- 1Department of Radiology, Ca' Foncello General Hospital, Treviso, Italy
- 2Department of Experimental and Clinical Biomedical Sciences, Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy
- 3Department of Experimental and Clinical Medicine, Division of Rheumatology, Azienda Ospedaliero-Universitaria Careggi and Scleroderma Unit, University of Florence, Florence, Italy
- 4Department of Experimental and Clinical Medicine, Careggi University Hospital, Florence, Italy
- 5Unit of Immunology, Rheumatology, Allergy and Rare Diseases, San Raffaele Scientific Institute, Istituti di Ricovero e Cura a Carattere Scientifico, Vita-Salute San Raffaele University, Milan, Italy
Objective: Although interstitial lung disease (ILD) is a major cause of morbidity and mortality in systemic sclerosis (SSc), its prognostication remains challenging. Given that CT represents the gold standard imaging technique in ILD assessment, a systematic review on chest CT findings as predictors of mortality or ILD progression in SSc-ILD was performed.
Materials and Methods: Three databases (Medline, Embase, and Web of Science) were searched to identify all studies analyzing CT mortality or ILD progression predictors in SSc-ILD, from inception to December 2020. ILD progression was defined by worsening of forced vital capacity and/or CT ILD findings. Manuscripts not written in English, with not available full-text, not focusing on SSc-ILD or with SSc-ILD not extrapolated, otherwise with overlap syndromes, pediatric patients, <10 cases or predictors other than CT features were excluded.
Results: Out of 3,513 citations, 15 full-texts (2,332 patients with SSc-ILD) met the inclusion criteria. ILD extent and extensive ILD, ILD densitometric analysis parameters, fibrotic extent and reticulation extent resulted as independent mortality predictors. Extensive ILD is also an independent predictor of death, need for supplemental oxygen or lung transplantation. Honeycombing extent is an independent risk factor for respiratory mortality. Independent predictors of ILD progression were not identified.
Conclusions: ILD extent and extensive ILD independently predict mortality in SSc-ILD on CT, as well as ILD densitometric analysis, fibrotic extent and reticulation extent. Extensive ILD is also a predictor of death, need for supplemental oxygen, or lung transplantation. Honeycombing extent predicts respiratory mortality. CT predictors of ILD progression need to be further investigated.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, PROSPERO, identifier: CRD420202005001.
Introduction
Interstitial lung disease (ILD) is one of the most common complications in systemic sclerosis (SSc) and is a leading cause of mortality (1–3). The screening for ILD is recommended in every patient with SSc (4) and chest CT, as the gold standard imaging technique for ILD assessment, plays a key role in both diagnosis and management (5). Characteristic features of ILD on CT in patients with SSc include ground glass opacities (GGO) and reticulations, with or without bronchiectasis (as signs of fibrosis), configuring a non-specific interstitial pneumonia (NSIP) pattern. Honeycomb lung and usual interstitial pneumonia (UIP) are also possible, though less common in SSc (6). The prevalence of CT-detected SSc-ILD may vary from 47 to 84%, depending on the subtype and the criteria used to define it. The disease course for patients with SSc-ILD is highly variable: it may range from a subclinical disease to a more aggressive phenotype. Thus, the identification of patients with rapidly progressive ILD is crucial to establish a more aggressive therapeutic approach (3). In light of the previously published data (7) on prognostic factors in SSc-ILD, in terms of mortality and disease progression, the aim of our systematic literature review was to collect and discuss available data on chest CT features as prognostic factors in patients with SSc-ILD.
Materials and Methods
Data Sources and Search
A systematic search was performed to identify all papers that investigated CT findings as predictor of mortality and/or ILD progression in SSc-ILD. Medline, Embase, and Web of Science databases were searched from the onset of each database to 31 December 2020. Two assessors (N.L. and M.O.) adopted predefined criteria to review all citations. Additionally, references from the selected articles and major reviews were also evaluated. Search terms and strategies are shown in Supplementary Table 1. The systematic review was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/), ID CRD42020205001.
Study Selection
Both assessors independently reviewed all manuscripts, screening titles and abstracts as first step, then evaluated full texts, looking for studies that identified CT features as predictors of mortality or ILD progression in SSc-ILD. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (http://prisma-statement.org/prismastatement/flowdiagram.aspx) was adopted. Exclusion criteria were: papers not written in English, no full text available, conditions other than SSc-ILD, works with overlap syndromes or SSc-ILD as part of the cohort but whose data could not be separately extracted, pediatric patients, a study population smaller than 10 patients with SSc-ILD, papers focusing on treatment effects only, studies on predictors other than CT features. ILD progression was defined as ILD radiologic progression in terms of extent or the worsening of forced vital capacity (FVC), as previously proposed in Winstone et al. (7). In addition, FVC decline was considered if the study clearly presented a cut-off to define worsening. There were no restrictions on study quality, design, or follow-up. Disagreements were resolved by consensus or, if not reached, through a third assessor (C.B.).
Quality Assessment, Data Extraction, Synthesis, and Analysis
The studies' quality was assessed with the Critical Appraisal Skills Program (http://www.casp-uk.net). Quality assessment was based on the method of population sampling, the definitions of SSc and ILD, how the outcome was ascertained and whether confounders were appropriately identified and accounted for in the statistical analysis. Risk of bias and applicability concerns was also evaluated following the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria (http://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/). The two assessors independently evaluated the study quality. Disagreements were resolved by consensus. They extracted the following data from selected papers and recorded them in pre-available forms: study design, subject characteristics and study results. Available anamnestic and clinical data (age, sex, smoking habit, serum auto-antibodies, FVC, and diffusion capacity for carbon monoxide) were recorded and summarized. Hazard ratios and odds ratios for predictors were collected, as well as p-values. Due to the heterogeneity of selected papers and results, a formal meta-analysis was not performed.
Results
Search Results and Study Characteristics
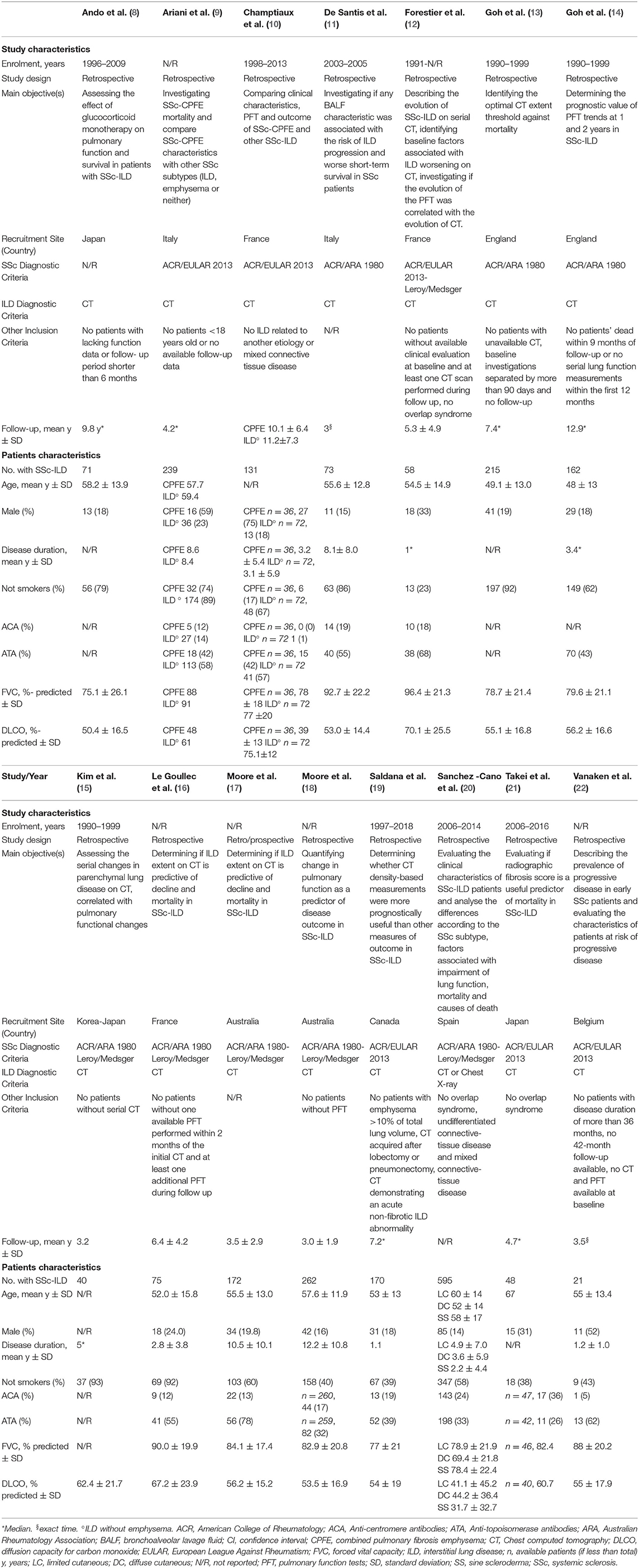
Out of 3,513 papers identified, 15 fulfilled inclusion criteria, for a total amount of 2,332 patients with SSc (range 21–595 patients per paper) (8–22). The detailed flow diagram of studies' selection is available in Figure 1. Papers that were not included because only partially meeting inclusion criteria, but relevant for the discussion, are listed in Supplementary Data 1, with the reason of rejection. All included studies were retrospective (8–16, 18–22) or retrospective analyses of prospectively collected data (17) and adopted CT for ILD diagnosis (8–19, 21, 22), except for one that utilized CT or chest X-ray (20). Prone acquisitions were additionally performed to confirm ILD presence in one study (12), while in one work prone scans could be part of the protocol, but only supine CT was analyzed (19). The other studies did not specify. SSc was variously defined, mainly by the 1980 American College of Rheumatology classification criteria (23). The follow-up was heterogeneous, with a mean duration ranging from 3.0 to 11.2 years (10, 12, 15–18) and a median ranging between 4.2 and 12.9 years (8, 9, 13, 14, 19, 21). Two studies followed-up the patients exactly for 1 (22) and 3 (11) years. Studies' quality is summarized in Supplementary Table 2 (Critical Appraisal Skills Program) and Supplementary Figure 1 (QUADAS-2 criteria). Considering patients with available data, mean age ranged from 48 to 67 years, 440/2,069 (21.3%) were men, and 1,546/2,309 (66.9%) were not-smokers. The mean disease duration ranged from 1.1 to 12.2 years in nine studies (9–11, 16–20, 22), the median ranged from 1.0 to 5.0 years in three (12, 14, 15). Anti-centromere antibodies were positive in 306/1,818 (16.8%) patients, and 788/1,974 (39.9%) were positive for anti-topoisomerase I antibodies. FVC ranged from 69.4 to 96.4% predicted and diffusion capacity for carbon monoxide ranged from 31.7 to 75.1% predicted. All characteristics of included studies and patients are summarized in Table 1.

Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram of studies' selection. CT, chest computed tomography; ILD, interstitial lung disease; SSc, systemic sclerosis.
Predictors of Mortality
The definitions of independent predictors were provided in the text. The definitions of all investigated CT predictors are reported in Supplementary Data 2.
Univariate Analysis
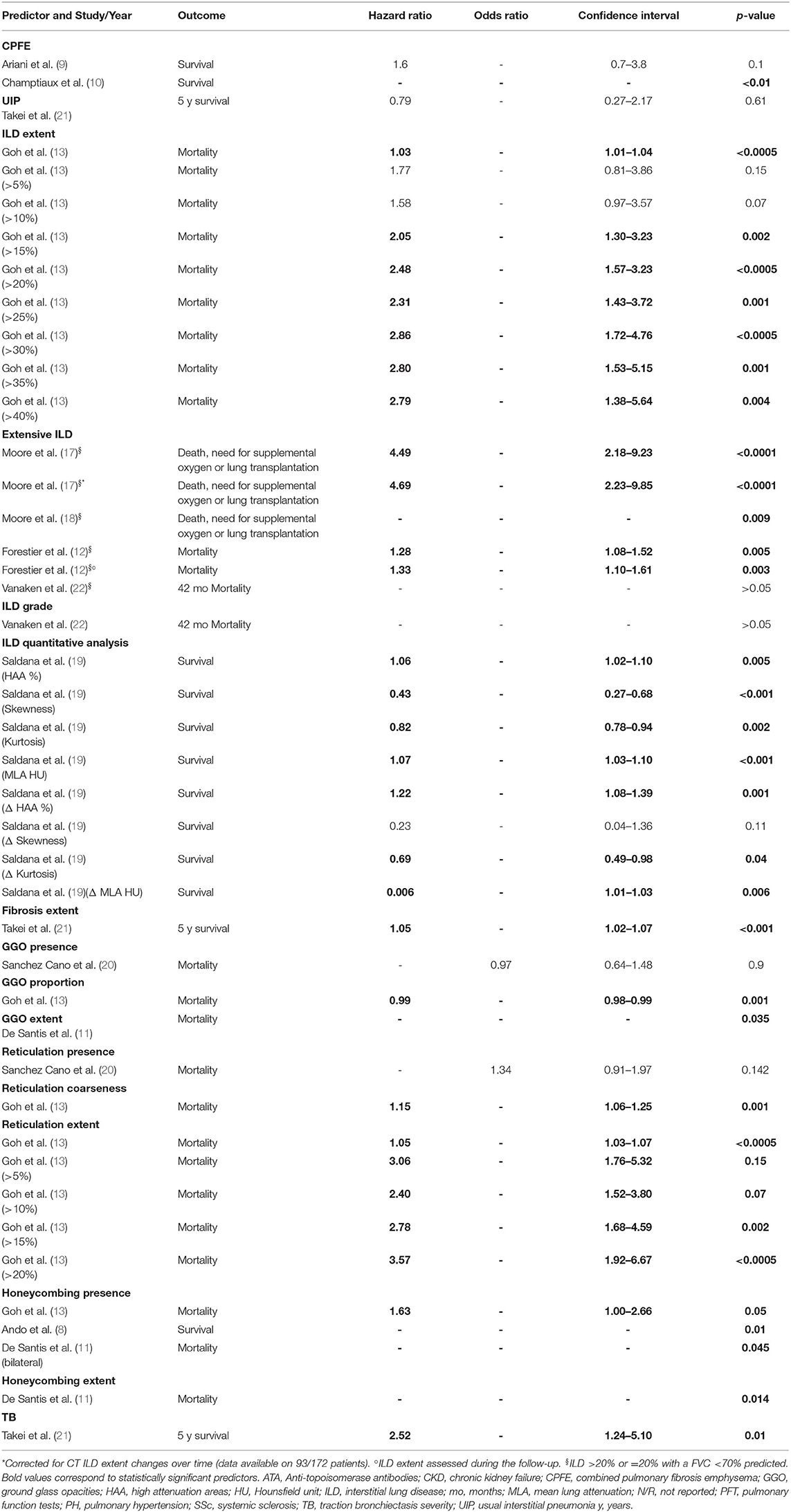
Univariate analyses were performed on combined pulmonary fibrosis emphysema (CPFE) and UIP patterns as manifestation of ILD against other patterns, ILD extent (also with threshold of 5, 10, 15, 20, 25, 30, 35, and 40%), extensive ILD, ILD grade, ILD densitometric analysis parameters, fibrosis extent, GGO presence, proportion and extent, reticulation presence, coarseness and extent (also with threshold of 5, 10, 15, and 20%), honeycombing presence and extent, traction bronchiectasis severity (9 papers, 1,084 patients) (8–13, 19–22). Extensive ILD was also analyzed with univariate analysis for mortality or need for supplemental oxygen or lung transplantation, as combined outcome, in two studies (17, 18). All studies expressed the risk as hazard ratio, except for one that adopted odds ratio, investigating GGO and reticulation presence (20).
CPFE, compared to ILD without emphysema, was not a significant predictor of mortality in Ariani et al. (9), whereas it was correlated with a higher mortality in Champtiaux et al. (10). UIP did not result as a significant predictor (21). ILD extent and the predefined thresholds from 15 to 40%, were significant mortality predictors (13). Extensive disease was a significant predictor in one (12) out of two studies (12, 22). Extensive ILD was also a significant predictor for death, need for supplemental oxygen, or lung transplantation (17, 18), even when adjusted for extent changes during the follow-up (17). However, this was not the case for ILD grade (22). All ILD quantitative analysis variables, except for skewness, were significant predictors (19). The fibrosis extent >14.2% was identified as the best cut-off for mortality prediction, resulting a significant risk factor (21). GGO presence was not a significant predictor (20), contrarily to GGO extent (11). GGO proportion resulted as a weak protective factor, with a hazard ratio of 0.99 (p-value 0.001). Moreover, reticulation coarseness and extent (also with threshold of 5, 10, 15, and 20%) were significant predictors of death (13), but not reticulation presence when was considered alone (20). Honeycombing presence and extent were associated with mortality (8, 11, 13). Lastly, traction bronchiectasis severity (21) was a significant predictor, as well. All univariate analyses are reported in Table 2.
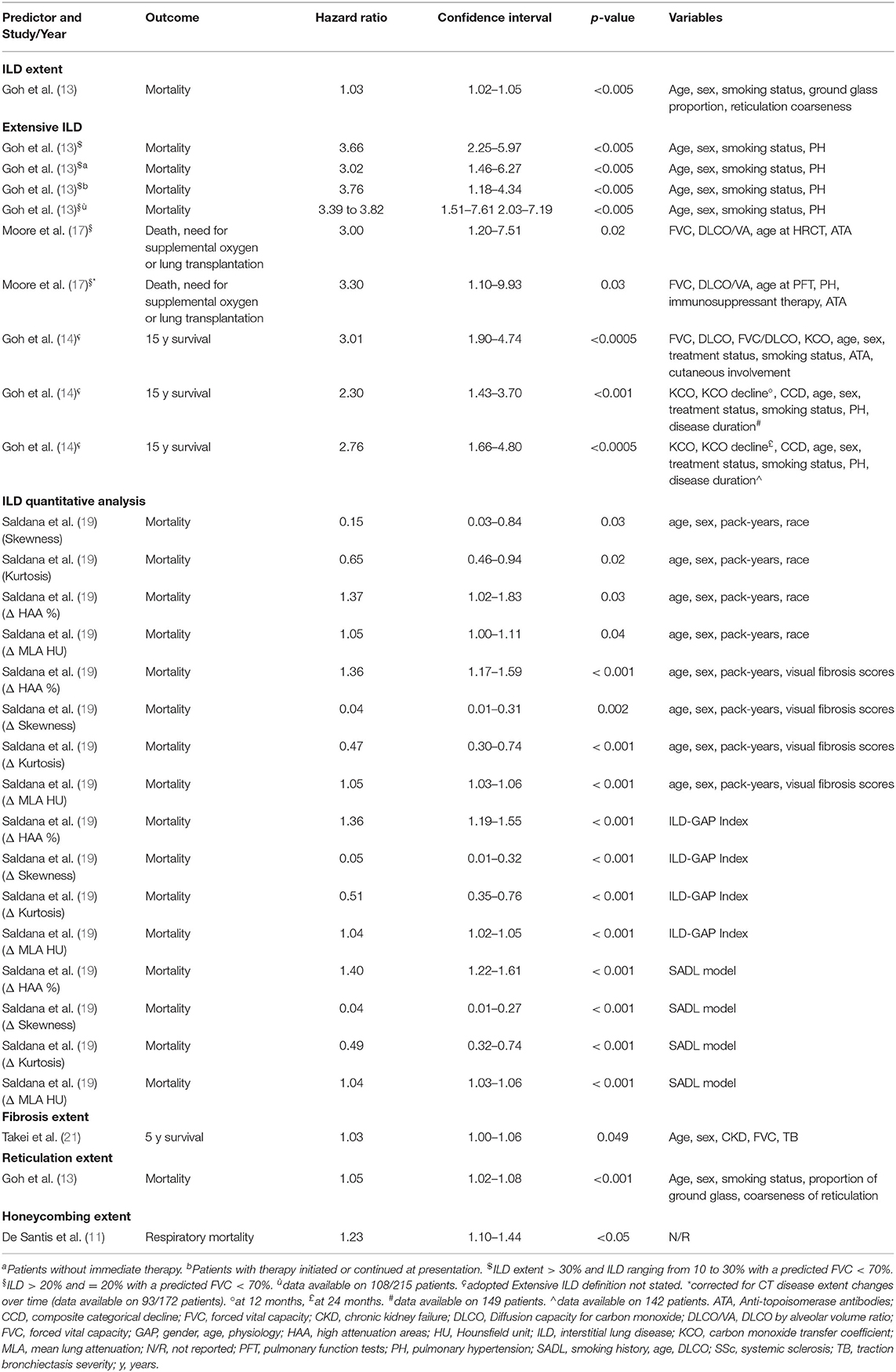
Multivariate Analysis and Independent Predictors
A multivariate analysis for the prediction of mortality in SSc-ILD subjects was performed in 8 studies and the potential CT mortality predictors were: CPFE (10), ILD extent (13, 14), extensive ILD (13, 14), ILD quantitative analysis parameters (19), fibrosis extent (21), GGO extent (11), reticulation extent (13), honeycombing presence (8), honeycombing extent (11) and traction bronchiectasis severity (21). Extensive ILD was also investigated as predictor of mortality, need for supplemental oxygen, or lung transplantation (17), as combined outcome. Honeycombing extent was also evaluated for respiratory mortality (11) in one study. Covariates for adjusted analysis were different among studies, although not detailed in two of them (10, 11).
While CPFE (10), GGO extent (11), honeycombing presence (8, 11), and traction bronchiectasis severity (21) did not result as independent predictors on multivariate analysis, other independent factors were identified, with the following definitions:
-ILD extent, in one study (13), defined as the mean of ILD percentages scored at 5 levels to the nearest 5%.
-Extensive ILD, defined, adopting the same scoring system for ILD extent, as 1) ILD extent >30% or ranging from 10 to 30% with a predicted FVC <70% or 2) ILD extent >20% or =20% with a predicted FVC <70%, investigated in two papers (13, 14). In addition, extensive ILD (>20% or =20% with a predicted FVC < 70%) was also an independent predictor of mortality, need for supplemental oxygen, or lung transplantation, even considering the patients who changed the CT score during the follow-up (17).
-Fibrosis extent (cut-off >14.2%), in one work (21), as the mean percentage of lung involvement due to reticulation and/or honeycombing, computed on six levels. Different thresholds of lung involvement were firstly tested, to define the best cut-off as mortality predictor.
-ILD densitometric analysis, in one study (19), with the following parameters obtained by software analysis: mean lung attenuation, skewness, kurtosis and the percentage of high attenuation areas. The changes between two serial CT of those parameters for each patient were also evaluated.
-Reticulation extent, in one work (13), as the mean of percentages scored at 5 levels, to the nearest 5%.
-Honeycombing extent, in one work, specifically addressing respiratory mortality (11), as the mean score computed for each lung lobe (0–5 for each lobe, depending on percentage of involvement).
All independent predictors are reported in Table 3 and all multivariate analyses are shown in Supplementary Table 3.
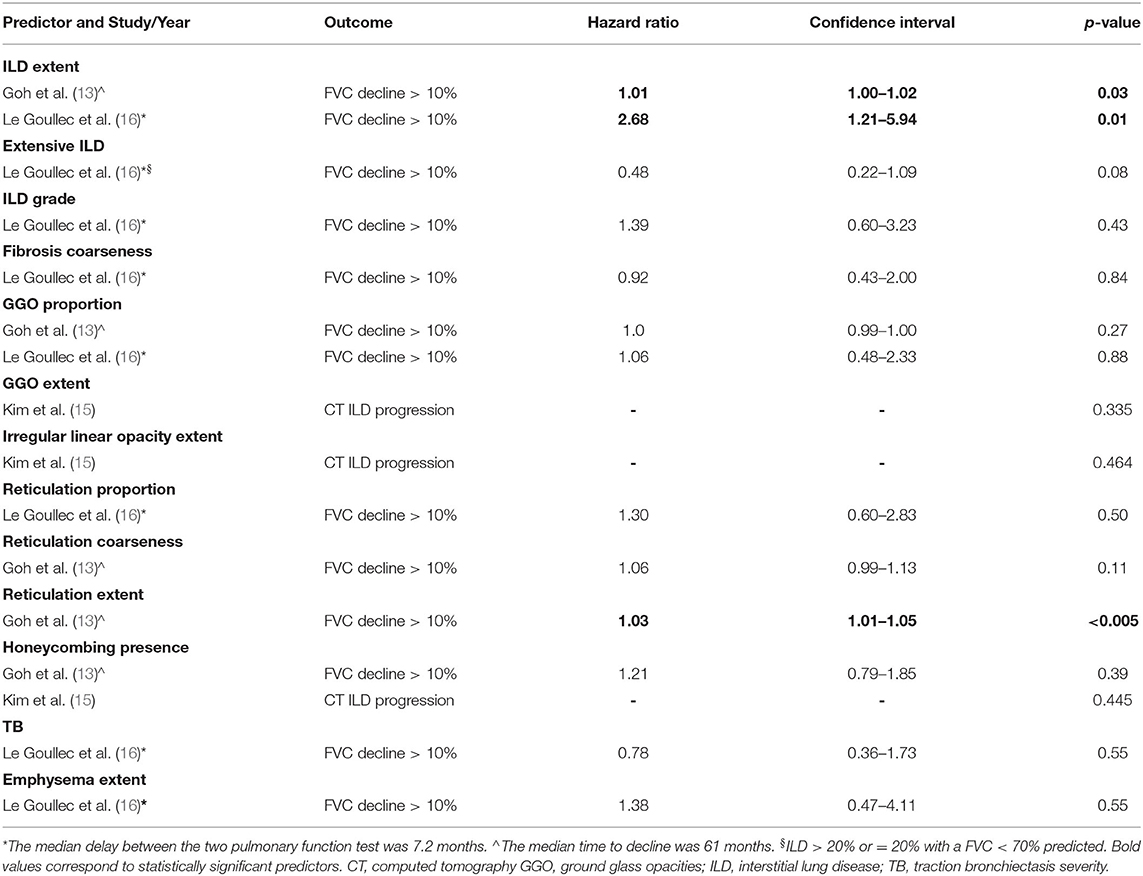
Predictors of ILD Progression
The predictors of ILD progression were investigated in 3 studies, only through univariate analysis (13, 15, 16). They included ILD extent, extensive ILD, ILD grade, fibrosis coarseness, GGO proportion and extent, irregular linear opacities extent, reticulation proportion and extent, honeycombing presence, traction bronchiectasis severity and emphysema extent (13, 15, 16). According to the included manuscripts, ILD progression was defined as progression of ILD extent on CT vs. stability (15) or FVC decline more than 10% predicted (6, 13). Considered timing intervals were the median delay between the two pulmonary function tests (7.2 months) (16) or the median time to decline (61 months) (13).
Among all the parameters, only ILD grade (13, 16) and reticulation extent (13) were statistically significant predictors of FVC decline (Table 4). None was a predictor of ILD progression on CT. The definitions of all investigated CT predictors are reported in Supplementary Data 2.
Discussion
Our results identified some CT features mainly related to the extent of disease as independent prognostic factors for mortality. In regards to the prediction of overall deaths, ILD extent (13) and extensive ILD represented independent predictors of mortality (13, 14), the latter also for a combined outcome including death, need for supplemental oxygen or lung transplantation (17). In particular, extensive ILD, among all variables, was the most investigated risk factor (6 studies, 3 with multivariable analyses) (12–14, 17, 18, 22). Thus, in our opinion, it could be confidently incorporated in future clinical trial settings, i.e. adopting the definition of extensive ILD as >20% or =20% with a FVC <70% predicted, that was suggested for clinical practice (13) and verified as independent predictor for both mortality (13) and the aforementioned combined outcome (17). However, these data don't match other studies conducted on connective tissue disease-related ILD (CTD-ILD), where ILD extent was not an independent mortality predictor (24–26). Actually, Vanaken et al. (22), on univariate analysis, did not identify extensive ILD as a significant predictor of death: nevertheless, this result could be related to the small sample of patients with SSc-ILD in their study. Moreover, the fibrosis extent resulted as an independent predictor of mortality in one study (21), contrarily to the data reported in another recent paper on patients with CTD-ILD (24). Among the various analysis of reticular features (presence, coarseness, extent), reticulation extent was the only independent predictor of mortality in the unique work that investigated this variable (specific thresholds were not tested on multivariate analysis) (13), differently to other studies on patients with CTD-ILD (24–26). Similarly, honeycombing extent was an independent risk factor for respiratory death, although not for overall mortality (11), whereas it resulted an independent mortality predictor in two works on CTD-ILD (24, 26).
Presumably, the discrepancies between SSc-ILD and CTD-ILD regarding honeycombing extent as prognostic factors could be explained by the fact that honeycombing is less common in patients with SSc-ILD. In fact, NSIP is the most common ILD pattern and it is usually sustained by reticulation with a lower frequency/extent of honeycombing. On the other hand, in other ILD, such as rheumatoid arthritis, UIP and honeycombing may be more frequent and could have had a major burden in the analysis of a CTD-ILD population (5). However, considering that honeycombing extent may have a prognostic role in respiratory mortality, we believe that the role of CT-ILD features should be investigated for this specific outcome. This could allow to split factors that provide prognostic information regarding mortality more directly caused by ILD from those generically associated to death, as expressions of the systemic disease. Moreover, further studies might confirm the prognostic value of those factors that were, respectively, evaluated only in one study, such as fibrosis extent, reticulation (overall mortality) and honeycombing extent (respiratory mortality).
On the other hand, the potential role of ILD densitometric analysis, that provides continuous quantitative parameters based on the software-based images assessments, remains debatable. In fact, histogram-based densitometry parameters resulted as independent risk factors for mortality (19), in particular their changes during the follow-up (Table 3). However, when adjusted for baseline and temporal changes in lung function, these automated data were not independently associated with death: in the opinion of the authors, this suggests that densitometric data may not provide additional information beyond what is provided by readily available physiological variables (19).
From our analysis, other CT features did not result as independent predictors of mortality. However, there are further considerations that are worth to be made. In particular, we believe that some CT features should be further investigated, as the results are available in one study only and/or the features could be investigated more in detail.
Firstly, in the overall SSc population, a CPFE pattern, namely the presence of emphysema together with ILD, is considered a mortality risk factor (9). However, among patients with SSc-ILD, CPFE did not resulted an independent predictor of shorter survival (10), while it may be considered a risk factor in ILD related to rheumatoid arthritis (27) and idiopathic pulmonary fibrosis (28). Interestingly, the presence of pulmonary hypertension was significantly associated with CPFE (44% patients with CPFE, 11% other ILD subjects, p < 0.0001) in one study (10), resulting an independent predictor of mortality. In our opinion, this suggests that the role of CPFE in SSc-ILD mortality could be deeper investigated, for example, considering also the extent of disease (i.e., of both emphysema and ILD) in comparison with ILD without emphysema. Moreover, taking into consideration also other conditions that may manifest in SSc-ILD, pleuroparenchymal fibroelastosis (PPFE) can be more common in SSc than in other CTD (29) and proved to determine a worse survival among SSc patients, irrespectively to its extent (30). However, the burden of PPFE only among SSC-ILD was not evaluated and should be addressed. Lastly, since CPFE and PPFE may be associated with different fibrotic patterns (28, 29), the influence of different associated ILD patterns could also be taken into consideration.
Then, the presence of an UIP pattern did not independently predict mortality in SSc-ILD (21). However, recently, the paper of Okamoto et al. (25), which was excluded from the analysis for some overlap patients, reported that UIP was a significant predictor of mortality on univariate analysis (a multivariate analysis was not performed), thus more data are desirable to confirm or reject UIP as a mortality predictor, preferably considering also the extent of disease. Moreover, in our review we did not find a comparison between UIP and NSIP in SSc-ILD. In fact, Takei et al. (21) compared UIP with all the other possible ILD patterns. A further selection of patients, based on the comparison between UIP and NSIP could be performed, as already suggested by Winstone et al. (7), since UIP is generally considered with worse prognosis while NSIP is the most common ILD pattern in SSc (6).
Lastly, bronchiectasis severity was investigated only in one study, resulting a predictor of mortality on univariate analysis but not confirmed as an independent prognostic factor (21). Bronchiectasis proved to be an independent risk factor for mortality in one out of two studies investigating patients with CTD-ILD (24, 26). Moreover, traction bronchiectasis inside GGO resulted as a predictor of mortality on multivariate analysis in one study on SSc-ILD (but with some overlaps) (25). Thus, we believe that also this CT feature need to be further investigated.
CT features as predictors of ILD progression were only analyzed on univariate analysis, with ILD (13, 16) and reticulation extent (16) resulting as significant risk factors for functional decline. Despite this, lacking a multivariate analysis, they cannot be confirmed as independent predictors. Moreover, CT features seem to be unable to predict ILD worsening on CT (15). Nevertheless, we have to note that only 3 studies investigated ILD progression (FVC decline or ILD increment on CT). Furthermore, we have also to specify that our review included only papers with a given definition of FVC decline or radiologic worsening, though accepting whatever formulation. We decided this criterion on a clinical basis: in fact, although statistically different trends of ILD progression of functional decline have been described and put in relation with CT features (10, 31–33), this may not represent a clinically meaningful worsening in pulmonary function or CT ILD features.
Our systematic review presents limitations, such as the variable methodologic quality of the included studies. Moreover, the heterogeneity of these studies makes it difficult to sum up, compare and discuss the whole data. Lastly, most predictors were evaluated only in one study.
Conclusions
ILD extent and, in particular, extensive ILD are CT mortality predictors in SSc-ILD. Extensive ILD may be also considered a predictor of death, need for supplemental oxygen, or lung transplantation, as a combined outcome. ILD quantitative analysis parameters, fibrosis extent and reticulation extent seem also able to provide prognostic information on mortality risk, as well as honeycombing extent for respiratory mortality, but further studies are desirable to confirm these data. The role of CT features in predicting ILD progression remains to be further investigated.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
NL conceived the idea, planned the work, screened the articles, assessed the studies' quality, extrapolated and collected data and drafted the first version of the manuscript. SC and MM-C conceived the idea, planned the work and edited the final version of the manuscript. EC, CN, LC, GM, and ST checked data collection and edited the final version of the manuscript. CB conceived the idea, planned the work, resolved the disagreements on studies' selection as third assessor and edited the final version of the manuscript. MO conceived the idea, planned the work, screened the articles, assessed the studies' quality, collected data and edited the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.807982/full#supplementary-material
Supplementary Figure 1. Risk of bias and applicability concerns, based on QUADAS-2 (http://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/).
Supplementary Data 1. Papers not included (only partially meeting inclusion criteria), but relevant for the discussion, grouped by reasons of rejection.
Supplementary Data 2. Definitions of investigated predictors.
Supplementary Table 1. Search strategy.
Supplementary Table 2. Study quality assessment, based on the Critical Appraisal Skill Program questionnaire for cohort studies (http://www.casp-uk.net).
Supplementary Table 3. Predictors of mortality in ILD-SSc, multivariate analysis.
Abbreviations
CPFE, combined pulmonary fibrosis emphysema; CTD, connective tissue disease; CT, chest computed tomography; GGO, ground glass opacities; FVC, forced vital capacity; ILD, interstitial lung disease; NSIP, non-specific interstitial pneumonia; SSc, systemic sclerosis; UIP, usual interstitial pneumonia.
References
1. Hoffmann-Vold AM, Allanore Y, Bendstrup E, Bruni C, Distler O, Maher TM, et al. The need for a holistic approach for SSc-ILD – achievements and ambiguity in a devastating disease. Respir Res. (2020) 21:197. doi: 10.1186/s12931-020-01459-0
2. Volkmann ER, Fischer A. Update on morbidity and mortality in systemic sclerosis-related interstitial lung disease. J Scleroderma Relat Disord. (2021) 6:11–20. doi: 10.1177/2397198320915042
3. Orlandi M, Lepri G, Damiani A, Barsotti S, Di Battista M, Codullo V, et al. Review one year in review 2020: systemic sclerosis. Clin Exp Rheumatol. (2020) 38 Suppl 125:3–17.
4. Roofeh D, Jaafar S, Vummidi D, Khanna D. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. (2019) 31:241–9. doi: 10.1097/BOR.0000000000000592
5. Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Barake R, Barsotti S, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. (2020) 2:e71–83. doi: 10.1016/S2665-9913(19)30144-4
6. Capobianco J, Grimberg A, Thompson BM, Antunes VB, Jasinowodolinski D, Meirelles GSP. Thoracic manifestations of collagen vascular diseases. RadioGraphics. (2012) 32:33–50. doi: 10.1148/rg.321105058
7. Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease. Chest. (2014)146:422–36. doi: 10.1378/chest.13-2626
8. Ando K, Motojima S, Doi T, Nagaoka T, Kaneko N, Aoshima M et al. Effect of glucocorticoid monotherapy on pulmonary function and survival in Japanese patients with scleroderma-related interstitial lung disease. Respir Investig. (2013) 51:69–75. doi: 10.1016/j.resinv.2012.12.002
9. Ariani A, Silva M, Bravi E, Parisi S, Saracco M, De Gennaro F, et al. Overall mortality in combined pulmonary fibrosis and emphysema related to systemic sclerosis. RMD Open. (2019) 5:e000820. doi: 10.1136/rmdopen-2018-000820
10. Champtiaux N, Cottin V, Chassagnon G, Chaigne B, Valeyre D, Nunes H, et al. Combined pulmonary fibrosis and emphysema in systemic sclerosis: a syndrome associated with heavy morbidity and mortality. Semin Arthritis Rheum. (2019) 49:98–104. doi: 10.1016/j.semarthrit.2018.10.011
11. De Santis M, Bosello SL, Peluso G, Pinnelli M, Alivernini S, Zizzo G, et al. Bronchoalveolar lavage fluid and progression of scleroderma interstitial lung disease: scleroderma interstitial lung disease. Clin Respir J. (2012) 6:9–17. doi: 10.1111/j.1752-699X.2010.00228.x
12. Forestier A, Le Gouellec N, Béhal H, Kramer G, Perez T, Sobanski V, et al. Evolution of high-resolution CT-scan in systemic sclerosis-associated interstitial lung disease: description and prognosis factors. Semin Arthritis Rheum. (2020) 50:1406–13. doi: 10.1016/j.semarthrit.2020.02.015
13. Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. (2008) 177:1248–54. doi: 10.1164/rccm.200706-877OC
14. Goh NS, Hoyles RK, Denton CP, Hansell DM, Renzoni EA, Maher TM, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. (2017) 69:1670–8. doi: 10.1002/art.40130
15. Kim EA, Johkoh T, Lee KS, Ichikado K, Koh EM, Kim TS, et al. Interstitial pneumonia in progressive systemic sclerosis: serial high-resolution CT findings with functional correlation. J Comput Assist Tomogr. (2001) 25:757–63. doi: 10.1097/00004728-200109000-00015
16. Le Gouellec N, Duhamel A, Perez T, Hachulla AL, Sobanski V, Faivre JB, et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS ONE. (2017) 12:e0181692. doi: 10.1371/journal.pone.0181692
17. Moore OA, Goh N, Corte T, Rouse H, Hennessy O, Thakkar V, et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology. (2013) 52:155–60. doi: 10.1093/rheumatology/kes289
18. Moore OA, Proudman SM, Goh N, Corte TJ, Rouse H, Hennessy O, et al. Quantifying change in pulmonary function as a prognostic marker in systemic sclerosis-related interstitial lung disease. Clin Exp Rheumatol. (2015) 33:S111–6.
19. Saldana DC, Hague CJ, Murphy D, Coxson HO, Tschirren J, Peterson S, et al. Association of computed tomography densitometry with disease severity, functional decline, and survival in systemic sclerosis-associated interstitial lung disease. Ann Am Thorac Soc. (2020) 17:813–20. doi: 10.1513/AnnalsATS.201910-741OC
20. Sánchez-Cano D, Ortego-Centeno N, Callejas JL, Fonollosa Plá V, Ríos-Fernández R, Tolosa-Vilella C, et al. Interstitial lung disease in systemic sclerosis: data from the spanish scleroderma study group. Rheumatol Int. (2018) 38:363–74. doi: 10.1007/s00296-017-3916-x
21. Takei R, Arita M, Kumagai S, Ito Y, Tokioka F, Koyama T, et al. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease: Radiographic fibrosis in SSc-ILD. Respirology. (2018) 23:385–91. doi: 10.1111/resp.13175
22. Vanaken L, Landini N, Lenaerts J, Claeys E, Lenaerts J, Wuyts WA, et al. Progressive lung fibrosis and mortality can occur in early systemic sclerosis patients without pulmonary abnormalities at baseline assessment. Clin Rheumatol. (2020) 39:3393–400. doi: 10.1007/s10067-020-05105-4
23. Masi AT. Preliminary criteria for the classification of systemic sclerosis (scleroderma). subcommittee for Scleroderma criteria of the American rheumatism association diagnostic and therapeutic criteria committee. Arthritis Rheum. (1980) 23:581–90. doi: 10.1002/art.1780230510
24. Jacob J, Bartholmai BJ, Rajagopalan S, Brun AL, Egashira R, Karwoski R, et al. Evaluation of computer-based computer tomography stratification against outcome models in connective tissue disease-related interstitial lung disease: a patient outcome study. BMC Med. (2016) 14:190. doi: 10.1186/s12916-016-0739-7
25. Okamoto M, Fujimoto K, Sadohara J, Furuya K, Kaieda S, Miyamura T, et al. A retrospective cohort study of outcome in systemic sclerosis-associated interstitial lung disease. Respir Investig. (2016) 54:445–53. doi: 10.1016/j.resinv.2016.05.004
26. Walsh SLF, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. (2014) 69:216–22. doi: 10.1136/thoraxjnl-2013-203843
27. Jacob J, Song JW, Yoon H-Y, Cross G, Barnett J, Woo WL, et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. EBioMedicine. (2018) 28:303–10. doi: 10.1016/j.ebiom.2018.01.038
28. Cottin V. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. (2005) 26:586–93. doi: 10.1183/09031936.05.00021005
29. Orlandi M, Landini N, Bruni C, Sambataro G, Nardi C, Bargagli E, et al. Pleuroparenchymal fibroelastosis in rheumatic autoimmune diseases: a systematic literature review. Rheumatology. (2020) 59:3645–56. doi: 10.1093/rheumatology/keaa451
30. Bonifazi M, Sverzellati N, Negri E, Jacob J, Egashira R, Moser J, et al. Pleuroparenchymal fibroelastosis in systemic sclerosis: prevalence and prognostic impact. Eur Respir J. (2020) 56:1902135. doi: 10.1183/13993003.02135-2019
31. Khanna D, Nagaraja V, Tseng C, Abtin F, Suh R, Kim G, et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis–associated interstitial lung disease trials. Arthritis Res Ther. (2015) 17:372. doi: 10.1186/s13075-015-0872-2
32. Khanna D, Tseng C-H, Farmani N, Steen V, Furst DE, Clements PJ, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the scleroderma lung study placebo group: natural history of lung physiology in SSc-ILD. Arthritis Rheum. (2011) 63:3078–85. doi: 10.1002/art.30467
Keywords: systemic sclerosis (scleroderma), interstitial lung disease (ILD), computed tomography, prognostic factors, mortality, disease progression
Citation: Landini N, Orlandi M, Bruni C, Carlesi E, Nardi C, Calistri L, Morana G, Tomassetti S, Colagrande S and Matucci-Cerinic M (2022) Computed Tomography Predictors of Mortality or Disease Progression in Systemic Sclerosis–Interstitial Lung Disease: A Systematic Review. Front. Med. 8:807982. doi: 10.3389/fmed.2021.807982
Received: 02 November 2021; Accepted: 06 December 2021;
Published: 27 January 2022.
Edited by:
Silvia Piantoni, ASST-Spedali Civili and University of Brescia, ItalyReviewed by:
Peter Korsten, University Medical Center Göttingen, GermanyVeronica Codullo, Hôpital Cochin, France
Copyright © 2022 Landini, Orlandi, Bruni, Carlesi, Nardi, Calistri, Morana, Tomassetti, Colagrande and Matucci-Cerinic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas Landini, bmlrb2xhbmRpbmlAaG90bWFpbC5pdA==
 Nicholas Landini
Nicholas Landini Martina Orlandi
Martina Orlandi Cosimo Bruni
Cosimo Bruni Edoardo Carlesi
Edoardo Carlesi Cosimo Nardi2
Cosimo Nardi2 Giovanni Morana
Giovanni Morana Sara Tomassetti
Sara Tomassetti Marco Matucci-Cerinic
Marco Matucci-Cerinic