
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med. , 24 December 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.806849
This article is part of the Research Topic Biofilm-Mediated Nosocomial Infections and its Association with Antimicrobial Resistance: Detection, Prevention, and Management View all 5 articles
 Aye Mya Sithu Shein1,2,3†
Aye Mya Sithu Shein1,2,3† Parichart Hongsing4,5†
Parichart Hongsing4,5† Shuichi Abe6
Shuichi Abe6 Sirirat Luk-in7
Sirirat Luk-in7 Naveen Kumar Devanga Ragupathi8,9,10
Naveen Kumar Devanga Ragupathi8,9,10 Dhammika Leshan Wannigama1,2,8,11*
Dhammika Leshan Wannigama1,2,8,11* Tanittha Chatsuwan1,2*
Tanittha Chatsuwan1,2*Urinary tract infection (UTI) is a considerable public health issue that threatens 150 million individuals globally each year, exhibiting a significant impact on the economy and quality of life in affected individuals (1, 2). UTI is especially prevalent among females, both in terms of occurrence and recurrence (2). Bacterial colonization and subsequent invasion in various parts of the urinary tract, combined with biofilm formation, induce uncomplicated mild UTI, chronic recurrent UTI, and complicated severe UTI which can lead to septicemia and renal failure, resulting in mortality rates of 20–40% among patients with underlying immunocompromised conditions, long-term urinary catheterization, and chronic kidney diseases (2–6).
Klebsiella pneumoniae is one of the most common causal microorganisms that causes UTI in clinical settings (7). Their abilities to construct biofilms in medical devices, including urinary catheters, as their critical step in their disease pathogenesis can result in biofilm-mediated antibiotic tolerance in these pathogens (7, 8). Moreover, worldwide emerging trends of multidrug-resistant (MDR) and pandrug-resistant (PDR) strains in K. pneumoniae make it challenging for clinicians to provide prompt and efficient therapy, imposing a considerable negative burden on patient's morbidity and mortality (9, 10). The growing incidences of antimicrobial resistance and biofilm-mediated antimicrobial tolerance in K. pneumoniae with limited treatment alternatives, combined with the failure to discover new antibiotics have triggered the reappraisal of colistin as a valid therapeutic option (11).
Colistin is a bactericidal cationic antibiotic that triggers increased membrane permeabilities and cell death via electrostatic interactions with lipid A of lipopolysaccharide (LPS) (11, 12). However, colistin-resistant K. pneumoniae has been continuously raised from <2 to 9% worldwide as a result of the current surge in colistin treatment administration (13, 14). Infections with colistin-resistant K. pneumoniae were also revealed to be independently associated with the excess of mortality in healthcare settings (15).
The intrinsic chromosome-mediated and acquired plasmid-mediated alteration of lipid A phosphate moieties in LPS with amino-4-deoxy-L-arabinose (L-Ara4N) and phosphoethanolamine (pEtN) weakens the electrostatic affinity of colistin to LPS, causing colistin resistance in K. pneumoniae (Figure 1) (10). The therapeutic efficacy of colistin become compromised as a consequence of increased genetic mutation and the dissemination of plasmid-mediated mcr genes, emphasizing the importance of exploring innovative alternative treatment strategies to address untreatable colistin-resistant K. pneumoniae related with UTI (Table 1). There for in this opinion we discuss potential effective treatment options for patients who suffer from chronic colistin-resistant Klebsiella pneumoniae urinary tract infection.
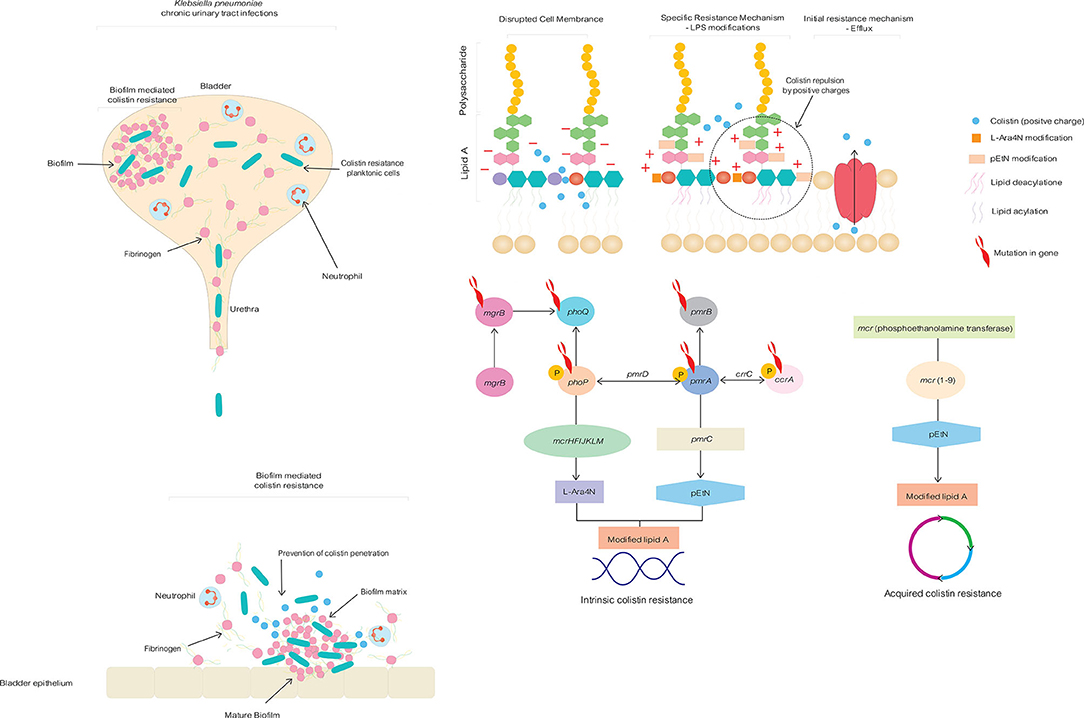
Figure 1. Intrinsic chromosome-mediated and acquired plasmid-mediated colistin resistance mechanisms in Klebsiella pneumoniae togeetrh with biofilm formation in chronic, life-changing urinary tract infection.
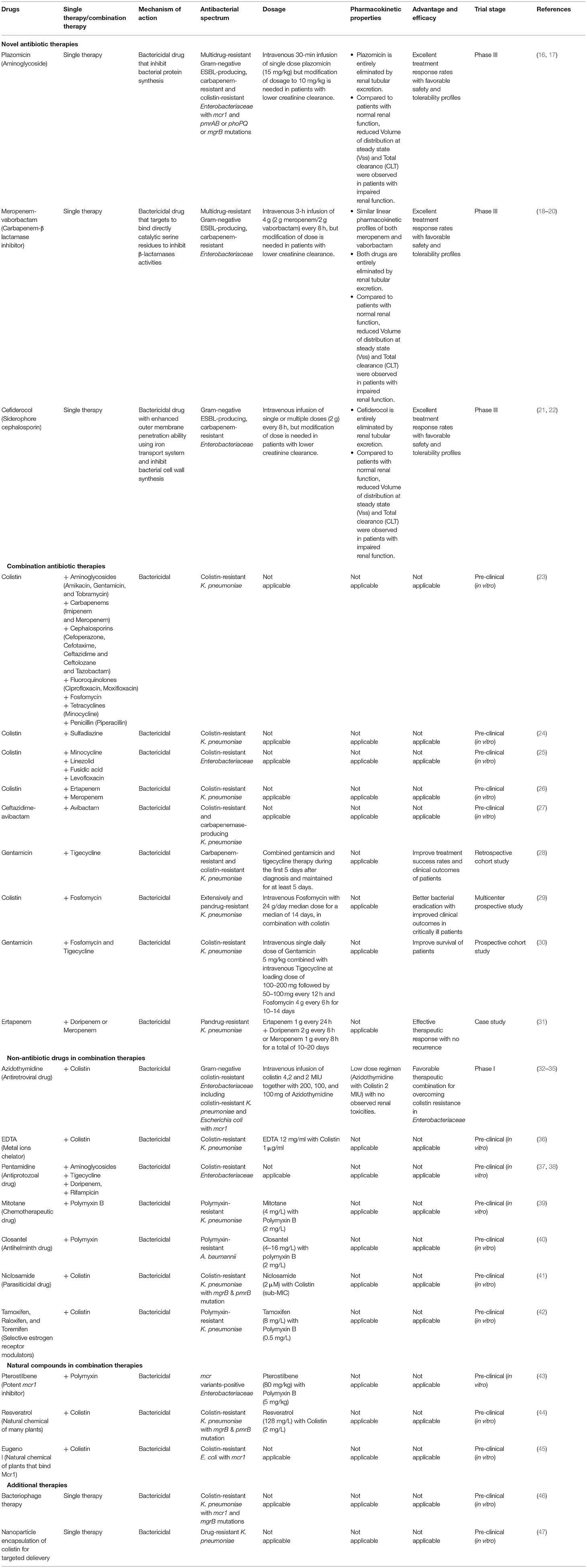
Table 1. Different therapeutic strategies to confront colistin resistance in chronic, life-changing colistin-resistant Klebsiella pneumoniae urinary tract infection.
Plazomicin, FDA approved new semisynthetic aminoglycoside for UTI, was reported to be efficacious against colistin-resistant Enterobacteriaceae with both plasmid-mediated mcr1 and chromosomal mutation of pmrAB or phoPQ or mgrB, although 10% of tested K. pneumoniae isolates demonstrated resistance to this novel medication (16).
Meropenem-vaborbactam, an innovative carbapenem-β lactamase inhibitor and FDA-approved medication for severe UTIs, has been reported to be effective against drug-resistant Enterobacteriaceae from all over the world (18, 19). However, the efficacy of this new antibiotic was reported to be decreased among KPC-producing K. pneumoniae strains lacking outer membrane porins and overexpressing AcrAB efflux pump (48).
Cefiderocol is another promising novel siderophore cephalosporin against drug-resistant K. pneumoniae due to its enhanced outer membrane penetration ability using iron transport system and intrinsic effective antimicrobial activities (21). Nevertheless, resistance issues were recorded following 21 days of initiating cefiderocol therapy (49).
Although these newly discovered medications proved useful as rescue therapy at different stages of clinical trial, one of the most efficacious approaches for limiting the evolution of resistance to newer medications and tackling colistin-resistant K. pneumoniae is utilizing the combination therapy which combines two or more antimicrobial throughout a treatment course (50).
Colistin in combination with different classes of antibiotics displayed substantial synergistic effects in suppressing the growth of colistin-resistant K. pneumoniae isolates in vitro (23). Sulfadiazine in combination with colistin had a remarkable in vitro synergy against colistin-resistant K. pneumoniae, independent of underlying colistin resistance mechanisms (24). According to previous study, colistin permeabilization on Gram-negative outer membrane allowed minocycline, linezolid, fusidic acid, and levofloxacin to generate significant in vitro synergistic interactions in the treatment of colistin-resistant Enterobacteriaceae (25). Recent study reported that combination therapy comprising double carbapenems and colistin displayed significant synergistic bactericidal effects against colistin-resistant K. pneumoniae in vitro (26). Ceftazidime-avibactam, recently approved for treating UTI can also be explored as a potential combination treatment with avibactam to overcome colistin-resistance in K. pneumoniae in vitro (27). Synergistic combinations that have been discovered to be effective in vitro are needed to investigate further for in vivo efficacies, pharmacokinetic/ pharmacodynamic characteristics, and subsequent clinical trials to evaluate their therapeutic usefulness.
According to retrospective cohort evaluation, combined gentamicin and tigecycline was significantly correlated with higher treatment success rates and survival in patients with colistin-resistant K. pneumoniae (28). A multicenter prospective study revealed that addressing extensively and pandrug-resistant K. pneumoniae with colistin-fosfomycin combination was significantly related with favorable clinical outcomes in critically ill patients (29). In septic shock patients with colistin-resistant K. pneumoniae, recent prospective cohort study found that targeted combination therapy with gentamicin. fosfomycin and tigecycline was significantly related with lower mortalities (30). According to a prior case study, administration of double carbapenem treatment was proven to be beneficial in eliciting an effective therapeutic response with no recurrence in patients with pandrug-resistant K. pneumoniae bacteremia and UTI (31).
However, previous single-center retrospective study revealed that using different combination therapies for colistin- and carbapenem-resistant K. pneumoniae did not contribute to a substantial improvement in patients survival (51). Moreover, combination therapy involving multiple antibiotics has been attributed to the risks of drug resistance, toxicities, bacterial superinfections, higher costs, and probable antagonism (52). Reutilizing the currently prescribed non-antibiotic drugs in combination therapy is another plausible repurposing approach for managing colistin resistance in K. pneumoniae uropathogens (53).
FDA-approved Metal ions chelator - EDTA was demonstrated to perform as an efficacious adjuvant for colistin to reverse colistin resistance both in in vitro and in vivo catheter-related biofilm infections of colistin-resistant K. pneumoniae (36).
Azidothymidine is an approved antiretroviral drug, and it also exhibits antibacterial activities against Enterobacteriaceae by acting on bacterial DNA synthesis (32). Previous studies demonstrated significant synergistic activities of azidothymidine for retaining therapeutic efficacies of colistin in colistin-resistant K. pneumoniae that express mcr-1, both in vitro and in vivo (33, 34).
Antiprotozoal pentamidine has been demonstrated to have antibacterial activity against carbapenem and colistin-resistant Enterobacteriaceae isolates both individually and in combination with other antibiotics including aminoglycosides, tigecycline, doripenem, and rifampicin (37). Their effective perturbant actions on bacterial outer membrane help the combined antibiotics to produce significant synergistic activities for increasing bacterial clearance in internal organs and enhancing survival in a systemic mouse infection model of colistin resistant Acinetobacter baumannii with pEtn-mediated LPS modification similar to that conferred by mcr-1 (38).
Mitotane is currently used chemotherapeutic agent for adrenocortical carcinoma. Whereas, mitotane monotherapy had limited antimicrobial activities, polymyxin B combined with mitotane therapy displayed substantial synergistic antibacterial effects for bacterial killing and impeding regrowth of polymyxin-resistant K. pneumoniae pathogens both in vitro and in vivo murine burn models due to permeabilization effects of polymyxin, which allows migration of mitotane into bacterial cells for its antibacterial effects (39).
Veterinary antihelminth closantel was observed to be ineffective when it was given as monotherapy in treating A. baumanii (40). However, with uncoupling oxidative phosphorylation activities of closantel, polymyxin was proved to recover its antimicrobial properties against polymyxin-resistant A. baumannii isolates and this combination drastically prevented the establishment of resistance in polymyxin-susceptible isolates (40).
Niclosamide, parasiticidal drugs for tapeworm infection, has previously been shown to increase negative surface charges of colistin-resistant K. pneumoniae with mutated mgrB and pmrB genes, allowing colistin to reactive against these clinical strains (41).
Additionally, colistin was reported to regain effectiveness against polymyxin-resistant K. pneumoniae when given in combination with membrane-active selective estrogen receptor modulators (SERM) such as tamoxifen, raloxifen, and toremifene by their combined synergistic effects in disrupting the outer membrane (42).
Antidepressants (amitriptyline, citalapram, and sertraline), antipsychotics (chlorpromazine and levopromazine), and statins (simvastatin) were discovered to possess synergistic activities with polymyxin in treatment of K. pneumoniae. Among these, citalopram, sertraline, and spironolactone were demonstrated to exert consistent synergistic effects in augmenting polymyxin activities (54).
Natural compounds used for other treatment purposes have also demonstrated promising outcomes as a prospective combination therapy for overcoming colistin resistance. Potent mcr1 inhibitory activities of natural compound - pterostilbene in combination with polymyxin as inhibitor-antibiotic combination significantly improved antimicrobial activities of polymyxin against different mcr variants-positive Enterobacteriaceae, resulting in lower bacterial-induced pathological damage in internal organs and better lifespan in treated mice (43).
Resveratrol, a natural chemical found in many plants, was discovered to augment colistin efficacy against a diverse panel of colistin-resistant K. pneumoniae strains with various resistance mechanisms involving the mgrB and pmrB mutations (44).
Previous research has also demonstrated combining eugenol and colistin showed a remarkable synergistic impact in lowering the colistin dose required to elicit antibacterial effects on colistin-resistant E. coli due to activities of eugenol in binding Mcr1 and suppressing mcr1 expression (45, 55).
Bacteriophage therapy is an additional therapeutic modality with favorable clinical outcomes that exploits the bacteriolytic activities of phage to target a variety of drug-resistant pathogens in infected individuals (10). In biofilm-producing colistin-resistant K. pneumoniae with mcr1 and mgrB mutations, higher sensitivities to phage were also reported both in vitro and in vivo, owing to better adherence of negative-charge phage to altered LPS of this pathogen (46).
Nanocarrier strategies are revolutionary therapies for combating drug-resistant pathogens by increasing penetration and concentration of drug at the infection site through targeted delivery approaches (56). Nanoparticle encapsulation of colistin has also shown to have stronger colistin counteracting activities against planktonic and biofilms of drug-resistant K. pneumoniae as compared to providing free colistin (47).
Another feasible alternative is to modify urinary catheters with antifouling coatings including hydrogels, polytetrafluoroethylene, polyzwitterions, and polyethylene glycol which limit bacterial colonization and subsequent biofilm formation in catheter-associated UTI by their repulsive properties (57). Moreover, silver nanoparticles in hydrogel composite were efficient antimicrobial coating in providing significant inhibitory effects against K. pneumoniae uropathogens (58). Various research on biocidal catheters, such as gentamicin-coated, nitric oxide-coated, nitrofurazone-impregnated, antimicrobial peptide-coated, and phage-impregnated catheters, have shown promising results in minimizing the growth and biofilm development of UTI-causing K. pneumoniae pathogens (59).
Increasing resistance to colistin in K. pneumoniae uropathogens due to chromosomal mutations and plasmid-mediated mcr genes result in chronic severe and recurrent UTI in clinical settings. Furthermore, they could serve as a source for pathogen dissemination in the healthcare environment, emphasizing the significance of discovering alternative therapeutic strategies to effectively combat colistin-resistant K. pneumoniae uropathogens. Although novel antibiotics including plazomicin, meropenem/vaborbactam, and cefiderocol have been demonstrated to be successful as rescue therapy for colistin-resistant K. pneumoniae, reports of emerging resistance to these newer antibiotics render them concerning for use as monotherapy. Instead of utilizing antibiotic monotherapy, combining two or more antibiotics or repurposing non-antibiotic medications or natural compounds in combination therapy is another promising approach for tackling colistin resistance in K. pneumoniae. Bacteriophage therapy, nanocarrier strategies and modification of urinary catheters are also designated to be used as future innovative treatment modalities for successful control of colistin-resistant K. pneumoniae uropathogens. While several in vitro and in vivo studies have revealed the potent therapeutic effects of various alternative strategies for addressing colistin-resistant K. pneumoniae, further clinical trial studies are required to investigate their therapeutic efficacies and safety in human patients.
AS: conception and writing the original draft of the manuscript. PH: conception, supervision, and writing the original draft of the manuscript. SA, SL-i, and NR: supervision, critical review, and editing of the manuscript. DW and TC: conception, supervision, critical review, and editing of the manuscript. All authors contributed to the article and approved the submitted version.
AS was supported under the Chulalongkorn University Graduate Scholarship Program for ASEAN Countries. DW was supported by Chulalongkorn University (Second Century Fund- C2F Fellowship), and the University of Western Australia (Overseas Research Experience Fellowship). The sponsor(s) had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. (2001) 183:S1–4. doi: 10.1086/318850
2. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, economic costs. Am J Med. (2002) 113:5–13. doi: 10.1016/S0002-9343(02)01054-9
3. Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, et al. Diagnosis and management for urosepsis. Int J Urol. (2013) 20:963–70. doi: 10.1111/iju.12200
4. Wannigama DL, Hurst C, Pearson L, Saethang T, Singkham-In U, Luk-In S, et al. Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms. Sci Rep. (2019) 9:6300. doi: 10.1038/s41598-019-42353-0
5. Phuengmaung P, Somparn P, Panpetch W, Singkham-In U, Wannigama DL, Chatsuwan T, et al. Coexistence of Pseudomonas aeruginosa with Candida albicans enhances biofilm thickness through alginate-related extracellular matrix but is attenuated by N-acetyl-l-cysteine. Front Cell Infect Microbiol. (2020) 10:594336. doi: 10.3389/fcimb.2020.594336
6. Wannigama DL, Hurst C, Hongsing P, Pearson L, Saethang T, Chantaravisoot N, et al. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann Clin Microbiol Antimicrob. (2020) 19:8. doi: 10.1186/s12941-020-00350-6
7. Amalaradjou MAR, Venkitanarayanan K. Role of bacterial biofilms in catheter-associated urinary tract infections (CAUTI) and strategies for their control. Recent Adv Field Urin Tract Infect. (2013) 10:1–32. doi: 10.5772/55200
8. Salzo A, Ripabelli G, Sammarco ML, Mariano A, Niro C, Tamburro M. Healthcare-associated infections and antibiotics consumption: a comparison of point prevalence studies and intervention strategies. Hosp Top. (2021) 99:140–50. doi: 10.1080/00185868.2021.1902758
9. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. (2017) 41:252–75. doi: 10.1093/femsre/fux013
10. Di Tella D, Tamburro M, Guerrizio G, Fanelli I, Sammarco ML, Ripabelli G. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect Drug Resist. (2019) 12:3783–95. doi: 10.2147/IDR S226416
11. Biswas S, Brunel J-M, Dubus J-C, Reynaud-Gaubert M, Rolain J-M. Colistin: an update on the antibiotic of the 21st century. Exp Rev Anti Infect Ther. (2012) 10:917–34. doi: 10.1586/eri.12.78
12. Luk-In S, Chatsuwan T, Kueakulpattana N, Rirerm U, Wannigama DL, Plongla R, et al. Occurrence of mcr-mediated colistin resistance in Salmonella clinical isolates in Thailand. Sci Rep. (2021) 11:14170. doi: 10.1038/s41598-021-93529-6
13. Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother. (2011) 66:2070–4. doi: 10.1093/jac/dkr239
14. Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. (2011) 55:593–9. doi: 10.1128/AAC.01020-10
15. Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. (2013) 19:E23–30. doi: 10.1111/1469-0691.12070
16. Denervaud-Tendon V, Poirel L, Connolly LE, Krause KM, Nordmann P. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. J Antimicrob Chemother. (2017) 72:2787–91. doi: 10.1093/jac/dkx239
17. Clark JA, Burgess DS. Plazomicin: a new aminoglycoside in the fight against antimicrobial resistance. Ther Adv Infect Dis. (2020) 7:2049936120952604. doi: 10.1177/2049936120952604
18. Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother. (2017) 61:e00567–17. doi: 10.1128/AAC.00567-17
19. Andrei S, Valeanu L, Chirvasuta R, Stefan M-G. New FDA approved antibacterial drugs: 2015-2017. Discoveries. (2018) 6. doi: 10.15190/d.2018.1
20. Burgos RM, Biagi MJ, Rodvold KA, Danziger LH. Pharmacokinetic evaluation of meropenem and vaborbactam for the treatment of urinary tract infection. Expert Opin Drug Metab Toxicol. (2018). 14:1007–21.
21. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis. (2019) 69:S538–43. doi: 10.1093/cid/ciz826
22. Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for Gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol. (2017) 57:584–91. doi: 10.1002/jcph.841
23. Ontong JC, Ozioma NF, Voravuthikunchai SP, Chusri S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE. (2021) 16:e0244673. doi: 10.1371/journal.pone.0244673
24. Okdah L, Le Page S, Olaitan AO, Dubourg G, Hadjadj L, Rolain JM. New therapy from old drugs: synergistic bactericidal activity of sulfadiazine with colistin against colistin-resistant bacteria, including plasmid-mediated colistin-resistant mcr-1 isolates. Int J Antimicrob Agents. (2018) 51:775–83. doi: 10.1016/j.ijantimicag.2018.01.027
25. Brennan-Krohn T, Pironti A, Kirby JE. Synergistic activity of colistin-containing combinations against colistin-resistant Enterobacteriaceae. Antimicrob Agents Chemother. (2018) 62:e00873–18. doi: 10.1128/AAC.00873-18
26. Erdem F, Abulaila A, Aktas Z, Oncul O. In vitro evaluation of double carbapenem and colistin combinations against OXA-48, NDM carbapenemase-producing colistin-resistant Klebsiella pneumoniae strains. Antimicrob Resist Infect Control. (2020) 9:70. doi: 10.1186/s13756-020-00727-4
27. Jayol A, Nordmann P, Poirel L, Dubois V. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. (2018) 73:542–4. doi: 10.1093/jac/dkx393
28. Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, et al. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. (2015) 70:905–13. doi: 10.1093/jac/dku432
29. Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, et al. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents. (2014) 43:52–9. doi: 10.1016/j.ijantimicag.2013.09.010
30. Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger I, Rivera Espinar F, Cano Á, Guzmán-Puche J, et al. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother. (2017) 61:e00406–17. doi: 10.1128/AAC.00406-17
31. Giamarellou H, Galani L, Baziaka F, Karaiskos I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant. Klebsiella pneumoniae. Antimicrob Agents Chemother. (2013) 57:2388–90. doi: 10.1128/AAC.02399-12
32. Hu Y, Liu Y, Coates A. Azidothymidine produces synergistic activity in combination with colistin against antibiotic-resistant enterobacteriaceae. Antimicrob Agents Chemother. (2019) 63. doi: 10.1128/AAC.01630-18
33. Loose M, Naber KG, Hu Y, Coates A, Wagenlehner FME. Serum bactericidal activity of colistin and azidothymidine combinations against mcr-1-positive colistin-resistant Escherichia coli. Int J Antimicrob Agents. (2018) 52:783–9. doi: 10.1016/j.ijantimicag.2018.08.010
34. Chang YT, Yang TY, Lu PL, Lin SY, Wang LC, Wang SF, et al. Combination of colistin and azidothymidine demonstrates synergistic activity against colistin-resistant, carbapenem-resistant Klebsiella pneumoniae. Microorganisms. (2020) 8. doi: 10.3390/microorganisms8121964
35. Naber K, Walker H, Mair S, McKenzie L, Upton C, Whitaker G. Safety and Pharmacokinetics of IV Azidothymidine alone and combined with Colistin Being Developed for Treatment of Carbapanem-and Colistin-Resistant Enterbacteriaceae (Phase 1-Study). Poster# 3265 In: European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Madrid, Spain), (2018), 21–4.
36. Shein AMS, Wannigama DL, Higgins PG, Hurst C, Abe S, Hongsing P, et al. Novel colistin-EDTA combination for successful eradication of colistin-resistant Klebsiella pneumoniae catheter-related biofilm infections. Sci Rep. (2021) 11:1–13. doi: 10.1038/s41598-021-01052-5
37. Cebrero-Cangueiro T, Álvarez-Marín R, Labrador-Herrera G, Smani Y, Cordero-Matía E, Pachón J, et al. In vitro activity of pentamidine alone and in combination with aminoglycosides, tigecycline, rifampicin, and doripenem against clinical strains of carbapenemase-producing and/or colistin-resistant enterobacteriaceae. Front Cell Infect Microbiol. (2018) 8:363. doi: 10.3389/fcimb.2018.00363
38. Stokes JM, Macnair CR, Ilyas B, French S, Côt é JP, Bouwman C, et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol. (2017) 2:17028. doi: 10.1038/nmicrobiol.2017.28
39. Tran TB, Wang J, Doi Y, Velkov T, Bergen PJ, Li J. Novel polymyxin combination with antineoplastic mitotane improved the bacterial killing against polymyxin-resistant multidrug-resistant gram-negative pathogens. Front Microbiol. (2018) 9:721. doi: 10.3389/fmicb.2018.00721
40. Tran TB, Cheah SE, Yu HH, Bergen PJ, Nation RL, Creek DJ, et al. Anthelmintic closantel enhances bacterial killing of polymyxin B against multidrug-resistant Acinetobacter baumannii. J Antibiot. (2016) 69:415–21. doi: 10.1038/ja.2015.127
41. Ayerbe-Algaba R, Gil-Marqués ML, Jiménez-Mejías ME, Sánchez-Encinales V, Parra-Millán R, Pachón-Ibáñez ME, et al. Synergistic activity of niclosamide in combination with colistin against colistin-susceptible and colistin-resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front Cell Infect Microbiol. (2018) 8:348. doi: 10.3389/fcimb.2018.00348
42. Hussein MH, Schneider EK, Elliott AG, Han M, Reyes-Ortega F, Morris F, et al. From breast cancer to antimicrobial: combating extremely resistant gram-negative “superbugs” using novel combinations of polymyxin B with selective estrogen receptor modulators. Microb Drug Resist. (2017) 23:640–50. doi: 10.1089/mdr.2016.0196
43. Zhou Y, Liu S, Wang T, Li H, Tang S, Wang J, et al. Pterostilbene, a potential MCR-1 inhibitor that enhances the efficacy of polymyxin B. Antimicrob Agents Chemother. (2018) 62. doi: 10.1128/AAC.02146-17
44. Cannatelli A, Principato S, Colavecchio OL, Pallecchi L, Rossolini GM. Synergistic activity of colistin in combination with resveratrol against colistin-resistant gram-negative pathogens. Front Microbiol. (2018) 9:1808. doi: 10.3389/fmicb.2018.01808
45. Wang YM, Kong LC, Liu J, Ma HX. Synergistic effect of eugenol with colistin against clinical isolated colistin-resistant Escherichia coli strains. Antimicrob Resist Infect Control. (2018) 7:17. doi: 10.1186/s13756-018-0303-7
46. Hao G, Chen AI, Liu M, Zhou H, Egan M, Yang X, et al. Colistin-resistance-mediated bacterial surface modification sensitizes phage infection. Antimicrob Agents Chemother. (2019) 63. doi: 10.1128/AAC.01609-19
47. Scutera S, Argenziano M, Sparti R, Bessone F, Bianco G, Bastiancich C, et al. Enhanced antimicrobial and antibiofilm effect of new colistin-loaded human albumin nanoparticles. Antibiotics. (2021) 10. doi: 10.3390/antibiotics10010057
48. Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. (2017) 61:e01443–17. doi: 10.1128/AAC.01443-17
49. Klein S, Boutin S, Kocer K, Fiedler MO, Störzinger D, Weigand MA, et al. Rapid development of cefiderocol resistance in carbapenem-resistant enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin Infect Dis. (2021). doi: 10.1093/cid/ciab511
50. Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. (2013) 31:177–84. doi: 10.1016/j.tibtech.2012.12.006
51. Kaur A, Gandra S, Gupta P, Mehta Y, Laxminarayan R, Sengupta S. Clinical outcome of dual colistin-and carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a single-center retrospective study of 75 cases in India. Am J Infect Control. (2017) 45:1289–91. doi: 10.1016/j.ajic.2017.06.028
52. Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. (2012) 25:450–70. doi: 10.1128/CMR.05041-11
53. Farha MA, Brown ED. Drug repurposing for antimicrobial discovery. Nat Microbiol. (2019) 4:565–77. doi: 10.1038/s41564-019-0357-1
54. Otto RG, Van Gorp E, Kloezen W, Meletiadis J, Van Den Berg S, Mouton JW. An alternative strategy for combination therapy: interactions between polymyxin B and non-antibiotics. Int J Antimicrob Agents. (2019) 53:34–9. doi: 10.1016/j.ijantimicag.2018.09.003
55. Singkham-In U, Higgins PG, Wannigama DL, Hongsing P, Chatsuwan T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS ONE. (2020) 15:e0243082. doi: 10.1371/journal.pone.0243082
56. Yeh Y-C, Huang T-H, Yang S-C, Chen C-C, Fang J-Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front Chem. (2020) 8:286. doi: 10.3389/fchem.2020.00286
57. Andersen MJ, Flores-Mireles AL. Urinary catheter coating modifications: the race against catheter-associated infections. Coatings. (2020) 10:23. doi: 10.3390/coatings10010023
58. Alshehri SM, Aldalbahi A, Al-Hajji AB, Chaudhary AA, In Het Panhuis M, Alhokbany N, et al. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr Polym. (2016) 138:229–36. doi: 10.1016/j.carbpol.2015.11.004
Keywords: Klebsiella pneumoniae, urinary tract infection, colistin-resistant, colistin-resistant Klebsiella pneumoniae, chronic infection, biofilm infections, chronic urinary infection
Citation: Shein AMS, Hongsing P, Abe S, Luk-in S, Ragupathi NKD, Wannigama DL and Chatsuwan T (2021) Will There Ever Be Cure for Chronic, Life-Changing Colistin-Resistant Klebsiella pneumoniae in Urinary Tract Infection? Front. Med. 8:806849. doi: 10.3389/fmed.2021.806849
Received: 01 November 2021; Accepted: 29 November 2021;
Published: 24 December 2021.
Edited by:
Aleksandra Barac, University of Belgrade, SerbiaReviewed by:
Bela Kocsis, Semmelweis University, HungaryCopyright © 2021 Shein, Hongsing, Abe, Luk-in, Ragupathi, Wannigama and Chatsuwan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dhammika Leshan Wannigama, ZGhhbW1pa2EubEBjaHVsYS5hYy50aA==; Tanittha Chatsuwan, dGFuaXR0aGEuY0BjaHVsYS5hYy50aA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.