
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 24 December 2021
Sec. Nephrology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.792744
This article is part of the Research TopicThe Kidney in Auto-Immune and Auto-Inflammatory Processes: Definitions, Mechanisms, and BiomarkersView all 17 articles
Central nervous system (CNS) is rarely involved in microscopic polyangiitis (MPA). Here, we report a 14-year-old girl with MPA who developed new-onset seizures with deterioration of renal function. Her brain CT scan and MRI showed concurrent complications of intracerebral hemorrhage and posterior reversible encephalopathy syndrome (PRES). She got remission with combinations of methylprednisolone pulse, plasma exchange, regular hemodialysis, antiseizure and antihypertension medications. Furthermore, it is crucial to exclude the adverse effect of medications such as corticosteroid and biological therapy. We searched the literatures, retrieved 6 cases of MPA with PRES and summarized their clinical characteristics.
Microscopic polyangiitis (MPA) is one classical type of antineutrophil cytoplasmic antibody (ANCA)-associated systemic vasculitis which mainly affects small vessels like arterioles, capillaries or venules. Unlike peripheral vasculitic neuropathy common in patients with ANCA-associated vasculitis (1), central nervous system (CNS) involvement was infrequent in MPA (2). Even so, it may lead to complications like ischemic infarction (3–5), intracerebral hemorrhage (6, 7), subarachnoid hemorrhage (8), ventricular hemorrhage (9), as well as posterior reversible encephalopathy syndrome (PRES) (10–15).
At the same time, treatment with high dose of corticosteroids and biological agents like rituximab, could also lead to CNS complications including PRES (16–19).
Herein, we reported a rare case of MPA who developed concurrent intracerebral hemorrhage and PRES.
A 14-year-old girl with no significant past medical history was admitted to the local hospital in January 2018 due to lower extremities edema. Her urine examination showed proteinuria (4.95 g/24 h) and microscopic hematuria, with serum creatinine value elevated to 2.29 mg/dl. She had positive result of perinuclear antineutrophilic cytoplasmic antibody (p-ANCA) (1:32) and the myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) was 81.7 RU/ml (Enzyme linked immunosorbent assay, normal range <10 RU/ml). Her anti-glomerular basement membrane (GBM) antibody was negative. Renal biopsy was performed and reported necrotizing crescentic glomerulonephritis with pauci-immune deposits. Imaging examinations, including computed tomography (CT) scans of head and chest, showed no abnormality. She was diagnosed as MPA and was then treated with three courses of plasma exchange, methylprednisolone pulse therapy (15 mg/kg/day for 3 days), followed by oral prednisone 1 mg/kg/day (tapered gradually after 6 weeks) and intravenous cyclophosphamide (500 mg/1.73 m2, once a month). Her serum creatinine value gradually decreased to 1.58 mg/dl and anti-MPO antibody turned to negative. She presented with a relapse six months later with her serum creatinine value increased to 3.98 mg/dl. She was admitted to our hospital on June 27, 2018. Physical examinations showed that blood pressure was 120/80 mmHg and there were no other remarkable abnormal findings on admission. She maintained with oral prednisone of 60 mg, received another 10 rounds of plasma exchange and two doses of rituximab (100 and 500 mg respectively, with an interval of one week). Three days after the second infusion of 500 mg rituximab, she was complicated with bacterial pneumonia and a further deterioration of renal function (serum creatinine level increased to 4.69 mg/dl. Ceftriaxone (1 g/day) and meropenem (0.5 g/day) were then used. Seventeen days later, she suddenly developed disorder of thought and auditory hallucination followed 10 min later by tonic-clonic seizures. Her blood pressure was 150/100 mmHg and the neurological examinations had no abnormality at that time. Her head CT scan showed a small amount of hemorrhage in the left temporal lobe (Figure 1). Brain magnetic resonance image (MRI) revealed multiple foci lesions under bilateral cortex of the superior frontal sulcus, parieto-occipital and watershed area was dominant, which suggested typical PRES features (Figures 2A1–4). The brain magnetic resonance angiography (MRA) was normal. The clinical findings timeline was presented in Figure 3.
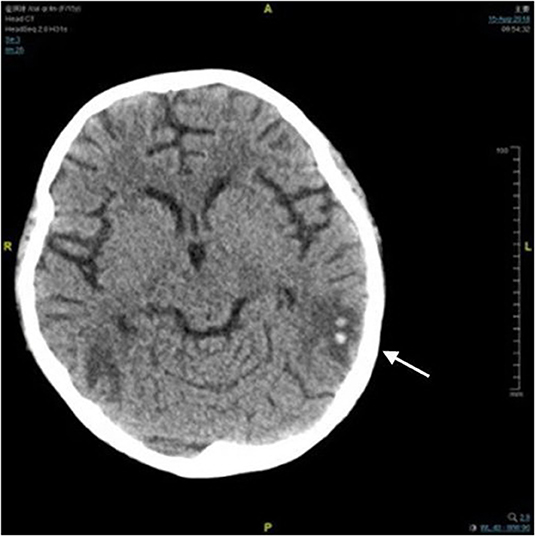
Figure 1. Computed tomography image of brain. There was a small amount of hemorrhage in the left temporal lobe (arrow).
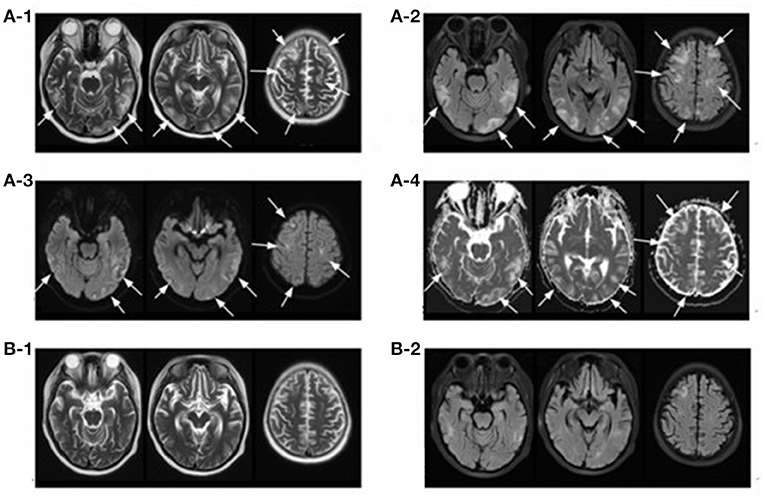
Figure 2. Magnetic resonance imaging (MRI) of brain. T2-weighted (A-1) and FLAIR (A-2) sequences depict hyper-intense lesions involving the bilateral cerebral hemispheres (occipital lobe, parietal lobe, temporal lobe and frontal lobe) and cerebral cortex and subcortex (arrows), most of which disappeared 17 days later (B-1,B-2). Diffusion-weighted magnetic resonance imaging (A-3) and apparent diffusion coefficient (A-4) sequences of the involved regions showed isointense or hyperintensity lesions (arrows).
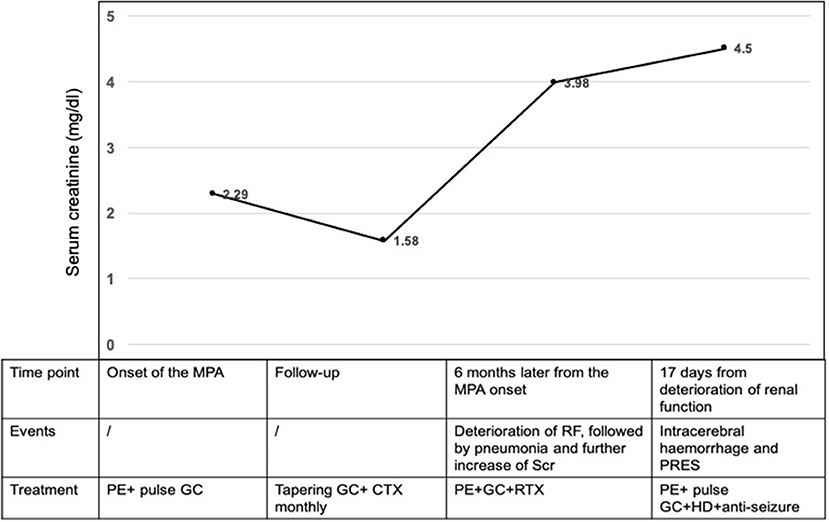
Figure 3. Clinical findings timeline. MPA, microscopic polyangiitis; GC, glucocorticoid; CTX, cyclophosphamide; PE, plasmapheresis; RTX, rituximab; RF, renal function; PRES, posterior reversible encephalopathy syndrome; HD, hemodialysis.
In our case, the head CT was normal before admission and she didn't have any other risk factors for cerebrovascular disease. Her new-onset of consciousness changing and seizures, accompanied by brain MRI showing vasogenic edema in bilateral cerebral hemispheres, indicating the classic characteristics of PRES. Based on the progressive advanced renal failure with severe microscopic hematuria, elevated erythrocyte sedimentation rate (ESR) and C-reactive protein level, her Birmingham Vasculitis Activity Score (BVAS) at the time of PRES was 21, which indicated high disease activity. Furthermore, receiving high dose corticosteroid, and cytotoxic agents such as rituximab, might contribute to hypervolemia and vasospasm, which promoted the development of PRES (19–22). But the interval between the rituximab regimen against MPA and the onset of PRES was more than two weeks, much longer than the interval reported by previous literature varying between 6 h to 8 days (19–22), which excluded its contribution. The pneumonia and blood pressure were under well-control and her hemodialysis was regular and efficient, which did not support the participation of infection and hypertension (23, 24). Thus, PRES in our patient might mainly attributed to MPA. As the intracerebral hemorrhage could happen in 15% of patients with PRES (17), we proposed that the PRES contributed to the intracranial hemorrhage. Therefore, treatment was aimed at MPA with administration of 200 mg methylprednisolone and initiation of another seven courses of plasma exchange, in combination with regular hemodialysis (three times per week), anti-seizure medication and well blood pressure control. The patient's mental symptoms resolved within 24 h.
The follow-up brain MRI after 17 days showed that the degree of gyrus swelling was reduced, most of the abnormal high signal shadows disappeared and multiple small ischemic lesions were found under bilateral frontal-parietal cortex (Figures 2B1,2). Unfortunately, her renal function did not recover with maintenance hemodialysis and oral prednisone gradually tapered after 6 weeks. Her peripheral-blood CD19+ B-cell counts decreased to 1/μl after two times of rituximab infusion and increased to 5/μl six months later when she received another time of rituximab infusion (100 mg) as part of maintenance therapy. She was content with her treatment since the disease was controlled and stabilized. No PRES relapsed and she received a deceased donor kidney transplantation in December 2019. She observed the strict and routine follow-up with treatments to prevent graft rejection, and her renal function and central nervous system condition kept stable until now.
We herein report a teenage girl with active MPA and advanced renal injury who was treated with corticosteroid and rituximab, then developed PRES with intracerebral hemorrhage presenting with consciousness disturbance and seizure. In most MPA cases, it was characterized by rapidly progressive glomerulonephritis, lung hemorrhage and interstitial pneumonitis, while the CNS involvement was reported less frequently (18).
Besides MPA, there were some other predisposed factors related to PRES should be distinguished in our patient: (1) The adverse effect of rituximab: The time interval between the infusion of rituximab and PRES in our patients was much longer than reported in previous literature which has been stated in the previous paragraph (19–22). Besides, there was no PRES recurrence during the re-administration of rituximab to the patient which helped to rule out the participation of RTX (19–22). (2) Renal failure: Renal failure could occur in up to 55–57% of the PRES cases reported in the literatures (25), in which the uremic toxins accumulation could lead to endothelial dysfunction and account for the development of PRES. Thus, renal failure may act as the “second-hit” in our case. Taken together, we proposed that PRES might be mainly attributed to MPA with participation of its associated renal dysfunction in the patient.
Furthermore, although the differentiation of which therapy actually helped the case was difficult, it was noticed that our treatment project to the patient was mainly targeted on MPA per se. The PRES was alleviated and did not relapse with the MPA stabilization which also supported our previous hypothesis that the patient's PRES might be the neurologic involvement of ANCA associated vasculitis.
Although not fully understood, endothelial dysfunction and abnormal cerebral blood flow were key factors in the pathophysiological changes underlying PRES (16, 24–26). MPA could induce inflammation within the vasculature of the CNS and result in ischemic or hemorrhagic damage to the brain parenchyma with resultant focal or generalized neurologic deficits (3–9). In PubMed, only 6 cases of MPA with PRES were reported (10–15) (More details in Table 1). In conclusion, five of them were females and one was male, with a mean age of 43.8 years (range 10–76 years). The duration from MPA onset to PRES varied from 2 days to 3 years. Two cases had hypertension at baseline and renal failure was found in all patients (serum creatinine value ranging from 1.2 to 24.48 mg/dL). All cases received immunosuppressive therapy such as high-dose steroids, plasma exchanges, etc. They responded well to the supportive therapy combining with hemodialysis, anti-seizure medication and antihypertensive drugs. During the follow-up, two cases presented with PRES recurrence, which might be attributed to quickly tapering of steroids, irregular hemodialysis, or relapse of vasculitis.
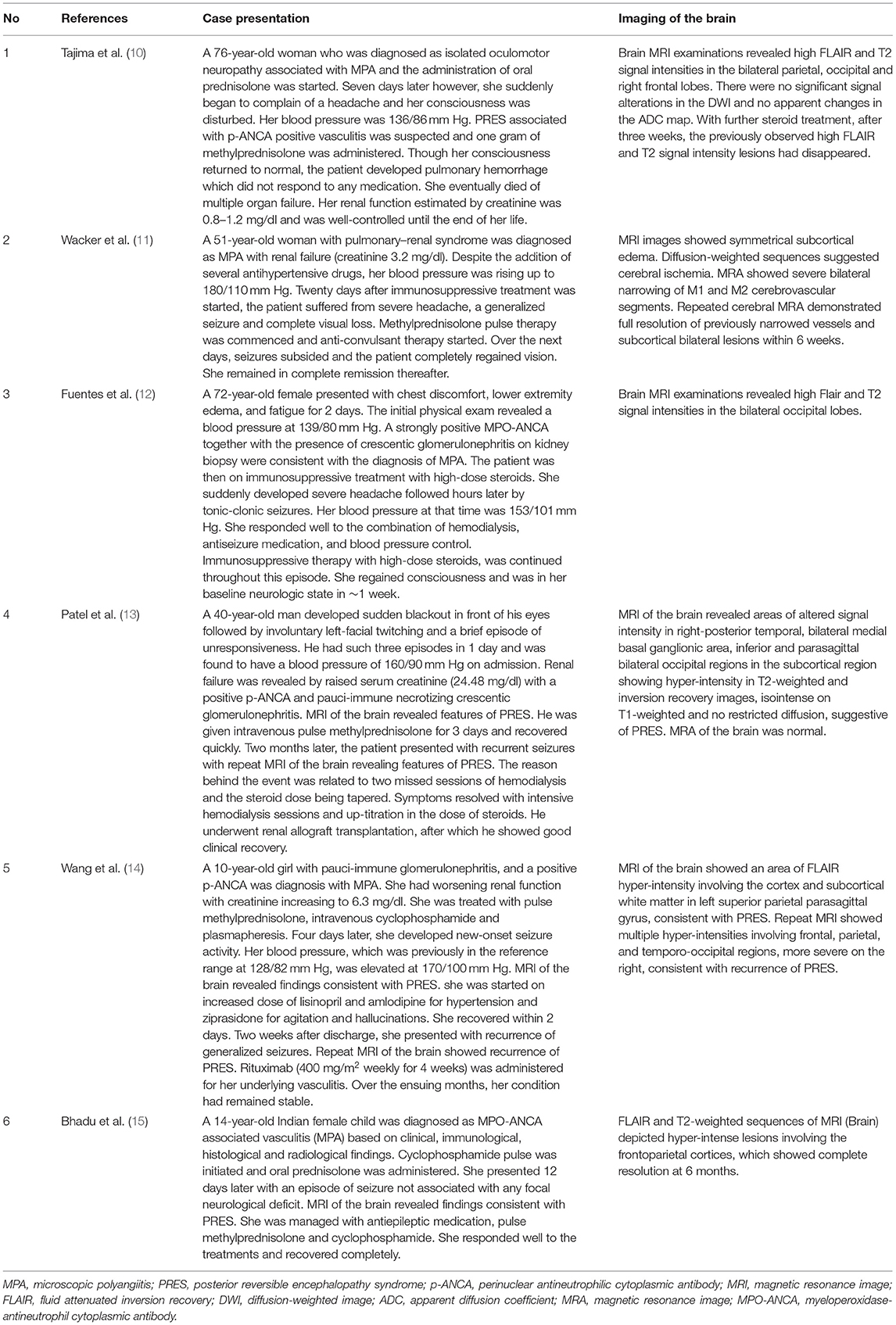
Table 1. Cases describing microscopic polyangiitis with posterior reversible encephalopathy syndrome.
The limitation of our case was that it was a pure clinical report and the causal relationship between MPA and PRES accompanied by intracerebral hemorrhage was not fully elucidated. Further laboratory studies like in vitro experiments or animal models, are needed.
In summary, this was a case which presented a MPA patient complicated with PRES. Through careful differential diagnosis. We excluded rituximab as the cause of PRES and the patient was well followed-up.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Peking University International Hospital, Beijing, China, does not require ethical approval for reporting individual cases. Written informed consent for patient information and images to be published was provided by the patient's legally authorized representative.
ZQ and FY: conceptualization, supervision, and writing—review and editing. JX, YD, ZQ, and FY: data curation and investigation. JX and YD: writing—original draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wludarczyk A, Szczeklik W. Neurological manifestations in ANCA-associated vasculitis – assessment and treatment. Expert Rev Neurother. (2016) 16:861–3. doi: 10.1586/14737175.2016.1165095
2. Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol. (2018) 9:1166. doi: 10.3389/fneur.2018.01166
3. Ku BD, Shin HY. Multiple bilateral non-hemorrhagic cerebral infarctions associated with microscopic polyangiitis. Clin Neurol Neurosurg. (2009) 111:904–6. doi: 10.1016/j.clineuro.2009.08.008
4. Chen NC, Lai PH, Fang HC, Chou KJ, Chen CL. Microscopic polyangiitis with an initial presentation of pontine infarction. Am J Med Sci. (2012) 344:163–5. doi: 10.1097/MAJ.0b013e3182536789
5. Ito Y, Suzuki K, Yamazaki T, Yoshizawa T, Ohkoshi N, Matsumura A. ANCA-associated vasculitis (AAV) causing bilateral cerebral infarction and subsequent intracerebral hemorrhage without renal and respiratory dysfunction. J Neurol Sci. (2006) 240:99–101. doi: 10.1016/j.jns.2005.07.003
6. Tan J, Hussain A, Daiwajna R, Chai LK, Lim E, Han A. Microscopic polyangiitis complicated by intracerebral hemorrhage and pulmonary hemorrhage in a pediatric patient. Am J Case Rep. (2013) 14:276–9. doi: 10.12659/AJCR.889064
7. Iglesias E, Eleftheriou D, Mankad K, Prabhakar P, Brogan PA. Microscopic polyangiitis presenting with hemorrhagic stroke. J Child Neurol. (2014) 29:Np1–4. doi: 10.1177/0883073813488661
8. Wang X, Wang J. Microscopic polyangiitis presenting as spontaneous subarachnoid haemorrhage. Nephrology. (2015) 20:110. doi: 10.1111/nep.12352
9. Chang JF, Lai YJ, Tsai CC, Peng YS. Intraventricular hemorrhage as a complication of microscopic polyangiitis. Kidney Int. (2015) 88:642–3. doi: 10.1038/ki.2015.10
10. Tajima Y, Matsumoto A. Reversible posterior leukoencephalopathy syndrome in p-ANCA-associated vasculitis. Internal Med (Tokyo, Japan). (2006) 45:1169–71. doi: 10.2169/internalmedicine.45.1839
11. Wacker J, Handschu R, Manger B, Schett G, Zwerina J. Sudden visual loss in a patient with microscopic polyangiitis. Rheumatology (Oxford, England). (2009) 48:1173–4. doi: 10.1093/rheumatology/kep193
12. Fuentes AG, Komarla A, Gomez JI. Posterior reversible encephalopathy syndrome in a patient with ANCA-associated vasculitis. Rheumatol Int. (2012) 32:2529–30. doi: 10.1007/s00296-010-1367-8
13. Patel UV, Patel NJ. Posterior reversible leukoencephalopathy syndrome as a presenting manifestation of p-ANCA-associated vasculitis. BMJ Case Rep. (2014) 2014. doi: 10.1136/bcr-2013-202022
14. Wang S, Habib S, Umer S, Reisman L, Raman V. Recurrent posterior reversible encephalopathy syndrome in a child with microscopic polyangiitis. J Clin Rheumatol. (2015) 21:113–4. doi: 10.1097/RHU.0000000000000222
15. Bhadu D, Kumar P, Malhotra KP, Sharma A, Sharma M, Srivastava D. Central nervous system vasculitis in pediatric microscopic polyangiitis. Acta Reumatol Portuguesa. (2016) 41:372–5.
16. Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, et al. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. (2017) 380:11–5. doi: 10.1016/j.jns.2017.06.044
17. Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. (2009) 30:1371–9. doi: 10.3174/ajnr.A1588
18. Koike H, Sobue G. Clinicopathological features of neuropathy in anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Nephrol. (2013) 17:683–5. doi: 10.1007/s10157-012-0767-3
19. Mavragani CP, Vlachoyiannopoulos PG, Kosmas N, Boletis I, Tzioufas AG, Voulgarelis M, et al. Case of reversible posterior leucoencephalopathy syndrome after rituximab infusion. Rheumatology (Oxford, England). (2004) 43:1450–1. doi: 10.1093/rheumatology/keh305
20. Sánchez-Carteyron A, Alarcia R, Ara JR, Martín J. Posterior reversible encephalopathy syndrome after rituximab infusion in neuromyelitis optica. Neurology. (2010) 74:1471–3. doi: 10.1212/WNL.0b013e3181dc1af3
21. Jaiswal A, Sabnani I, Baran DA, Zucker MJ. A unique case of rituximab-related posterior reversible encephalopathy syndrome in a heart transplant recipient with posttransplant lymphoproliferative disorder. Am J Transplant. (2015) 15:823–6. doi: 10.1111/ajt.13021
22. Mondal S, Goswami RP, Sinha D, Basu K, Das S, Ghosh P, et al. Posterior reversible encephalopathy syndrome in a patient with lupus nephritis on rituximab therapy: a challenge to find the offender. Lupus. (2016) 25:445–6. doi: 10.1177/0961203315607648
23. Racchiusa S, Mormina E, Ax A, Musumeci O, Longo M, Granata F. Posterior reversible encephalopathy syndrome (PRES) and infection: a systematic review of the literature. Neurol Sci. (2019) 40:915–22. doi: 10.1007/s10072-018-3651-4
24. Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. (2018) 89:14–20. doi: 10.1136/jnnp-2017-316225
25. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. (2015) 14:914–25. doi: 10.1016/S1474-4422(15)00111-8
Keywords: microscopic polyangiitis, central nervous system, intracerebral hemorrhage, posterior reversible encephalopathy syndrome, case report
Citation: Xu J, Ding Y, Qu Z and Yu F (2021) Posterior Reversible Encephalopathy Syndrome in a Patient With Microscopic Polyangiitis: A Case Report and Literature Review. Front. Med. 8:792744. doi: 10.3389/fmed.2021.792744
Received: 11 October 2021; Accepted: 07 December 2021;
Published: 24 December 2021.
Edited by:
Gian Marco Ghiggeri, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Prasanta Padhan, Kalinga Institute of Medical Sciences (KIMS), IndiaCopyright © 2021 Xu, Ding, Qu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Qu, amFuZXF1ODJAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.