
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 12 January 2022
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.796615
This article is part of the Research Topic Primary Sjögren's Syndrome: From Immunological to Rheumatological Aspects View all 8 articles
 Cheng-You Wang1,2†
Cheng-You Wang1,2† Jung-Nien Lai3,4
Jung-Nien Lai3,4 Chin-Hsiu Liu5,6
Chin-Hsiu Liu5,6 Kai-Chieh Hu6,7
Kai-Chieh Hu6,7 Kai-Lun Sheu2,8,9,10†
Kai-Lun Sheu2,8,9,10† James Cheng-Chung Wei8,11,12*
James Cheng-Chung Wei8,11,12*Purpose: Previous studies have shown that metformin exhibits an anti-inflammatory effect and may decrease the risk of incidental diabetes. But the effect of metformin on incidental Sjögren's syndrome is unknown. The aim of the study was to examine the association between metformin exposure and Sjögren's syndrome in diabetic patients.
Methods: The dataset in this retrospective cohort study was obtained from the National Health Insurance Research Database (2000–2013) in Taiwan. In total, 15,098 type 2 diabetic patients under metformin treatment and an equivalent number without metformin treatment matched for comparison were included. The primary endpoint was the incidence of Sjogren's syndrome. Univariate and multivariate Cox proportional hazards models were used for data analysis. A subgroup analysis and sensitivity test were also performed.
Results: The incidence rate of Sjögren's syndrome in non-metformin controls was 40.83 per 100,000 person-years and 16.82 per 100,000 person-years in metformin users. The adjusted hazard ratio (aHR) in diabetic patients under metformin treatment was 0.46 (95% CI, 0.23 to 0.92). In subgroup analysis, men had a lower risk of developing Sjögren's syndrome than women [aHR = 0.15, 95% CI = (0.05, 0.41)]. After prescribing metformin to type 2 diabetic patients aged 60 years or more, those patients had a lower risk of developing Sjögren's syndrome [aHR = 0.34, 95% CI = (0.12, 0.96)].
Conclusion: In this large population-based cohort study, metformin exposure was associated with a reduced risk of developing Sjögren's syndrome in type 2 diabetic patients.
Sjögren's syndrome (SS) is a chronic systemic autoimmune disorder characterized by lymphocytic infiltrates of the affected exocrine gland with various manifestations (1). In addition to dry eye as the most common symptom affecting more than 95% of SS patients, sleep disturbance, dysphagia, oral candidiasis, joint inflammatory, and neurological and multi-organ manifestation have also been reported (2–6). The global prevalence of SS is about 0.2% in the adult population with a male/female ratio of 1:9 according to the classification criteria of the American-European Consensus Group (AECG) (7). Numerous factors, including genes, environment, viruses, and hormones might trigger the progression of the disease mediated particularly by T and B lymphocytes (8–10). Elevated B-cell activating factor (BAFF) level plays an especially important role in the maturation of irregular B cells in exocrine glands (11). Due to the aggravating symptoms and non-negligible life-threatening comorbidities, many studies have been dedicated to developing effective treatments. However, traditional immunosuppressives which are effective in other autoimmune diseases seem to be an unsuccessful therapeutic strategy in SS (12). Numerous biological agents such as rituximab, belimumab, and abatacept have been reported to be effective in patients with SS, except TNF inhibitors (13).
Metformin, an oral anti-hyperglycemic agent, is a first-line therapy for type 2 diabetes mellitus (DM) due to an improvement of insulin sensitivity and a decrease in glucose production (14). Furthermore, survival benefits associated with metformin use in numerous types of cancer have been reported, including colorectal cancer, neck cancer, and non-small-cell lung cancer (15–17). Recently, metformin has shown a new benefit in autoimmune diseases due to its anti-inflammatory and immune-modulatory mechanisms (18). Although several studies have investigated the association between metformin and autoimmune disease, few studies have focused on the impact of metformin on SS. Accordingly, we aimed to investigate whether metformin would be beneficial in reduction of SS in type 2 diabetic patients in the nationwide retrospective cohort study by using the National Health Insurance Research Database (NHIRD).
This study used the Longitudinal Health Insurance Database (LHID), which was randomly sampled from the National Health Insurance Research Database (NHIRD) derived from a single-payer system for healthcare launched in Taiwan in 1995, and 99.9% of Taiwan's population was enrolled. The LHID was released with anonymous and encrypted identifiers for preserving privacy and consisted of comprehensive medical records of one million beneficiaries involving diagnoses of diseases, inpatient and outpatient services, and details of the use of prescription drugs, operations and investigations. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) was used in assigning codes to diagnoses. The study was approved by the Research Ethics Committee at the China Medical University and Hospital [CMUH104-REC2-115(CR-6)].
Patients with at least one inpatient or two outpatient claims of type 2 diabetes mellitus (ICD-9-CM: 250 except 250.x1 and 250.x3) were enrolled (19). Patients who received metformin after the diagnosis of type 2 diabetes mellitus were assigned to the case cohort, and the index date was defined as the date when patients were first prescribed metformin between 2000 and 2012. Type 2 diabetes mellitus patients without metformin treatment were randomly selected and matched with metformin users for the index year, 5-year age group, gender, baseline comorbidities, and other anti-diabetic drugs in a ratio of 1:1 by propensity score matching. The end date of follow-up was the onset date of Sjögren's syndrome (ICD-9-CM: 710.2), the date of withdrawal or death, or December 31st, 2013 (20, 21). The study excluded (1) patients aged <20 years, (2) patients who were diagnosed with Sjögren's syndrome before index dates, (3) patients who had missing data on gender, (4) patients whose follow-up duration was 0 or less, (5) patients whose index dates were not between enrollment dates and end of study, and (6) metformin users whose treatment duration was 0 or less. Figure 1 displays the flowchart of the study population selection.
Baseline comorbidities considered included cirrhosis (ICD-9-CM: 571), hypertension (ICD-9-CM: 401–405), hyperlipidemia (ICD-9-CM: 272.0–272.4), asthma (ICD-9-CM: 493), chronic obstruction pulmonary disease (ICD-9-CM: 490–496), coronary artery disease (ICD-9-CM: 410–414), anxiety (ICD-9-CM: 300), alcohol-related disorders (ICD-9-CM: 291, 303), tobacco use disorder (ICD-9-CM: 305.1), and autoimmune diseases (ICD-9-CM: 710.0, 710.1, 710.3, 710.4, 714.0, 714.30, 714.31, 714.32, 714.33). Patients diagnosed with the comorbidities should have at least one inpatient or two outpatient claims. Other anti-diabetic drugs considered included DPP-4 inhibitors, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, and insulin. All medications should be prescribed after the diagnosis of type 2 diabetes mellitus.
Descriptive statistics of demographics, comorbidities, and medications were summarized by counts and percentages for the categorical variables and means and standard deviations (SDs) for the continuous variables. The distributions of demographic, comorbidities, and medications between the case and comparison cohorts were compared using standardized mean differences (SMDs). When a SMD was <0.1 in an absolute value, a negligible difference between the two cohorts for the variables was identified. Cumulative incidence rates of events were calculated based on the Kaplan-Meier method, and the log-rank test was used to compare the difference in time-to-event distributions between the case and comparison cohorts. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were estimated using univariate Cox proportional hazards models; adjusted hazard ratios (aHRs) with 95% CIs were estimated by multivariate Cox proportional hazards models with the covariates of age, gender, comorbidities, and medications. Significant levels of 0.05 were used. To test the proportional assumption for the multivariate Cox regression model, a Wald chi-squared test was performed. Data were analyzed using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Table 1 shows the demographic characteristics, comorbidities and other anti-diabetic drugs in the propensity score matched cohorts with and without metformin among type 2 diabetic patients. The average age of metformin users was 61.54 ± 14.51 years, and males accounted for 50.43% of the users. In the profiles of baseline comorbidities and medications, there were no obvious differences between non-metformin and metformin users.
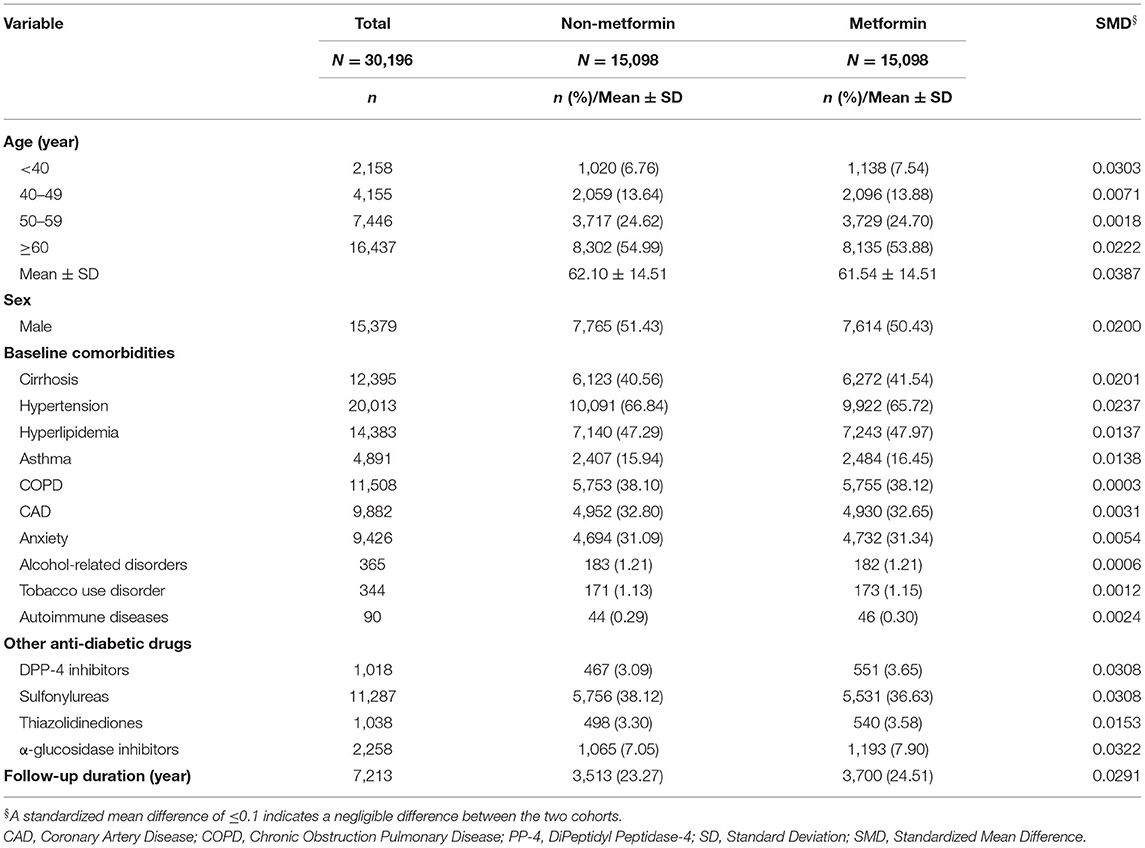
Table 1. Demographic characteristics, comorbidities, and other anti-diabetic drugs in type 2 diabetic patients with and without metformin.
Table 2 shows Cox regression analyses of Sjögren's syndrome associated with metformin, demographics, baseline comorbidities, and other anti-diabetic drugs in type 2 diabetic patients. The incidence rate of Sjögren's syndrome in non-metformin users was 40.83 per 100,000 person-years; and the incidence rate of Sjögren's syndrome in metformin users was 16.82 per 100,000 person-years. It was shown that metformin could reduce the risk of Sjögren's syndrome in type 2 diabetic patients [aHR = 0.46, 95% CI = (0.23, 0.92)]. When compared to women, men had a lower risk of developing Sjögren's syndrome [aHR = 0.15, 95% CI = (0.05, 0.41)]. In the baseline comorbidities, type 2 diabetic patients with cirrhosis were at a higher risk of developing Sjögren's syndrome [aHR = 2.26, 95% CI = (1.13, 4.54)].
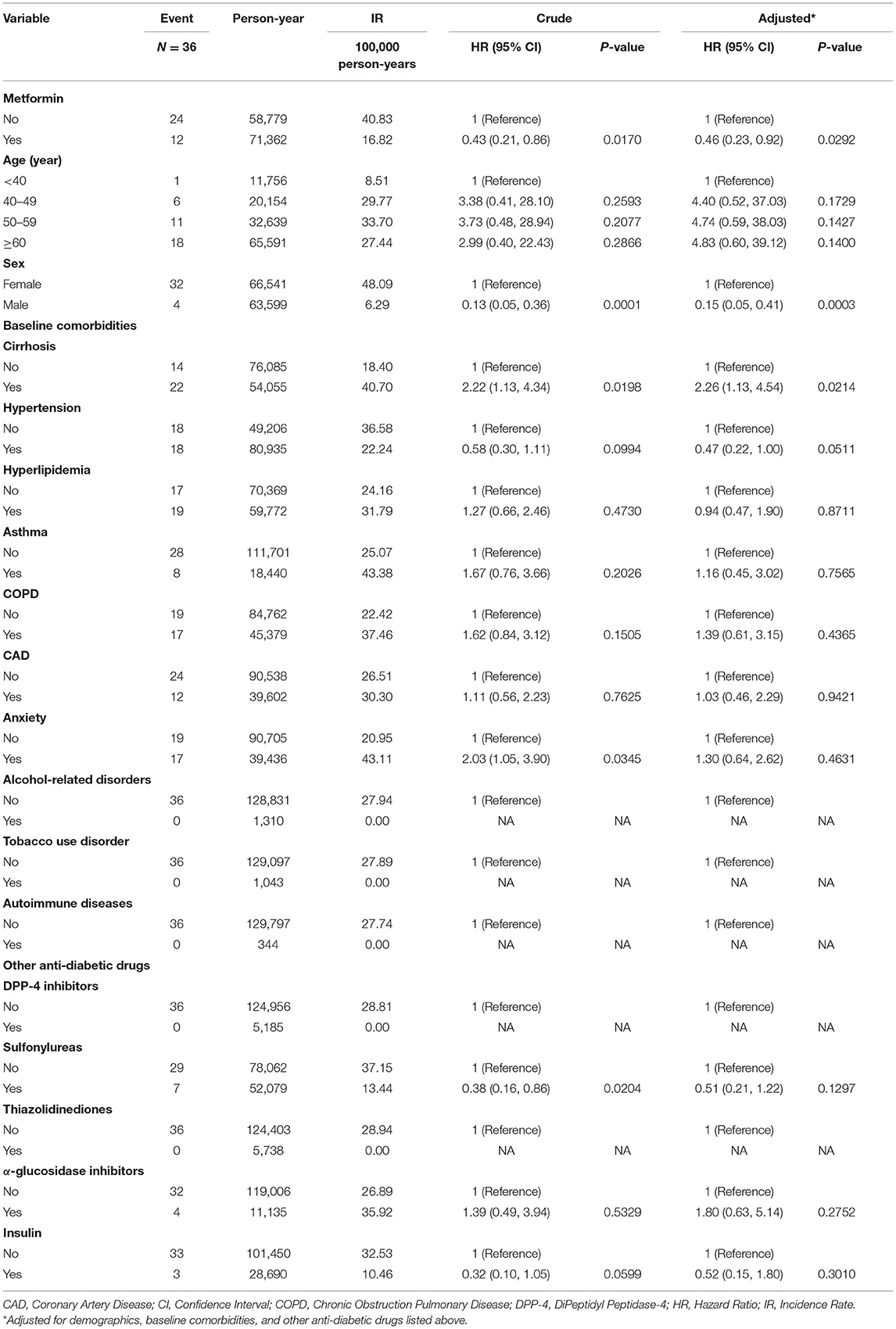
Table 2. Cox regression analyses of Sjögren's syndrome associated with metformin, demographics, baseline comorbidities, and other anti-diabetic drugs in type 2 diabetic patients.
Table 3 shows comparison of incidence of Sjögren's syndrome in type 2 diabetic patients with and without metformin stratified by demographics, baseline comorbidities, and other anti-diabetic drugs. After prescribing metformin to type 2 diabetic patients aged 60 years or more, those patients had a lower risk of developing Sjögren's syndrome [aHR = 0.34, 95% CI = (0.12, 0.96)].
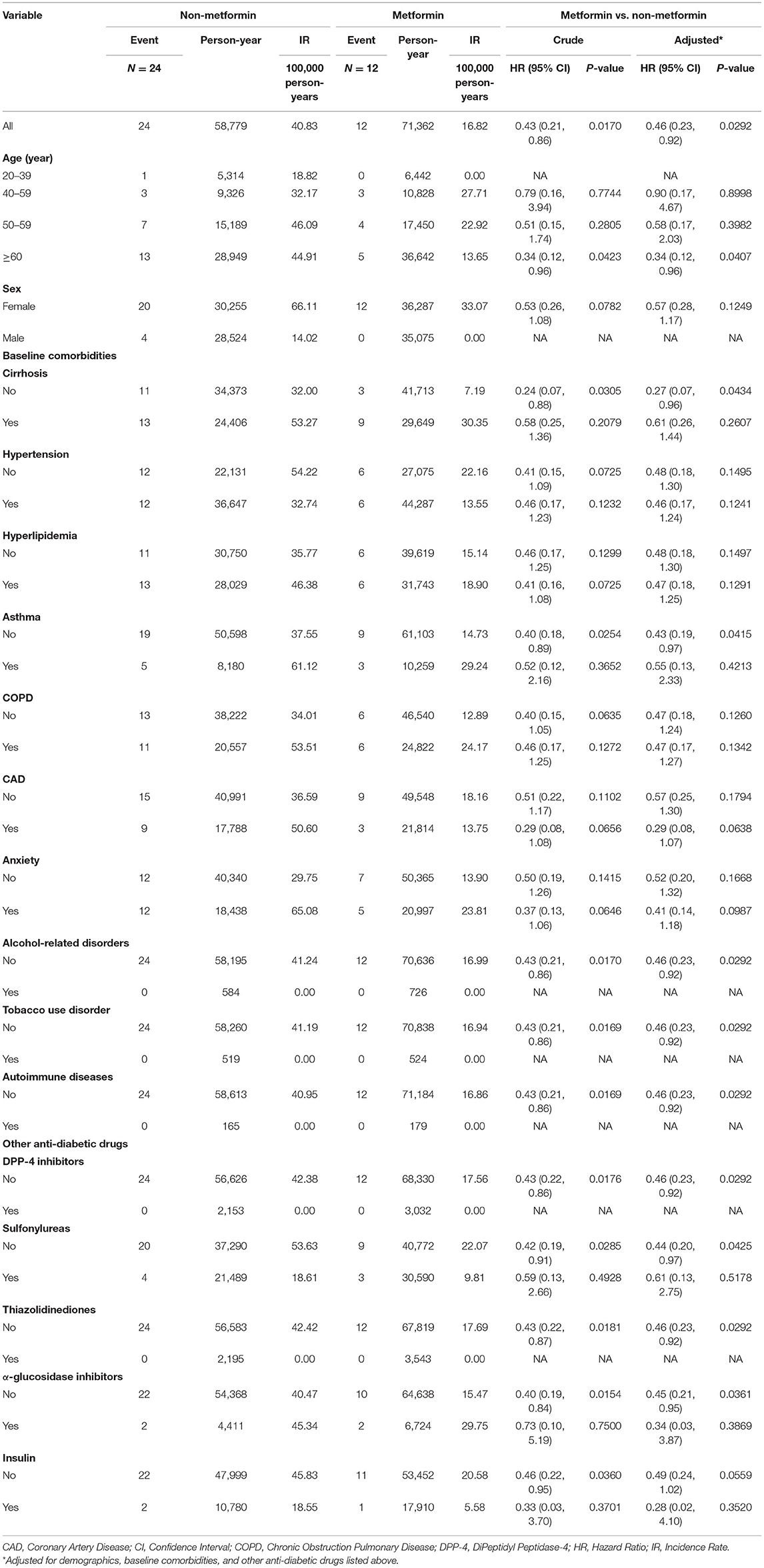
Table 3. Comparisons of incidence of Sjögren's syndrome in type 2 diabetic patients with and without metformin stratified by demographics, baseline comorbidities, and other anti-diabetic drugs.
Table 4 shows Cox regression analyses of Sjögren's syndrome associated with different treatment duration and cumulative doses of metformin in type 2 diabetic patients. When treatment duration of metformin was 90 days or more, the risk of Sjögren's syndrome decreased in type 2 diabetic patients with metformin in contrast to those without metformin [aHR = 0.27, 95% CI = (0.10, 0.71)]. On the other hand, when cumulative doses of metformin was 45,000 mg or more, the risk of Sjögren's syndrome also decreased in type 2 diabetic patients with metformin in contrast to those without metformin [aHR = 0.30, 95% CI = (0.12, 0.74)].
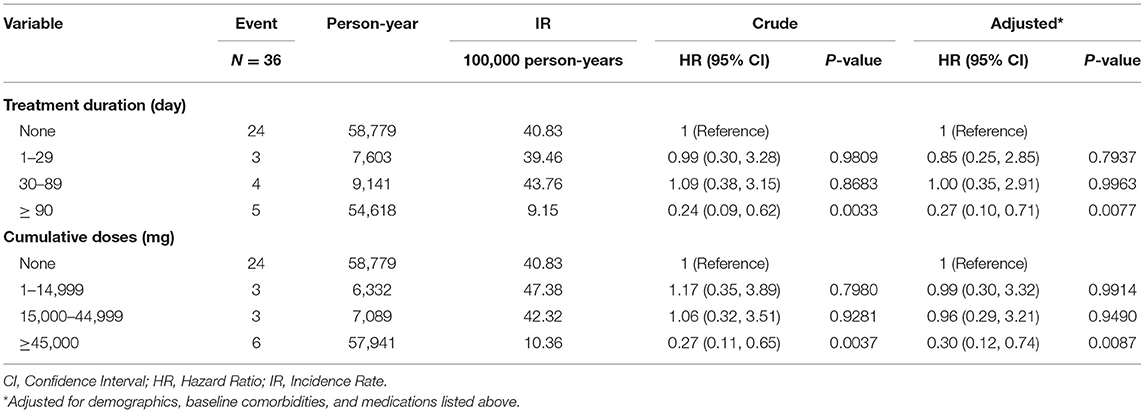
Table 4. Cox regression analyses of Sjögren's syndrome associated with different treatment duration and cumulative doses of metformin in type 2 diabetic patients.
Figure 2 depicts the cumulative incidence of Sjögren's syndrome curves in type 2 diabetic patients with and without metformin. The resulting p-value for the log rank test between the curves of two cohorts was <0.05, and the case cohort was more likely to have a lower risk of developing Sjögren's syndrome than the comparison cohort.
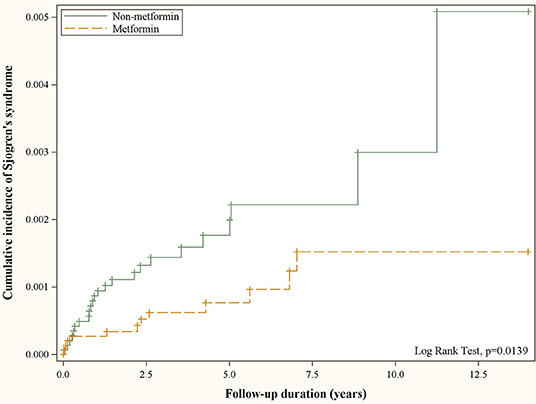
Figure 2. Cumulative incidence of Sjögren's syndrome in patients with and without metformin use obtained using the Kaplan–Meier method.
Table 5 shows the proportionality assumption test for the multivariate Cox regression model in Table 2. We generated the time dependent covariates by creating interactions of the predictors and a natural logarithmic function of follow-up duration and included these in the multivariate model used in Table 2. The result shows that we could not reject the proportionality assumption in the case (p-value = 0.3679).

Table 5. Test of proportional hazard assumption for the multivariate Cox regression model used in Table 2.
In this nationwide population-based cohort study, we found that diabetic patients exposed to metformin had a reduced risk of SS compared to those without metformin use [aHR = 0.46, 95% CI = (0.23, 0.92)]. In subgroup analysis, type 2 diabetic patients aged 60 years or more had a lower risk of developing Sjögren's syndrome under metformin use [aHR = 0.34, 95% CI = (0.12, 0.96)].
Several studies have revealed the novel use of metformin for its anti-inflammatory and immune-modulatory effects (22). Recently, distinct benefits between various autoimmune diseases and the use of metformin have been noted in observational cohort studies of psoriasis, multiple sclerosis, myasthenia gravis, ankylosing spondylitis, and rheumatoid arthritis (RA) (23–26). Metformin may also reduce all-cause mortality and admission rate among patients with autoimmune disease (27). Data from several animal models, including experimental autoimmune encephalomyelitis, collagen antibody-induced arthritis, inflammatory bowel disease and Roquinsan/san model of systemic lupus erythematosus, strongly supported the immune-modulatory effect of metformin with abilities to suppress T helper (Th)17 cells, promote regulatory T (Treg) cells production, or reduce autoreactive marginal B cells and geminal center formation (28, 29). At the molecular level, these immuno-modulatory effects of metformin were characterized by an increased activation of 5'-AMP-activated protein kinase (AMPK) with subsequent decrease in phosphorylation of mammalian target of rapamycin (mTOR) and the signal transducer and activator of transcription (STAT) 3 pathway. Another study also demonstrated that metformin could inhibit the proliferation of human RA-fibrobalst-like synoviocytes through cell cycle arrest by regulating the insulin-like growth factor receptor/phosphoinositide kinase 3/ protein kinase B/ m-TOR pathway (30). Moreover, the indirect effects of metformin on anti-inflammation might also be induced by an improvement in hyperglycemic episodes, weight reduction, and lipid control after its prescription (31). Our longitudinal population-based study provides strong evidence of the reduced risk of SS in metformin-treated patients with type 2 diabetes mellitus. Although few large-scale studies have investigated the new use of this old drug in SS, there are two available studies that support our results (32, 33). A significant decrease in the ratio of Th17/Treg cells in peripheral blood with the improvement of clinical symptoms was observed in patients with SS after metformin treatment (32). In addition, a murine model of SS revealed that metformin could ameliorate salivary gland inflammation by downregulating the expression of interleukin (IL)-6, tumor necrosis factor-α, and IL-17 in situ, maintaining the balance between effector T and Treg cells and controlling B cells differentiation (33).
In our study, medications including other anti-diabetic drugs between the case and comparison cohorts were compared. Dipeptidyl peptidase-4 inhibitors (DPP4i), Sulfonylureas, Thiazolidinediones, α-glucosidase and insulin may not reduce the incidence of SS. However, a retrospective cohort study showed that DPP4i might reduce the incidence of autoimmune disorders in type 2 DM patients with HR 0.56 (95% CI 0.53–0.60; P < 0.001) (34). The underlying mechanism might be attributed to the important role of CD26/DPP4 in T cell development and memory T cell generation (35). A case report also showed that the use of gliclazide might induce the insulin autoimmune syndrome (36). Further studies are necessary to investigate the underlying mechanism.
Our study possesses several strengths compared to previous studies. To the best of our knowledge, this is the first worldwide population-based study that revealed the association between metformin and reduced risk of SS. The nationwide database covering >99% of the population avoided selection bias. Furthermore, a previous animal model study supports our hypothesis and correlates well with our current results (33). Second, due to the aggravating symptoms and life-threatening comorbidities of SS, many traditional immunotherapy and biological medicines have been investigated. However, the results of these traditional medicines on SS have not been on a par with the marked efficacy seen in treating other autoimmune diseases such as RA and systemic lupus erythematosus (12). Therefore, our findings alternatively offer a preclinical background that the new use of metformin could be reconsidered in clinical trials designed to prove its efficacy in patients with SS due to its well-established safety profile and low cost.
This study has several limitations. First, the incidence of SS in metformin users, in contrast to non-metformin users, was not significant in the subgroup analysis of age even though the overall aHR was significant. There might be some confounding factors that could affect the risk assessment of SS. Different aspects of individual lifestyle, including smoking, alcohol use, daily diet plan, coexisting autoimmune diseases, were all possible confounders. Second, although the sample size in our study is large (N = 30,196), the number of events are very small (N = 24). Any misclassification in the outcome variable can easily change the p-value of the adjusted hazard ratio to >0.05. Our study focused on SS in the Taiwan database diagnosed between 2000 and 2013. Misclassification bias might have existed due to the modification of the diagnosis criteria in SS; for instance, the inclusion of salivary gland ultrasonography in the 2016 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria slightly increased the sensitivity from 87.4 to 91.1% (37). Third, the association between the mean daily metformin dose and the risk of SS should be elucidated in further studies. Forth, the manuscript lacked detailed information on patient characteristics at baseline, including past/family history, signs and symptoms of SS, organ manifestation, and concomitant use of treatments.
In conclusion, this 13-year, nationwide, population-based retrospective study demonstrated that type 2 diabetic patients with metformin treatment is associated with a reduced risk of developing SS. Further studies are required to strengthen the result in clinical trial, and to examine the underlying mechanism.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
C-YW: drafting the work. J-NL: substantial contributions to the conception or design of the work. K-CH: the acquisition, analysis, or interpretation of data for the work. K-LS and C-HL: revising it critically for important intellectual content. JW: providing final revision and agreement to all aspects of this research and manuscript in order to ensure the ultimate accuracy and quality of this work. All authors contributed to the article and approved the submitted version.
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-109-231), Tseng-Lien Lin Foundation, Taichung, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Hackett KL, Gotts ZM, Ellis J, Deary V, Rapley T, Ng WF, et al. An investigation into the prevalence of sleep disturbances in primary Sjögren's syndrome: a systematic review of the literature. Rheumatology. (2017) 56:570–80. doi: 10.1093/rheumatology/kew443
3. Voulgarelis M, Moutsopoulos HM. Mucosa-associated lymphoid tissue lymphoma in Sjögren's syndrome: risks, management, and prognosis. Rheumat Dis Clin N Am. (2008) 34:921–33, viii. doi: 10.1016/j.rdc.2008.08.006
4. López-Pintor RM, Fernández Castro M, Hernández G. Oral involvement in patients with primary Sjögren's syndrome. Multidisciplinary care by dentists and rheumatologists. Reumatol Clin. (2015) 11:387–94. doi: 10.1016/j.reumae.2015.03.014
5. Ramos-Casals M, Brito-Zerón P, Seror R, Bootsma H, Bowman SJ, Dörner T, et al. Characterization of systemic disease in primary Sjögren's syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology. (2015) 54:2230–8. doi: 10.1093/rheumatology/kev200
6. Alunno A, Carubbi F, Bartoloni E, Cipriani P, Giacomelli R, Gerli R. The kaleidoscope of neurological manifestations in primary Sjögren's syndrome. Clin Exp Rheumatol. (2019) 37 (Suppl. 118):192–98.
7. Westhoff G, Zink A. [Epidemiology of primary Sjörgren's syndrome]. Zeitschrift Rheumatol. (2010) 69:41–9. doi: 10.1007/s00393-009-0518-3
8. Kang HI, Fei HM, Saito I, Sawada S, Chen SL, Yi D, et al. Comparison of HLA class II genes in Caucasoid, Chinese, and Japanese patients with primary Sjögren's syndrome. J Immunol. (1993) 150:3615–23.
9. Jonsson R, Moen K, Vestrheim D, Szodoray P. Current issues in Sjögren's syndrome. Oral Dis. (2002) 8:130–40. doi: 10.1034/j.1601-0825.2002.02846.x
10. Ishimaru N, Arakaki R, Yoshida S, Yamada A, Noji S, Hayashi Y. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjögren's syndrome-like autoimmune exocrinopathy. J Exp Med. (2008) 205:2915–27. doi: 10.1084/jem.20080174
11. Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren's syndrome. Nat Rev Rheumatol. (2010) 6:529–37. doi: 10.1038/nrrheum.2010.118
12. Mavragani CP, Moutsopoulos NM, Moutsopoulos HM. The management of Sjögren's syndrome. Nat Clin Pract Rheumatol. (2006) 2:252–61. doi: 10.1038/ncprheum0165
13. Mavragani CP, Moutsopoulos HM. Sjögren's syndrome: old and new therapeutic targets. J Autoimmunity. (2020) 110:102364. doi: 10.1016/j.jaut.2019.102364
14. Yang X, Xu Z, Zhang C, Cai Z, Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim Biophys Acta. (2017) 1863:1984–90. doi: 10.1016/j.bbadis.2016.09.019
15. Tseng CH. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: a retrospective cohort analysis. Diabetes Metab. (2017) 43:438–45. doi: 10.1016/j.diabet.2017.03.004
16. Lee DJ, McMullen CP, Foreman A, Huang SH, Lu L, Xu W, et al. Impact of metformin on disease control and survival in patients with head and neck cancer: a retrospective cohort study. J Otolaryngol Head neck Surg. (2019) 48:34. doi: 10.1186/s40463-019-0348-5
17. Chuang MC, Yang YH, Tsai YH, Hsieh MJ, Lin YC, Lin CK, et al. Survival benefit associated with metformin use in inoperable non-small cell lung cancer patients with diabetes: a population-based retrospective cohort study. PLoS ONE. (2018) 13:e0191129. doi: 10.1371/journal.pone.0191129
18. Ursini F, Russo E, Pellino G, D'Angelo S, Chiaravalloti A, De Sarro G, et al. Metformin and autoimmunity: a “new deal” of an old drug. Front Immunol. (2018) 9:1236. doi: 10.3389/fimmu.2018.01236
19. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formosan Med Assoc. (2005) 104:157–63. doi: 10.29828/JFMA.200503.0002
20. Chen H-H, Perng W-T, Chiou J-Y, Wang Y-H, Huang J-Y, Wei JC-C. Risk of dementia among patients with Sjogren's syndrome: a nationwide population-based cohort study in Taiwan. Semin Arthritis Rheumat. (2019) 48:895–9. doi: 10.1016/j.semarthrit.2018.06.007
21. Liang Y-T, Leong P-Y, Wei JC-C. Questions on the bidirectional relationship between primary Sjögren Syndrome and Non-Hodgkin Lymphoma. J Rheumatol. (2021) 48:620. doi: 10.3899/jrheum.201352
22. Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. (2016) 119:652–65. doi: 10.1161/CIRCRESAHA.116.308445
23. Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. (2016) 73:520–8. doi: 10.1001/jamaneurol.2015.4807
24. Cui Y, Chang L, Wang C, Han X, Mu L, Hao Y, et al. Metformin attenuates autoimmune disease of the neuromotor system in animal models of myasthenia gravis. Int Immunopharmacol. (2019) 75:105822. doi: 10.1016/j.intimp.2019.105822
25. Qin X, Jiang T, Liu S, Tan J, Wu H, Zheng L, et al. Effect of metformin on ossification and inflammation of fibroblasts in ankylosing spondylitis: an in vitro study. J Cell Biochem. (2018) 119:1074–82. doi: 10.1002/jcb.26275
26. Naffaa ME, Rosenberg V, Watad A, Tiosano S, Yavne Y, Chodick G, et al. Adherence to metformin and the onset of rheumatoid arthritis: a population-based cohort study. Scand J Rheumatol. (2020) 49:173–80. doi: 10.1080/03009742.2019.1695928
27. Lin C-Y, Wu C-H, Hsu C-Y, Chen T-H, Lin M-S, Lin Y-S, et al. Reduced mortality associated with the use of metformin among patients with autoimmune diseases. Front Endocrinol. (2021) 12:641635. doi: 10.3389/fendo.2021.641635
28. Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, et al. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol. (2016) 292:58–67. doi: 10.1016/j.jneuroim.2016.01.014
29. Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. (2019) 11:2393–402.
30. Chen K, Lin ZW, He SM, Wang CQ, Yang JC, Lu Y, et al. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother. (2019) 115:108875. doi: 10.1016/j.biopha.2019.108875
31. Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. (2015) 15:196–205. doi: 10.2174/1871530315666150316124019
32. Sun X, Yao H, He J, Chai G, Wei L, Xie J, et al. AB0535 Effect of metformin on the absolute number of cd4+ t cell subsets in patients with primary sjogren's syndrome. Ann Rheumat Dis. (2018) 77:1425–5. doi: 10.1136/annrheumdis-2018-eular.2255
33. Kim J-W, Kim S-M, Park J-S, Hwang S-H, Choi J, Jung K-A, et al. Metformin improves salivary gland inflammation and hypofunction in murine Sjögren's syndrome. Arthritis Res Ther. (2019) 21:136. doi: 10.1186/s13075-019-1904-0
34. Chen YC, Chen TH, Sun CC, Chen JY, Chang SS, Yeung L, et al. Dipeptidyl peptidase-4 inhibitors and the risks of autoimmune diseases in type 2 diabetes mellitus patients in Taiwan: a nationwide population-based cohort study. Acta Diabetol. (2020) 57:1181–92. doi: 10.1007/s00592-020-01533-5
35. Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol. (2016) 185:1–21. doi: 10.1111/cei.12781
36. Feng X, Yuan L, Hu Y, Zhu Y, Yang F, Jiang L, et al. Gliclazide-induced insulin autoimmune syndrome: a rare case report and review on literature. Endocr Metab Immune Disord Drug Targets. (2016) 16:230–4. doi: 10.2174/1871530316666161223144558
37. Le Goff M, Cornec D, Jousse-Joulin S, Guellec D, Costa S, Marhadour T, et al. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren's syndrome. Arthritis Res Ther. (2017) 19:269. doi: 10.1186/s13075-017-1475-x
Keywords: metformin, Sjögren's syndrome, retrospective, cohort, National Health Insurance Research Database (NHIRD)
Citation: Wang C-Y, Lai J-N, Liu C-H, Hu K-C, Sheu K-L and Wei JC-C (2022) Metformin Use Was Associated With Reduced Risk of Incidental Sjögren's Syndrome in Patients With Type 2 Diabetes: A Population-Based Cohort Study. Front. Med. 8:796615. doi: 10.3389/fmed.2021.796615
Received: 17 October 2021; Accepted: 13 December 2021;
Published: 12 January 2022.
Edited by:
Xiao-Mei Li, The First Affiliated Hospital of University of Science and Technology of China (USTC), ChinaReviewed by:
Chun Li, Peking University People‘s Hospital, ChinaCopyright © 2022 Wang, Lai, Liu, Hu, Sheu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.