategies.
NMP, which ideally provides a physiological environment, is a more attractive platform for kidney ex-vivo therapeutics. Not surprisingly, most of the experimental studies used physiological temperature for kidney graft therapy. Long-term warm perfusion is crucial to enhance kidney tissue repair and regeneration (62, 79). SNMP was also found to be effective, especially for gas delivery (90). However, none of these strategies have been adopted in clinical practice yet. The results of the first clinical trial of short-term end-ischemic NMP should answer some questions and probably even open the window of opportunities for kidney perfusion therapeutics (69).
Nevertheless, the optimal protocol, especially for long-term perfusion, still needs to be established including, optimal timing, perfusion settings, oxygen partial pressure or perfusate composition. Moreover, the discussion regarding kidney evaluation parameters during perfusion, which is crucial to observe the graft state during therapy, is still ongoing The rationale for the use of perfusion readings, such as pressure, flow and resistance, is based on the kidney structure, which is rich in capillary network with filtration function. The release of vasoconstrictors from capillaries following the ischemic and inflammatory insults, determines accumulation of erythrocytes and microthrombosis, leading to a diminished flow and increased resistance in the graft. The hypoxia directly activates single-layer endothelium cells, favoring a pro-coagulant and pro-inflammatory phenotype of the kidney vasculature, with consequent disruption of the blood flow, increased leukocyte infiltration, and a further decline in kidney function. On the basis of kidney structure and physiology, perfusion readings are considered as a valuable tool to assess kidney viability before implantation. On the other hand, the relative predictive value of perfusion parameters is low and they cannot be considered as the only criteria to determine, whether to transplant the kidney. In general, there is likely no universal perfusate marker or universal perfusion parameter. However, the best combination of different parameters, sensitive and specific kidney quality score, is an objective of the current research (22). The optimal timing and duration of the different treatments during kidney perfusion also requires further investigation. Due to the short action or life-span of many agents, it might be necessary to continue some therapies systemically after implantation.
The main limitation of the aforementioned studies is that the results mostly reveal the expression of different molecular markers, and thorough investigation of the treatment impact on kidney function after implantation, clinical alloimmune response and transplant outcomes is lacking. Most of the experiments only evaluated the preservation stage or used short ex-vivo reperfusion models, which might not reflect the real conditions after implantation. Therefore, more animal models of kidney implantation and longer follow-up periods are necessary before kidney perfusion therapeutics clinical studies. Lastly, implementation of novel gene and cell therapies might be challenging due to ethical regulation, logistics (e.g., MSC retrieval and growth), and high cost. On the other hand, the application of those therapies ex-vivo via the machine perfusion platform is more ethically acceptable and less risky than systemic recipient or donor treatment. Therefore, machine perfusion might facilitate the adoption of novel techniques and therapies into clinical practice.
Conclusions
The rapid development of kidney ex-vivo machine perfusion techniques and strategies started a new era in kidney Tx. Many (pre-) clinical studies on ex-vivo kidney perfusion using various modalities have already demonstrated its safety and feasibility. Further, IRI and graft immunogenicity may be reduced, and both regeneration and repair may be promoted. Kidney perfusion therapeutics offers an opportunity to overcome the challenges faced with ECD or DCD kidneys, increase the pool of transplantable organs and improve outcomes after implantation. Nevertheless, further extensive research is necessary to transfer these techniques from the experimental to clinical stage.
To date criteria for kidney machine perfusion to improve graft survival/function are still pending. Clinical trials are warranted to define organs that need ex-vivo machine perfusion before transplantation for optimal graft function. These data would allow a cost-effective use of machine perfusion.
Author Contributions
PSt was responsible for the study concept design and critical revision of the drafted manuscript. RZ, PM, PSc, BL, and KS were responsible for the literature review, interpretation, and drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eurotransplant–Statistics. (2020). Available online at: https://statistics.eurotransplant.org/index.php?search_type=waiting+list&search_organ=&search_region=&search_period=by+year+chart&search_characteristic=&search_text= (accessed November 22, 2021).
2. Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. (2013) 381:727–34. doi: 10.1016/S0140-6736(12)61685-7
3. Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. (2010) 376:1303–11. doi: 10.1016/S0140-6736(10)60827-6
4. Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD–fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. (2009) 4:1827–31. doi: 10.2215/CJN.02270409
5. Jochmans I, Akhtar MZ, Nasralla D, Kocabayoglu P, Boffa C, Kaisar M, et al. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant. (2016) 16:2545–55. doi: 10.1111/ajt.13778
6. Weissenbacher A, Vrakas G, Nasralla D, Ceresa CDL. The future of organ perfusion and re-conditioning. Transpl Int. (2019) 32:586–97. doi: 10.1111/tri.13441
7. Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours' ice storage. Lancet. (1969) 2:1219–22. doi: 10.1016/S0140-6736(69)90753-3
8. Watkins GM, Prentiss NA, Couch NP. Successful 24-hour kidney preservation with simplified hyperosmolar hyperkalemic perfusate. Transplant Proc. (1971) 3:612–5.
9. Ploeg RJ, Goossens D, McAnulty JF, Southard JH, Belzer FO. Successful 72-hour cold storage of dog kidneys with UW solution. Transplantation. (1988) 46:191–6. doi: 10.1097/00007890-198808000-00002
10. Moers C, Smits JM, Maathuis M-HJ, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. (2009) 360:7–19. doi: 10.1056/NEJMoa0802289
11. O'Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg. (2013) 100:991–1001. doi: 10.1002/bjs.9169
12. Martínez Arcos L, Fabuel Alcañiz JJ, Gómez Dos Santos V, Burgos Revilla FJ. Functional results of renal preservation in hypothermic pulsatile machine perfusion versus cold preservation: systematic review and meta-analysis of clinical trials. Transplant Proc. (2018) 50:24–32. doi: 10.1016/j.transproceed.2017.12.005
13. Peng P, Ding Z, He Y, Zhang J, Wang X, Yang Z. Hypothermic machine perfusion versus static cold storage in deceased donor kidney transplantation: a systematic review and meta-analysis of randomized controlled trials. Artif Organs. (2019) 43:478–89. doi: 10.1111/aor.13364
14. Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. (2019) 3:CD011671. doi: 10.1002/14651858.CD011671.pub2
15. Elliott TR, Nicholson ML, Hosgood SA. Normothermic kidney perfusion: an overview of protocols and strategies. Am J Transplant. (2021) 21:1382–90. doi: 10.1111/ajt.16307
16. Kargaard A, Sluijter JPG, Klumperman B. Polymeric siRNA gene delivery - transfection efficiency versus cytotoxicity. J Control Release. (2019) 316:263–91. doi: 10.1016/j.jconrel.2019.10.046
17. Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost. (2010) 36:537–49. doi: 10.1055/s-0030-1255448
18. Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage. (2004) 27:185–90. doi: 10.1016/j.jpainsymman.2003.06.010
19. Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. (2010) 114:e107–16. doi: 10.1159/000262318
20. Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FKF, Franquesa M, et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. (2013) 22:2825–35. doi: 10.1089/scd.2013.0193
21. Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. (2012) 3:297. doi: 10.3389/fimmu.2012.00297
22. De Beule J, Jochmans I. Kidney perfusion as an organ quality assessment tool-are we counting our chickens before they have hatched? J Clin Med. (2020) 9:E879. doi: 10.3390/jcm9030879
23. Gallinat A, Fox M, Lüer B, Efferz P, Paul A, Minor T. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation. (2013) 96:538–42. doi: 10.1097/TP.0b013e31829c24e2
24. Mohr A, Brockmann JG, Becker F. HTK-N: modified histidine-tryptophan-ketoglutarate solution-a promising new tool in solid organ preservation. Int J Mol Sci. (2020) 21:6468. doi: 10.3390/ijms21186468
25. von Horn C, Wilde B, Rauen U, Paul A, Minor T. Use of the new preservation solution Custodiol-MP for ex vivo reconditioning of kidney grafts. Artif Organs. (2021) 45:1117–23. doi: 10.1111/aor.13951
26. Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. (1968) 278:608–10. doi: 10.1056/NEJM196803142781108
27. Belzer FO, Southard JH. The future of kidney preservation. Transplantation. (1980) 30:161–5. doi: 10.1097/00007890-198009000-00001
28. Zhong Z, Lan J, Ye S, Liu Z, Fan L, Zhang Y, et al. Outcome improvement for hypothermic machine perfusion versus cold storage for kidneys from cardiac death donors. Artif Organs. (2017) 41:647–53. doi: 10.1111/aor.12828
29. Jochmans I, Moers C, Smits JM, Leuvenink HGD, Treckmann J, Paul A, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. (2010) 252:756–64. doi: 10.1097/SLA.0b013e3181ffc256
30. Treckmann J, Moers C, Smits JM, Gallinat A, Maathuis M-HJ, van Kasterop-Kutz M, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int. (2011) 24:548–54. doi: 10.1111/j.1432-2277.2011.01232.x
31. Deng R, Gu G, Wang D, Tai Q, Wu L, Ju W, et al. Machine perfusion versus cold storage of kidneys derived from donation after cardiac death: a meta-analysis. PLoS ONE. (2013) 8:e56368. doi: 10.1371/journal.pone.0056368
32. Bathini V, McGregor T, McAlister VC, Luke PPW, Sener A. Renal perfusion pump vs. cold storage for donation after cardiac death kidneys: a systematic review. J Urol. (2013) 189:2214–20. doi: 10.1016/j.juro.2012.11.173
33. Jiao B, Liu S, Liu H, Cheng D, Cheng Y, Liu Y. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS ONE. (2013) 8:e81826. doi: 10.1371/journal.pone.0081826
34. Lam VWT, Laurence JM, Richardson AJ, Pleass HCC, Allen RDM. Hypothermic machine perfusion in deceased donor kidney transplantation: a systematic review. J Surg Res. (2013) 180:176–82. doi: 10.1016/j.jss.2012.10.055
35. Bellini MI, Nozdrin M, Yiu J, Papalois V. Machine perfusion for abdominal organ preservation: a systematic review of kidney and liver human grafts. J Clin Med. (2019) 8:E1221. doi: 10.3390/jcm8081221
36. Stone JP, Sevenoaks H, Sjöberg T, Steen S, Yonan N, Fildes JE. Mechanical removal of dendritic cell-generating non-classical monocytes via ex vivo lung perfusion. J Heart Lung Transplant. (2014) 33:864–9. doi: 10.1016/j.healun.2014.03.005
37. Schlegel A, Kron P, Graf R, Clavien P-A, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. (2014) 260:931–7; discussion 937–938. doi: 10.1097/SLA.0000000000000941
38. Yuan X, Theruvath AJ, Ge X, Floerchinger B, Jurisch A, García-Cardeña G, et al. Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int. (2010) 23:561–70. doi: 10.1111/j.1432-2277.2009.01047.x
39. Chatauret N, Coudroy R, Delpech PO, Vandebrouck C, Hosni S, Scepi M, et al. Mechanistic analysis of nonoxygenated hypothermic machine perfusion's protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am J Transplant. (2014) 14:2500–14. doi: 10.1111/ajt.12904
40. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. (2011) 17:1391–401. doi: 10.1038/nm.2507
41. Kaminski J, Delpech P-O, Kaaki-Hosni S, Promeyrat X, Hauet T, Hannaert P. Oxygen consumption by warm ischemia-injured porcine kidneys in hypothermic static and machine preservation. J Surg Res. (2019) 242:78–86. doi: 10.1016/j.jss.2019.04.015
42. Kox J, Moers C, Monbaliu D, Strelniece A, Treckmann J, Jochmans I, et al. The benefits of hypothermic machine preservation and short cold ischemia times in deceased donor kidneys. Transplantation. (2018) 102:1344–50. doi: 10.1097/TP.0000000000002188
43. Patel K, Smith TB, Neil DAH, Thakker A, Tsuchiya Y, Higgs EB, et al. The effects of oxygenation on ex vivo kidneys undergoing hypothermic machine perfusion. Transplantation. (2019) 103:314–22. doi: 10.1097/TP.0000000000002542
44. Kron P, Schlegel A, Muller X, Gaspert A, Clavien P-A, Dutkowski P. Hypothermic oxygenated perfusion: a simple and effective method to modulate the immune response in kidney transplantation. Transplantation. (2019) 103:e128–36. doi: 10.1097/TP.0000000000002634
45. Venema LH, Brat A, Moers C, 't Hart NA, Ploeg RJ, Hannaert P, et al. Effects of oxygen during long-term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation. (2019) 103:2057–64. doi: 10.1097/TP.0000000000002728
46. Darius T, Gianello P, Vergauwen M, Mourad N, Buemi A, De Meyer M, et al. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia1003 reperfusion autotransplant model. Am J Transplant. (2019) 19:752–62. doi: 10.1111/ajt.15100
47. Thuillier R, Allain G, Celhay O, Hebrard W, Barrou B, Badet L, et al. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J Surg Res. (2013) 184:1174–81. doi: 10.1016/j.jss.2013.04.071
48. Buchs J-B, Lazeyras F, Ruttimann R, Nastasi A, Morel P. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion. (2011) 26:159–65. doi: 10.1177/0267659110387184
49. Jochmans I, Brat A, Davies L, Hofker HS, van de Leemkolk FEM, Leuvenink HGD, et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double-blind, paired, phase 3 trial. Lancet. (2020) 396:1653–62. doi: 10.1016/S0140-6736(20)32411-9
50. Husen P, Boffa C, Jochmans I, Krikke C, Davies L, Mazilescu L, et al. Oxygenated End1016 hypothermic machine perfusion in expanded criteria donor kidney transplant: a randomized clinical trial. JAMA Surg. (2021) 156:517–25. doi: 10.1001/jamasurg.2021.0949
51. Hosgood S, Harper S, Kay M, Bagul A, Waller H, Nicholson ML. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br J Surg. (2006) 93:879–84. doi: 10.1002/bjs.5381
52. Hosgood SA, Barlow AD, Yates PJ, Snoeijs MGJ, van Heurn ELW, Nicholson ML. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res. (2011) 171:283–90. doi: 10.1016/j.jss.2010.01.027
53. Harper S, Hosgood S, Kay M, Nicholson M. Leucocyte depletion improves renal function during reperfusion using an experimental isolated haemoperfused organ preservation system. Br J Surg. (2006) 93:623–9. doi: 10.1002/bjs.5324
54. Yang B, Hosgood SA, Harper SJF, Nicholson ML. Leucocyte depletion improves renal function in porcine kidney hemoreperfusion through reduction of myeloperoxidase+ cells, caspase-3, IL-1β, and tubular apoptosis. J Surg Res. (2010) 164:e315–24. doi: 10.1016/j.jss.2010.07.044
55. Kirby BS, Hanna G, Hendargo HC, McMahon TJ. Restoration of intracellular ATP production in banked red blood cells improves inducible ATP export and suppresses RBC1033 endothelial adhesion. Am J Physiol Heart Circ Physiol. (2014) 307:H1737–44. doi: 10.1152/ajpheart.00542.2014
56. Hod EA. Red blood cell transfusion-induced inflammation: myth or reality. ISBT Sci Ser. (2015) 10:188–91. doi: 10.1111/voxs.12108
57. Rapido F, Brittenham GM, Bandyopadhyay S, La Carpia F, L'Acqua C, McMahon DJ, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. (2017) 127:375–82. doi: 10.1172/JCI90837
58. Bagul A, Hosgood SA, Kaushik M, Kay MD, Waller HL, Nicholson ML. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg. (2008) 95:111–8. doi: 10.1002/bjs.5909
59. Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. (2013) 182:153–60. doi: 10.1016/j.jss.2012.08.001
60. Brasile L, Buelow R, Stubenitsky BM, Kootstra G. Induction of heme oxygenase-1 in kidneys during ex vivo warm perfusion. Transplantation. (2003) 76:1145–9. doi: 10.1097/01.TP.0000081044.37318.E3
61. Hameed AM, Lu DB, Patrick E, Xu B, Hu M, Chew YV, et al. Brief normothermic machine perfusion rejuvenates discarded human kidneys. Transplant Direct. (2019) 5:e502. doi: 10.1097/TXD.0000000000000944
62. Brasile L, Stubenitsky BM, Booster MH, Lindell S, Araneda D, Buck C, et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation. (2002) 73:897–901. doi: 10.1097/00007890-200203270-00011
63. Stone JP, Ball AL, Critchley WR, Major T, Edge RJ, Amin K, et al. Ex vivo normothermic perfusion induces donor-derived leukocyte mobilization and removal prior to renal transplantation. Kidney Int Rep. (2016) 1:230–9. doi: 10.1016/j.ekir.2016.07.009
64. Hosgood SA, Moore T, Kleverlaan T, Adams T, Nicholson ML. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J Transl Med. (2017) 15:216. doi: 10.1186/s12967-017-1314-5
65. Ferdinand JR, Hosgood SA, Moore T, Ferro A, Ward CJ, Castro-Dopico T, et al. Cytokine absorption during human kidney perfusion reduces delayed graft function-associated inflammatory gene signature. Am J Transplant. (2021) 21:2188–99. doi: 10.1111/ajt.16371
66. Hosgood SA, Barlow AD, Hunter JP, Nicholson ML. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg. (2015) 102:1433–40. doi: 10.1002/bjs.9894
67. Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. (2013) 13:1246–52. doi: 10.1111/ajt.12179
68. Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. (2016) 16:3282–5. doi: 10.1111/ajt.13906
69. Hosgood SA, Saeb-Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. (2017) 7:e012237. doi: 10.1136/bmjopen-2016-012237
70. Vallant N, Wolfhagen N, Sandhu B, Hamaoui K, Cook T, Pusey C, et al. A comparison of pulsatile hypothermic and normothermic ex vivo machine perfusion in a porcine kidney model. Transplantation. (2021) 105:1760–70. doi: 10.1097/TP.0000000000003599
71. Blum MF, Liu Q, Soliman B, Dreher P, Okamoto T, Poggio ED, et al. Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death. J Surg Res. (2017) 216:35–45. doi: 10.1016/j.jss.2017.04.008
72. Kaths JM, Cen JY, Chun YM, Echeverri J, Linares I, Ganesh S, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant. (2017) 17:957–69. doi: 10.1111/ajt.14059
73. Kaths JM, Echeverri J, Linares I, Cen JY, Ganesh S, Hamar M, et al. Normothermic ex vivo kidney perfusion following static cold storage-brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant. (2017) 17:2580–90. doi: 10.1111/ajt.14294
74. Kaths JM, Spetzler VN, Goldaracena N, Echeverri J, Louis KS, Foltys DB, et al. Normothermic ex vivo kidney perfusion for the preservation of kidney grafts prior to transplantation. J Vis Exp. (2015) 101:e52909. doi: 10.3791/52909
75. Kaths JM, Echeverri J, Goldaracena N, Louis KS, Chun Y-M, Linares I, et al. Eight-Hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation. (2016) 100:1862–70. doi: 10.1097/TP.0000000000001299
76. Kaths JM, Hamar M, Echeverri J, Linares I, Urbanellis P, Cen JY, et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am J Transplant. (2018) 18:580–9. doi: 10.1111/ajt.14491
77. Hamar M, Urbanellis P, Kaths MJ, Kollmann D, Linares I, Ganesh S, et al. Normothermic ex vivo kidney perfusion reduces warm ischemic injury of porcine kidney grafts retrieved after circulatory death. Transplantation. (2018) 102:1262–70. doi: 10.1097/TP.0000000000002245
78. Urbanellis P, Hamar M, Kaths JM, Kollmann D, Linares I, Mazilescu L, et al. Normothermic ex vivo kidney perfusion improves early DCD graft function compared with hypothermic machine perfusion and static cold storage. Transplantation. (2020) 104:947–55. doi: 10.1097/TP.0000000000003066
79. Brasile L, Henry N, Orlando G, Stubenitsky B. Potentiating renal regeneration using mesenchymal stem cells. Transplantation. (2019) 103:307–13. doi: 10.1097/TP.0000000000002455
80. Weissenbacher A, Lo Faro L, Boubriak O, Soares MF, Roberts IS, Hunter JP, et al. Twenty-four-hour normothermic perfusion of discarded human kidneys with urine recirculation. Am J Transplant. (2019) 19:178–92. doi: 10.1111/ajt.14932
81. Weissenbacher A, Huang H, Surik T, Lo Faro ML, Ploeg RJ, Coussios CC, et al. Urine recirculation prolongs normothermic kidney perfusion via more optimal metabolic homeostasis-a proteomics study. Am J Transplant. (2021) 21:1740–53. doi: 10.1111/ajt.16334
82. Weissenbacher A, Voyce D, Ceresa CDL, Soares MF, Roberts IS, Hunter JP, et al. Urine recirculation improves hemodynamics and enhances function in normothermic kidney perfusion. Transplant Direct. (2020) 6:e541. doi: 10.1097/TXD.0000000000000985
83. Pool MBF, Hamelink TL, van Goor H, van den Heuvel MC, Leuvenink HGD, Moers C. Prolonged ex-vivo normothermic kidney perfusion: the impact of perfusate composition. PLoS ONE. (2021) 16:e0251595. doi: 10.1371/journal.pone.0251595
84. Adams TD, Patel M, Hosgood SA, Nicholson ML. Lowering perfusate temperature from 37°C to 32°C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct. (2017) 3:e140. doi: 10.1097/TXD.0000000000000655
85. Hoyer DP, Gallinat A, Swoboda S, Wohlschläger J, Rauen U, Paul A, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int. (2014) 27:1097–106. doi: 10.1111/tri.12389
86. Bhattacharjee RN, Ruthirakanthan A, Sun Q, Richard-Mohamed M, Luke S, Jiang L, et al. Subnormothermic oxygenated perfusion optimally preserves donor kidneys ex vivo. Kidney Int Rep. (2019) 4:1323–33. doi: 10.1016/j.ekir.2019.05.013
87. Bhattacharjee RN, Patel SVB, Sun Q, Jiang L, Richard-Mohamed M, Ruthirakanthan A, et al. Renal protection against ischemia reperfusion injury: hemoglobin-based oxygen carrier-201 versus blood as an oxygen carrier in ex vivo subnormothermic machine perfusion. Transplantation. (2020) 104:482–9. doi: 10.1097/TP.0000000000002967
88. Urcuyo D, Blum MF, Liu Q, Nassar A, Buccini LD, Diago Uso T, et al. Development of a prolonged warm ex vivo perfusion model for kidneys donated after cardiac death. Int J Artif Organs. (2017) 40:265–71. doi: 10.5301/ijao.5000586
89. Brasile L, Green E, Haisch C. Ex vivo resuscitation of kidneys after postmortem warm ischemia. ASAIO J. (1997) 43:M427–30. doi: 10.1097/00002480-199709000-00014
90. Juriasingani S, Ruthirakanthan A, Richard-Mohamed M, Akbari M, Aquil S, Patel S, et al. Subnormothermic perfusion with H2S donor AP39 improves DCD porcine renal graft outcomes in an ex vivo model of kidney preservation and reperfusion. Biomolecules. (2021) 11:446. doi: 10.3390/biom11030446
91. Juriasingani S, Akbari M, Chan JY, Whiteman M, Sener A. H2S supplementation: a novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide. (2018) 81:57–66. doi: 10.1016/j.niox.2018.10.004
92. Minor T, von Horn C. Rewarming injury after cold preservation. Int J Mol Sci. (2019) 20:E2059. doi: 10.3390/ijms20092059
93. Minor T, von Horn C, Gallinat A, Kaths M, Kribben A, Treckmann J, et al. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am J Transplant. (2020) 20:1192–5. doi: 10.1111/ajt.15647
94. von Horn C, Minor T. Improved approach for normothermic machine perfusion of cold stored kidney grafts. Am J Transl Res. (2018) 10:1921–9. Available online at: www.ajtr.org/ISSN:1943-8141/AJTR0075884
95. Gallinat A, Lu J, von Horn C, Kaths M, Ingenwerth M, Paul A, et al. Transplantation of cold stored porcine kidneys after controlled oxygenated rewarming. Artif Organs. (2018) 42:647–54. doi: 10.1111/aor.13096
96. von Horn C, Zlatev H, Kaths M, Paul A, Minor T. Controlled oxygenated rewarming compensates for cold storage-induced dysfunction in kidney grafts. Transplantation. (2021). doi: 10.1097/TP.0000000000003854 [Epub ahead of print].
97. Gregorini M, Corradetti V, Pattonieri EF, Rocca C, Milanesi S, Peloso A, et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J Cell Mol Med. (2017) 21:3381–93. doi: 10.1111/jcmm.13249
98. Pool M, Eertman T, Sierra Parraga J, 't Hart N, Roemeling-van Rhijn M, Eijken M, et al. Infusing mesenchymal stromal cells into porcine kidneys during normothermic machine perfusion: intact MSCs can be traced and localised to glomeruli. Int J Mol Sci. (2019) 20:E3607. doi: 10.3390/ijms20143607
99. Pool MBF, Vos J, Eijken M, van Pel M, Reinders MEJ, Ploeg RJ, et al. Treating ischemically damaged porcine kidneys with human bone marrow- and adipose tissue-derived mesenchymal stromal cells during ex vivo normothermic machine perfusion. Stem Cells Dev. (2020) 29:1320–30. doi: 10.1089/scd.2020.0024
100. Lohmann S, Pool MBF, Rozenberg KM, Keller AK, Moers C, Møldrup U, et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: a porcine renal autotransplantation study. Am J Transplant. (2021) 21:2348–59. doi: 10.1111/ajt.16473
101. Thompson ER, Bates L, Ibrahim IK, Sewpaul A, Stenberg B, McNeill A, et al. Novel delivery 1230 of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Am J Transplant. (2021) 21:1402–14. doi: 10.1111/ajt.16100
102. Yuzefovych Y, Valdivia E, Rong S, Hack F, Rother T, Schmitz J, et al. Genetic engineering of the kidney to permanently silence MHC transcripts during ex vivo organ perfusion. Front Immunol. (2020) 11:265. doi: 10.3389/fimmu.2020.00265
103. Yang B, Hosgood SA, Nicholson ML. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation. (2011) 91:501–7. doi: 10.1097/TP.0b013e318207949f
104. Moser MAJ, Arcand S, Lin H-B, Wojnarowicz C, Sawicka J, Banerjee T, et al. Protection of the transplant kidney from preservation injury by inhibition of matrix metalloproteinases. PLoS ONE. (2016) 11:e0157508. doi: 10.1371/journal.pone.0157508
105. Diuwe P, Domagala P, Durlik M, Trzebicki J, Chmura A, Kwiatkowski A. The effect of the use of a TNF-alpha inhibitor in hypothermic machine perfusion on kidney function after transplantation. Contemp Clin Trials. (2017) 59:44–50. doi: 10.1016/j.cct.2017.05.013
106. Hameed AM, Lu DB, Burns H, Byrne N, Chew YV, Julovi S, et al. Pharmacologic targeting of renal ischemia-reperfusion injury using a normothermic machine perfusion platform. Sci Rep. (2020) 10:6930. doi: 10.1038/s41598-020-63687-0
107. Brasile L, Glowacki P, Castracane J, Stubenitsky BM. Pretransplant kidney-specific treatment to eliminate the need for systemic immunosuppression. Transplantation. (2010) 90:1294–8. doi: 10.1097/TP.0b013e3181ffba97
108. Tietjen GT, Hosgood SA, DiRito J, Cui J, Deep D, Song E, et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci Transl Med. (2017) 9:eaam6764. doi: 10.1126/scitranslmed.aam6764
109. DiRito JR, Hosgood SA, Reschke M, Albert C, Bracaglia LG, Ferdinand JR, et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am J Transplant. (2021) 21:161–73. doi: 10.1111/ajt.16148
110. Nghiem DD, Olson PR, Sureshkumar KK. Role of pulsatile perfusion with tissue plasminogen activator in deceased donor kidneys with extensive glomerular thrombosis. Transplant Proc. (2009) 41:29–31. doi: 10.1016/j.transproceed.2008.08.150
111. Woodside KJ, Goldfarb DA, Rabets JC, Sanchez EQ, Lebovitz DJ, Schulak JA, et al. Enhancing kidney function with thrombolytic therapy following donation after cardiac death: a multicenter quasi-blinded prospective randomized trial. Clin Transplant. (2015) 29:1173–80. doi: 10.1111/ctr.12647
112. Sedigh A, Larsson R, Brännström J, Magnusson P, Larsson E, Tufveson G, et al. Modifying the vessel walls in porcine kidneys during machine perfusion. J Surg Res. (2014) 191:455–62. doi: 10.1016/j.jss.2014.04.006
113. Sedigh A, Nordling S, Carlsson F, Larsson E, Norlin B, Lübenow N, et al. Perfusion of porcine kidneys with macromolecular heparin reduces early ischemia reperfusion injury. Transplantation. (2019) 103:420–7. doi: 10.1097/TP.0000000000002469
114. Hamaoui K, Gowers S, Boutelle M, Cook TH, Hanna G, Darzi A, et al. Organ pretreatment with cytotopic endothelial localizing peptides to ameliorate microvascular thrombosis and perfusion deficits in ex vivo renal hemoreperfusion models. Transplantation. (2016) 100:e128–39. doi: 10.1097/TP.0000000000001437
115. Hosgood SA, Bagul A, Kaushik M, Rimoldi J, Gadepalli RS, Nicholson ML. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg. (2008) 95:1060–7. doi: 10.1002/bjs.6174
116. Smith SF, Adams T, Hosgood SA, Nicholson ML. The administration of argon during ex vivo normothermic perfusion in an experimental model of kidney ischemia-reperfusion injury. J Surg Res. (2017) 218:202–8. doi: 10.1016/j.jss.2017.05.041
117. Bhattacharjee RN, Richard-Mohamed M, Sun Q, Haig A, Aboalsamh G, Barrett P, et al. CORM-401 reduces ischemia reperfusion injury in an ex vivo renal porcine model of the donation after circulatory death. Transplantation. (2018) 102:1066–74. doi: 10.1097/TP.0000000000002201
118. Yang B, Hosgood SA, Bagul A, Waller HL, Nicholson ML. Erythropoietin regulates apoptosis, inflammation and tissue remodelling via caspase-3 and IL-1β in isolated hemoperfused kidneys. Eur J Pharmacol. (2011) 660:420–30. doi: 10.1016/j.ejphar.2011.03.044
119. Yang C, Hosgood SA, Meeta P, Long Y, Zhu T, Nicholson ML, et al. Cyclic helix B peptide in preservation solution and autologous blood perfusate ameliorates ischemia-reperfusion injury in isolated porcine kidneys. Transplant Direct. (2015) 1:e6. doi: 10.1097/TXD.0000000000000515
120. Huijink TM, Venema LH, Posma RA, de Vries NJ, Westerkamp AC, Ottens PJ, et al. Metformin preconditioning and postconditioning to reduce ischemia reperfusion injury in an isolated ex vivo rat and porcine kidney normothermic machine perfusion model. Clin Transl Sci. (2021) 14:222–30. doi: 10.1111/cts.12846
121. Moser MAJ, Sawicka K, Sawicka J, Franczak A, Cohen A, Bil-Lula I, et al. Protection of the transplant kidney during cold perfusion with doxycycline: proteomic analysis in a rat model. Proteome Sci. (2020) 18:3. doi: 10.1186/s12953-020-00159-3
122. Nakladal D, Buikema H, Romero AR, Lambooy SPH, Bouma J, Krenning G, et al. The (R)-enantiomer of the 6-chromanol derivate SUL-121 improves renal graft perfusion via antagonism of the α1-adrenoceptor. Sci Rep. (2019) 9:13. doi: 10.1038/s41598-018-36788-0
123. Snoeijs MGJ, Vaahtera L, de Vries EE, Schurink GWH, Haenen GRMM, Peutz-Kootstra CJ, et al. Addition of a water-soluble propofol formulation to preservation solution in experimental kidney transplantation. Transplantation. (2011) 92:296–302. doi: 10.1097/TP.0b013e3182247b78
124. Bogensperger C, Hofmann J, Messner F, Resch T, Meszaros A, Cardini B, et al. Ex vivo mesenchymal stem cell therapy to regenerate machine perfused organs. Int J Mol Sci. (2021) 22:5233. doi: 10.3390/ijms22105233
125. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
126. Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. (2013) 91:32–9. doi: 10.1038/icb.2012.64
127. Sierra-Parraga JM, Eijken M, Hunter J, Moers C, Leuvenink H, Møller B, et al. Mesenchymal stromal cells as anti-inflammatory and regenerative mediators for donor kidneys during normothermic machine perfusion. Stem Cells Dev. (2017) 26:1162–70. doi: 10.1089/scd.2017.0030
128. Kuo TK, Hung S-P, Chuang C-H, Chen C-T, Shih Y-RV, Fang S-CY, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. (2008) 134:2111–21.e1–3. doi: 10.1053/j.gastro.2008.03.015
129. Lindoso RS, Collino F, Bruno S, Araujo DS, Sant'Anna JF, Tetta C, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. (2014) 23:1809–19. doi: 10.1089/scd.2013.0618
130. György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. (2011) 68:2667–88. doi: 10.1007/s00018-011-0689-3
131. Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett. (2015) 168:154–8. doi: 10.1016/j.imlet.2015.06.007
132. Konala VBR, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. (2016) 18:13–24. doi: 10.1016/j.jcyt.2015.10.008
133. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. (2003) 14 (Suppl. 1):S55–61. doi: 10.1097/01.ASN.0000067652.51441.21
134. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. (2012) 307:1169–77. doi: 10.1001/jama.2012.316
135. Reinders MEJ, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. (2013) 2:107–11. doi: 10.5966/sctm.2012-0114
136. Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille M-H, Grégoire C, et al. Infusion of third-party mesenchymal stromal cells after kidney transplantation: a phase I-II, open-label, clinical study. Kidney Int. (2019) 95:693–707. doi: 10.1016/j.kint.2018.08.046
137. Gregorini M, Bosio F, Rocca C, Corradetti V, Valsania T, Pattonieri EF, et al. Mesenchymal stromal cells reset the scatter factor system and cytokine network in experimental kidney transplantation. BMC Immunol. (2014) 15:44. doi: 10.1186/s12865-014-0044-1
138. Sierra-Parraga JM, Munk A, Andersen C, Lohmann S, Moers C, Baan CC, et al. Mesenchymal stromal cells are retained in the porcine renal cortex independently of their metabolic state after renal intra-arterial infusion. Stem Cells Dev. (2019) 28:1224–35. doi: 10.1089/scd.2019.0105
139. Najar M, Raicevic G, Fayyad-Kazan H, De Bruyn C, Bron D, Toungouz M, et al. Impact of different mesenchymal stromal cell types on T-cell activation, proliferation and migration. Int Immunopharmacol. (2013) 15:693–702. doi: 10.1016/j.intimp.2013.02.020
140. Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. (2015) 6:560. doi: 10.3389/fimmu.2015.00560
141. Sindberg GM, Lindborg BA, Wang Q, Clarkson C, Graham M, Donahue R, et al. Comparisons of phenotype and immunomodulatory capacity among rhesus bone-marrow-derived mesenchymal stem/stromal cells, multipotent adult progenitor cells, and dermal fibroblasts. J Med Primatol. (2014) 43:231–41. doi: 10.1111/jmp.12122
142. Eggenhofer E, Popp FC, Mendicino M, Silber P, Van't Hof W, Renner P, et al. Heart grafts tolerized through third-party multipotent adult progenitor cells can be retransplanted to secondary hosts with no immunosuppression. Stem Cells Transl Med. (2013) 2:595–606. doi: 10.5966/sctm.2012-0166
143. Soeder Y, Loss M, Johnson CL, Hutchinson JA, Haarer J, Ahrens N, et al. First-in-Human case study: multipotent adult progenitor cells for immunomodulation after liver transplantation. Stem Cells Transl Med. (2015) 4:899–904. doi: 10.5966/sctm.2015-0002
144. Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. (2014) 5:148. doi: 10.3389/fimmu.2014.00148
145. Luk F, de Witte SFH, Korevaar SS, Roemeling-van Rhijn M, Franquesa M, Strini T, et al. Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev. (2016) 25:1342–54. doi: 10.1089/scd.2016.0068
146. Jacobs SA, Pinxteren J, Roobrouck VD, Luyckx A, van't Hof W, Deans R, et al. Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transplant. (2013) 22:1915–28. doi: 10.3727/096368912X657369
147. Sierra Parraga JM, Rozenberg K, Eijken M, Leuvenink HG, Hunter J, Merino A, et al. Effects of normothermic machine perfusion conditions on mesenchymal stromal cells. Front Immunol. (2019) 10:765. doi: 10.3389/fimmu.2019.00765
148. Contreras JL, Vilatoba M, Eckstein C, Bilbao G, Anthony Thompson J, Eckhoff DE. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. (2004) 136:390–400. doi: 10.1016/j.surg.2004.05.015
149. Zhang X, Zheng X, Sun H, Feng B, Chen G, Vladau C, et al. Prevention of renal ischemic injury by silencing the expression of renal caspase 3 and caspase 8. Transplantation. (2006) 82:1728–32. doi: 10.1097/01.tp.0000250764.17636.ba
150. Zheng X, Zang G, Jiang J, He W, Johnston NJ, Ling H, et al. Attenuating ischemia1251 reperfusion injury in kidney transplantation by perfusing donor organs with siRNA cocktail solution. Transplantation. (2016) 100:743–52. doi: 10.1097/TP.0000000000000960
151. Yang C, Zhao T, Zhao Z, Jia Y, Li L, Zhang Y, et al. Serum-stabilized naked caspase-3 siRNA protects autotransplant kidneys in a porcine model. Mol Ther. (2014) 22:1817–28. doi: 10.1038/mt.2014.111
152. Foster DJ, Brown CR, Shaikh S, Trapp C, Schlegel MK, Qian K, et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther. (2018) 26:708–17. doi: 10.1016/j.ymthe.2017.12.021
153. Sondhi D, Stiles KM, De BP, Crystal RG. Genetic modification of the lung directed toward treatment of human disease. Hum Gene Ther. (2017) 28:3–84. doi: 10.1089/hum.2016.152
154. Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, Jansson K, Höffler K, Yuzefovych Y, et al. Immunoengineering of the vascular endothelium to silence MHC expression during normothermic ex vivo lung perfusion. Hum Gene Ther. (2019) 30:485–96. doi: 10.1089/hum.2018.117
155. Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO, et al. Risks associated with lentiviral vector exposures and prevention strategies. J Occup Environ Med. (2016) 58:1159–66. doi: 10.1097/JOM.0000000000000879
156. Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. (2015) 14:239–47. doi: 10.1038/nrd4503
157. Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. (2012) 338:903–10. doi: 10.1126/science.1226338
158. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. (2015) 33:941–51. doi: 10.1038/nbt.3330
159. Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. (2008) 105:14265–70. doi: 10.1073/pnas.0805135105
160. Devalliere J, Chang WG, Andrejecsk JW, Abrahimi P, Cheng CJ, Jane-wit D, et al. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. (2014) 28:908–22. doi: 10.1096/fj.13-238527
161. Shao L, Wu D, Zhang P, Li W, Wang J, Su G, et al. The significance of microthrombosis and fgl2 in no-reflow phenomenon of rats with acute myocardial ischemia/reperfusion. Clin Appl Thromb Hemost. (2013) 19:19–28. doi: 10.1177/1076029612437577
162. McCall SJ, Tuttle-Newhall JE, Howell DN, Fields TA. Prognostic significance of microvascular thrombosis in donor kidney allograft biopsies. Transplantation. (2003) 75:1847–52. doi: 10.1097/01.TP.0000063126.88887.68
163. Ng JCY, Leung M, Landsberg D. Evaluation of heparin anticoagulation protocols in post-renal transplant recipients (EHAP-PoRT study). Can J Hosp Pharm. (2016) 69:114–21. doi: 10.4212/cjhp.v69i2.1538
164. Ripert T, Menard J, Schoepen Y, Nguyen P, Rieu P, Staerman F. Preventing graft thrombosis after renal transplantation: a multicenter survey of clinical practice. Transplant Proc. (2009) 41:4193–6. doi: 10.1016/j.transproceed.2009.07.106
165. Nghiem DD, Olson PR, Sureshkumar KK. Significance of microvascular thrombosis in renal allografts: role of ex vivo thrombolytic therapy. Clin Transplant. (2007) 21:172–6. doi: 10.1111/j.1399-0012.2006.00616.x
166. Gok MA, Shenton BK, Buckley PE, Peaston R, Cornell C, Soomro N, et al. How to improve the quality of kidneys from non-heart-beating donors: a randomised controlled trial of thrombolysis in non-heart-beating donors. Transplantation. (2003) 76:1714–9. doi: 10.1097/01.TP.0000093834.05766.FD
167. Rijken DC, Seifried E, Barrett-Bergshoeff MM, Dooijewaard G. Plasminogen activation at low temperatures in plasma samples containing therapeutic concentrations of tissue-type plasminogen activator or other thrombolytic agents. Thromb Haemost. (1990) 64:47–52. doi: 10.1055/s-0038-1647252
168. Garcia-Criado FJ, Eleno N, Santos-Benito F, Valdunciel JJ, Reverte M, Lozano-Sánchez FS, et al. Protective effect of exogenous nitric oxide on the renal function and inflammatory response in a model of ischemia-reperfusion. Transplantation. (1998) 66:982–90. doi: 10.1097/00007890-199810270-00003
169. Kurata H, Takaoka M, Kubo Y, Katayama T, Tsutsui H, Takayama J, et al. Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol. (2005) 517:232–9. doi: 10.1016/j.ejphar.2005.05.026
170. Nakao A, Choi AMK, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. (2006) 10:650–71. doi: 10.1111/j.1582-4934.2006.tb00426.x
171. Vummaleti SVC, Branduardi D, Masetti M, De Vivo M, Motterlini R, Cavalli A. Theoretical insights into the mechanism of carbon monoxide (CO) release from CO-releasing molecules. Chemistry. (2012) 18:9267–75. doi: 10.1002/chem.201103617
172. Lobb I, Jiang J, Lian D, Liu W, Haig A, Saha MN, et al. Hydrogen sulfide protects renal grafts against prolonged cold ischemia-reperfusion injury via specific mitochondrial actions. Am J Transplant. (2017) 17:341–52. doi: 10.1111/ajt.14080
173. Lobb I, Davison M, Carter D, Liu W, Haig A, Gunaratnam L, et al. Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol. (2015) 194:1806–15. doi: 10.1016/j.juro.2015.07.096
174. Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology. (2006) 11:306–12. doi: 10.1111/j.1440-1797.2006.00585.x
175. El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. (2000) 275:223–8. doi: 10.1074/jbc.275.1.223
176. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. (2017) 60:1577–85. doi: 10.1007/s00125-017-4342-z
177. De Broe ME, Kajbaf F, Lalau J-D. Renoprotective effects of metformin. Nephron. (2018) 138:261–74. doi: 10.1159/000481951
178. Mohsin AA, Chen Q, Quan N, Rousselle T, Maceyka MW, Samidurai A, et al. Mitochondrial complex i inhibition by metformin limits reperfusion injury. J Pharmacol Exp Ther. (2019) 369:282–90. doi: 10.1124/jpet.118.254300
179. Kunugi S, Shimizu A, Kuwahara N, Du X, Takahashi M, Terasaki Y, et al. Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Lab Invest. (2011) 91:170–80. doi: 10.1038/labinvest.2010.174
180. Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. (2008) 73:863–9. doi: 10.1038/sj.ki.5002715
181. Yan Q, Sui W, Wang B, Zou H, Zou G, Luo H. Expression of MMP-2 and TIMP-1 in renal tissues of patients with chronic active antibody-mediated renal graft rejection. Diagn Pathol. (2012) 7:141. doi: 10.1186/1746-1596-7-141
182. Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, et al. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J Nephrol. (2013) 2:84–9. doi: 10.5527/wjn.v2.i3.84
183. Hajmousa G, Vogelaar P, Brouwer LA, van der Graaf AC, Henning RH, Krenning G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials. (2017) 119:43–52. doi: 10.1016/j.biomaterials.2016.12.008
184. Vogelaar PC, Roorda M, de Vrij EL, Houwertjes MC, Goris M, Bouma H, et al. The 6-hydroxychromanol derivative SUL-109 ameliorates renal injury after deep hypothermia and rewarming in rats. Nephrol Dial Transplant. (2018) 33:2128–38. doi: 10.1093/ndt/gfy080
185. Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Antioxidant protection of propofol and its recycling in erythrocyte membranes. Am J Respir Crit Care Med. (2002) 165:54–60. doi: 10.1164/ajrccm.165.1.2010134
186. Aarts L, van der Hee R, Dekker I, de Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. (1995) 357:83–5. doi: 10.1016/0014-5793(94)01337-Z
187. Träger K, Fritzler D, Fischer G, Schröder J, Skrabal C, Liebold A, et al. Treatment of post1431 cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs. (2016) 39:141–6. doi: 10.5301/ijao.5000492
Keywords: kidney transplantation, ex-vivo machine perfusion, ex-vivo therapy, graft preservation, kidney preconditioning, ischemia-reperfusion injury, graft rejection, organ regeneration
Citation: Zulpaite R, Miknevicius P, Leber B, Strupas K, Stiegler P and Schemmer P (2021) Ex-vivo Kidney Machine Perfusion: Therapeutic Potential. Front. Med. 8:808719. doi: 10.3389/fmed.2021.808719
Received: 03 November 2021; Accepted: 06 December 2021;
Published: 24 December 2021.
Edited by:
Ondrej Viklicky, Institute for Clinical and Experimental Medicine (IKEM), CzechiaReviewed by:
Maria Irene Bellini, Sapienza University of Rome, ItalyMiha Arnol, University Medical Centre Ljubljana, Slovenia
Copyright © 2021 Zulpaite, Miknevicius, Leber, Strupas, Stiegler and Schemmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Stiegler, cGhpbGlwcC5zdGllZ2xlckBtZWR1bmlncmF6LmF0
†ORCID: Ruta Zulpaite orcid.org/0000-0002-2528-8704
Bettina Leber orcid.org/0000-0002-2101-7560
Peter Schemmer orcid.org/0000-0002-4192-6155
 Ruta Zulpaite
Ruta Zulpaite Povilas Miknevicius1,2
Povilas Miknevicius1,2 Peter Schemmer
Peter Schemmer