- Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Background: The HAT2CH2 score has been evaluated for predicting new onset atrial fibrillation, but never for adverse systemic thromboembolic events (STE) in elderly. We aimed to evaluate the HAT2CH2 score and comparing to atrial high rate episodes (AHRE) ≥24 h for predicting STE in older patients with cardiac implantable electronic devices (CIED) implantation.
Methods: We retrospective enrolled 219 consecutive patients ≥ 65 years of age undergoing CIED implantation. The primary endpoint was subsequent STE. For all patients in the cohort, the CHA2DS2-VASc, C2HEST, mC2HEST, HAVOC, HAT2CH2 scores and AHRE ≥ 24 h were determined. AHRE was defined as > 175 bpm lasting ≥ 30 s. Multivariate Cox regression analysis with time-dependent covariates was used to determine variables associated with independent risk of STE.
Results: The median patient age was 77 years, and 61.2% of the cohort was male. During follow-up (median, 35 months), 16 STE occurred (incidence rate, 2.51/100 patient-years; 95% CI, 1.65–5.48). Multiple Cox regression analysis showed that the HAT2CH2 score (HR, 3.405; 95% CI, 2.272–5.104; p < 0.001) was an independent predictor for STE. The optimal HAT2CH2 score cutoff value was 3, with the highest Youden index (AUC, 0.907; 95% CI, 0.853–0.962; p < 0.001). The STE rate increased with increasing HAT2CH2 score (p < 0.001).
Conclusions: This study is the first to show the prognostic value of the HAT2CH2 score for STE occurrence in older patients with CIEDs.
Key Points
- The HAT2CH2 score predicts STE in older patients with CIED and without prior atrial fibrillation.
- HAT2CH2 score of 0–2 indicates low-risk, 3–5: medium-risk, and 6–7: high-risk, for STE events.
Why Does This Paper Matter?
Our study shows the prognostic value of HAT2CH2 score for STE occurrence in older patients with CIEDs.
Introduction
A variety of cardiac implantable electronic devices (CIED) are used in the elderly, including permanent pacemakers (PPM) (1), cardiac resynchronization therapy (CRT) (2), and implantable cardioverter defibrillators (ICD) (2). Atrial high-rate episodes (AHRE), commonly detected by CIED, are an important risk factor for new-onset atrial fibrillation (3) The latest European Society of Cardiology guidelines (3) regarding non-valvular atrial fibrillation (AF) state that CIED-detected AHRE > 5–6 min and > 180 bpm increase the risk for systemic thromboembolic events (STE). They recommend that AHRE ≥ 24 h should be closely monitored and treated. The CHA2DS2-VASc score is used for risk stratification of STE (4); however, its utility in non-valvular AF patients is controversial, primarily because this vascular scoring system does not include AF-related parameters (4). One meta-analysis showed that the discrimination power of the CHA2DS2-VASc score in predicting STE is modest and is similar in the presence or absence of non-valvular AF (4). Thus, more accurate STE prediction models are needed for assessing non-valvular AF and older patients with CIEDs.
The HAT2CH2 score, based on patient age and the presence of hypertension, stroke or transient ischemic attack, chronic obstructive pulmonary disease (COPD), and heart failure, was developed in 2010 for identifying patients who are likely to progress to sustained forms of AF in the near future (5). Studies have investigated the use of HAT2CH2 scores for predicting AF in cancer patients (6), in patients after coronary bypass surgery (7), and emergency-department patients (8). Other scoring systems for predicting new AF, including C2HEST (9), mC2HEST (10), and HAVOC (11, 12), have demonstrated acceptable discriminating power. For older patients with CIEDs, a small number of studies have investigated the performance of the HAT2CH2, C2HEST, mC2HEST, and HAVOC scoring systems for predicting new-onset atrial fibrillation and STE.
The present study aims to determine the performance of HAT2CH2 score for predicting STE and to compare this performance to that of AHRE ≥ 24 h and other scoring systems (CHA2DS2-VASc, C2HEST, mC2HEST, and HAVOC) in older patients with CIEDs and no history of AF.
Methods
Consecutive patients ≥18 years of age who underwent CIED implantation (Medtronic: dual chamber PPM, dual chamber ICD, CRTP, or CRTD) in the Cardiology Department of National Cheng Kung University Hospital from January 2015 to April 2021 were retrospectively included.
Ethical Considerations
The protocol for this cohort study was reviewed and approved by the Ethics Committee of National Cheng Kung University Hospital and conducted according to guidelines of the International Conference on Harmonization for Good Clinical Practice (B-ER-108-278). All included patients provided signed informed consent for data to be used for later publication at the time of the implantation procedures.
Data Collection and Definitions
Patient medical histories and data regarding co-morbidities and echocardiographic parameters were collected from medical records for retrospective evaluation. Diabetes mellitus was defined as the presence of symptoms and casual plasma glucose concentration ≥ 200 mg/dL, fasting plasma glucose concentration ≥ 126 mg/dL, 2-h plasma glucose concentration ≥ 200 mg/dL from a 75-g oral glucose tolerance test, or taking medication for diabetes mellitus. Hypertension was defined as in-office systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or taking antihypertensive medication. Dyslipidemia was defined as low-density lipoprotein ≥ 140 mg/dL, high-density lipoprotein <40 mg/dL, triglycerides ≥ 150 mg/dL, or taking medication for dyslipidemia. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 for at least 3 months. The primary endpoint for this study was the occurrence of STE after the date of CIED implantation, including stroke or transient ischemic attack (TIA) diagnosed by experienced neurologists based on clinical symptoms and brain imaging; pulmonary embolism diagnosed by experienced cardiologists based on clinical symptoms and chest imaging. TIA was defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction. Pulmonary embolism was diagnosed using computed tomography pulmonary angiography or pulmonary angiography. For each outcome, only the first event of that outcome in a given patient was included. For the composite outcome, only the first event in a given patient was included.
AHRE data were extracted from the devices via telemetry at each office visit (every 3–6 months). AHRE electrograms were reviewed by at least one experienced electrophysiologist, who carefully considered the possibility that the AHRE included lead noise or artifacts, far-field R-waves, or paroxysmal supraventricular tachycardia and thus visually confirmed AF in the detected AHRE. Atrial sensitivity was programmed to 0.3 mV with bipolar sensing of Medtronic devices. AHRE was defined as heart rate > 175 bpm and at least 30 s of atrial tachyarrhythmia recorded by the device on any day during the study period.
Scoring System Assessments
CHA2DS2-Vasc score (4): Range from 0 to 9. History of heart failure, hypertension, diabetes, vascular disease, age 65–74 years, and female sex each is calculated as 1 point; 75 years or older and prior stroke, TIA, or thromboembolism each is calculated as 2 points; C2HEST score (9): Range from 0 to 8. C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point); mC2HEST score (10): Range from 0 to 8. C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age 65–74 years, 1 point; age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point); HAVOC score (11): H: hypertension (2 points); A: age (age ≥ 75 years, 2 points); V: valvular heart disease (2 points), peripheral vascular disease (1 point); O: obesity (1 point); C: congestive heart failure (4 points) and coronary artery disease (2 points). HAT2CH2 score (5): Range from 0 to 7. Hypertension, 1 point; age >75 years, 1 point; stroke or transient ischemic attack, 2 points; chronic obstructive pulmonary disease (COPD), 1 point; and heart failure, 2 points.
Statistical Analysis
Categorical variables are presented as percentages, and continuous variables are presented as the mean and standard deviation for normally distributed values or medians and as interquartile interval (IQI) for non-normally distributed values. Normal distribution for continuous variables was assessed using the Kolmogorov–Smirnov method. Pearson's chi-square test or Fisher's exact-test was used to determine differences in baseline characteristics for categorical variables, and a two-sample Student's t-test or Mann–Whitney U-test was used to analyze continuous variables. Survival was estimated using the Kaplan–Meier method, and differences in survival were evaluated using the log-rank test. Multivariate Cox regression analysis was used to identify variables associated with STE occurrence, reported as hazard ratios (HR) with 95% confidence intervals (CI). Parameters with p < 0.05 in univariable analysis and age, gender, body mass index, were entered into multivariable analysis. The receiver-operating characteristic (ROC) area under the curve (AUC) of the HAT2CH2 score and the associated 95% confidence interval (CI) was evaluated for association with future STE after CIED implantation. The optimal cutoff values with the highest Youden index were chosen based on the results of ROC curve analysis and used to evaluate the associated values of the HAT2CH2 score for determining STE. For all comparisons, p < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical package version 23.0 (SPSS Inc. Chicago, IL, USA).
Results
Between January 1, 2014 and April, 2021, a total of 453 consecutive patients who underwent Medtronic CIED implantation at National Cheng Kung University Hospital were recruited. Patients with previous AF (n = 105) and those age <65 years (n = 129) were excluded. The final analysis included 219 patients, of which 16 had experienced STE.
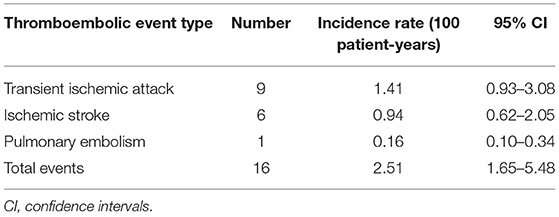
The median follow-up period was 35 months after CIED implantation. Table 1 shows patient baseline demographic and clinical characteristics according to the presence or absence of STE. The median age was 77 years, and 61.2% were men. Most patients were not obese. The types of CIED included dual chamber PPM (173; 79.0%), dual chamber ICD (66; 21.0%); CRTP (23; 7.3%); and CRTD (5; 1.6%). The most common indication for CIED implantation was sick sinus syndrome (53.9%), followed by atrioventricular block (25.1%) (Table 1). We observed an overall atrial pacing median of 33.5% and ventricular pacing median of 4.3%. High percentages of hypertension (92.2%), hyperlipidemia (86.3%), diabetes (52.1%), and CKD (39.7%) suggest a relatively high risk of STE for the entire study cohort. Ninety-two patients (42.0%) received antiplatelet agents and 21 patients (9.6%) received anticoagulants. Data regarding the type and incidence of STEs are reported in Table 2. The total number of STEs was 16 [incidence rate (IR), 2.51/100 patient-years; 95% CI, 1.65–5.48] (Table 2).
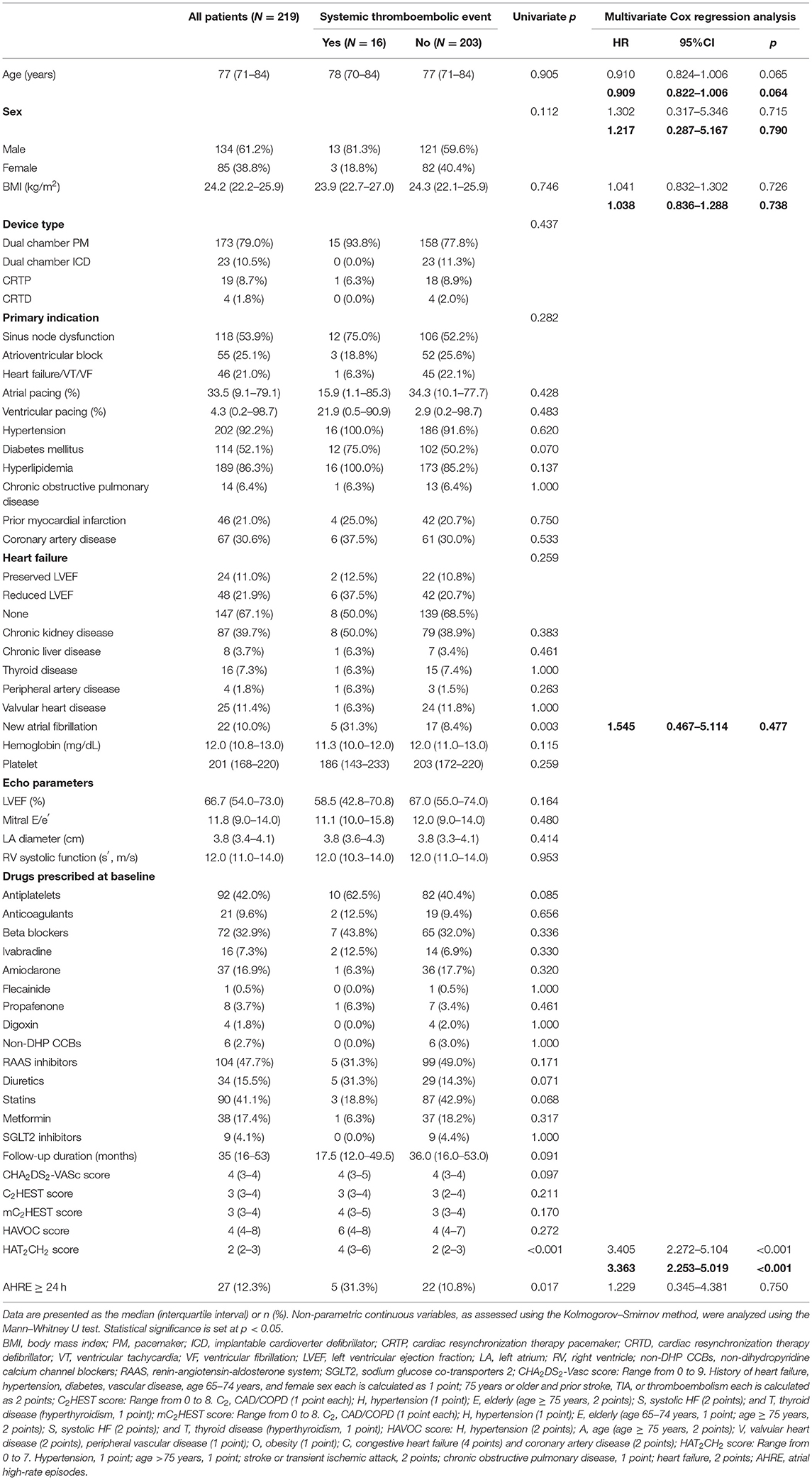
Table 1. Baseline characteristics of the overall study group and with/without systemic thromboembolic events.
Univariate Analysis and Multivariate Cox Regression Analysis to Identify Independent Predictors of STE
Univariate analysis revealed that STE occurrence was significantly associated with AHRE ≥ 24 h, new AF, and HAT2CH2 score (Table 1). Multivariate Cox regression analysis showed that only the HAT2CH2 score was independently associated with STE (HR, 3.405; 95% CI, 2.272–5.104; p < 0.001). Twenty-two patients (10.0%) had new AF. Only the HAT2CH2 score differed significantly between those with and without new AF (p = 0.025) (Supplementary Table 1).
ROC-AUC Determination of AHRE and HAT2CH2 Score Cutoff Values for Factors Predictive of Future STE and Survival Analysis
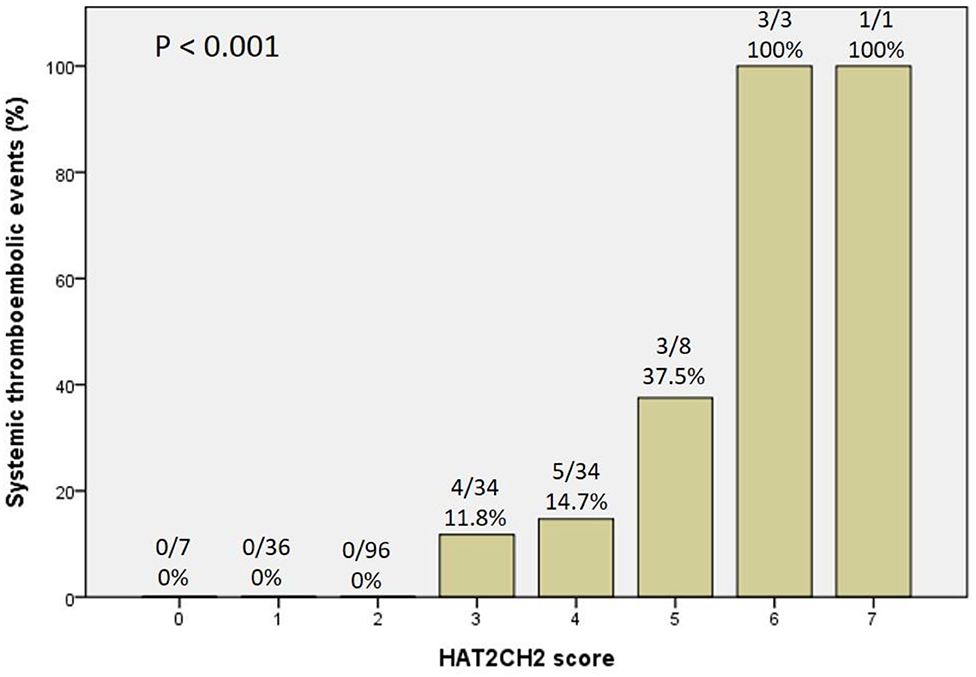
The optimal HAT2CH2 score cutoff value predictive of future STE was determined to be 3 according to the highest Youden index (sensitivity, 100.0%; specificity, 80.0%; AUC, 0.907; 95% CI, 0.853–0.962; p < 0.001) (see Figure 1). Kaplan–Meier curves depict the cumulative survival rates without STE according to HAT2CH2 score groups of 0–7. Patients with a HAT2CH2 score of 4–7 had a higher risk for NE development than did those with a HAT2CH2 score of 0–3 (log-rank test, p < 0.001) (see Figure 2). The rate of NE occurrence significantly increased with increasing HAT2CH2 score, as follows: 0–2 (0%), 3 (11.8%), 4 (14.7%), 5 (37.5%), to 6–7 (100%) (p < 0.001) (see Figure 3). We further classified our study population as very low risk (HAT2CH2 score, 0–2), low risk (HAT2CH2 score, 3), medium risk (HAT2CH2 score, 4), high risk (HAT2CH2 score, 5), and very high risk (HAT2CH2 score, 6–7).
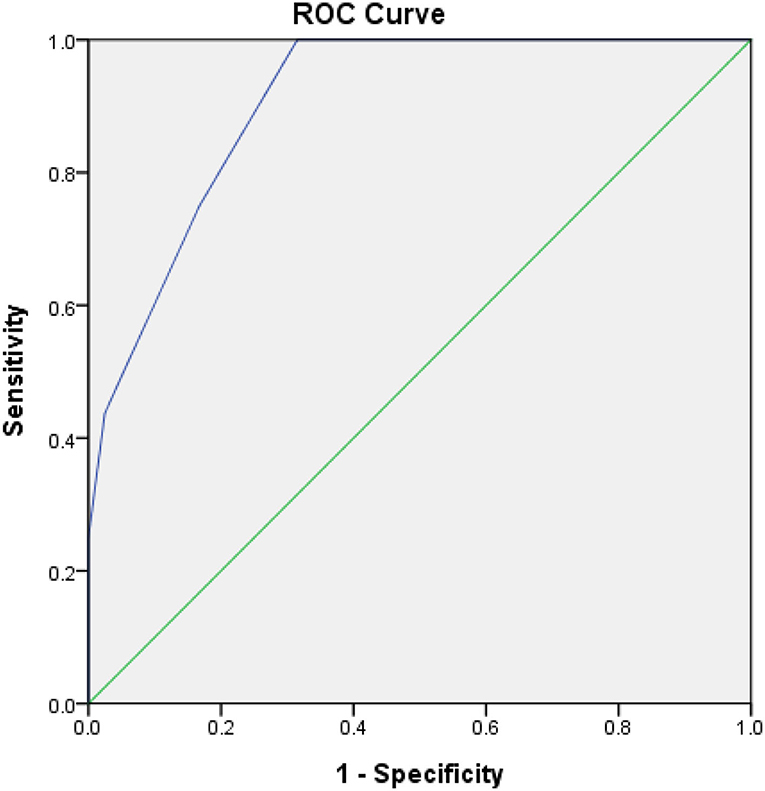
Figure 1. Receiver-operating characteristic curve analysis of the HAT2CH2 score in patients with CIEDs with subsequent systemic thromboembolic events. HAT2CH2 score: optimal cutoff value with the highest Youden index, 3; sensitivity, 100.0%; specificity, 80.0%; AUC, 0.907; 95% CI, 0.853–0.962; p < 0.001.
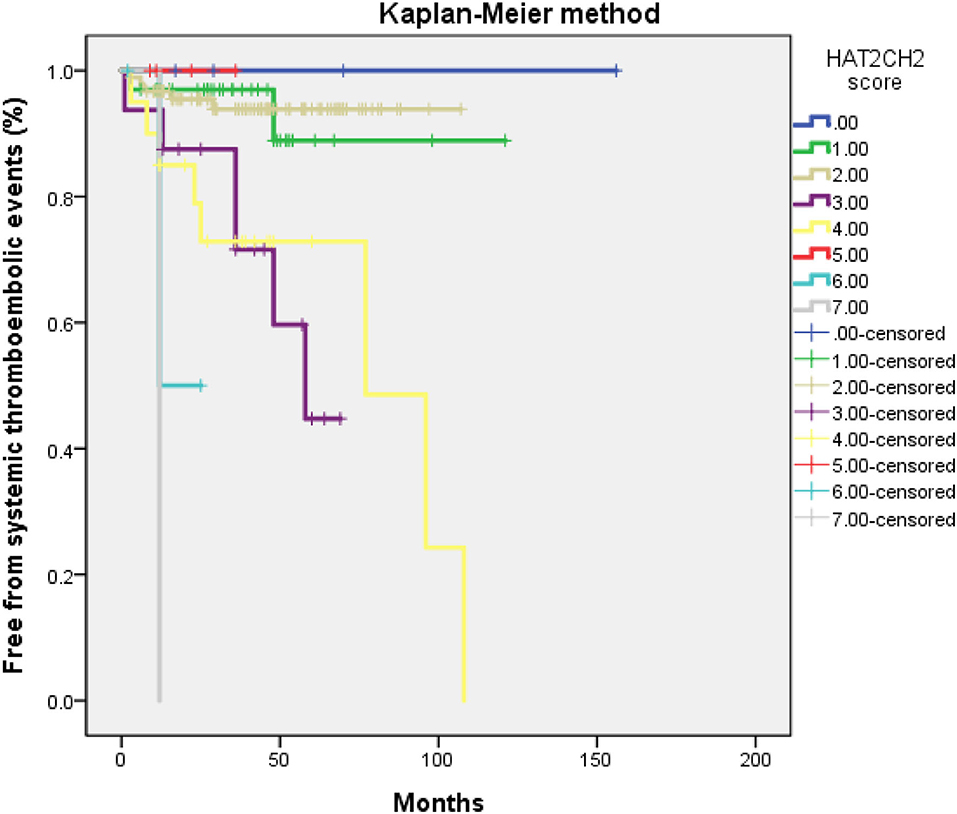
Figure 2. Kaplan–Meier curves depicting the cumulative survival rates free from systemic thromboembolic events with respect to HAT2CH2 scores (0–7; log-rank p < 0.001).
Discussion
The main finding of this study is that the HAT2CH2 score is significantly and independently associated with STE in a population of older Taiwanese patients with CIED and no history of AF. The optimal HAT2CH2 score cutoff value for predicting subsequent STE was 3, with 100% sensitivity and 80% specificity. These results suggest that comprehensive assessment of older patients with CIED to determine the HAT2CH2 score may be warranted to allow for early, aggressive therapy to prevent STE.
The present study was conducted because the performance of several AF-predicting scoring systems (CHA2DS2-VASc, HAT2CH2, C2HEST, mC2HEST, and HAVOC) for predicting subsequent STE in older patients with CIED had not been confirmed. Of these scoring systems, CHA2DS2-VASc is recommended for predicting the risk of STE in patients with non-valvular AF (3). However, some studies report that the discrimination power of the CHA2DS2-VASc score for predicting STE in patients without non-valvular AF is insufficient (4, 13, 14). Melgaard et al. (15) showed the predictive accuracy of the CHA2DS2-VASc score for STE in patients with heart failure and without AF was modest (C-statistic, 0.64; 95% CI, 0.61–0.67). A meta-analysis by Siddiqi et al. (4) including 9 studies of patients (n = 453,747) with non-valvular AF and 10 studies of patients (n = 138262) without non-valvular AF reports that the discrimination power is modest (C-statistic, 0.67; 95% CI, 0.64–0.70) (4). Furthermore, Hu et al. used data from the Taiwan Health Insurance Research Database, reporting that for patients with venous thromboembolism (n = 56 996), the area under the curve of ROC for CHA2DS2-VASc score for predicting STE was 0.66, which also is modest (13). Therefore, more accurate models are needed for predicting STE in patients without AF.
To the best of our knowledge, the current study is the first to show that the HAT2CH2 score more accurately predicts the risk of STE in patients with CIED than does the CHA2DS2-VASc, C2HEST, mCHEST, or HAVOC score (Table 1); the AUC of the ROC curve is 0.907, indicating excellent discrimination (Figure 1). We also proposed an STE risk stratification scheme based on the HAT2CH2 score (0–2, low risk; 3–5, medium risk; and 6–7, high risk) (Figure 3). Validation of this scheme in an external population is needed to confirm the accuracy of HAT2CH2 scores for predicting STE in patients with CIED.
We also observed that of the scoring systems investigated here, only the HAT2CH2 score could independently predict new-onset AF in this study population. The HAT2CH2 score includes COPD as one point instead of diabetes or vascular disease included as one point in the CHA2DS2-VASc score. Thus, different diseases have different effects on the risk of STE in patients with CIED. For example, systemic inflammation and oxidative stress are associated with COPD and promote platelet hyperactivity and cerebral vascular dysfunction (14), and COPD increases the risk of STE, independent of other shared risk factors of cardiovascular disease (16). Additional prospective studies are needed to elucidate the possible mechanisms underlying COPD-related STE risk and to identify effective preventive interventions.
Current guidelines recommend that patients with AHRE ≥ 24 h should be regarded as having the same risk of STE as those with clinical AF (3). A recent systemic review and meta-analysis also demonstrated that AHRE detected by CIED in patients without prior AF would increase the risks of stroke and clinical AF (17). Therefore, in the current study, we included AHRE ≥ 24 h (Table 1) and new AF (Table 1) in the multivariate Cox regression analysis for predicting STE. However, the HAT2CH2 score was still the only independent predictor of STE in our cohort. Although the proportion of STE associated with AF increases progressively with age (18), the cause of STE in a large proportion of patients is not cardioembolic. Non-cardioembolic risk factors for STE include atherosclerotic intracranial arterial stenosis (19), carotid atherosclerotic stenosis (20), and complex aortic plaque (21); these conditions share more risk factors with the HAT2CH2 score parameters than with the AHRE ≥ 24 h or AF. Therefore, the HAT2CH2 score provides a more comprehensive evaluation of STE risk factors in this population.
Limitations
The present study has several limitations. First, this was a single-center, retrospective, observational study with a relatively small number of older patients with CIED in a hospital setting, and all patients were Taiwanese. As a result, causality cannot be inferred between the HAT2CH2 score and STE, and the presence of confounding factors cannot be ruled out. Also, the results may not be generalizable to other populations. Prospective multicenter studies with larger cohorts are required to confirm the results of this study. Second, this study did not investigate the nature of heart rhythms at the time of STE onset. Third, because the patient data were analyzed retrospectively, we could not confirm that patients started anticoagulants for the treatment of CIED-detected AHRE, although these patients were not excluded because no significant difference were found between anticoagulants use and the presence (2, 12.5%) or absence (19, 9.4%) of STE (p = 0.656) (Table 1). Fourthly, it is necessary to take into consideration the older patients' heterogeneity in the current retrospective study in terms of frailty and dependence, undergoing a detailed multidimensional geriatric assessment before CIED therapy should be a critically important step. Finally, not all National Institutes of Health Stroke Scale was available in all patients with STE will limit the power to detect the severity of STE.
Conclusions
STEs are not uncommon in older patients after CIED implantation. The HAT2CH2 score is an independent predictor of STE risk in this population. Our results suggest that for patients with CIED and no history of AF, determination of the HAT2CH2 score may be warranted to allow for early, aggressive therapy to prevent STE, especially in those with HAT2CH2 score ≥ 3.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of National Cheng Kung University Hospital. All patients provided signed informed consent at the time of CIED implantation for later use of their data in publications.
Author Contributions
J-YC: conception and design, data analysis and interpretation, statistical analysis, drafting and finalizing the manuscript, and critical revision of the manuscript for important intellectual content. T-WC and W-DL: data acquisition. All authors contributed to the article and approved the submitted version.
Funding
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under contract MOST 110-2218-E-006-017.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Convergence CT for assistance with English editing of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.786779/full#supplementary-material
Abbreviations
AF, atrial fibrillation; AHRE, atrial high-rate episodes; AMI, acute myocardial infarction; CIEDs, cardiac implantable electronic devices; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; NE, neurologic events; TIA, transient ischemic attacks.
References
1. Gregoratos G. Permanent pacemakers in older persons. J Am Geriatr Soc. (1999) 47:1125–35. doi: 10.1111/j.1532-5415.1999.tb05239.x
2. Sandhu A, Levy A, Varosy PD, Matlock D. Implantable cardioverter-defibrillators and cardiac resynchronization therapy in older adults with heart failure. J Am Geriatr Soc. (2019) 67:2193–9. doi: 10.1111/jgs.16099
3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehab648
4. Siddiqi TJ, Usman MS, Shahid I, Ahmed J, Khan SU, Ya'qoub L, et al. Utility of the CHA2DS2-VASc score for predicting ischaemic stroke in patients with or without atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol. (2021). doi: 10.1093/eurjpc/zwab018. [Epub ahead of print].
5. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. (2010) 55:725–31. doi: 10.1016/j.jacc.2009.11.040
6. Hu WS, Lin CL. Comparison of CHA2 DS2-VASc, CHADS2 and HATCH scores for the prediction of new-onset atrial fibrillation in cancer patients: a nationwide cohort study of 760,339 study participants with competing risk analysis. Atherosclerosis. (2017) 266:205–11. doi: 10.1016/j.atherosclerosis.2017.10.007
7. Emren V, Aldemir M, Duygu H, Kocabaş U, Tecer E, Cerit L, et al. Usefulness of HATCH score as a predictor of atrial fibrillation after coronary artery bypass graft. Kardiol Pol. (2016) 74:749–53. doi: 10.5603/KP.a2016.0045
8. Barrett TW, Self WH, Wasserman BS, McNaughton CD, Darbar D. Evaluating the HATCH score for predicting progression to sustained atrial fibrillation in ED patients with new atrial fibrillation. Am J Emerg Med. (2013) 31:792–7. doi: 10.1016/j.ajem.2013.01.020
9. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, et al. A simple clinical risk score (C2 HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. (2019) 155:510–8. doi: 10.1016/j.chest.2018.09.011
10. Li YG, Bai J, Zhou G, Li J, Wei Y, Sun L, et al. Refining age stratum of the C2 HEST score for predicting incident atrial fibrillation in a hospital-based Chinese population. Eur J Intern Med. (2021) 90:37–42. doi: 10.1016/j.ejim.2021.04.014
11. Kwong C, Ling AY, Crawford MH, Zhao SX, Shah NH. A clinical score for predicting atrial fibrillation in patients with cryptogenic stroke or transient ischemic attack. Cardiology. (2017) 138:133–40. doi: 10.1159/000476030
12. Ntaios G, Perlepe K. Lambrou D, Sirimarco G, Strambo D, Eskandari A, et al. External performance of the HAVOC score for the prediction of new incident atrial fibrillation. Stroke. (2020) 51:457–61. doi: 10.1161/STROKEAHA.119.027990
13. Hu WS, Lin CL. The predictive role of CHA2DS2-VASc score between venous thromboembolism and ischemic stroke: a large-scale cohort study. J Hypertens. (2018) 36:628–33. doi: 10.1097/HJH.0000000000001539
14. Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond). (2016) 130:1039–350. doi: 10.1042/CS20160043
15. Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. (2015) 314:1030–8. doi: 10.1001/jama.2015.10725
16. Kim YR, Hwang IC, Lee YJ, Ham EB, Park DK, Kim S. Stroke risk among patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clinics (São Paulo). (2018) 73:e177. doi: 10.6061/clinics/2018/e177
17. Doundoulakis I, Gavriilaki M, Tsiachris D, Arsenos P, Antoniou CK, Dimou S, et al. Atrial high-rate episodes in patients with devices without a history of atrial fibrillation: a systemic review and meta-analysis. Cardiovasc Drugs Ther. (2021). doi: 10.1007/s10557-021-07209-8. [Epub ahead of print].
18. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly: the Framingham Study. Arch Intern Med. (1987) 147:1561–4. doi: 10.1001/archinte.147.9.1561
19. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. (2013) 12:1106–14. doi: 10.1016/S1474-4422(13)70195-9
20. Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke. (2007) 38:1470–5. doi: 10.1161/STROKEAHA.106.477091
21. Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators. J Am Coll Cardiol. (1998) 31:1622–6. doi: 10.1016/S0735-1097(98)00146-6
Keywords: atrial high-rate episodes, cardiac implantable electronic device, elderly, HAT2CH2 score, neurologic events (NE), transient ischemic attacks (TIA)
Citation: Chen J-Y, Chen T-W and Lu W-D (2021) HAT2CH2 Score Predicts Systemic Thromboembolic Events in Elderly After Cardiac Electronic Device Implantation. Front. Med. 8:786779. doi: 10.3389/fmed.2021.786779
Received: 30 September 2021; Accepted: 10 December 2021;
Published: 24 December 2021.
Edited by:
Tzvi Dwolatzky, Technion Israel Institute of Technology, IsraelReviewed by:
Leonardo Bencivenga, University of Naples Federico II, ItalyMaria Gavriilaki, University General Hospital of Thessaloniki AHEPA, Greece
Copyright © 2021 Chen, Chen and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ju-Yi Chen, anV5aUBuY2t1LmVkdS50dw==; orcid.org/0000-0003-2760-9978
 Ju-Yi Chen
Ju-Yi Chen Tse-Wei Chen
Tse-Wei Chen