- 1Sackler Faculty of Medicine, Department of Epidemiology and Preventive Medicine, School of Public Health, Tel Aviv University Ramat Aviv, Tel Aviv, Israel
- 2Central Virology Laboratory, Ministry of Health, Tel Hashomer, Israel
- 3Department of Pediatrics, Hillel Yaffe Medical Center, Hadera, Israel
- 4Saint Vincent de Paul-French Hospital, Nazareth, Israel
- 5Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, MD, United States
Objectives: To examine the association between Helicobacter pylori seroprevalence and serum pepsinogens (PGs) as markers of gastric inflammation), with high neutralizing antibody titers to poliovirus type 1 and 3 vaccine strains among children age 3–4 years, subsequent to sub-clinical infection acquired during a wild-type poliovirus type 1 outbreak in Israel.
Methods: A serosurvey was conducted among 336 children aged 5–17 years who were vaccinated with both inactivated polio vaccine and oral polio vaccines. H. pylori serum IgG antibodies and PG concentrations were measured using ELISA. Neutralizing antibodies to poliovirus vaccine strains were measured and children with a titer ≥1:8 were considered immune. High-level immunity was defined as having a serum NA titer >1:2048. Propensity score inverse weighting was used to account for confounders.
Results: Neutralizing antibodies titers ≥1:8 to poliovirus type 1 and 3 vaccine strains were found in 99.4 and 98.2% of the children, respectively. An inverse association was found between H. pylori seropositivity accompanied by PGI:PGII ratio ≤6.5 (marker of gastric inflammation) and high-level immunity to poliovirus type 1: OR 0.39 (95% CI 0.68–0.91), p = 0.027. The association between H. pylori seropositivity of CagA virulent phenotype and polio high immunity was not significant. The association between H. pylori seropositivity and high neutralizing antibodies to type 3 poliovirus was of low magnitude and not significant.
Conclusions: H. pylori seroprevalence accompanied by evidence of gastric inflammation was inversely correlated with high titers of neutralizing antibodies to poliovirus in children from a population with near universal polio immunity.
Introduction
Polioviruses comprise 3 serotypes that multiply in the human intestine; in a minority of infected persons these can cause paralytic disease, poliomyelitis (1). Since the World Health Assembly adopted a resolution for the worldwide eradication of polio in 1988, the number of polio cases worldwide has declined by nearly 99.9%. Both wild poliovirus type 2 (WPV2) and WPV3 have officially been certified as globally eradicated (no cases of WPV2 have been documented since 1999, and none of WPV3 since 2012). Only two countries (Afghanistan and Pakistan) are currently affected by WPV1 (2, 3). The remarkable progress toward polio eradication is attributed to successful childhood vaccination with the oral polio vaccine (OPV) and the injectable inactivated polio vaccine (IPV) (4).
Immunization with OPV is simple and practical; it generates both systemic humoral (serum antibodies) and intestinal (secretory IgA) immunity. Vaccination with OPV induces superior intestinal immunity than IPV; thus, it can prevent the transmission of wild type viruses. Despite these advantages, OPV poses a risk, albeit very low, of vaccine-associated paralytic poliomyelitis (VAPP) (4). IPV is highly safe and induces high titers of serum antibodies (4). After the certification of WPV2 eradication, a global switch from trivalent OPV (tOPV, containing vaccine virus types 1, 2, and 3) to bivalent OPV (bOPV, containing types 1 and 3) was completed in 2016 (3, 4). At least two doses of IPV in routine immunization are also recommended in countries that vaccinate with OPV only (4, 5) to reduce the risk of VAPP. Presently, polio vaccination is based mainly on OPV (with one dose of IPV) in most low-middle income countries, while IPV is mainly used in high-income countries (4).
The threat remains of poliomyelitis re-emerging from the final global foci to spread elsewhere. This is evidenced by the silent circulation of WPV1 in 2013–2014 in the southern region of Israel, which was identified through environmental surveillance. No clinical cases of paralytic poliomyelitis were detected and molecular analysis found the virus to be similar to viruses circulating in Pakistan and from sewage samples in Egypt (6, 7). This alarming event occurred on the background of using only IPV vaccination during 2005–2013, and consistently high national coverage of polio vaccines in Israel. The silent poliovirus reintroduction, which lasted over 1 year in Israel (2013–2014), was successfully interrupted consequent to major interventions implemented by the Israeli Ministry of Health. These included mass administration of bOPV to stimulate intestinal immunity in all birth cohorts of children under age 9 years, the birth cohorts that were subject to the IPV-only vaccination program implemented during 2005–2013. In addition, OPV vaccination supplementary to IPV was reintroduced at ages 6 and 18 months, as the updated polio immunization policy in Israel (6, 8).
Oral enteric vaccines have demonstrated lower immunogenicity and efficacy in persons living in low-middle income countries, in contrast to persons from high-income countries (9). This was first reported in the 1960s and 1970s, in publications that showed OPV to be markedly less immunogenic in infants in India and other developing countries (10–13). This phenomenon was partly explained by the presence of the immunodominant attenuated type 2 poliovirus in the vaccine, which negatively affected the immune response to types 1 and 3 poliovirus in the vaccine, even as it elicited strong type 2 seroconversions (13); and to low vaccine take due to concurrent enteric infections at the time of vaccination (11, 14). An association was found of diminished immunogenicity and belonging to low socioeconomic status communities (14). Moreover, significantly lower seroconversion rates were found for poliovirus types 1, 2, and 3 following immunization with IPV in infants from Puerto Rico, compared to US infants (15). This suggested differences between the two populations, in the immune response to injectable poliovirus vaccines, as well.
Helicobacter pylori, a bacterium that persistently colonizes the stomach, is acquired in early childhood and causes asymptomatic gastritis; in a minority of infected persons peptic ulcers develop (16), and gastric cancer usually in later adulthood (17). Cytotoxin-associated gene A (CagA) antigen is main virulence factor of H. pylori. The cag pathogenicity island of H. pylori encodes for a type-IV secretory apparatus through which CagA antigen is inserted into the host cell [reviewed by Surbaum and Michetti (18)]. Infection with H. pylori CagA positive strains was linked with increased risk for peptic ulcers, premalignant gastric lesions and gastric cancer (17, 19). H. pylori have additional antigens such as VacA, Omp and NapA and others, however only a few of these antigens showed positive associations with gastric cancer (20–22). Additionally, the association between CagA sero-positivity and gastric cancer was of greater magnitude than other antigens (20, 21). Following adjustment for the presence of other antigens, CagA remained the only antigen associated with an increased risk of gastric cancer (21).
We previously showed that infection with H. pylori might affect immune responses to live oral enteric vaccines, such as Vibrio cholerae vaccine CVD 103-HgR (23) and Salmonella Typhi vaccine CVD 908-htrA (24). Specifically, the immune response was diminished in young Chilean children vaccinated with CVD 103-HgR (23), and enhanced in Malian adults vaccinated with CVD 103-HgR (25), and in US adults vaccinated with CVD 908-htrA (24). Given these discrepancies and on the background of the 2013–2014 silent outbreak with WPV1 in Israel during 2013–2014, the aim of the current study was to examine the association of H. pylori seroprevalence, and serum pepsinogens (PGs, as markers of gastric inflammation) with the neutralizing antibodies to polio vaccine strains in school-age children.
Materials and Methods
Study Design and Population
A seroepidemiological study was conducted among a convenience sample of children aged 5–17 years from northern Israel. Jewish and Arab children were enrolled from Hadera sub-district who attended Hillel Yaffe Medical Center, and from the area of Nazareth city who attended the French Hospital in Nazareth. Children with known immunosuppressive conditions were excluded.
The coverage of OPV vaccination during the 2013–2014 campaign was 79% in the Hadera sub-district and 90% in the northern region of Israel, including Nazareth. Parents of eligible children were interviewed in their native language (Hebrew or Arabic) regarding sociodemographic characteristics and children's health status and medical history.
Definition of the Study Variables
The Dependent Variables
Titers of neutralizing antibody against poliovirus types 1 and 3 vaccine strains were measured using a standard microneutralization assay (26). Children with antibody titer lower than 1:8 were considered unimmunized and unprotected; it is encouraging that only a few children had such low titers. Therefore, we defined the dependent variables, namely high immune response to poliovirus vaccine strains, as having a titer of neutralizing antibodies >1:2,048. This value corresponded to the 60th and 40th percentiles of neutralizing antibody titers against poliovirus type 1 and type 3 vaccine strains, respectively.
The Main Independent Variables
The main independent variables were H. pylori immunoglobulin G (IgG) seropositivity and serum pepsinogens (PGs) as markers of gastric inflammation (27–29). Children were classified as (1) H. pylori positive-CagA positive if they had H. pylori IgG antibodies and CagA IgG antibodies; (2) H. pylori positive-CagA negative if they had H. pylori IgG antibodies, but lacked CagA IgG antibodies; and (3) H. pylori negative if they lacked H. pylori IgG antibodies. We focused on CagA rather than other antigens, given its strong association with gastric pathology, which is well-established than other H. pylori antigens.
An additional classification was based on the combination of H. pylori seropositivity with a PGI:PGII ratio ≤6.5 [lowest quartile]. This ratio serves as an indication for severe gastritis, and is similar to clinically relevant mean values in children with gastritis established by endoscopy (27–29). Accordingly, children were grouped as (1) H. pylori positive and PGI:PGII ≤6.5 (more severe gastritis); (2) H. pylori positive and PGI:PGII>6.5 (without severe gastritis) and (3) H. pylori negative if they lacked H. pylori IgG antibodies.
Covariates
Data were collected on the child's age (in years, continuous variable), sex, population group (Jews or Arabs), maternal age (in years, continuous variable), the number of maternal schooling years (continuous variable), the number of siblings, and the birth order of the child. A household crowding index was calculated by dividing the number of persons living in the household by the number of rooms in the household. These variables might be related to H. pylori infection, and were considered as potential confounders.
Laboratory Methods
Blood samples were obtained from the children centrifuged immediately upon collection, and sera were kept at −20°C until testing. Sera were tested for the presence of H. pylori IgG antibodies using commercial ELISA kits (Enzygnost® Anti-Helicobacter pylori II/IgG kit, Siemens Diagnostics GmbH, Marburg, Germany) according to the manufacturer's instructions. Sensitivity and specificity values of the kit among children are within the range of 92–97% (30). The detection of H. pylori IgG serum antibodies using this kit has been shown to strongly correlate with the detection of H. pylori antigen in stool samples using the monoclonal antigen enzyme immunoassay (correlation coefficient = 0.70, p < 0.001) (Muhsen K., unpublished). H. pylori seropositive sera were tested for IgG antibodies against recombinant CagA protein, using an in-house ELISA protocol (17, 31). Concentrations of serum PGI and PGII were measured using ELISA kits (Biohit Inc., Helsinki, Finland), according to the manufacturer's instructions.
The levels of neutralizing serum antibodies against poliovirus type 1 and 3 were measured at the polio reference laboratory at the Central Virology Laboratory of the Ministry of Health, Israel. The titer of neutralizing antibody against poliovirus type 1 and type 3 vaccine strains was measured in a standard microneutralization assay (26, 32–34), using live attenuated polioviruses (Sabin 1 and Sabin 3).
Ethical Aspects
The Institutional Review Boards of the Hillel Yaffe Medical Center and the French Hospital, and the ethics committee of Tel Aviv University approved the study protocol. The parents signed a written informed consent.
Statistical Methods
The study sample was described using means and SD for continuous variables, medians and interquartile range for variables with skewed distribution, and frequencies and percentages for categorical variables.
Sociodemographic differences between children who were H. pylori seropositive and negative were examined using the chi square test and Fisher's Exact test as appropriate, and the Student's t test for continuous variables and the Mann Whitney test for variables with skewed distribution. Correlations of neutralizing serum antibody titers to poliovirus type 1 and type 3 vaccine strains with the independent variables were assessed using Spearman's correlation coefficient. Differences between children with H. pylori seropositivity and negativity, in their having high immune response to poliovirus type 1 and type 3 vaccine strains (see definitions above), were assessed using the chi square test.
To account for potential confounders, we used the inverse propensity score treatment weighting approach (35, 36). We created 3 propensity scores for each main independent variable (35–39). For H. pylori seropositivity (positive or negative), a propensity score was created using the predicted probability from the binary logistic regression model. For H. pylori/CagA seropositivity (3 categories as outlined above), a propensity score was created using the predicted probability of H. pylori/CagA seropositivity from a multinomial logistic regression model with the abovementioned covariates. Inverse probability weights were calculated using the created propensity score by weighting each participant in H. pylori/CagA positivity categories (H. pylori positive/CagA positive, H. pylori positive/CagA negative or H. pylori negative) inversely to his/her probability of being classified into these specific categories. A similar approach was followed for the H. pylori seropositivity/PGI:PGII ratio. By using the inverse weights, we created pseudo-populations in which H. pylori categories were balanced in the distribution of these covariates.
We assessed the association between H. pylori seropositivity and high immunity to polioviruses vaccine strains (yes or no) using weighted generalized estimating equations with binary logistic regression, providing a robust variance estimator (39). We also conducted unweighted multivariable logistic regression models that adjusted for confounders using the conventional approach. Similar models were constructed for the main independent variables H. pylori/CagA seropositivity and H. pylori seropositivity/PGI:PGII ratio. ORs and 95% CIs were obtained from these models.
P-value <0.05 was considered statistically significant. The data were analyzed using SPSS software version 25 (IBM, Armonk, New York, USA).
Missing values for the study variables were negligible (<3%); therefore, we performed complete case analysis.
Results
The Study Population According to H. pylori Seropositivity
Overall, 336 children (63.1% males) were enrolled in the study, of whom 98 (29.2%) were Jewish children and the rest were Arab. The participants' age was in the range of 4.8–17.3 years, with a mean age of 10.7 [standard deviation [SD] 2.3]. Overall, 137 (40.8%) children tested positive for H. pylori IgG serum antibody; among these, 51 (37.2%) also tested positive for CagA (virulent phenotype) IgG antibody.
The variables serum pepsinogen (PG) I and PGII levels and the PGI:PGII ratio (measures of gastric inflammation) did not follow normal distributions (p < 0.001 by Kolmogorov-Smirnov test). The median of serum PGI and PGII concentration was significantly higher among H. pylori seropositive than seronegative children, while the median PGI:PGII ratio was lower among H. pylori seropositive children (Supplementary Table 1).
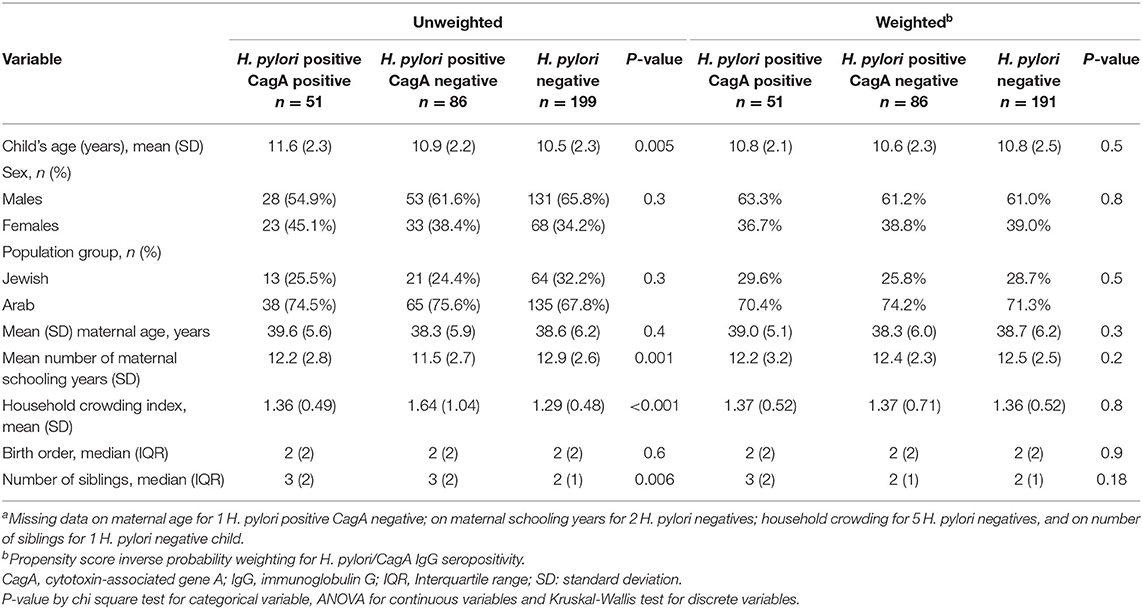
The unweighted analysis showed that H. pylori seropositive children were older than seronegative children: 11.1 years and 10.5 years, respectively (p = 0.007). The mean number of maternal schooling years was lower among H. pylori seropositive than seronegative children: 11.8 and 12.8 years, respectively (p < 0.001). The mean crowded index and the mean number of siblings was higher among H. pylori seropositive than seronegative children. These differences were balanced in the weighted analysis (Supplementary Table 2). Similar socio-demographic differences were found between the H. pylori/CagA positivity groups and the H. pylori positivity/PGI:PGII ratio groups (proxy for gastric inflammation); these were balanced in the weighted analyses (Table 1 and Supplementary Table 3).
Description of the Immune Response Against Poliovirus Types 1 and 3 Vaccine Strains
A serum neutralizing antibody titer of <1:8 to poliovirus type 1 vaccine strain was found in only 2 (0.6%) children and to poliovirus type 3 vaccine strain in 4 (1.2%) participants. These were considered unimmunized. The remaining participants had a titer ≥1:8 and were considered immunized (Figure 1).
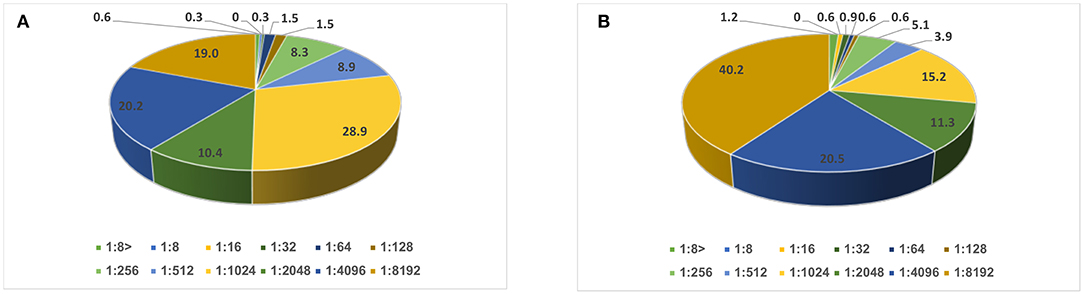
Figure 1. Distribution of neutralizing antibody titer (in percentages): (A) against poliovirus type 1 and (B) type 3 vaccine strains among school age children (N = 336).
Significant (p < 0.001) negative correlations were found between the child's age and neutralizing antibody titers against poliovirus types 1 and 3 vaccine strains (Spearman's correlation coefficients −0.30 and −0.23, respectively). No significant correlations were found between neutralizing antibody titers and other sociodemographic factors (Supplementary Table 4).
Association of H. pylori Seropositivity, Serum Pepsinogens, and Neutralizing Antibody Titer Against Poliovirus Type 1 and 3 Vaccine Strains
In the unweighted analysis, H. pylori IgG seropositivity was evident among 46/132 (34.8%) children with high-level immunity to poliovirus type 1 (neutralizing antibody titer >1:2,048) compared to 92/205 (44.9%) among children with neutralizing antibody titer ≤1:2,048, [OR 0.66 (95% CI 0.42–1.03)], but this association was not statistically significant p = 0.062. A significant and stronger association was found for the CagA-positive phenotype [OR 0.48 (95% CI 0.25–0.95)], p = 0.035, and for H. pylori positivity with PGI:PGII ≤6.5 (a proxy of gastric inflammation) [OR 0.41 (95% CI 0.19–0.88)], p = 0.021. In the inverse propensity score weighted analysis, these associations were attenuated; but the association with H. pylori positivity with PGI:PGII ≤6.5 remained statistically significant [OR 0.43 (95% CI 0.19–0.97)], p = 0.041 (Table 2). Associations of H. pylori IgG seropositivity, and of H. pylori IgG seropositivity according to CagA phenotype and PGI:PGII ≤6.5, with high-level immunity to poliovirus type 3 vaccine strains were of weaker magnitude and mostly non-significant (Table 3). Including the variable “age” in the weighted models slightly strengthened the inverse association between H. pylori/PGI:PGII ≤6.5 and high immunity to poliovirus type 1 [OR 0.39 (95% 0.68–0.91), p = 0.027]. Each one-year increase in a child's age was associated with a 14%-21% lower odds of having high immunity levels to both poliovirus type 1 and 3 (Table 4). The results were similar in unweighted multivariable logistic regression models that controlled for the child's age, the number of maternal schooling years, household crowding and the number of siblings (Table 5).
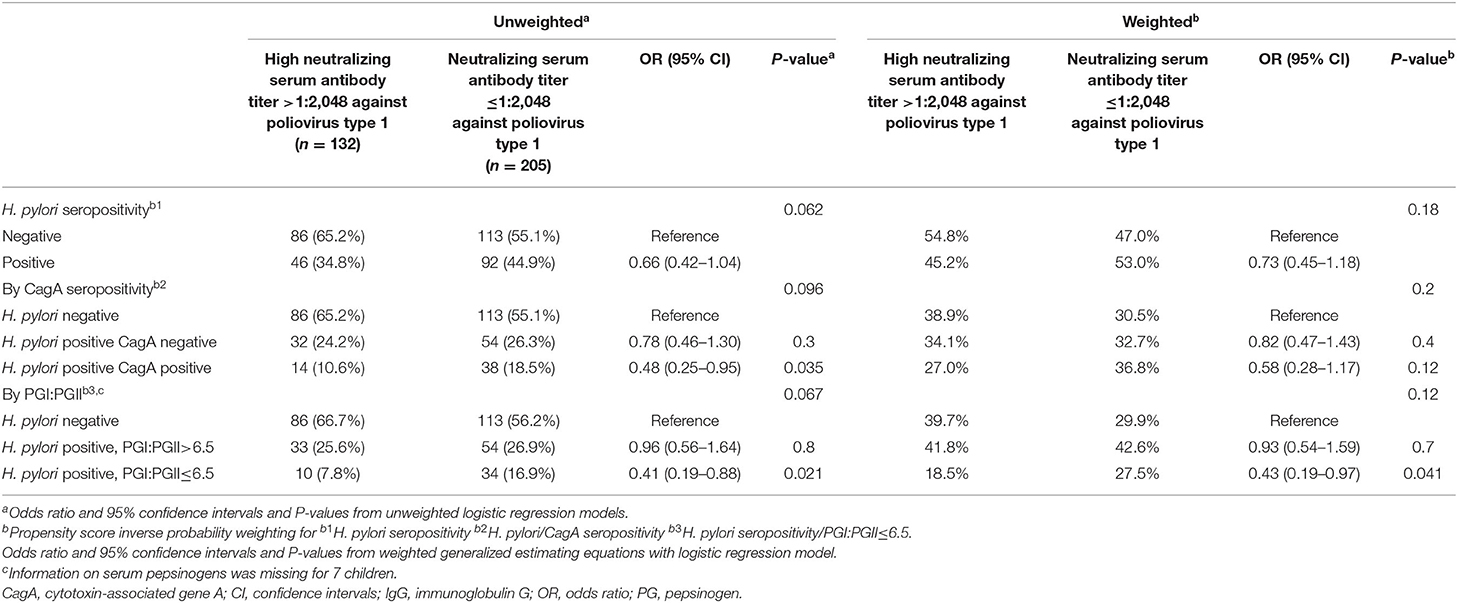
Table 2. Associations of H. pylori IgG seropositivity and serum pepsinogens, with high serum neutralizing antibody level against poliovirus type 1 vaccine strain.

Table 3. Associations of H. pylori IgG seropositivity and serum pepsinogens, with high serum neutralizing antibody level against poliovirus type 3 vaccine strain.
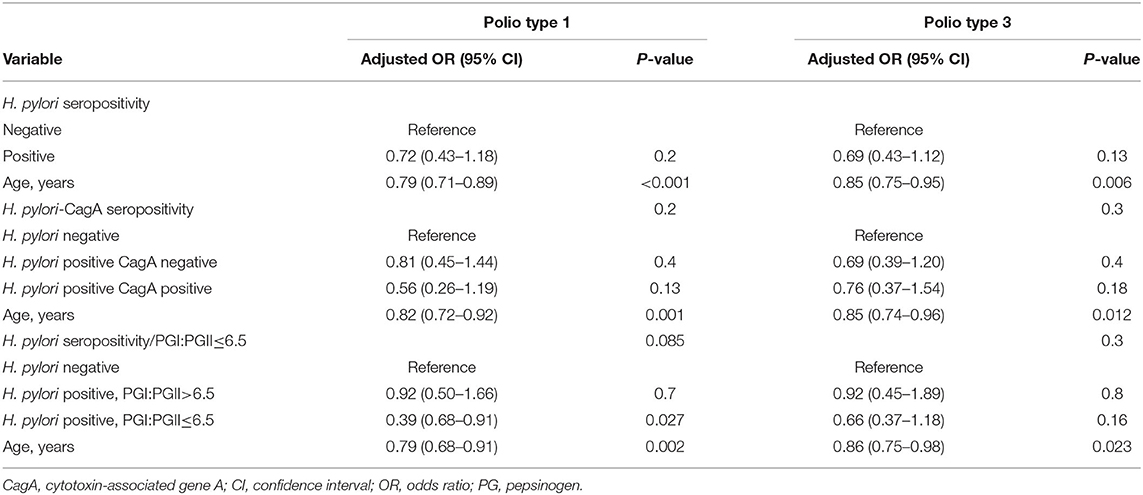
Table 4. Age-adjusted propensity score inverse probability weighted models of the association between H. pylori seropositivity and high serum neutralizing antibody level (a titer >1:2,048) to poliovirus type 1 and 3 vaccine strains.
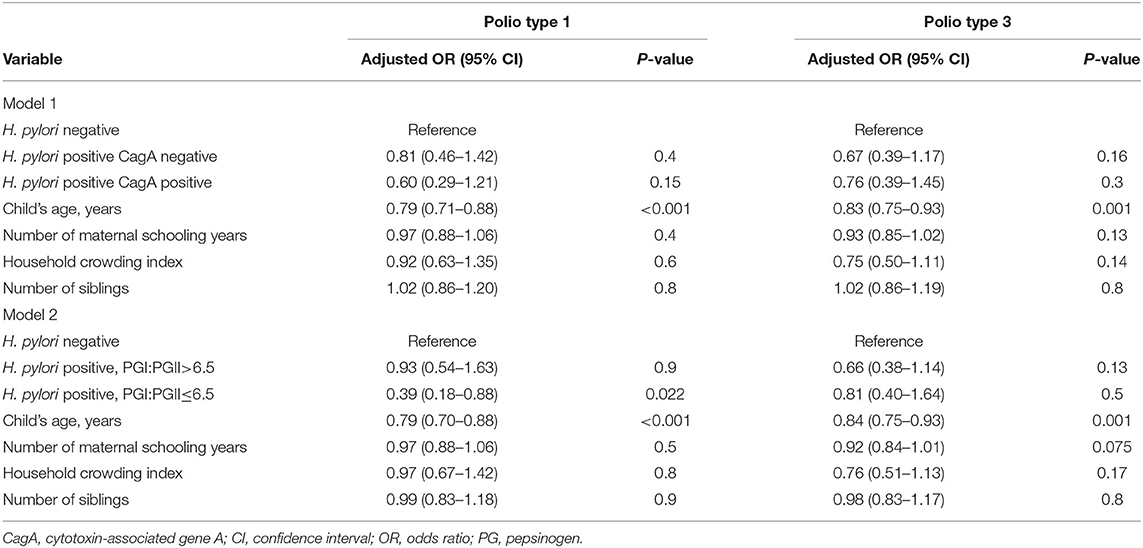
Table 5. Unweighted multivariable logistic regression models of factors associated with high serum neutralizing antibody level (a titer >1:2,048) against poliovirus type 1 and 3 vaccine strains.
In an additional analysis, to minimize the impact of outlier observations we excluded from the analysis 14 children (4.2%) with low neutralizing antibody titers (≤1:128) against poliovirus type 1 vaccine strain, relatively to the rest of the study sample. Neutralizing antibody titer (as a continuous variable) against poliovirus type 1 vaccine strain was significantly lower among H. pylori sero-positive participants with evidence of gastric inflammation (PGI:PGII <6.5) compared to H. pylori sero-negative children: median levels 1:2,048 vs. 1:1,024, p = 0.038 by Mann Whitney U test. No significant difference (p = 0.16) was found between the groups in the median levels of neutralizing antibody titer against poliovirus type 3 vaccine strain (the median was 1:4,096 in both groups).
Discussion
An inverse association was found between H. pylori seroprevalence accompanied with gastric inflammation, as evident by the PGI:PGII ratio, and between high neutralizing to poliovirus type 1 vaccine strain among school age children. This association was consistent and robust while using various analytical approaches to account for confounders. The association between overall H. pylori sero-prevalence and high immunity to poliovirus type 1 vaccine strain was not significant (p = 0.062).
Overall, the study population had very high immunity levels to polioviruses. This finding is in agreement with previous seroepidemiological studies from Israel (8, 40) and represents the high vaccination coverage (~95%) of poliovirus vaccines in Israel (8).
The observed inverse association between H. pylori seroprevalence/PGI:PGII ratio and high immunity to polioviruses corroborates our previous study in Chile. There we reported an inverse association between H. pylori sero-prevalence and the immune response to the live attenuated oral cholera vaccine CVD 103-HgR among children aged 6 months to 4 years. However, in older children aged 5–9 years in the same study (23), we found a positive association, as well as in Malian adults (25). Moreover, we found a positive association between H. pylori seroprevalence and the humoral immune response to S. Typhi vaccine CVD 908-htrA in US adults (24). Notably, the immune response to poliovirus in the current study results from vaccination with both intramuscular IPV in early childhood and OPV at school age, during the silent circulation of the wild type poliovirus 1. This limits direct comparison between the observations presented herein and those of previous studies on oral enteric vaccines (23, 24). Collectively, our observations suggest that H. pylori seropositivity is related to the immune response to enteric vaccines, regardless of the administration route. Moreover, our findings might have implications to children receiving combined OPV/IPV vaccination schedule, as it is the case in Israel, suggesting that the immune response elicited by combined OPV/IPV vaccines, although in general is high, might be somewhat blunted by H. pylori-associated gastric inflammation. The apparently contradictory observations support the notion that the association of H. pylori seroprevalence with the immune response to enteric vaccines might vary according to age, i.e., between children and adults. This might be explained by age-related differences in H. pylori-induced gastritis. H. pylori infection is acquired in early childhood (41), and causes chronic gastritis (42). Although most infected persons remain asymptomatic, the severity of gastric inflammation increases with age, including histopathological changes, even in the absence of symptoms (43). Antrum-predominant-H. pylori gastritis, which typically occurs in children, might enhance the secretion of gastric acid. This may explain the inverse association between the infection and an enhanced immune response to poliovirus, since the response is sensitive to acid (44, 45) if the impact is via OPV. Corpus-predominant gastritis causes hypochlorhydria, which usually occurs in adults (42). This might explain the enhanced immune response to the S. Typhi and cholera vaccines in adults (24, 25), to acid sensitive pathogens (46, 47).
In the current study, being H. pylori sero-positive and having gastric inflammation as measured by PGI:PGII <6.5 was significantly related to high immunity to poliovirus type 1 vaccine strain. However, the association with overall H. pylori sero- positivity was not significant (p=0.062), possibly due to limited power. These findings also might suggest that H. pylori might plays a role in the immune response to poliovirus vaccine only in a portion of the infected children.
Notably, lower immune response to OPV, measured in terms of seroconversion, in populations from developing countries, was studied in the 1960s and 1970s, especially the immune response to the trivalent OPV (12). This phenomenon was partly attributed to low vaccine uptake due to simultaneous enteric infections at the time of vaccination (11, 14), and the presence of the immunodominant attenuated type 2 poliovirus, which interfered with the response to types 1 and 3 in the vaccine (13). A study conducted by Swartz et al. (14), during 1969–1970 in Tel Aviv metropolitan, showed lower immune response to the trivalent OPV among infants who were vaccinated during the summer than the winter, among infants with vs. without concurrent enteric infections at the time of immunization, and among infants from low vs. high socioeconomic status (14).
H. pylori infection affects the microbiome of the stomach and gut (48, 49). A recent study showed a significant effect of enteric infections on the immune response to OPV in children from India, while the gut microbiome did not have any impact in this population (50). In contrast, another study, conducted in the USA, showed that the gut microbiome might affect the immune response to both oral and parental polio vaccines (51, 52). These studies did not assess the role of H. pylori infection. The gut microbiome might be a possible link between H. pylori infection and the adaptive immune response to vaccines. Among infants, the immune response to IPV might be reduced in relation to maternal poliovirus antibody levels (53). In adults and adolescents, increased age was associated with lower levels of polioviruses sero-positivity in Italy (54). In our study, older children had lower likelihood of having high immunity to poliovirus type 1 and type 3 vaccine strains.
Our study has several strengths. First, we used various markers to characterize H. pylori infection, including the measurement of antibodies against CagA (a virulence attribute) and serum PGs as markers of gastric inflammation. Second, we assessed several potential confounders. Third, we utilized various analytical approaches to control for confounders, all of which yielded comparable results. Lastly, the study population sample had near universal immunity to polioviruses. This enabled an exceptional opportunity to assess the relation between chronic infection and attaining the high population level of immunity to polio that is needed to prevent the re-emergence of wild type polioviruses.
Our study has some limitations, including the inability to distinguish between immunity resulting from OPV vs. IPV and between antibodies stimulated by polio vaccines vs. by clinically asymptomatic infection with wild poliovirus. Moreover, the study sample comprised a high proportion of Arab children (70% of the sample), which is not representative of the general population in Israel. However, the sample was representative of the population in the northern region of Israel, where vaccination coverage is higher than in the rest of the country. This, however, is not expected to affect our findings of inverse associations of H. pylori seroprevalence plus gastric inflammation and high-level immunity to poliovirus. These associations are generalizable to other populations.
In conclusion, H. pylori seroprevalence, accompanied by gastric inflammation, evidenced by a low PGI:PGII ratio, was inversely related to the presence of high titers of serum neutralizing antibody to poliovirus vaccines, in school age children from a population with a high background prevalence of antibodies to poliovirus.
Data Availability Statement
The datasets presented in this article are not readily available because ethical and legal restrictions apply. Requests to access the datasets should be directed to Khitam Muhsen, a211aHNlbkB0YXVleC50YXUuYWMuaWw=.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Boards of the Hillel Yaffe Medical Center and the French Hospital, and the Ethics Committee of Tel Aviv University approved the study protocol. The parents signed a written informed consent. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
KM and ML contributed equally to the study as co-PIs and senior authors on this manuscript, planned the study, made substantial contribution to it design, acquired funding, and drafted the manuscript. EK, LB, NE, and MW directed its implementation. EK, LB, NE, KM, and ML contributed substantially to acquisition of data and blood samples. LB and MW were responsible for the laboratory work. KM, LB, and ML were responsible for data analysis. All authors made substantial contributions to interpretation of findings, approved the submitted version of the manuscript, agreed to be personally accountable for the author's own contributions and accuracy of data presented in the manuscript, and contributed to writing and substantive revisions.
Funding
This study was funded by the United States-Israel Binational Science Foundation (BSF) (PIs KM and ML) grant number 2015361.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very thankful to Professor Guillermo I. Perez-Perez and Professor Martin J. Blaser from New York University School of Medicine, New York, NY, USA for providing the recombinant CagA antigen. This work was undertaken in partial fulfillment of the requirements for an M.Sc. degree of LB, Sackler Faculty of Medicine, Tel Aviv University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.797719/full#supplementary-material
References
1. Cohen JI. Enterovirus, parechovirus, and reovirus infections. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison's Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill Education (2018).
2. Lickness JS, Gardner T, Diop OM, Chavan S, Jorba J, Ahmed J, et al. Surveillance to track progress toward polio eradication - worldwide, 2018–2019. MMWR Morb Mortal Wkly Rep. (2020) 69:623–9. doi: 10.15585/mmwr.mm6920a3
3. Greene SA, Ahmed J, Datta SD, Burns CC, Quddus A, Vertefeuille JF, et al. Progress toward polio eradication - worldwide, January 2017-March 2019. MMWR Morb Mortal Wkly Rep. (2019) 68:458–62. doi: 10.15585/mmwr.mm6820a3
4. Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Fut Microbiol. (2015) 10:791–808. doi: 10.2217/fmb.15.19
5. WHO. WHO Recommendations for Routine Immunization - Summary Tables Internet 2021 Available online at: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/who-recommendations-for-routine-immunization—summary-tables (accessed December 18, 2021).
6. Kopel E, Kaliner E, Grotto I. Lessons from a public health emergency–importation of wild poliovirus to Israel. N Engl J Med. (2014) 371:981–3. doi: 10.1056/NEJMp1406250
7. Shulman LM, Gavrilin E, Jorba J, Martin J, Burns CC, Manor Y, et al. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel, 2013. Eurosurveillance. (2014) 19:13–20. doi: 10.2807/1560-7917.ES2014.19.7.20709
8. Kaliner E, Kopel E, Anis E, Mendelson E, Moran-Gilad J, Shulman LM, et al. The Israeli public health response to wild poliovirus importation. Lancet Infect Dis. (2015) 15:1236–42. doi: 10.1016/S1473-3099(15)00064-X
9. Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. (2010) 8:129. doi: 10.1186/1741-7007-8-129
10. John TJ. Oral polio vaccination of children in the tropics. II. Antibody response in relation to vaccine virus infection. Am J Epidemiol. (1975) 102:414–21. doi: 10.1093/oxfordjournals.aje.a112180
11. John TJ, Christopher S. Oral polio vaccination of children in the tropics. III. Intercurrent enterovirus infections, vaccine virus take and antibody response. Am J Epidemiol. (1975) 102:422–8. doi: 10.1093/oxfordjournals.aje.a112181
12. John TJ, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. (1972) 96:263–9. doi: 10.1093/oxfordjournals.aje.a121457
13. Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. (1991) 13:926–39. doi: 10.1093/clinids/13.5.926
14. Swartz TA, Skalska P, Gerichter CG, Cockburn WC. Routine administration of oral polio vaccine in a subtropical area. Factors possibly influencing sero-conversion rates. J Hyg. (1972) 70:719–26. doi: 10.1017/S0022172400022567
15. Dayan GH, Thorley M, Yamamura Y, Rodriguez N, McLaughlin S, Torres LM, et al. Serologic response to inactivated poliovirus vaccine: a randomized clinical trial comparing 2 vaccination schedules in Puerto Rico. J Infect Dis. (2007) 195:12–20. doi: 10.1086/508427
16. Nomura AMY, Perez-Perez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. (2002) 155:1054–9. doi: 10.1093/aje/155.11.1054
17. Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. (1995) 55:2111–5.
18. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. (2002) 347:1175–86. doi: 10.1056/NEJMra020542
19. Ching CK, Wong BC, Kwok E, Ong L, Covacci A, Lam SK. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. (1996) 91:949–53.
20. Song H, Michel A, Nyrén O, Ekström AM, Pawlita M, Ye W. A CagA-independent cluster of antigens related to the risk of noncardia gastric cancer: associations between Helicobacter pylori antibodies and gastric adenocarcinoma explored by multiplex serology. Int J Cancer. (2014) 134:2942–50. doi: 10.1002/ijc.28621
21. Fernández de Larrea-Baz N, Pérez-Gómez B, Michel A, Romero B, Lope V, Pawlita M, et al. Helicobacter pylori serological biomarkers of gastric cancer risk in the MCC-Spain case-control Study. Cancer Epidemiol. (2017) 50(Pt A):76–84. doi: 10.1016/j.canep.2017.08.002
22. Shakeri R, Malekzadeh R, Nasrollahzadeh D, Pawlita M, Murphy G, Islami F, et al. Multiplex H. pylori serology and risk of gastric cardia and noncardia adenocarcinomas. Cancer Res. (2015) 75:4876–83. doi: 10.1158/0008-5472.CAN-15-0556
23. Muhsen K, Lagos R, Reymann MK, Graham DY, Pasetti MF, Levine MM. Age-dependent association among Helicobacter pylori infection, serum pepsinogen levels and immune response of children to live oral cholera vaccine CVD 103-HgR. PLoS ONE. (2014) 9:e83999. doi: 10.1371/journal.pone.0083999
24. Muhsen K, Pasetti MF, Reymann MK, Graham DY, Levine MM. Helicobacter pylori infection affects immune responses following vaccination of Typhoid-naive US adults with attenuated Salmonella Typhi oral vaccine CVD 908-htrA. J Infect Dis. (2014) 209:1452–8. doi: 10.1093/infdis/jit625
25. Muhsen K, Sow SO, Tapia MD, Haidara FC, Reymann M, Asato V, et al. Pre-existing Helicobacter pylori serum IgG enhances the vibriocidal antibody response to CVD 103-HgR live oral cholera vaccine in Malian adults. Sci Rep. (2020) 10:16871. doi: 10.1038/s41598-020-71754-9
26. Albrecht P, van Steenis G, van Wezel AL, Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis. (1984) 6(Suppl. 2):S540–4. doi: 10.1093/clinids/6.Supplement_2.S540
27. Kalach N, Legoedec J, Wann AR, Bergeret M, Dupont C, Raymond J. Serum levels of pepsinogen I, pepsinogen II, and gastrin-17 in the course of Helicobacter pylori gastritis in pediatrics. J Pediatr Gastroenterol Nutr. (2004) 39:568–9. doi: 10.1097/00005176-200411000-00025
28. de Angelis GL, Cavallaro LG, Maffini V, Moussa AM, Fornaroli F, Liatopoulou S, et al. Usefulness of a serological panel test in the assessment of gastritis in symptomatic children. Dig Dis. (2007) 25:206–13. doi: 10.1159/000103886
29. Guariso G, Basso D, Bortoluzzi CF, Meneghel A, Schiavon S, Fogar P, et al. GastroPanel: evaluation of the usefulness in the diagnosis of gastro-duodenal mucosal alterations in children. Clin Chim Acta. (2009) 402:54–60. doi: 10.1016/j.cca.2008.12.014
30. Kindermann A, Konstantopoulos N, Lehn N, Demmelmair H, Koletzko S. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol. (2001) 39:3591–6. doi: 10.1128/JCM.39.10.3591-3596.2001
31. Muhsen K, Sinnereich R, Beer-Davidson G, Nassar H, Abu Ahmed W, Cohen D, et al. Correlates of infection with Helicobacter pylori positive and negative cytotoxin-associated gene A phenotypes among Arab and Jewish residents of Jerusalem. Epidemiol Infect. (2019) 147:e276. doi: 10.1017/S0950268819001456
32. Green MS, Handsher R, Cohen D, Melnick JL, Slepon R, Mendelsohn E, et al. Age differences in immunity against wild and vaccine strains of poliovirus prior to the 1988 outbreak in Israel and response to booster immunization. Vaccine. (1993) 11:75–81. doi: 10.1016/0264-410X(93)90342-U
33. Grotto I, Handsher R, Gdalevich M, Mimouni D, Huerta M, Green MS, et al. Decline in immunity to polio among young adults. Vaccine. (2001) 19:4162–6. doi: 10.1016/S0264-410X(01)00165-7
34. Swartz TA, Green MS, Handscher R, Sofer D, Cohen-Dar M, Shohat T, et al. Intestinal immunity following a combined enhanced inactivated polio vaccine/oral polio vaccine programme in Israel. Vaccine. (2008) 26:1083–90. doi: 10.1016/j.vaccine.2007.12.021
35. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34:3661–79. doi: 10.1002/sim.6607
36. Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
37. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ-Brit Med J. (2019) 367:l5657. doi: 10.1136/bmj.l5657
38. Spreeuwenberg MD, Bartak A, Croon MA, Hagenaars JA, Busschbach JJV, Andrea H, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms an Introduction from a case study in mental health. Med Care. (2010) 48:166–74. doi: 10.1097/MLR.0b013e3181c1328f
39. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. (2016) 35:5642–55. doi: 10.1002/sim.7084
40. Moran-Gilad J, Kaliner E, Gdalevich M, Grotto I. Public health response to the silent reintroduction of wild poliovirus to Israel, 2013–2014. Clin Microbiol Infect. (2016) 22(Suppl. 5):S140–5. doi: 10.1016/j.cmi.2016.06.018
41. Muhsen K, Jurban M, Goren S, Cohen D. Incidence, age of acquisition and risk factors of Helicobacter pylori infection among Israeli Arab infants. J Trop Pediatr. (2012) 58:208–13. doi: 10.1093/tropej/fmr068
42. Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. (2009) 136:1863–73. doi: 10.1053/j.gastro.2009.01.073
43. Ganga-Zandzou PS, Michaud L, Vincent P, Husson MO, Wizla-Derambure N, Delassalle EM, et al. Natural outcome of Helicobacter pylori infection in asymptomatic children: a two-year follow-up study. Pediatrics. (1999) 104(2 Pt 1):216–21. doi: 10.1542/peds.104.2.216
44. Salo RJ, Cliver DO. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch Virol. (1976) 52:269–82. doi: 10.1007/BF01315616
45. Ohka S. [Analysis of dissemination pathways for poliovirus]. Uirusu. (2009) 59:107–14. doi: 10.2222/jsv.59.107
47. Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. (1972) 13:251–6. doi: 10.1136/gut.13.4.251
48. Benavides-Ward A, Vasquez-Achaya F, Silva-Caso W, Aguilar-Luis MA, Mazulis F, Urteaga N, et al. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res Notes. (2018) 11:468. doi: 10.1186/s13104-018-3565-5
49. Lapidot Y, Reshef L, Cohen D, Muhsen K. Helicobacter pylori and the intestinal microbiome among healthy school-age children. Helicobacter. (2021) 26:e12854. doi: 10.1111/hel.12854
50. Praharaj I, Parker EPK, Giri S, Allen DJ, Silas S, Revathi R, et al. Influence of nonpolio enteroviruses and the bacterial gut microbiota on oral poliovirus vaccine response: a study from South India. J Infect Dis. (2019) 219:1178–86. doi: 10.1093/infdis/jiy568
51. Huda MN, Ahmad SM, Alam MJ, Khanam A, Kalanetra KM, Taft DH, et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics. (2019) 143:e20181489. doi: 10.1542/peds.2018-1489
52. Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, et al. Stool microbiota and vaccine responses of infants. Pediatrics. (2014) 134:e362–72. doi: 10.1542/peds.2013-3937
53. Jia S, Tang R, Li G, Hu Y, Liang Q. The effect of maternal poliovirus antibodies on the immune responses of infants to poliovirus vaccines. BMC Infect Dis. (2020) 20:641. doi: 10.1186/s12879-020-05348-1
Keywords: poliovirus vaccine, neutralizing antibodies, H. pylori, cytotoxin-associated gene A, pepsinogen
Citation: Badran Abu Zher L, Weil M, Kassem E, Elias N, Levine MM and Muhsen K (2022) Relationship Between Helicobacter pylori IgG Seroprevalence and the Immune Response to Poliovirus Vaccine Among School-Age Children From a Population With Near-Universal Immunity Level. Front. Med. 8:797719. doi: 10.3389/fmed.2021.797719
Received: 19 October 2021; Accepted: 22 December 2021;
Published: 20 January 2022.
Edited by:
Jian Wu, Zhejiang University, ChinaReviewed by:
John Modlin, Dartmouth College, United StatesGuanghua Zhai, Nanjing Medical University, China
Copyright © 2022 Badran Abu Zher, Weil, Kassem, Elias, Levine and Muhsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khitam Muhsen, a211aHNlbkB0YXVleC50YXUuYWMuaWw=
†These authors have contributed equally to this work and share senior authorship
 Layaly Badran Abu Zher1
Layaly Badran Abu Zher1 Khitam Muhsen
Khitam Muhsen