
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 10 January 2022
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.791689
 Daniel R. Machin1,2,3
Daniel R. Machin1,2,3 Heather L. Clifton2
Heather L. Clifton2 D. Walter Wray1,2,4
D. Walter Wray1,2,4 Tracy M. Frech1,5
Tracy M. Frech1,5 Anthony J. Donato1,2,4,6*
Anthony J. Donato1,2,4,6*Systemic sclerosis (SSc) is a rare, auto-immune disease with variably progressive fibrosis of the skin and internal organs, as well as vascular dysfunction. Recently, we demonstrated a decrement in exercising skeletal muscle blood flow and endothelium-dependent vasodilation in SSc, but the mechanisms responsible for these impairments have not been investigated. Thus, we sought to determine if acute administration of tetrahydrobiopterin (BH4), an essential cofactor for endothelial nitric oxide synthase (eNOS), would improve hyperemia and brachial artery vasodilation during progressive handgrip exercise in SSc. Thirteen patients with SSc (63 ± 11 years) participated in this placebo-controlled, randomized, double-blind, crossover study. Tetrahydrobiopterin (10 mg/kg) administration resulted in a ~4-fold increase in circulating BH4 concentrations (P < 0.05). Cardiovascular variables at rest were unaffected by BH4 (P > 0.05). During handgrip exercise, BH4 administration increased brachial artery blood flow (placebo: 200 ± 87; BH4: 261 ± 115 ml/min; P < 0.05) and vascular conductance (placebo: 2.0 ± 0.8; BH4: 2.5 ± 1.0 ml/min/mmHg; P < 0.05), indicating augmented resistance artery vasodilation. Tetrahydrobiopterin administration also increased brachial artery vasodilation in response to exercise (placebo: 12 ± 6; BH4: 17 ± 7%; P < 0.05), resulting in a significant upward shift in the slope relationship between Δ brachial artery vasodilation and Δ shear rate (placebo: 0.030 ± 0.007; BH4: 0.047 ± 0.007; P < 0.05) that indicates augmented sensitivity of the brachial artery to vasodilate to the sustained elevations in shear rate during handgrip exercise. These results demonstrate the efficacy of acute BH4 administration to improve both resistance and conduit vessel endothelial function in SSc, suggesting that eNOS recoupling may be an effective strategy for improving vasodilatory capacity in this patient group.
Systemic sclerosis (SSc, scleroderma) is a rare auto-immune disease that is characterized by variably progressive fibrosis of the skin and internal organs, as well as an attenuated exercise capacity (1). Despite variability in the extent of organ involvement among SSc patients, the presence of enhanced peripheral vascular resistance is nearly universal (2). Indeed, a meta-analysis has reported that peripheral arterial vasodilatory capacity, as determined by flow-mediated dilation, is diminished in patients with SSc (3). Additionally, we have shown that the reactive hyperemic response during the flow-mediated dilation testing is also blunted in this population (4). Although the attenuated exercise capacity in SSc patients (1) is often linked to cardiopulmonary abnormalities (5, 6), exercise capacity remains impaired in SSc patients without central hemodynamic impairments (7), suggesting that impairments in peripheral vascular control play an important role in exercise intolerance in this patient group.
We have recently demonstrated marked impairments in the peripheral vascular response to exercise in SSc patients (8). Compared to healthy controls, we observed a ~35% reduction in forearm blood flow and vascular conductance during progressive handgrip exercise in SSc patients, demonstrating a clear disease-related impairment in “exercise hyperemia” in this patient group. This decrement in resistance vessel responsiveness was accompanied by blunted dilation of the brachial artery in response to the sustained elevation in shear rate present during exercise, suggesting that conduit vessel endothelium-dependent vasodilation is also diminished in SSc patients. Considering the widespread prevalence of elevated peripheral vascular resistance and diminished vasodilatory capacity in SSc, it is likely that dysfunctional peripheral arteries contribute to the attenuated exercise capacity in this population.
In addition to peripheral vascular dysfunction, blood concentrations of oxidative stress and damage markers are elevated in patients with SSc (9, 10). The vascular endothelium is particularly vulnerable to oxidative damage (11), as increases in oxidative stress result in endothelial dysfunction (12) that can impair the peripheral vascular response to exercise (13). Indeed, we have shown that elevated blood oxidative stress markers accompany brachial arterial endothelial dysfunction during handgrip exercise in SSc patients (8). Therefore, if brachial artery endothelial dysfunction during exercise is due to elevated systemic oxidative stress then it is likely that the downstream resistance artery vasodilatory dysfunction may also be due to oxidative stress-induced endothelial dysfunction.
Low endothelial tetrahydrobiopterin (BH4) bioavailability is one potential source of oxidative stress in SSc patients. Tetrahydrobiopterin is an essential cofactor for endothelial nitric oxide synthase (eNOS) (14) that is critical for maintaining nitric oxide (NO) bioavailability in the vascular endothelium (15). An insufficient endothelial concentration of BH4 results in “uncoupled” eNOS that no longer produces NO, but produces superoxide instead (16). Increased superoxide can lead to peroxynitrite formation, which, in turn, oxidizes BH4 to its inactive form, leading to further eNOS uncoupling, greater superoxide formation, and reduced NO bioavailability (17). We have recently reported that acute BH4 administration augments resting brachial artery flow-mediated dilation and reactive hyperemia in SSc patients (18). Currently, it is unknown if exogenous BH4 administration can improve the peripheral vascular response to progressive handgrip exercise in SSc patients. Therefore, we sought to examine the peripheral vascular response to progressive handgrip exercise after acute oral BH4 administration in patients with SSc. Progressive handgrip exercise was employed in this study, as it incorporates a small muscle mass, thus, requiring a fraction of maximal cardiac output, limiting the impact of central hemodynamic factors (19). We hypothesized that, compared to placebo, acute BH4 administration would augment exercise-induced hyperemia, our primary endpoint, as well as the brachial artery vasodilation to increases in shear rate that occur during handgrip exercise.
Written informed consent was obtained prior to participation after an explanation of the nature, benefits, and risks of the study. All procedures were approved by the institutional review board of the University of Utah and Salt Lake City Veterans Affairs Medical Center (IRB# 38705), which serves as the ethics committee.
Thirteen patients with SSc were recruited from the University of Utah SSc Clinic to participate in this study. Patients were previously diagnosed with SSc, by 2013 classification criteria (20). Body mass index was calculated from height and body mass. The clinical features of patients with SSc that were recorded for SSc disease duration, cardiovascular-acting medications, SSc-related vasculopathy medical history (i.e., pulmonary arterial hypertension, scleroderma renal crisis, and/or digital ulcer), antinuclear antibody, and SSc-specific antibody status. None of the participants had diabetes mellitus, overt cardiovascular disease, or met criteria for another inflammatory overlap rheumatic disease.
A placebo-controlled, randomized, double-blind, crossover experimental design was employed with a washout period of at least 5 days before crossing over into the alternate study drug condition. Treatment randomization was performed by DWW using coin flip and kept confidential from the remainder of the research team. On the experimental days, patients reported to the laboratory after having consumed a standardized breakfast and oral BH4 (10 mg/kg) or placebo in tablet form 5 h prior to their arrival. Main known side effects of BH4, such as headache and rhinorrhea, and participants were monitored for these side effects. Others have shown that this dose of oral BH4 administration effectively increases circulating BH4 concentrations (21, 22). Participants were instructed to abstain from food (not including the standardized breakfast), alcohol, caffeine, and exercise for ≥12 h prior to arrival. Additionally, vasodilatory medications were discontinued 12 h prior to study visit. In premenopausal women, measurements were performed during the early follicular phase of the menstrual cycle. All measurements were made under quiet, comfortable, ambient (~22°C) laboratory conditions at the same time of day to eliminate any diurnal effects. The primary endpoint measure was brachial artery blood flow in response to progressive handgrip exercise measured via ultrasound Doppler. Secondary endpoint measures were forearm vascular conductance and brachial artery vasodilation in response to progressive handgrip exercise measured via ultrasound Doppler.
After collection of a venous blood sample, participants were instrumented for assessment of heart rate and arterial blood pressure. Static intermittent handgrip exercise was then performed at 1 Hz, as described previously (19). Participants were encouraged to perform rapid contractions with the goal of limiting contraction time to <25% of the duty cycle. Participants exercised at 15, 30, and 45% of their maximum voluntary contraction. Each exercise stage was performed for 3 min with a 2-min break allotted between each workload.
Heart rate was monitored with a three-lead electrocardiogram, recorded in duplicate on the data acquisition device (Biopac, Goleta, CA) and ultrasound Doppler (Logic 7, GE Medical Systems, Milwaukee, WI). Mean arterial blood pressure (MAP) was measured in the non-exercising contralateral arm by auscultation of the brachial artery (Tango+, SunTech, Morrisville, NC). Simultaneous measurements of brachial artery blood velocity and vessel diameter were performed using a linear array transducer operating in duplex mode, with imaging frequency of 14 MHz and Doppler frequency of 5 MHz. The brachial artery was insonated approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle. All measurements were obtained with the probe appropriately positioned to maintain an insonation angle of ≤ 60°. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Angle-corrected, time-average, and intensity-weighted mean blood velocity values were calculated using commercially available software (Logic 7). Brachial artery vasodilation was determined offline from end-diastolic, ECG R-wave-triggered images collected from the ultrasound Doppler using automated edge-detection software (Medical Imaging Applications, Coralville, IA). Ultrasound Doppler measurements were performed continuously, with the last 60 s of each exercise intensity used for the determination of limb blood flow.
Mean arterial blood pressure was calculated according to the equation: MAP (mmHg) = systolic blood pressure · 1/3 + diastolic blood pressure · 2/3. Shear rate was calculated according to the equation: shear rate (s−1) = blood velocity · 4/vessel diameter. Brachial artery blood flow was calculated as per the equation: blood flow (ml/min) = (blood velocity · π · [vessel diameter/2]2 · 60). Brachial artery vascular conductance was calculated according to the equation: blood flow/MAP.
Blood was collected in EDTA plasma tubes containing 1 mM dithiothreitol (DTE) in 0.9% saline to give a final concentration of 0.1 mM DTE. After blood collection, tubes were centrifuged at 3,000 RPM for 15 min at 4°C, and plasma was collected and stored at −80°C. To quantify BH4 concentrations, plasma was thawed and extracted using differential oxidation with iodine using the Fukushima-Nixon method (23), which enables measurement of total biopterin and BH4. Under acidic conditions, BH4 and 7,8-dihydrobiopterin (BH2) are oxidized to biopterin. Under alkaline conditions only BH2 is oxidized to biopterin, while BH4 undergoes side-chain cleavage to form pterin. Tetrahydrobiopterin levels was quantified by calculating the difference in biopterin content between the two oxidation reactions. Prior to iodine oxidation, the plasma was deproteinized by adding 250 μl of 1 M trichloroacetic acid to 1 ml plasma. This was incubated in the dark at 4°C for 15 min and centrifuged at 20,000 g for 15 min at 4°C. HPLC of biopterin was performed on an Acquity Arc system with a CORTECS®C18, 2.7 μm column (4.6 × 150 mm) with a Vanguard® pre-column at a column temperature of 40°C. All were from Waters Corporation. Samples were run isocratically with 15 mM potassium phosphate buffer, pH 6.4 at a flow rate of 0.8 ml/min. A Waters 2475 Multi λ Fluorescence Detector was used to detect biopterin with excitation set to 350 nm and an emission setting of 440 nm.
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Paired t-tests were used to compare differences in participant characteristics, cardiovascular variables at rest. A two-way repeated-measures ANOVA was used to evaluate differences between placebo and BH4 during exercise, and a least significant difference paired t-test identified the means that were significantly different. Univariate linear regression analysis was performed to confirm associations between Δ brachial artery vasodilation and Δ shear rate. Multiple linear regression was performed to model the relationships of placebo and BH4 with Δ brachial artery vasodilation to Δ shear rate. Slopes were compared by applying analysis of covariance (ANCOVA) to the straight lines obtained by the regression methods (24). Statistical significance was set at P < 0.05 for all analyses. Data are presented as mean ± SD.
Participant characteristics, including SSc-related vasculopathy medical history, are presented in Table 1. Among these participants, SSc disease duration ranged from 1 to 36 years with a mean 7 ± 11 years. Nearly all the SSc patients (85%) were prescribed calcium channel blockers as well as other vasodilatory medications, all of which were discontinued 12 h prior to the study visits. The majority of the SSc patients had a history of digital ulcers (54%), while two of these patients with a positive digital ulcer history also had pulmonary arterial hypertension or scleroderma renal crisis. Antinuclear antibody testing was previously performed in all but one of the SSc patients. All the SSc patients that were tested were positive for antinuclear antibodies and the majority (58%) were positive for anti-centromeric antibodies.
Tetrahydrobiopterin administration increased plasma BH4 concentrations ~4-fold compared with placebo (placebo: 10.9 ± 2.2; BH4: 39.5 ± 13.0 nmol/L; P < 0.05). Blood markers of oxidative stress, antioxidant capacity, and inflammation in this cohort have been published elsewhere (18), and were unchanged between placebo and BH4 conditions.
There was no effect of BH4 on handgrip maximum voluntary contraction (placebo: 17.9 ± 7.2; BH4: 17.4 ± 6.1 kg; P > 0.05). Similarly, cardiovascular variables were unaffected by acute BH4 administration at rest (Table 2). No patient reported any side effect or adverse events in response to acute BH4 administration.
Heart rate and MAP increased with each workload in response to handgrip exercise but were not different between placebo and BH4 (P > 0.05; Table 2; Figure 1A). At each handgrip workload, exercise-induced brachial artery blood velocity was greater after acute BH4 compared to placebo (P < 0.05; Table 2). Exercise-induced brachial artery blood flow was ~28-32% greater after acute BH4 administration compared to placebo (P < 0.05; Figure 1B). Because MAP was not different between placebo and BH4, like blood flow, exercise-induced vascular conductance was ~28–34% greater at each handgrip workload after BH4 compared to placebo (P < 0.05; Figure 1C). Individual peak brachial artery blood flow and vascular conductance responses to handgrip exercise are presented in Figure 2. Eleven of the 13 participants had at least a 10% increase in peak brachial artery blood flow and vascular conductance after BH4 compared to placebo.
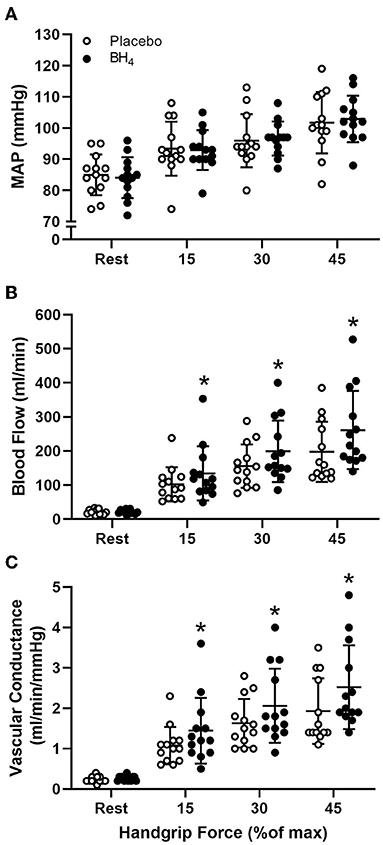
Figure 1. Mean arterial pressure [MAP (A)], brachial artery blood flow (B), and brachial artery vascular conductance (C) at rest and during progressive handgrip exercise in patients with systemic sclerosis after placebo (white circles) and tetrahydrobiopterin (BH4; black circles). *P < 0.05, significantly different than placebo. All data are presented as mean ± SD.
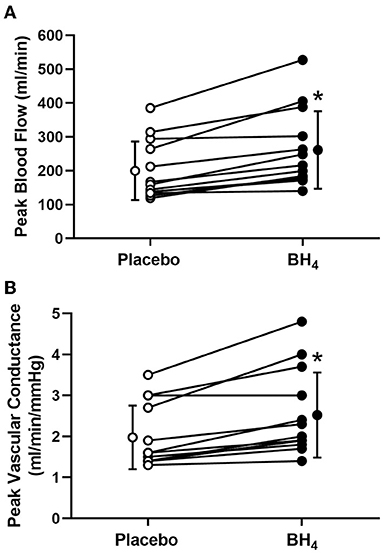
Figure 2. Individual peak brachial artery blood flow (A) and vascular conductance (B) in response to progressive handgrip exercise in patients with systemic sclerosis after placebo (white circles) and tetrahydrobiopterin (BH4; black circles). *P < 0.05, significantly different than placebo. Group data are presented as mean ± SD.
In response to handgrip exercise, brachial artery lumen diameter increased at each exercise workload (Table 2). Acute BH4 administration resulted in a ~45–70% greater vasodilatory response at each handgrip workload (P < 0.05; Figure 3). Exercise-induced shear rate was also significantly at each handgrip workload (P < 0.05; Table 2). Despite elevations in shear rate after BH4, Δ brachial artery vasodilation normalized to Δ shear rate was ~49–60% greater at each handgrip workload after BH4 administration compared to placebo (P < 0.05; Figure 4A), resulting in a significant upward shift in slope of the relationship between Δ brachial artery vasodilation and Δ shear rate (P < 0.05; Figure 4B).
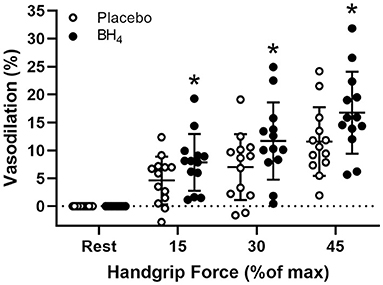
Figure 3. Brachial artery vasodilation during progressive handgrip exercise in patients with systemic sclerosis after placebo (white circles) and tetrahydrobiopterin (BH4; black circles). *P < 0.05, significantly different than placebo. All data are presented as mean ± SD.
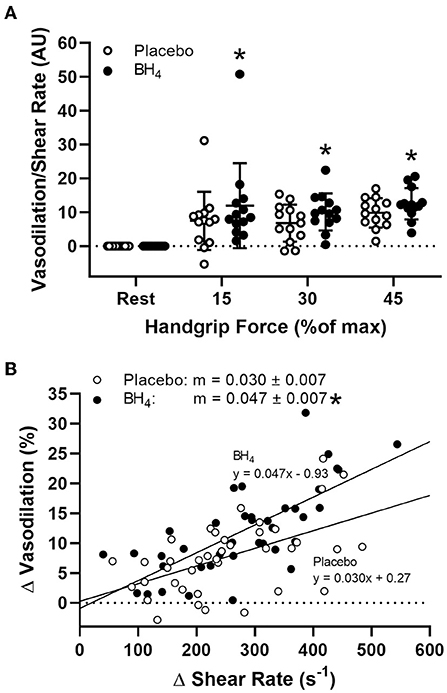
Figure 4. Brachial artery vasodilation normalized to increases in shear rate (A) during progressive handgrip exercise in patients with systemic sclerosis after placebo (white circles) and tetrahydrobiopterin (BH4; black circles). Brachial artery vasodilation to sustained increases in shear rate after placebo and BH4 (B). After acute BH4 administration, patients with systemic sclerosis had a significantly higher slope (m) compared to placebo. *P < 0.05, significantly different than placebo. All data are presented as mean ± SD.
The current study provides evidence that acute oral BH4 administration ameliorates the dysfunctional peripheral vascular response to exercise in SSc patients. The main findings are that, compared to placebo, BH4 administration increased circulating BH4 concentrations by ~4-fold, and had a positive effect on our primary endpoint, exercise-induced hyperemia, resulting in a greater brachial artery blood flow at all exercise workloads, indicating an improvement in resistance artery vasodilatory function. Acute BH4 administration also augmented vascular conductance, as well as the sensitivity of the brachial artery to vasodilate to the sustained elevations in shear rate that occur during handgrip exercise, indicating improved conduit artery endothelial function. Taken together, these results demonstrate the efficacy of acute BH4 administration to improve both resistance and conduit vessel endothelial function in SSc patients, suggesting that eNOS recoupling may be an effective strategy for improving vasodilatory capacity in this patient group.
In the current study, we observed that acute oral BH4 administration augments exercise-induced hyperemia in patients with SSc. Because BH4 had minimal effects on blood pressure at rest or during exercise, the magnitude of improvement in exercise-induced brachial artery vascular conductance was nearly identical to the improvement in exercise-induced hyperemia (Figure 1). The ability of resistance arteries to vasodilate during exercise is demonstrated by increases in blood flow and vascular conductance (25), both of which are partially mediated by NO (26, 27). Thus, one of the likely mechanisms for BH4-mediated improvements in exercise-induced blood flow and vascular conductance in this study are due to augmented resistance artery endothelial function. Although the role of NO in the regulation of exercising skeletal muscle blood flow continues to be debated, using an identical protocol to that employed in the present study, our group has previously identified a significant contribution of this pathway to the overall hyperemic response during handgrip exercise in young, healthy adults (19). Specific to SSc, we have recently shown that acute BH4 administration results in a slight, but significant, increase in brachial artery peak reactive hyperemia after 5 min of distal cuff occlusion in SSc patients (18). Like exercise hyperemia, the entirety of the reactive hyperemic response also cannot be attributed to NO-mediated resistance artery vasodilation, although NO does have a slight impact on the magnitude of reactive hyperemia (28–30). Still, as we did not employ any NO blockade in the current study, an improvement in resistance artery endothelial function via BH4-mediated improvement in NO signaling cannot definitively be shown in the current study.
During progressive handgrip exercise, stepwise increases in steady-state brachial artery blood flow result in sustained elevations in shear rate (31). It is now well-accepted that changes in shear rate are the stimulus for conduit arterial endothelium-dependent dilation (32). Therefore, brachial artery vasodilation during progressive handgrip exercise can be used as a measurement of endothelium-dependent dilation. Our group has utilized this ‘sustained stimulus flow-mediated dilation' in a variety of populations to detect endothelial dysfunction in both health and disease (8, 13, 33, 34), and importantly, have identified the NO-dependent nature of brachial artery vasodilation using this experimental model (19).
In SSc patients, we have reported that acute BH4 administration augments brachial artery flow-mediated dilation, which support the findings of the current study by demonstrating the beneficial effects of BH4 using a different model of conduit artery endothelium-dependent dilation (18). Therefore, it is likely that improved exercise-induced brachial artery vasodilation after acute BH4 administration in SSc patients observed in the present study (Figure 3) was the consequence of an NO-dependent improvement in endothelial function.
Given the known stimulus-response relationship between shear stress and endothelium-dependent vasodilation (35), it could be argued that augmented brachial artery vasodilation following BH4 administration was simply the consequence of a greater shear stimulus, as BH4 also increases exercise-induced blood flow. To explore this possibility, we normalized brachial artery vasodilation to changes in shear rate. When responses are viewed in this manner, a clear treatment effect of BH4 remains, as quantified by a steeper slope of vasodilation normalized to shear rate compared to placebo (Figure 4). This is particularly important given our previous findings that SSc patients demonstrate impaired brachial artery vasodilation to increases in shear rate during handgrip exercise (8) and in response to reactive hyperemia following ischemic cuff occlusion (4), and is suggestive that BH4 administration may act to partially restore conduit vessel endothelial function in this patient group. These findings both confirm and extend reported improvements in conduit (36–39), resistance (40–44), and coronary (45, 46) artery vasodilatory function with BH4 administration in a variety of populations, showing for the first time BH4-mediated improvements also occur during exercise.
We have previously observed that elevated blood oxidative stress markers accompany the impaired vascular response to handgrip exercise in SSc patients (8). Although the etiology of disease-related increases in oxidative stress and damage in this population is not well-understood, deficits in endothelial BH4 may be a significant contributor, as uncoupled eNOS produces superoxide rather than NO (16). At supraphysiological concentrations, BH4 has been reported to have antioxidant properties, acting as a free radical scavenger in vitro (47, 48). However, it is unlikely that improvements in the vascular response to exercise with BH4 observed in the present study were due to an antioxidant effect, as BH4-mediated superoxide scavenging is not a major reaction in vivo (49). It is also unlikely that BH4 acted directly to reduce vascular free radical concentration. In fact, there is no data that suggests BH4 administration can acutely lower oxidative stress in any human population, and we have previously reported no changes in blood oxidative stress markers after BH4 administration in SSc patients (18). Thus, the most likely mechanism by which vascular function improved in the present study is a decline in superoxide concentration due to greater NO synthesis (50) that reflects eNOS recoupling. Although it appears acute BH4 administration does not lower oxidative stress to a magnitude of physiological significance, it should be noted that chronic BH4 supplementation does lower blood oxidative damage markers in hypercholesterolemic individuals (51). Thus, the long-term effects of BH4 are likely due to a chronic increase in BH4 bioavailability that permits greater eNOS coupling, thereby chronically lowering superoxide production and mitigating subsequent oxidative damage.
It is unlikely that BH4-mediated improvements were due to greater smooth muscle vasoreactivity, as others have reported endothelium-independent dilation to sublingual nitroglycerin to be unchanged with acute BH4 administration (36–40, 43, 44). Furthermore, we have previously observed no changes in brachial artery vasodilation or peak blood flow in response to sublingual nitroglycerin after acute BH4 administration in SSc patients (18). Taken together, these studies suggest that the beneficial effects of BH4 toward the vascular response to exercise come through improvements in endothelial function.
In contrast to handgrip exercise, there was no noticeable BH4-related effect on any cardiovascular variable at rest. There have also been no reported effects of acute BH4 administration on resting cardiovascular variables in other populations (36–39, 44). Thus, it is likely that the vascular improvements with BH4 are only exerted or noticeable when eNOS activation is elevated, such as in response to the increases in shear rate that arise with exercise or other physiological states that result in augmented blood flow.
We have shown that acute BH4 administration improves both resistance and conduit artery vasodilatory function during exercise in patients with SSc. Tetrahydrobiopterin-mediated improvements were achieved despite no changes in blood oxidative stress markers, which we and others have shown to be elevated in SSc patients. Taken together, these results imply that improvements in the peripheral vascular response to exercise in SSc patients with BH4 were due to an improved endothelial function that was likely achieved though BH4-mediated eNOS recoupling. Moreover, because we did not observe any changes in cardiovascular hemodynamics or blood pressure at rest, these data provide the first initial evidence for the safe and effective use of BH4 in SSc. Thus, future studies are warranted to assess the chronic effects BH4 and its role as an add-on therapy to normally prescribed medications in this population (i.e., calcium channel blockers) in SSc patients.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by Institutional Review Board of the University of Utah and Salt Lake City Veterans Affairs Medical Center (IRB# 38705). The patients/participants provided their written informed consent to participate in this study.
DRM, HLC, and TMF: performed experiments. DRM: prepared figures. DRM, HLC, DWW, and AJD: drafted manuscript. All authors analyzed data, conception, design of research, interpreted results of experiments, edited, revised manuscript, and approved the final version of manuscript.
This work was supported by awards from the National Institutes of Health (R00 AT010017, R01 AG040297, P01 HL091830, R21 AG043952, K02 AG045339, and K23 AR067889) and the U.S. Department of Veterans Affairs (I01 RX001697, I01 CX001183, I01 RX001311, and I01 CX002152).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blom-Bülow B, Jonson B, Brauer K. Factors limiting exercise performance in progressive systemic sclerosis. Semin Arthritis Rheum. (1983) 13:174–81. doi: 10.1016/0049-0172(83)90004-5
2. LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol. (2001) 28:1573–6.
3. Meiszterics Z, Timar O, Gaszner B, Faludi R, Kehl D, Czirjak L, et al. Early morphologic and functional changes of atherosclerosis in systemic sclerosis-a systematic review and meta-analysis. Rheumatology (Oxford). (2016) 55:2119–30. doi: 10.1093/rheumatology/kew236
4. Frech T, Walker AE, Barrett-O'Keefe Z, Hopkins PN, Richardson RS, Wray DW, et al. Systemic sclerosis induces pronounced peripheral vascular dysfunction characterized by blunted peripheral vasoreactivity and endothelial dysfunction. Clin Rheumatol. (2015) 34:905–13. doi: 10.1007/s10067-014-2834-5
5. Alkotob ML, Soltani P, Sheatt MA, Katsetos MC, Rothfield N, Hager WD, et al. Reduced exercise capacity and stress-induced pulmonary hypertension in patients with scleroderma. Chest. (2006) 130:176–81. doi: 10.1378/chest.130.1.176
6. Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Troster N, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. (2009) 180:881–6. doi: 10.1164/rccm.200904-0563OC
7. de Oliveira NC, dos Santos Sabbag LM, Ueno LM, de Souza RB, Borges CL, de Sa Pinto AL, et al. Reduced exercise capacity in systemic sclerosis patients without pulmonary involvement. Scand J Rheumatol. (2007) 36:458–61. doi: 10.1080/03009740701605889
8. Machin DR, Clifton HL, Garten RS, Gifford JR, Richardson RS, Wray DW, et al. Exercise-induced brachial artery blood flow and vascular function is impaired in systemic sclerosis. Am J Physiol Heart Circ Physiol. (2016) 311:H1375–81. doi: 10.1152/ajpheart.00547.2016
9. Allanore Y, Borderie D, Lemarechal H, Ekindjian OG, Kahan A. Acute and sustained effects of dihydropyridine-type calcium channel antagonists on oxidative stress in systemic sclerosis. Am J Med. (2004) 116:595–600. doi: 10.1016/j.amjmed.2003.11.022
10. Ogawa F, Shimizu K, Muroi E, Hara T, Hasegawa M, Takehara K, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology. (2006) 45:815–8. doi: 10.1093/rheumatology/kel012
11. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. (2000) 87:840–4. doi: 10.1161/01.res.87.10.840
12. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. (2001) 104:2673–8. doi: 10.1161/hc4601.099485
13. Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O'Keefe Z, Ives SJ, et al. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol. (2016) 310:H765–74. doi: 10.1152/ajpheart.00817.2015
14. Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial function. Eur Heart J. (1998) 19(Suppl G):G3–8.
15. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. (1991) 43:109–42.
16. Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. (1997) 237:340–4. doi: 10.1006/bbrc.1997.7069
17. Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci. (2007) 113:47–63. doi: 10.1042/CS20070108
18. Machin DR, Clifton HL, Richardson RS, Wray DW, Donato AJ, Frech TM. Acute oral tetrahydrobiopterin administration ameliorates endothelial dysfunction in systemic sclerosis. Clin Exp Rheumatol. (2017) 35:167.
19. Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, et al. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol. (2011) 300:H1101–7. doi: 10.1152/ajpheart.01115.2010
20. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. (2013) 65:2737–47. doi: 10.1002/art.38098
21. Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab. (2004) 81:45–51. doi: 10.1016/j.ymgme.2003.09.014
22. Pierce GL, Jablonski KL, Walker AE, Seibert SM, DeVan AE, Black SM, et al. Tetrahydrobiopterin supplementation enhances carotid artery compliance in healthy older men: a pilot study. Am J Hypertens. (2012) 25:1050–4. doi: 10.1038/ajh.2012.70
23. Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. (1980) 102:176–88. doi: 10.1016/0003-2697(80)90336-x
25. Katz SD, Yuen J, Bijou R, LeJemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. (1997) 82:1488–92. doi: 10.1152/jappl.1997.82.5.1488
26. Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. (1995) 488:259–65. doi: 10.1113/jphysiol.1995.sp020964
27. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. (2004) 557:599–611. doi: 10.1113/jphysiol.2004.061283
28. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. (1996) 81:1807–14. doi: 10.1152/jappl.1996.81.4.1807
29. Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, et al. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension. (1998) 32:9–15. doi: 10.1161/01.hyp.32.1.9
30. Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res. (2013) 113:1023–32. doi: 10.1161/CIRCRESAHA.113.301675
31. Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem. (1990) 184:193–9. doi: 10.1016/0003-2697(90)90668-y
32. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. (2007) 102:1510–9. doi: 10.1152/japplphysiol.01024.2006
33. Ratchford SM, Clifton HL, La Salle DT, Broxterman RM, Lee JF, Ryan JJ, et al. Cardiovascular responses to rhythmic handgrip exercise in heart failure with preserved ejection fraction. J Appl Physiol (1985). (2020) 129:1267–76. doi: 10.1152/japplphysiol.00468.2020
34. Donato AJ, Uberoi A, Bailey DM, Walter Wray D, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. (2010) 298:H671–8. doi: 10.1152/ajpheart.00761.2009
35. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. (2005) 568:357–69. doi: 10.1113/jphysiol.2005.089755
36. Ueda S, Matsuoka H, Miyazaki H, Usui M, Okuda S, Imaizumi T. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol. (2000) 35:71–5. doi: 10.1016/s0735-1097(99)00523-9
37. Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. (2005) 568:1057–65. doi: 10.1113/jphysiol.2005.092734
38. Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. (2008) 22:401–7. doi: 10.1038/sj.jhh.1002329
39. Maki-Petaja KM, Day L, Cheriyan J, Hall FC, Ostor AJ, Shenker N, et al. Tetrahydrobiopterin supplementation improves endothelial function but does not alter aortic stiffness in patients with rheumatoid arthritis. J Am Heart Assoc. (2016) 5:e002762. doi: 10.1161/JAHA.115.002762
40. Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. (1997) 99:41–6. doi: 10.1172/JCI119131
41. Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase. Circ Res. (2000) 86:E36–41. doi: 10.1161/01.RES.86.2.e36
42. Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. (2000) 43:1435–8. doi: 10.1007/s001250051551
43. Setoguchi S, Hirooka Y, Eshima K, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol. (2002) 39:363–8. doi: 10.1097/00005344-200203000-00007
44. Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. (2006) 186:390–5. doi: 10.1016/j.atherosclerosis.2005.07.025
45. Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. (2000) 35:173–8. doi: 10.1097/00005344-200002000-00001
46. Setoguchi S, Mohri M, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J Am Coll Cardiol. (2001) 38:493–8. doi: 10.1016/s0735-1097(01)01382-1
47. Hyun J, Komori Y, Chaudhuri G, Ignarro LJ, Fukuto JM. The protective effect of tetrahydrobiopterin on the nitric oxide-mediated inhibition of purified nitric oxide synthase. Biochem Biophys Res Commun. (1995) 206:380–6. doi: 10.1006/bbrc.1995.1052
48. Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res. (1995) 23:419–30. doi: 10.3109/10715769509065263
49. Vasquez-Vivar J, Whitsett J, Martasek P, Hogg N, Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic Biol Med. (2001) 31:975–85. doi: 10.1016/S0891-5849(01)00680-3
50. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. (2002) 362:733–9. doi: 10.1042/0264-6021:3620733
Keywords: exercise, systemic sclerosis, arterial function, physiology, blood flow, vasodilation, handgrip
Citation: Machin DR, Clifton HL, Wray DW, Frech TM and Donato AJ (2022) Tetrahydrobiopterin Administration Augments Exercise-Induced Hyperemia and Endothelial Function in Patients With Systemic Sclerosis. Front. Med. 8:791689. doi: 10.3389/fmed.2021.791689
Received: 08 October 2021; Accepted: 08 December 2021;
Published: 10 January 2022.
Edited by:
Francesco Del Galdo, University of Leeds, United KingdomCopyright © 2022 Machin, Clifton, Wray, Frech and Donato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony J. Donato, dG9ueS5kb25hdG9AdXRhaC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.