
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 04 January 2022
Sec. Hematology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.789972
This article is part of the Research Topic Immune Thrombocytopenia (ITP) - Diagnosis and Treatment View all 8 articles
COVID-19 vaccine-induced thrombotic thrombocytopenia (VITT) is a rare complication of adenoviral vector (ChAdOx1 nCoV-19) vaccine administration. It is presented as thrombocytopenia and thrombotic manifestations in various sites, especially in cerebral veins. Pulmonary emboli have been reported rarely. We present a case of a young male patient who developed severe thrombocytopenia and pulmonary embolism 12 days after the first dose of the vaccine. Severe thrombocytopenia, skin hematomas, and segmental pulmonary emboli were detected. Anti-platelet factor 4 (aPF-4) antibody was highly positive supporting the diagnosis of VITT. Prompt treatment with fondaparinux, intravenous immunoglobulin, and prednisone led to a marked improvement of clinical condition and thrombocytes count. We report the first known case of VITT in Slovakia.
COVID-19 is a global pandemic disease with a high morbidity and mortality and a deleterious impact on the human population. To date, more than 4.6 million people globally have died from COVID-19 (1). Hence, the massive vaccination efforts represent the only way to mitigate the negative consequences of this pandemic. Several vaccines have been developed to date, with various common and rare adverse effects (2).
It is known that the overall risk of thrombosis and thromboembolic complications due to COVID-19 is increased (3, 4). On the other hand, there are reports on thrombotic complications after vaccination against this infection, especially following the administration of adenovirus-based vaccines. Since March 2021, several cases of uncommon vaccine-related thrombotic events associated with thrombocytopenia have been reported, in particular after the ChAdOx1 nCov-19 vaccine administration (5–8). These complications have been termed vaccine-induced thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), or vaccine associated thrombotic thrombocytopenia (VATT) (9). This severe condition is characterized by unusual location of thrombosis, mostly in cerebral veins, however other sites of thrombosis or pulmonary embolism have been published. The mortality rate of VITT is high, reaching up to 25% (7).
The overall risk for VITT development following administration of the AstraZeneca vaccine is low (10.9 cases per million doses). To date, several hundreds of cases have been reported. Incidence of pulmonary embolism occurs in 0.08 people per million and it is more common in patients over 65 years (10).
In this report we refer to the first known case of a young patient with VITT manifested by severe thrombocytopenia, skin hematomas, and pulmonary embolism in Slovakia.
A 31-year-old man was admitted to the emergency unit because of multiple skin hematomas and severe thrombocytopenia. His family history as well as previous medical history were unremarkable and insignificant regarding venous thromboembolism. The patient did not have a history of autoimmune disease or COVID-19 infection.
In April 2021, the patient was vaccinated with the first dose of ChAdOx1 nCov-19 vaccine. After vaccination he developed fever and headache that disappeared within 2 days. On the seventh day after vaccination, his headache returned and on the twelfth day he presented skin hemorrhage and mild transient dyspnea. Because of its progression and laboratory finding of severe thrombocytopenia, he was referred to the hospital.
On physical examination, the patient was afebrile, eupneic, and with multiple skin hematomas on the chest wall and both lower extremities (Figure 1). No petechias were found. His blood pressure was 155/95 mmHg, heart rate was 96/min, and oxygen saturation value was normal (96%).
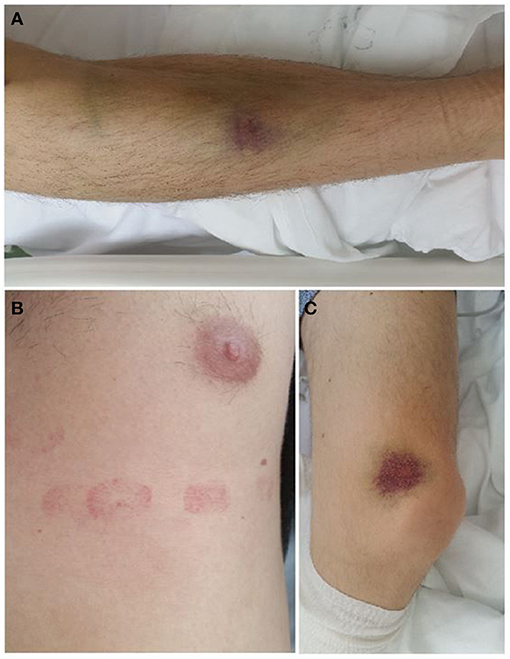
Figure 1. Skin hematomas on the chest wall and lower extremities. (A) Right foreleg (B) Left side of the chest wall (C) Medial side of the left knee joint.
Laboratory investigations revealed severe thrombocytopenia of 25 x 109/L (normal 150-400 x 109/L) whereas erythrocytes and leukocytes count were in normal range. Serum D dimer concentration was markedly elevated 39.198 mg/L (normal 0.03-0.5 mg/L) while serum fibrinogen concentration was slightly decreased 0.87 g/L (normal 1.8-3.5 g/L). Prothrombin time (PT) and activated partial thromboplastin time (APTT) were not significantly changed.
Biochemical evaluation demonstrated mild hyperbilirubinemia 41.3 umol/L (normal 5-21 umol/L) and slightly elevated hepatic enzymes, i.e., AST 0.98 ukat/L (normal 0.05-0.85 ukat/L), ALT 1.54 ukat/L (normal 0.05-0.85 ukat/L), and GMT 1.37 ukat/L (normal 0.05-0.92 ukat/L).
Anti-platelet factor 4 IgG (aPF4G) (Antibodies-Online Cat# ABIN351496, RRID:AB_10825453) measured by AESKULISA HIT II Kit was highly positive with the serum concentration of 38.67 U/ml (ref: <12 U/ml), supporting the diagnosis of vaccine-induced thrombotic thrombocytopenia (VITT). RT-PCR test for the presence of SARS-Cov-2 was negative at the time of admission. SARS-CoV-2 Total antibodies were not detected (0.105, normal cutoff index 0.000-0.999) and SARS-CoV-2 spike protein (S) antibodies (Leinco Technologies Cat# LT3500, RRID:AB_2893953) were elevated (32.38 U/ml, normal 0.00-0.8 U/ml).
To rule out other possible causes of thrombotic thrombocytopenia, we also measured ADAMTS13 (MGI Cat# 3708874, RRID:MGI:3708874), which was found to be in normal range (Table 1). Results of immunological evaluation are shown in Table 1. Complement profile did not show signs of dysregulation or consumption. Slight elevation of terminal pathway activation marker was found.
X ray of the chest did not detect any pathological changes, while abdominal ultrasound demonstrated cholecystolithiasis with small stone inside gallbladder. On echocardiography there were no significant changes in cardiac morphology or function. No signs of right ventricle hypertrophy or dilation were found. Ejection fraction was 65%. Due to mild dyspnea in previous medical history and markedly elevated serum D dimer concentration, CT pulmonary angiography was performed. It revealed an emboli in the segmental pulmonary arteries bilaterally. CT cerebral venography did not demonstrate cerebral venous thrombosis and Doppler ultrasound of both legs did not detect deep vein thrombosis of peripheral leg veins.
Based on these results we assumed the diagnosis was a vaccine-induced immune thrombotic thrombocytopenia and we initiated treatment with subcutaneous fondaparinux 2.5-7.5 mg daily, intravenous immunoglobulin (IVIG) of dose 1 g/kg, and prednisone 1 mg/kg per os. Within a few days after treatment, platelet count rapidly increased up to 103 x 109/L and serum D dimer concentration decreased to 1.34 mg/L. Clinical condition of the patient rapidly improved and we registered regression of skin hematomas. On the ninth day of hospitalization, the patient was discharged from the hospital.
In this report, we describe the first case of VITT identified and demonstrated in the Slovak Republic. VITT is a serious complication of ChAdOx1 nCoV-19 vaccine. Clinically it mimics autoimmune heparin-induced thrombocytopenia (HIT), which also presents with raised anti-PF4-antibodies (2, 11). Data indicate that the vaccine triggers formation of anti-platelet factor 4 (anti-PF4) immunoglobulin G which activates platelet aggregation resulting in a clinical manifestation of thrombosis and thrombocytes consumption (12, 13). Another possible mechanism includes adenoviral vector entry into megakaryocytes with the subsequent expression of spike protein on platelet surfaces leading to platelet activation by the vector (10). The positivity of anti-PF4 antibody in our patient favors the proposed autoimmune mechanism.
VITT manifests most often with unusual thromboses, but sometimes also with usual thromboses, like deep vein thrombosis of legs or pulmonary embolism (PE). Thrombotic manifestations of VITT were reported in various sites, such as cerebral, abdominal, i.e., splenic, renal, and hepatic veins (6–8, 14). Cerebral venous thrombosis is probably the most common site of thrombosis and occurs in 38-80% of reported cases (9).
Severe thrombocytopenia becomes clinically evident usually within 5-30 days after vaccine administration. In the study of Pavord et al., authors identified 170 definite cases of VITT, all from patients who received the first dose of ChAdOx1 nCoV-19 vaccine. Clinical manifestation presented 5-48 days after vaccination. There was no sex preponderance and known or detectable risk factors. Overall mortality was 22%, and was the highest in patients with intracranial bleeding (15).
In our patient, the first symptoms of skin bleeding appeared approximately on the twelfth day after receiving the ChAdOx1 nCoV-19 vaccine. Except for tachycardia, he did not present any other symptoms of thromboembolic disease. Laboratory findings, i.e., thrombocytopenia, increased serum D dimer concentration, lower fibrinogen concentration, and positive anti-PF4 antibodies were typical for VITT diagnosis. CT pulmonary angiography was realized with positive finding of pulmonary embolism in segmental pulmonary arteries bilaterally. Other investigations did not reveal thrombosis as the source of pulmonary embolism. Therefore, we assumed the primary pulmonary thrombosis should also be considered in our patient. This situation has been described in patients with COVID-19 disease (16–18).
Treatment of VITT should be managed like HIT syndrome and should be started immediately. High dose IVIG should be administered with non-heparin antithrombotic treatment by anticoagulant (fondaparinux, danaparoid, or argatroban). Glucocorticoids and plasma exchange are also therapeutic options to reduce anti-PF4 antibodies levels (10, 19–21). Platelet transfusion is contraindicated and can be considered only in severe bleeding complications (22). Timeline of patient's results initially and after treatment is shown in Table 2.
This case report highlights the potentially life-threatening complication associated with ChAdOx1 nCoV-19 vaccine. Data from the last 6 months showed that VITT may be more frequent than has been reported in previous studies. There are still some uncertainties about this pathologic condition and future studies could help us to identify prognostic markers for VITT and to improve outcomes of the disease.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
IL: conception and design of the work. MI, IT, and ZP: substantial contributions to the acquisition of data for the work. MI, IT, L'I, ZP, and IL: substantial contributions to the analysis of data for the work and interpretation of data for the work. IL, MI, and L'I: drafting the work. IL and ZP: revising the draft of the work critically for important intellectual content and final approval of the version to be published. All authors approved the final version.
This work was supported by the grants from National Office for Innovation and Research (2020-1.1.6-JOVO-2021-00013) for the research in the laboratory of ZP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the patient for agreeing to the study and providing his case history. We would like to thank the Department of Internal Medicine and Hematology of Semmelweis University for their cooperation and acknowledged with many thanks to analysis of expert laboratory and support from Adrienne Fehér and Zsófia Szabó.
1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. (2021). Available online at: https://covid19.who.int/ (accessed September 19, 2021).
2. Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun. (2021) 121:102663. doi: 10.1016/j.jaut.2021.102663
3. Schulman S, Hu Y, Konstantinides S. Venous thromboembolism in COVID-19. Thromb Haemost. (2020) 120:1642–53. doi: 10.1055/s-0040-1718532
4. Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. (2021) 76:412–20. doi: 10.1136/thoraxjnl-2020-216243
5. Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. (2021) 384:2124–30. doi: 10.1056/NEJMoa2104882
6. Turi MC, Spitaleri F, Gori AM, Parruti G, Rogolino AA, Albani A, et al. A case of vaccine-induced immune thrombotic thrombocytopenia with massive artero-venous thrombosis. Blood Transfus. (2021) 19:343–6. doi: 10.2450/2021.0131-21
7. Hocking J, Chunilal SD, Chen VM, Brighton T, Nguyen J, Tan J, et al. The first known case of vaccine-induced thrombotic thrombocytopenia in Australia. Med J Aust. (2021) 215:19–20. doi: 10.5694/mja2.51135
8. Cliff-Patel N, Moncrieff L, Ziauddin V. Renal vein thrombosis and pulmonary embolism secondary to vaccine-induced thrombotic thrombocytopenia (VITT). Eur J Case Rep Intern Med. (2021) 8:002692. doi: 10.12890/2021_002692
9. Arepally GM, Ortel TL. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. (2021) 138:293–8. doi: 10.1182/blood.2021012152
10. Elalamy I, Gerotziafas G, Alamowitch S, Laroche JP, Van Dreden P, Ageno W, et al. SARS-CoV-2 vaccine and thrombosis: an expert consensus on vaccine-induced immune thrombotic thrombocytopenia. Thromb Haemost. (2021) 121:982–91. doi: 10.1055/a-1499-0119
11. McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. (2021) 121:102662. doi: 10.1016/j.jaut.2021.102662
12. Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. (2021). doi: 10.1097/CCM.0000000000005211. [Epub ahead of print].
13. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. (2021) 596:565–9. doi: 10.1038/s41586-021-03744-4
14. Abou-Ismail MY, Moser KA, Smock KJ, Lim MY. Vaccine-induced thrombotic thrombocytopenia following Ad26.COV2.S vaccine in a man presenting as acute venous thromboembolism. Am J Hematol. (2021) 96:E346–9. doi: 10.1002/ajh.26265
15. Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. (2021) 385:1680–9. doi: 10.1056/NEJMoa2109908
16. Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. (2020) 120:1230–2. doi: 10.1055/s-0040-1712097
17. Al Rawahi B, BaTaher H, Jaffer Z, Al-Balushi A, Al-Mazrouqi A, Al-Balushi N. Vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine-a case report. Res Pract Thromb Haemost. (2021) 5:e12578. doi: 10.1002/rth2.12578
18. Micco PD, Camporese G, Cardillo G, Lodigiani C, Carannante N, Annunziata A, et al. Pathophysiology of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) and vaccine-induced thrombocytopenic thrombosis (VITT) and their diagnostic approach in emergency. Medicina. (2021) 57:997. doi: 10.3390/medicina57100997
19. Gattringer T, Gressenberger P, Gary T, Wölfler A, Kneihsl M, Raggam RB. Successful management of vaccine-induced immune thrombotic thrombocytopenia-related cerebral sinus venous thrombosis after ChAdOx1 nCov-19 vaccination. Stroke Vasc Neurol. (2021) svn-2021-001142. doi: 10.1136/svn-2021-001142
20. Lai C, Ko W, Chen C, Chen P, Huang Y, Lee P, et al. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Rev Vaccines. (2021) 20:1027–35. doi: 10.1080/14760584.2021.1949294
21. Brandao GM, Junqueira DR, Rollo HA, Sobreira ML. Pentasaccharides for the treatment of deep vein thrombosis. Cochrane Database Syst Rev. (2017) 12:CD011782. doi: 10.1002/14651858.CD011782.pub2
Keywords: COVID-19, vaccine-induced thrombotic thrombocytopenia, pulmonary embolism, adenoviral vector vaccine, hemorrhagia
Citation: Ihnatko M, Truchla I, Ihnatková L’, Prohászka Z and Lazúrová I (2022) Case Report: A Case of COVID Vaccine-Induced Thrombotic Thrombocytopenia Manifested as Pulmonary Embolism and Hemorrhagia. A First Reported Case From Slovakia. Front. Med. 8:789972. doi: 10.3389/fmed.2021.789972
Received: 05 October 2021; Accepted: 23 November 2021;
Published: 04 January 2022.
Edited by:
Tomás José Gonzalez López, Burgos University Hospital, SpainReviewed by:
Gianluca Di Micco, Ospedale Buon Consiglio Fatebenefratelli, ItalyCopyright © 2022 Ihnatko, Truchla, Ihnatková, Prohászka and Lazúrová. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivica Lazúrová, aXZpY2EubGF6dXJvdmFAdXBqcy5zaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.