
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 10 January 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.783626
 Junyan Jin1†
Junyan Jin1† Runsong Sun2†
Runsong Sun2† Tingting Mu1†
Tingting Mu1† Taiyi Jiang1
Taiyi Jiang1 Lili Dai1
Lili Dai1 Hongyan Lu3
Hongyan Lu3 Xianlong Ren3
Xianlong Ren3 Jing Chen3
Jing Chen3 Jingrong Ye3
Jingrong Ye3 Lijun Sun1
Lijun Sun1 Hao Wu1
Hao Wu1 Tong Zhang1
Tong Zhang1 Huachun Zou4*
Huachun Zou4* Bin Su1*
Bin Su1*Background: The use of post-exposure prophylaxis (PEP) is effective in reducing HIV risk, but it is underused by men who have sex with men (MSM) due to certain psychological and sociostructural factors. This article assessed the awareness and use of PEP among MSM in an effort to increase the visibility and uptake of PEP among at-risk populations.
Methods: We conducted a systematic literature search of the PubMed, Web of Science, PsycINFO, and Google Scholar electronic databases. Studies were screened for inclusion, and relevant data were abstracted, assessed for bias, and synthesized. Pooled effect estimates were calculated using random effects meta-analysis, meta-regression and subgroup analysis, and a qualitative review and risk of bias assessment were performed (PROSPERO, CRD42019123815).
Results: Twenty eligible studies involving 12,579 MSM were included in the meta-analysis. The pooled estimate of the proportions of MSM who were aware of PEP was modest at 59.9% (95% CI: 50.5~68.7) and that of MSM who previously used PEP was very low at 4.9% (95% CI: 2.4~9.8). PEP awareness showed no clear change over time, while PEP use significantly changed over time. Multiple factors affected awareness, including educational attainment, race/ethnicity, levels of HIV stigma, access to condoms, and so on. Many factors could potentially impede or facilitate the use of PEP, such as income, lack of PEP information, and partnership.
Conclusion: We observed that PEP is an underused HIV prevention strategy among MSM and that once MSM become aware of PEP, the majority are willing to use it if they are supported appropriately in terms of a range of individual, social, and structural barriers.
Systematic Review Registration: http://www.cdr.york.ac.uk/prospero, PROSPERO [CRD42019123815].
Gay men, bisexual men, and other men who have sex with men (MSM) are a critical population at risk for HIV/AIDS throughout the world (1). The HIV prevalence among MSM exceeds 10% in many regions, which is disproportionately high compared to the general population (2). In many highly developed countries where the overall HIV epidemic is in decline, there have been re-emergent epidemics among MSM (3). In low- and middle-income settings, the epidemic of HIV among MSM was expanding (4).
Chemoprophylaxis for HIV prevention, including pre- and post-exposure prophylaxis (PrEP/PEP), has emerged as an important component of HIV prevention efforts in recent years (5). PEP is a 4-week combination antiretroviral treatment beginning within 72 h of initial exposure or potential exposure to human body fluids possibly infected with HIV (6). PEP was initially used for the prevention of occupational HIV exposure, and recently, research hotspots have demonstrated its safety and feasibility in non-occupational incidents among high-risk populations such as MSM (7). However, PEP has been shown to be underused by those who experience non-occupational exposure (8–10).
Relatively low awareness and willingness to use PEP was prevalent among MSM. Some researchers found that most MSM (88.3%) in London had heard of PEP (11), but more studies showed that awareness of PEP was relatively low among that population (12–14), and a lower percentage (24%) of individuals at risk were aware of the proper timing of effective PEP treatment (15). One study showed that of the responders, 42.5% was aware of PEP, and 59.9% expressed interest in receiving PEP in the future, if required (16). A meta-analysis revealed that only 67.2% of MSM were willing to complete the full 28-day uptake of antiretroviral drugs prescribed for PEP (17). Another meta-analysis showed that the pooled PEP uptake rate among high-risk MSM was only 5.0% (14). Many factors may hinder PEP uptake among MSM, including experiences of racism, homophobia, and HIV stigma (18, 19), suggesting that PEP awareness and uptake may be influenced by certain psychological and sociostructural factors. Therefore, increased attention should be devoted to effectively increasing the use of PEP (20) and reducing HIV incidence among MSM (21).
The AIDS epidemic is hidden to some degree because of continued stigma, discrimination, and violence (22). To reduce new HIV infections, it is essential for medical and health services to expand to cover MSM as a key population. In an effort to understand how to raise the visibility and uptake of PEP within at-risk populations, we conducted a systematic review and meta-analysis that assessed PEP awareness and use among MSM, and we sought to examine factors associated with that.
This article was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.cdr.york.ac.uk/prospero, CRD42019123815). The work was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (23) and its protocols (24). The PRISMA checklist is reported in Supplementary Table 1 of the Supplementary Materials.
Comprehensive searches were conducted in the PubMed, Web of Science, PsycINFO, and Google Scholar databases to identify relevant articles related to awareness and use of PEP among MSM. The search strings were intersections of disease-, population-, PEP-, and outcome-related terms (Supplementary Table 2 of Supplementary Materials). Additional searches were conducted in the Cochrane Library. To identify additional relevant citations, the reference lists of published reviews and journal articles were also screened. All searches were limited to English peer-reviewed journal articles. The original bibliographic searches for PsycINFO and PubMed were conducted on June 15, 2021. The original search for Web of Science, Google Scholar, and Cochrane Library were conducted on June 16, 2021. The updated searches for PsycINFO, PubMed, and Web of Science were conducted on November 19, 2021, while for Google Scholar and Cochrane Library on November 20, 2021.
Studies were considered eligible if they met all of the following inclusion criteria: (1) conducted primary data collection; (2) were studies related to PEP use for HIV prevention; (3) reported data about gay and bisexual men, regardless of age, country, and HIV status; (4) reported history, awareness, or intention of PEP use among MSM; and (5) appeared in English-language, peer-reviewed journal articles. Studies that reported data about MSM and other populations, such as transgender people or sex workers, were included, but only data related to MSM were considered and abstracted. Studies were identified as ineligible if they were (1) case reports; (2) longitudinal studies or trend studies; (3) unpublished book chapters, theses, dissertations, or articles; and (4) non-original research, secondary reports, review articles, or theoretical framework articles.
EndNote (version 6) was adopted throughout the process of study selection. First, titles and abstracts were preliminarily screened to exclude irrelevant studies. Second, full-text versions of selected papers were assessed independently to ensure that all inclusion criteria were met. Disagreement in the process of study selection was resolved by discussion with a third reviewer. The two researchers used standardized Microsoft Excel spreadsheets to extract the following information: authors, year of publication, country of study, study design, settings, study populations, outcomes, and factors. Interview quotes relating to awareness or use of PEP were also collected to aid conceptual understanding of qualitative findings.
The primary outcomes of interest were measures of PEP awareness and previous use or potential usage intention (Supplementary Table 3 in Supplementary Materials). All available data were pooled and synthesized using a combination of single-rate meta-analysis and a narrative synthesis approach. For quantitative studies, a random effects model was used to quantitatively summarize the pooled event rates (ERs) of PEP awareness and previous PEP use. Random effects meta-regression was used to assess the relationship between year of data collection and PEP awareness or use. Subgroup analysis was performed to determine the potential influence of the following covariate: level of national economic development.
A synthesis of factors determining PEP awareness and use was performed and classified into individual, social, or structural layers based on a socioecological model (25, 26). Qualitative data were drawn to provide context for the quantitative findings as recommended (27, 28) by identifying participant perspectives about factors that might affect awareness and previous use or potential intention of use of PEP among MSM. Thematic analysis was used to abstract, sort, compare, and categorize data to construct a set of emerging descriptive themes from relevant quotes. Themes were then used to construct a conceptual framework of awareness and previous use or potential usage intention of PEP in the individual, social, and structural domains.
Two independent reviewers used the Cochrane Collaboration tool to assess the risk of different types of biases, including recruitment, sampling, attrition and non-response, social desirability, and researcher (29). The potential risk of impact of biases on the robustness of the included findings was also discussed (see Supplementary Table 4 in Supplementary Materials).
Of a total of 3,004 titles, 402 were further assessed for inclusion, and 20 were included in the final review (Figure 1). The initial screening excluded studies that did not specifically focus on HIV, PEP, and duplicates, leaving a total of 402 citations. Subsequently, 382 citations were excluded after screening the abstracts and full papers. Several papers focusing on other population groups (e.g., heterosexual, sex workers, health care workers, and transgender populations) were identified through the search and were excluded (n = 129). Additional exclusions were due to poor relevance of outcomes (e.g., cost effectiveness, medication adherence; n = 174), failure to segregate results by population (n = 16), and reviews or non-peer reviewed articles (n = 63).
Overall, 20 eligible studies published between 2007 and 2021 were included in the meta-analysis, involving 12,579 MSM. Supplementary Table 5 in the Supplementary Materials presents the key characteristics of the studies included in the review. Of the included studies, 19 were quantitative, and one was a mixed-method study. The two-phase mixed-method study (30) included a cross-sectional survey with a self-administered questionnaire and a prospective descriptive study using an in-depth interview, but data needed were collected in the quantitative survey phase. Another study used a longitudinal cohort study to evaluate the impact of HIV leadership programs, and its baseline survey provided the data we needed (31). All of the remaining quantitative studies were cross-sectional surveys.
The included studies used consistent definitions of PEP for MSM with different terms, such as non-occupational PEP (nPEP) or PEP after sexual exposure (PEPSE). Given that many persons did not know or knew little about PEP, an introductory explanation of PEP may have been provided to participants during the survey.
All 20 studies reporting awareness of PEP employed a simple binary question asking participants whether they were aware (or had heard) of PEP, except for one study (32) that asked whether a medical treatment existed that could reduce the chance of becoming HIV-positive.
Meta-analysis of the 20 studies found that the pooled estimate of the proportions of MSM who were aware of PEP was 59.9% (95% CI: 50.5~68.7) (Figure 2). Awareness of PEP in these studies was inconsistent, ranging from 22.09% among MSM in Guangxi, China (33), to 88.27% among MSM in London who use geosocial networking smartphone applications (11). Seven studies reported higher awareness than 70% among gay men in Israel (15), young MSM of color in the United States (34), MSM users of geosocial networking applications in England (11), non-HIV-positive MSM in Italy (32), sexually active MSM living with HIV in France (35), MSM in 22 Sub-Saharan African countries (36), and among MSM college students in three cities of China (37). Four studies reported much lower levels of awareness (<40%) among MSM in China (33); MSM tested in community centers in Portugal (38) MSM receiving rapid HIV testing in Spain (39), and among black MSM in the United States (13). Nine studies reported modest PEP awareness: 41.23% among men engaging in condomless anal sex with men in the United States (8), 42.5% among MSM in Beijing, China (16), 46.67% among gay and bisexual men in California (40), 50% among MSM in Brazil (41), 56.70% among sexually active MSM in Vancouver, Canada (31), 57.06% among MSM in Vancouver, Canada (42), 60.22% among Thai MSM (30), 60.6% among MSM in four cities of China (43), and 65.77% among HIV-positive MSM in England (44). There was significant heterogeneity between studies (Q statistic = 1990.122, I2 = 99.045; p < 0.001).
Two studies equated awareness with knowledge of PEP (32, 40, 45). However, self-reported awareness did not necessarily reflect a precise understanding of PEP. Several studies checked whether self-reported knowledge about PEP was accurate or evaluated PEP knowledge in MSM. Those MSM who were aware of PEP had adequate knowledge about PEP; however, less than half of them correctly answered that PEP needs to be taken for 28 days (30, 34). Among 94 MSM who had heard of PEP, 11% were concerned about side effects; most (68%) felt they would know how to get PEP, and nearly two-thirds anticipated that they would not be able to afford it (8). Only 40% of those who reported being aware of PEP (24% of total participants) were aware that the time window for an effective PEP is 72 h (15).
Bivariate meta-regression showed that temporal changes could not explain PEP awareness (slope = −0.031, 95% CI:-0.038~-0.024; p < 0.001): the minimum value of PEP awareness was collected in 2016 (33), and the maximum value was also gleaned in 2016 (11). Subgroup analysis revealed that developed countries PEP awareness had no differences compared to that in developing countries (Q statistic = 0.865; p = 0.352): fourteen studies from developed countries found that the point estimate of proportions of MSM who were aware of PEP was 51.9% (95% CI: 50.9~53), and six studies from developing countries found the point estimate was 52.9% (95% CI: 51.1~54.7). In addition, the studies had no significant publication bias in the awareness subgroup analysis (intercept = 8.544, 95% CI:−2.2~19.29, p = 0.112).
In addition to overall awareness, seven studies explored other factors that were correlated with awareness of PEP among MSM. Table 1 illustrates the range of factors affecting MSM's awareness of PEP documented in the included studies. These factors, which could potentially impede or facilitate participant's awareness of PEP, were conceptually divided into different categories within the individual, social (including partners, families, and communities), and structural domains (health systems and legal factors). In the individual domain, low educational attainment, unemployment, having casual partners, and closeted bisexual were negative factors; high-level education, white race/ethnicity, gay sexual identity, knowing the HIV status of one's self, higher personal sexual altruism, metropolitan resident, higher annual income, using the internet as the main way of meeting partners, having sex with an HIV-positive male partner, and having unprotected anal sex were positive factors. How age, drug use, and number of partners exert influence on awareness of PEP is uncertain.
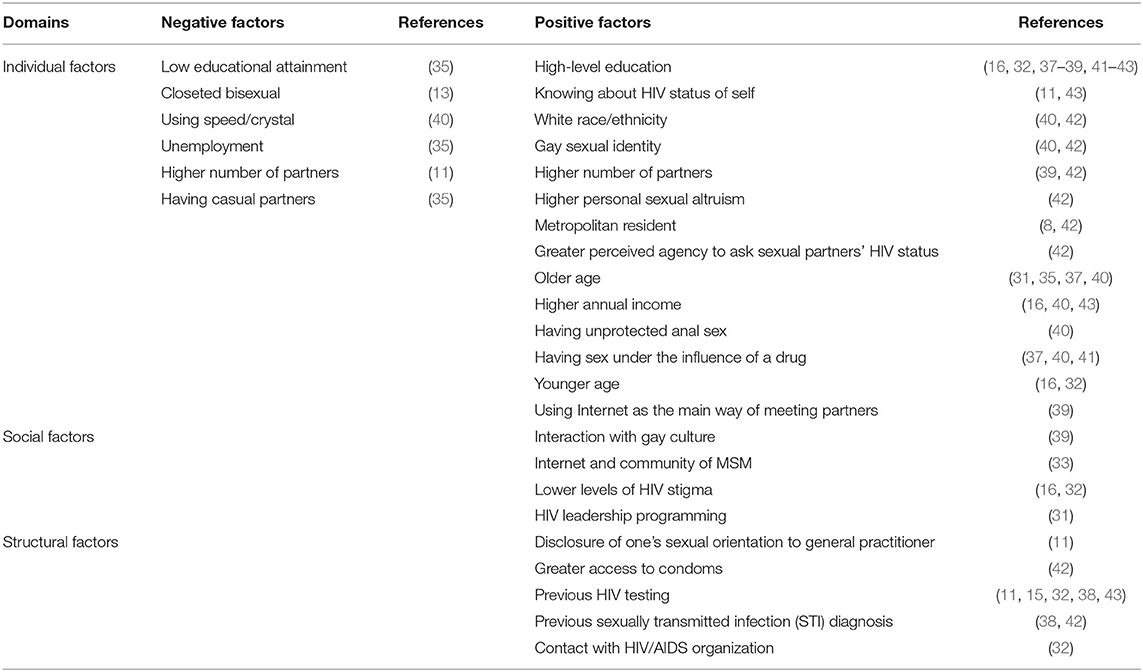
Table 1. Factors affecting awareness of HIV post-exposure prophylaxis (PEP) among men who have sex with men.
Social factors associated with greater awareness among MSM included more interaction with gay culture (39), lower levels of HIV stigma (32), and HIV leadership programming attendance (31). One study conducted logistic regressions predicting awareness of PEP and found that location was the only significant predictor: compared to Puerto Rican participants, those in Pittsburgh were 5.7 times more likely to have heard of PEP, and those in Boston were 10.1 times more likely to have heard of PEP (8).
Regarding structural factors, greater access to condoms (42), more frequent contact with HIV/AIDS organizations (32), previous sexually transmitted infection (STI) diagnosis (42), HIV testing (32, 34, 35, 43), and disclosure of one's sexual orientation to their general practitioner (11) were associated with having heard of PEP.
Thirteen studies assessed quantitative proportions of participants who had used PEP. All of them used a simple binary question, asking participants whether they had used or taken PEP in the past. Although the question was asked of all MSM participants in some studies (11, 13, 30, 39), we calculated the proportion of MSM who had used PEP among subsamples who were aware of PEP. Meta-analysis of the thirteen studies found that 4.9% (95% CI: 2.4~9.8) of MSM had used PEP (Figure 3). Previous use of PEP in these studies was low and inconsistent, ranging from 1.19% among HIV-negative MSM in Vancouver, Canada (42) to 40.74% among MSM in 22 Sub-Saharan African countries. Most studies reported much <10% of respondents that indicated previous use, with the exception of three studies that reported previous use of 40.74% among 22 Sub-Saharan African countries (36), 11.86% among young MSM of color in the United States (34), and 27.37% in London (11). As expected, there was a high level of heterogeneity between these studies (Q statistic = 656.703, I2 = 98.173, p < 0.001).
One study reported a qualitative assessment of willingness to use PEP, asking participants “If you have risk behaviors for HIV infection, will you seek PEP?,” and 416 participants (92.4%) answered yes (30). Another study (8) showed that participants were especially likely to say they would use PEP in the future, scoring an average of 9.1 (on a 10-point scale, with 10 being extremely likely), but did not report the proportion of MSM participants who were very willing to use PEP. One study used a single item with a 5-point Likert-type scale to assess willingness to use PEP and reported 461 (73.06%) MSM participants who were very-to-extremely likely to use PEP (45).
Bivariate meta-regression showed that years of data collection could predict PEP use (slope = −0.031, 95% CI:−0.038~-0.024; p < 0.001): PEP use among MSM significantly increased from 4.18% in 2006 to 30.01% in 2016 (11). Subgroup analysis revealed that PEP use in developing countries was significantly higher than that in developed countries (Q statistic = 96.77; p < 0.001): eight studies from developed countries found that the point estimate of proportions of MSM who previously used PEP was 5.4% (95% CI: 4.8~6.1), and five studies from developing countries found the point estimate was 13.2% (95% CI: 11.5~15). The studies had no significant publication bias in the use subgroup analysis (intercept = −7.260, 95% CI:−18.18~3.66, p = 0.171).
In addition to the overall previous use of PEP or willingness to use PEP, our review provides comprehensive information regarding barriers and facilitating factors of its use. Table 2 illustrates the range of factors influencing MSM's previous use or potential use of PEP mentioned in the eligible studies. Several studies explored factors that were associated with previous use of PEP among MSM. In the individual domain, concern about side effects (8) and lack of PEP information (11, 34) were barriers, whereas having adequate knowledge about PEP (30), having been involved in high-risk sexual intercourse (39), being circumcised (30), and using methamphetamine (11) were facilitating factors. Associations between past PEP use and club drug use have been tested for HIV in the past year, and recent sexually transmitted infection (STI) diagnoses have not persisted (11). In the social domain, being in a relationship (11) was a facilitating factor. In the structural domain, cost emerged as an important barrier to the use of PEP in some cities in the United States (8) or sometimes in Thailand, where PEP is not available free of charge (30).
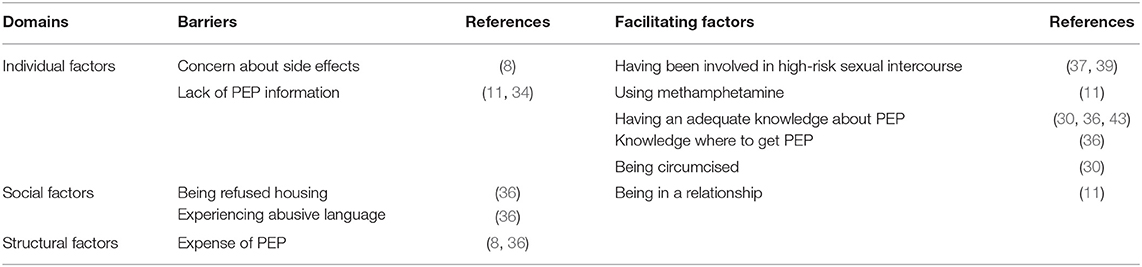
Table 2. Factors affecting the use of HIV post-exposure prophylaxis (PEP) among men who have sex with men.
Factors associated with an intention to take PEP included awareness of PEP, HIV knowledge with > 80% correct answers, absence of penetrative anal sex in the past 3 months, and circumcision (30). Partnered men's willingness to use PEP was positively associated with having an individual income < $30,000 USD and serosorting within the relationship (sexual partner selection on the basis of HIV status), whereas it was not clear why higher investment in couple relationships and greater age differences between primary partners were barriers (45).
The finding of modest PEP awareness may be because high-income countries possess an apparent advantage with coverage of antiretroviral therapy, HIV diagnosis among people living with HIV, and access to health care services (3). MSM, as a key population for HIV prevention, have recently received a great deal of attention, especially in cities of high-income countries. For example, a trend study in Australia found that awareness of PEP among gay men significantly increased from 23% in 2001 to 64% in 2010 (46). In Europe, researchers saw an opportunity to provide global leadership at the regional scale-up of comprehensive AIDS prevention interventions for MSM (47).
In many low- and middle-income countries, post-exposure prophylaxis is not yet a routine service, and there is relatively little research on it. This review lacks data from low- and middle-income settings. Most included studies (8/20) were conducted in the United States, England, and Canada, which limited the extent to which the findings may be applicable to MSM in low- and middle-income countries because factors that drive trends in HIV prevention among MSM may be very different among them. On the one hand, a body of evidence reports that MSM are at marked risk for HIV infection in low- and middle-income countries in Asia, Africa, Latin America, and Eastern Europe (48, 49). On the other hand, MSM are a markedly underserved and underresourced population in low- and middle-income settings such as Latin America (50) and Africa (51). There is an urgent need to better understand awareness and use of PEP, as well as to improve HIV prevention among MSM in low- and middle-income countries.
Appropriate use of PEP is extremely low compared with HIV prevalence and high-risk behavior among MSM. After high-risk exposure to HIV, very few MSM take PEP. A study found that only three had used PEP among 228 men engaging in condomless anal sex with men (8). Even if some MSM had used or were willing to use PEP, they may not adhere to completing the PEP regimen. A meta-analysis revealed that adherence to the full 28-day course of antiretroviral drugs prescribed for PEP was only 67.2% (17). In some countries, PEP services often depend on emergency physicians and specialists (IDs) in HIV/sexually transmitted infection clinics, which can potentially hinder the use of PEP (52, 53). Recently, one study showed that the mean knowledge scores were low for health care workers in campus clinics and tertiary hospitals (54). Some strategies are required to improve the appropriate use of PEP for HIV prevention among MSM when at risk. PrEP and PEP are highly validated HIV prevention tools and are part of a wide range of prevention measures, including HIV testing, condom use, and screening and treatment for sexually transmitted infections (55). We should train community physicians in risk assessment, medication adherence assessment, and offline communication through online social media and offline posters, cards and brochures to promote PEP knowledge and inform them of ways to seek services (56). The completion and use of PEP has been shown to vary depending on the drug regimen. In many countries, tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) is the preferred backbone drug (57, 58). However, in the MSM population, the vast majority of MSM prefer to use a single dose of PEP as opposed to a multitablet regimen formulation, and single-dose regimens show better adherence (59, 60). An optimized regimen with good tolerability and simplified use improves PEP adherence. We only conducted subgroup analysis of very limited geographic factors, which is one of the limitations of this review. MSM included in our meta-analysis are diverse, with different ages, educational attainment, races/ethnicities, annual incomes, sexual identities, HIV statuses, and so on. Sociodemographic comparisons could provide further insights into contextual determinants of awareness or use of PEP. As mentioned earlier, the reported proportions of PEP awareness and use were highly heterogeneous. The diversity of recruitment methods and data collection settings may have also influenced the precision of our estimates, and other factors, such as differences in MSM participants and their settings, could also contribute to bias. Therefore, we used the random effects model rather than the fixed effects model, as the former provides more conservative estimates, such as wider 95% confidence intervals (CIs).
This review provides information regarding factors associated with PEP awareness, which is crucial for the promotion of treatment-seeking behavior after sexual exposure and reduction of the HIV transmission rate among MSM. In particular, this review examined a series of individual, social, and structural factors that may influence this awareness. These findings about individual factors suggest that PEP education and information should be prioritized for MSM if they have a low educational level, are unemployed, have casual partners, are ethnic minorities, and are closeted bisexual men. In the social domain, lower levels of HIV stigma and more interaction with gay culture were positively associated with higher awareness, but other factors were not investigated sufficiently, such as stigmatization of PEP and homosexual orientation and reaction or support from partners, peers, and family. In the structural domain, access to condoms, HIV testing, STI diagnosis, and primary providers or general practitioners having accurate knowledge about MSM behavior and sexual orientation could play important roles in awareness of PEP. Stigma from peers, partners, and family as well as health care providers continues to be perceived by MSM (61, 62), and criminalization of same-sex relations may limit uptake of prevention services among MSM (1), so it is essential to integrate strategies to mitigate stigma related to sexual orientation and create an MSM-friendly environment, in addition to providing PEP information and education.
Our review also provides timely and comprehensive information regarding motivations for and barriers to PEP use, but many factors have not been fully discussed. Although PEP is effective in reducing HIV transmission, it may also exacerbate high-risk sexual behavior. The relationship between PEP use and high-risk sexual behaviors is unclear. PEP may exacerbate high-risk sexual behaviors, also known as sexual risk behavior disinhibition PEP (15, 63). However, some studies have shown no significant correlation between the two (64–66). In this regard, it is necessary to conduct research in the future to evaluate the potential impact of PEP on the sexual behavior of key populations. Except for concern about side effects, lack of PEP information, and high-risk behaviors, several other individual-based demographic factors, including levels of educational attainment, age, race/ethnicity, residency, and sexual orientation, could influence PEP use but were not well researched. Individual behaviors of MSM, such as engaging in condomless sex, are often shaped in social contexts. In addition to the relationship and cost of PEP, other key social and structural factors, including reaction or support from relatives and friends, stigmatization in health facilities, concern for quality of treatment, and a lack of confidentiality and privacy protection, should be further explored. As MSM increasingly turn to the internet to find the community and meet partners, internet-based platforms are becoming salient social environments for MSM and could offer opportunities for HIV prevention (67).
Notwithstanding several limitations, our review points to several considerations for future PEP research, policy, and clinical practice. (1) To better understand global AIDS prevention, awareness and use of PEP among MSM in low- and middle-income countries and areas should be surveyed. (2) A fuller understanding of the reasons for PEP awareness and use among MSM is needed. (3) To make significant progress in AIDS prevention and control, the barriers found in our review and other potential barriers should be addressed.
Post-exposure prophylaxis is important not only to reduce HIV transmission in MSM, but also in other high-risk populations. Examples include heterosexual populations, injection drug users, bisexual populations, and sex workers (43, 68). Some studies show that MSM report higher awareness of PEP than other at-risk groups for HIV (12). A study in Brazil found that MSM participants were more likely to have knowledge about PEP than heterosexual male participants (41). Awareness of PEP may be associated with the age, higher education, and income of these MSM in the high-risk population. In conclusion, PEP has been shown to be safe and efficacious in reducing the risk of HIV acquisition (69, 70), and demonstration projects are becoming increasingly being implemented (71–73). This review reveals that awareness of PEP among MSM is generally modest, ranging from 22.09 to 88.27%. However, PEP is underused, with a pooled estimate of 4.3% among MSM. Despite the currently low previous use of PEP, this review contributes to this evidence base by demonstrating that MSM are willing to use PEP when they become aware of it and that they should obtain various sources of support to cope with all kinds of barriers mentioned in this article. Programs aimed at introducing or promoting the usage of PEP need to be based on context-specific evidence, such as potential demand and user preferences, and need to be supported by enabling policies and legal framework environments.
BS affirms that this article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
RS, HZ, and BS conceptualized the study. JJ, RS, TM, and TJ searched the literature, selected studies, and extracted the data. JJ, RS, TM, LD, HL, XR, JC, JY, LS, HW, TZ, and HZ contributed to the analysis, interpretation of the data, and provided important scientific input. JJ, RS, and BS analyzed the findings and wrote the first draft of the manuscript. BS supervised the whole study. All authors collaboratively discussed key decisions throughout the course of the review, provided critical feedback on preliminary manuscript and interpretation of results, and approved the final version.
This work was supported by the National Natural Science Foundation of China (NSFC, 81772165 and 81974303 to BS, 82072271 to TZ, 81703278 to HZ), the NSFC-NIH Biomedical collaborative research program (81761128001 to HW), the Climbing the peak (Dengfeng) Talent Training Program of Beijing Hospitals Authority (DFL20191701 to TZ), Special Project of Scientific Research and Cultivation of Beijing Centers for Disease Prevention and Control/Center for Preventive Medicine Research (2020-BJYJ-14 to XR), and Beijing Key Laboratory for HIV/AIDS Research (BZ0089). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.783626/full#supplementary-material
PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; nPEP, non-occupational PEP; PEPSE, PEP after sexual exposure; MSM, Men who have sex with men; HIV, Human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; PROSPERO, Prospective Register of Systematic Reviews; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ER, event rates; STD, sexually transmitted disease; STI, sexually transmitted infection; CIs, confidence intervals.
1. Beyrer C, Baral SD, Collins C, Richardson ET, Sullivan PS, Sanchez J, et al. The global response to HIV in men who have sex with men. Lancet. (2016) 388:198–206. doi: 10.1016/S0140-6736(16)30781-4
2. Beyrer C, Sullivan P, Sanchez J, Baral SD, Collins C, Wirtz AL, et al. The increase in global HIV epidemics in MSM. AIDS. (2013) 27:2665–78. doi: 10.1097/01.aids.0000432449.30239.fe
3. Sullivan PS, Jones JS, Baral SD. The global north: HIV epidemiology in high-income countries. Curr Opin HIV AIDS. (2014) 9:199–205. doi: 10.1097/COH.0000000000000039
4. Beyrer C, Baral SD, Walker D, Wirtz AL, Johns B, Sifakis F. The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: diversity and consistency. Epidemiol Rev. (2010) 32:137–51. doi: 10.1093/epirev/mxq011
5. Caceres CF, Koechlin F, Goicochea P, Sow PS, O'Reilly KR, Mayer KH, et al. The promises and challenges of pre-exposure prophylaxis as part of the emerging paradigm of combination HIV prevention. J Int AIDS Soc. (2015) 18:19949. doi: 10.7448/IAS.18.4.19949
6. Almeda J, Casabona Barbara J, Simon B, Gerard M, Rey D, Puro V, et al. Proposed recommendations for the management of HIV post-exposure prophylaxis after sexual, injecting drug or other exposures in Europe. Euro Surveill. (2004) 9:5–6. doi: 10.2807/esm.09.06.00471-en
7. Lu TY, Mao X, Peng EL, Li JM, Geng WQ, Jiang YJ, et al. Bibliometric analysis on research hotspots on HIV post-exposure prophylaxis related articles in the world, 2000-2017. Zhonghua Liu Xing Bing Xue Za Zhi. (2018) 39:1501–6. doi: 10.3760/cma.j.issn.0254-6450.2018.11.016
8. Dolezal C, Frasca T, Giguere R, Ibitoye M, Cranston RD, Febo I, et al. Awareness of post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP) is low but interest is high among men engaging in condomless anal sex with men in boston, Pittsburgh, and san juan. AIDS Educ Prev. (2015) 27:289–97. doi: 10.1521/aeap.2015.27.4.289
9. Krakower DS, Jain S, Mayer KH. Antiretrovirals for primary HIV prevention: the current status of pre- and post-exposure prophylaxis. Curr HIV/AIDS Rep. (2015) 12:127–38. doi: 10.1007/s11904-014-0253-5
10. Jain S, Oldenburg CE, Mimiaga MJ, Mayer KH. Subsequent HIV infection among men who have sex with men who used non-occupational post-exposure prophylaxis at a Boston community health center: 1997-2013. AIDS Patient Care STDS. (2015) 29:20–5. doi: 10.1089/apc.2014.0154
11. Goedel WC, Hagen D, Halkitis PN, Greene RE, Griffin-Tomas M, Brooks FA, et al. Post-exposure prophylaxis awareness and use among men who have sex with men in London who use geosocial-networking smartphone applications. AIDS Care. (2017) 29:579–86. doi: 10.1080/09540121.2016.1259455
12. Walters SM, Rivera AV, Starbuck L, Reilly KH, Boldon N, Anderson BJ, et al. Differences in awareness of pre-exposure prophylaxis and post-exposure prophylaxis among groups at-risk for HIV in New York state: New York city and Long Island, NY, 2011-2013. J Acquir Immune Defic Syndr. (2017) 75:S383–91. doi: 10.1097/QAI.0000000000001415
13. Watson RJ, Fish JN, Allen A, Eaton L. Sexual identity disclosure and awareness of HIV prevention methods among black men who have sex with men. J Sex Res. (2018) 55:975–83. doi: 10.1080/00224499.2017.1375452
14. Wang ZY, Yuan TW, Fan S, Qian HZ, Li PY, Zhan YW, et al. HIV Nonoccupational postexposure prophylaxis among men who have sex with men: a systematic review and meta-analysis of global data. AIDS Patient Care STDS. (2020) 34:193–204. doi: 10.1089/apc.2019.0313
15. Leshin D, Olshtain-Pops K, Moses A, Elinav H. Limited awareness of the effective timing of HIV post-exposure prophylaxis among people with high-risk exposure to HIV. Eur J Clin Microbiol Infect Dis. (2019) 38:779–84. doi: 10.1007/s10096-019-03476-4
16. Sun Y, Li G, Lu H. Awareness and use of nonoccupational HIV post-exposure prophylaxis and factors associated with awareness among MSM in Beijing, China. PLoS ONE. (2021) 16:e0255108. doi: 10.1371/journal.pone.0255108
17. Ford N, Irvine C, Shubber Z, Baggaley R, Beanland R, Vitoria M, et al. Adherence to HIV postexposure prophylaxis: a systematic review and meta-analysis. AIDS. (2014) 28:2721–7. doi: 10.1097/QAD.0000000000000505
18. Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WL, Wilson PA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. (2012) 380:341–8. doi: 10.1016/S0140-6736(12)60899-X
19. Wolitski RJ, Fenton KA. Sexual Health, HIV, and sexually transmitted infections among gay, bisexual, and other men who have sex with men in the United States. AIDS Behav. (2011) 15:S9–S17. doi: 10.1007/s10461-011-9901-6
20. Morgan E, Skaathun B, Lancki N, Jimenez AD, Ramirez-Valles J, Bhatia R, et al. Trends in HIV Risk, testing, and treatment among MSM in Chicago 2004-2014: implications for HIV elimination planning. J Urban Health. (2017) 94:699–709. doi: 10.1007/s11524-017-0175-9
21. Hall HI, Song R, Tang T, An Q, Prejean J, Dietz P, et al. HIV trends in the United States: diagnoses and estimated incidence. JMIR Public Health Surveill. (2017) 3:e8. doi: 10.2196/publichealth.7051
22. Stahlman S, Beyrer C, Sullivan PS, Mayer KH, Baral SD. Engagement of gay men and other men who have sex with men (MSM) in the response to HIV: a critical step in achieving an AIDS-free generation. AIDS Behav. (2016) 20:330–40. doi: 10.1007/s10461-016-1388-8
23. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
24. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
25. Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. (1996) 10:282–98. doi: 10.4278/0890-1171-10.4.282
26. Mburu G, Ram M, Oxenham D, Haamujompa C, Iorpenda K, Ferguson L. Responding to adolescents living with HIV in Zambia: a social–ecological approach. Child Youth Serv Rev. (2014) 45:9–17. doi: 10.1016/j.childyouth.2014.03.033
27. Howe KR. Mixed methods, triangulation, and causal explanation. J Mix Methods Res. (2012) 6:89–96. doi: 10.1177/1558689812437187
28. Pope C, May N, Popay J. How can we synthesize qualitative and quantitative evidence for healthcare policy-makers and managers. Healthc Manage Forum. (2006) 19:27–31. doi: 10.1016/S0840-4704(10)60079-8
29. Higgins J, Green S. Cochrane Handjournal for Systematic Reviews of Interventions Version 5.0.1. Cochrane Collaborat. (2008) 187–234. doi: 10.1002/9780470712184
30. Chomchey N, Woratanarat T, Hiransuthikul N, Lertmaharit S, Lohsoonthorn V, Teeratakulpisarn N, et al. Factors associated with intention to take non-occupational HIV post-exposure prophylaxis among Thai men who have sex with men. J Virus Erad. (2017) 3:128–39. doi: 10.1016/S2055-6640(20)30331-9
31. Closson K, Chown S, Armstrong HL, Wang L, Bacani N, Ho D, et al. HIV leadership programming attendance is associated with PrEP and PEP awareness among young, gay, bisexual, and other men who have sex with men in Vancouver, Canada. BMC Public Health. (2019) 19:429. doi: 10.1186/s12889-019-6744-y
32. Prati G, Zani B, Pietrantoni L, Scudiero D, Perone P, Cosmaro L, et al. PEP and TasP awareness among Italian MSM, PLWHA, and high-risk heterosexuals and demographic, behavioral, and social correlates. PLoS ONE. (2016) 11:e0157339. doi: 10.1371/journal.pone.0157339
33. Zeng Z, Liu H, Xu J, Lan G, Wang L, Yin W. Demand for non-occupational post-exposure prophylaxis and its influencing factors among 344 men who have sex with men in Guangxi. Chin J AIDS STD. (2017) 23:620–4. doi: 10.13419/j.cnki.aids.2017.07.12
34. Koblin BA, Usher D, Nandi V, Tieu HV, Bravo E, Lucy D, et al. Post-exposure prophylaxis awareness, knowledge, access and use among three populations in New York City, 2016-17. AIDS Behav. (2018) 22:2718–32. doi: 10.1007/s10461-018-2175-5
35. Rey D, Anne-Déborah B, Peretti-Watel P, Obadia Y, Spire B. Awareness of non-occupational HIV postexposure prophylaxis among French people living with HIV:the need for better targeting. AIDS. (2007) 21:S71–6. doi: 10.1097/01.aids.0000255088.44297.26
36. Isano S, Wong R, Logan J, El-Halabi S, El-Khatib Z. Barriers to post exposure prophylaxis use among men who have sex with men in sub-Saharan Africa: an online cross-sectional survey. Prev Med Rep. (2020) 19:101100. doi: 10.1016/j.pmedr.2020.101100
37. Han J, Li J, Wang KR, Jiang TJ, Song B, Wang H, et al. [Status and influencing factors of knowledge awareness and service acceptance of HIV non-occupational post-exposure prophylaxis of men who have sex with men among college students among three cities of China, 2019]. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine]. (2020) 54:1220–6. doi: 10.3760/cma.j.cn112150-20200310-00302
38. Simoes D, Meireles P, Rocha M, Freitas R, Aguiar A, Barros H. Knowledge and use of PEP and PrEP among key populations tested in community centers in Portugal. Front Public Health. (2021) 9:673959. doi: 10.3389/fpubh.2021.673959
39. Fernandez-Balbuena S, Belza MJ, Castilla J, Hoyos J, Rosales-Statkus ME, Sanchez R, et al. Awareness and use of nonoccupational HIV post-exposure prophylaxis among people receiving rapid HIV testing in Spain. HIV Med. (2013) 14:252–7. doi: 10.1111/j.1468-1293.2012.01056.x
40. Liu AY, Kittredge PV, Vittinghoff E, Raymond HF, Ahrens K, Matheson T, et al. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. (2008) 47:241–7. doi: 10.1097/QAI.0b013e31815e4041
41. Sousa LRM, Elias HC, Fernandes NM, Gir E, Reis RK. Knowledge of PEP and PrEP among people living with HIV/aids in Brazil. BMC Public Health. (2021) 21:64. doi: 10.1186/s12889-020-10135-3
42. Lin SY, Lachowsky NJ, Hull M, Rich A, Cui Z, Sereda P, et al. Awareness and use of nonoccupational post-exposure prophylaxis among men who have sex with men in Vancouver, Canada. HIV Med. (2016) 17:662–73. doi: 10.1111/hiv.12369
43. Hou J, Wu Y, Xie L, Meng S, Fu R, Zheng H, et al. Post-exposure prophylaxis: an underutilized biomedical HIV prevention method among gay, bisexual and other men who have sex with men in China. AIDS Care. (2020) 32:1573–80. doi: 10.1080/09540121.2020.1742864
44. Joshi M, Basra A, McCormick C, Webb H, Pakianathan M. Post-exposure prophylaxis after sexual exposure (PEPSE) awareness in an HIV-positive cohort. Int J STD AIDS. (2014) 25:67–9. doi: 10.1177/0956462413491734
45. Mitchell JW, Sophus AI, Petroll AE. HIV-negative partnered men's willingness to use non-occupational post-exposure prophylaxis and associated factors in a US sample of HIV-negative and HIV-discordant male couples. LGBT Health. (2016) 3:146–52. doi: 10.1089/lgbt.2015.0065
46. Zablotska IB, Prestage G, Holt M, Poynten M, de Wit J, Guy R, et al. Australian gay men who have taken nonoccupational postexposure prophylaxis for HIV are in need of effective HIV prevention methods. JAIDS. (2011) 58:424–8. doi: 10.1097/QAI.0b013e318230e885
47. Stromdahl S, Hickson F, Pharris A, Sabido M, Baral S, Thorson A. A systematic review of evidence to inform HIV prevention interventions among men who have sex with men in Europe. Euro surveillance. (2015) 20:15. doi: 10.2807/1560-7917.ES2015.20.15.21096
48. van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS. (2009) 4:300–7. doi: 10.1097/COH.0b013e32832c3bb3
49. Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middleincome countries 2000–2006 a systematic review. PLoS Med. (2007) 4:e339. doi: 10.1371/journal.pmed.0040339
50. Global HIV Prevention Working Group. Bringing HIV prevention to scale: an urgent global priority. Global HIV Prevention Working Group (2007).
51. Zahn R, Grosso A, Scheibe A, Bekker LG, Ketende S, Dausab F, et al. Human rights violations among men who have sex with men in southern africa: comparisons between legal contexts. PLoS ONE. (2016) 11:e0147156. doi: 10.1371/journal.pone.0147156
52. Teo AKJ, Tai BC, Chio MT, La HH. A mixed methods study of non-occupational post-exposure prophylaxis at an STI clinic in Singapore: Five-year retrospective analysis and providers' perspectives. PLoS ONE. (2018) 13:e0202267. doi: 10.1371/journal.pone.0202267
53. Malinverni S, Libois A, Gennotte AF, La Morte C, Mols P. Prescription of non-occupational post-exposure HIV prophylaxis by emergency physicians: an analysis on accuracy of prescription and compliance. PLoS ONE. (2016) 11:e0153021. doi: 10.1371/journal.pone.0153021
54. Qianqian Y, Chunhui L, Xun H, Anhua W, Yuhua C, Yaowang W, et al. Knowledge of occupational exposure to HIV among healthcare workers in college campus clinics and tertiary hospitals. AIDS Care. (2021) doi: 10.1080/09540121.2021.1981815. [Epub ahead of print].
55. Phanuphak N, Gulick RM. HIV treatment and prevention 2019: current standards of care. Curr Opin HIV AIDS. (2020) 15:4–12. doi: 10.1097/COH.0000000000000588
56. Wu Y, Zhu Q, Zhou Y, Liang S, Li R, Liang N, et al. Implementation of HIV non-occupational post-exposure prophylaxis for men who have sex with men in 2 cities of Southwestern China. Medicine. (2021) 100:e27563. doi: 10.1097/MD.0000000000027563
57. Chauveau M, Billaud E, Bonnet B, Merrien D, Hitoto H, Bouchez S, et al. Tenofovir DF/emtricitabine/rilpivirine as HIV post-exposure prophylaxis: results from a multicentre prospective study. J Antimicrob Chemother. (2019) 74:1021–7. doi: 10.1093/jac/dky547
58. Ford N, Shubber Z, Calmy A, Irvine C, Rapparini C, Ajose O, et al. Choice of antiretroviral drugs for postexposure prophylaxis for adults and adolescents: a systematic review. Clin Infect Dis. (2015) 60:S170–6. doi: 10.1093/cid/civ092
59. Massud I, Ruone S, Zlotorzynska M, Haaland R, Mills P, Cong ME, et al. Single oral dose for HIV pre or post-exposure prophylaxis: user desirability and biological efficacy in macaques. EBioMedicine. (2020) 58:102894. doi: 10.1016/j.ebiom.2020.102894
60. Foster R, McAllister J, Read TR, Pierce AB, Richardson R, McNulty A, et al. Single-tablet emtricitabine-rilpivirine-tenofovir as HIV postexposure prophylaxis in men who have sex with men. Clin Infect Dis. (2015) 61:1336–41. doi: 10.1093/cid/civ511
61. Ayala G, Makofane K, Santos GM, Beck J, Do TD, Hebert P, et al. Access to basic HIV-related services and PrEP acceptability among men who have sex with men worldwide: barriers, facilitators, and implications for combination prevention. J Sex Transm Dis. (2013) 2013:953123. doi: 10.1155/2013/953123
62. Chakrapani V, Newman PA, Shunmugam M, Mengle S, Varghese J, Nelson R, et al. Acceptability of HIV pre-exposure prophylaxis (PrEP) and implementation challenges among men who have sex with men in india: a qualitative investigation. AIDS Patient Care STDS. (2015) 29:569–77. doi: 10.1089/apc.2015.0143
63. Jain S, Mayer KH. Practical guidance for nonoccupational postexposure prophylaxis to prevent HIV infection: an editorial review. AIDS. (2014) 28:1545–54. doi: 10.1097/QAD.0000000000000301
64. Poynten IM, Jin F, Mao L, Prestage GP, Kippax SC, Kaldor JM, et al. Nonoccupational postexposure prophylaxis, subsequent risk behaviour and HIV incidence in a cohort of Australian homosexual men. AIDS. (2009) 23:1119–26. doi: 10.1097/QAD.0b013e32832c1776
65. Waldo CR, Stall RD, Coates TJ. Is offering post-exposure prevention for sexual exposures to HIV related to sexual risk behavior in gay men? Aids. (2000) 14:1035–9. doi: 10.1097/00002030-200005260-00016
66. Schechter M, do Lago RF, Mendelsohn AB, Moreira RI, Moulton LH, Harrison LH. Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. (2004) 35:519–25. doi: 10.1097/00126334-200404150-00010
67. Young LE, Fujimoto K, Schneider JA. HIV prevention and sex behaviors as organizing mechanisms in a facejournal group affiliation network among young black men who have sex with men. AIDS Behav. (2018) 22:3324–34. doi: 10.1007/s10461-018-2087-4
68. Logie CH, Wang Y, Lalor P, Williams D, Levermore K. Pre and post-exposure prophylaxis awareness and acceptability among sex workers in Jamaica: a cross-sectional study. AIDS Behav. (2021) 25:330–43. doi: 10.1007/s10461-020-02972-5
69. Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. (1995) 270:1197–9. doi: 10.1126/science.270.5239.1197
70. Otten RA, Smith DK, Adams DR, Pullium JK, Jackson E, Kim CN, et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol. (2000) 74:9771–5. doi: 10.1128/JVI.74.20.9771-9775.2000
71. Minas B, Laing S, Jordan H, Mak DB. Improved awareness and appropriate use of non-occupational post-exposure prophylaxis (nPEP) for HIV prevention following a multi-modal communication strategy. BMC Public Health. (2012) 12:7. doi: 10.1186/1471-2458-12-906
72. Bakshi M, Malhotra R, Bhola R, Gupta A, Pawah S, Kumar H. Post-exposure prophylaxis awareness for HIV in India. Clin Epidemiol Glob Health. (2015) 3:S107–13. doi: 10.1016/j.cegh.2015.06.001
Keywords: post-exposure prophylaxis, MSM, awareness, meta-analysis, HIV
Citation: Jin J, Sun R, Mu T, Jiang T, Dai L, Lu H, Ren X, Chen J, Ye J, Sun L, Wu H, Zhang T, Zou H and Su B (2022) Awareness and Use of Post-exposure Prophylaxis for HIV Prevention Among Men Who Have Sex With Men: A Systematic Review and Meta-Analysis. Front. Med. 8:783626. doi: 10.3389/fmed.2021.783626
Received: 26 September 2021; Accepted: 16 December 2021;
Published: 10 January 2022.
Edited by:
Chiara de Waure, University of Perugia, ItalyReviewed by:
Guo-Cui Wu, Anhui Medical University, ChinaCopyright © 2022 Jin, Sun, Mu, Jiang, Dai, Lu, Ren, Chen, Ye, Sun, Wu, Zhang, Zou and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huachun Zou, em91aHVhY2h1bkBtYWlsLnN5c3UuZWR1LmNu; Bin Su, Ymluc3VAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.