- 1Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Hepatology, Wuxi Fifth People's Hospital, Wuxi, China
- 3Department of Oncology, The Second People's Hospital of Yancheng City, Yancheng, China
- 4Department of Urology, People's Hospital of Dongtai City, Dongtai, China
Exosomes, the important carriers between cells, can carry proteins, micro ribonucleic acids (miRNAs), long non-coding RNAs (lncRNAs) and other molecules to mediate cellular information transduction. They also play an important role in the pathogenesis, prognosis and treatment of viral hepatitis and its associated liver diseases. Several studies have reported that viral hepatitis and its associated liver diseases, including hepatitis A, B, C and E; hepatic fibrosis and hepatocellular carcinoma, were closely associated with exosomes. Exploring the role of exosomes in viral hepatitis and associated liver diseases will enhance our understanding of these diseases. Therefore, this review mainly summarised the role of exosomes in viral hepatitis and its associated liver diseases to identify new strategies for liver diseases in clinical practise.
Introduction
Exosomes, firstly discovered in 1980, are circular or elliptical membrane vesicles of endocytic origin with a diameter of ~30–150 nm, which are released into the extracellular environment after the fusion of the polycystins and plasma membranes (1). Exosomes are secreted by different body cells, including fat, dendritic, T, B, stem and tumour cells, which could be found in blood, urine and cerebrospinal fluid (2–5). Exosomes are produced by plasmacytoid dendritic cells (pDCs) and released after the fusion of multivesicular bodies (MVB) with the plasma membrane. They are composed of proteins, peptides, lipids, messenger ribonucleic acids (mRNAs), microRNAs (miRNAs), deoxyribonucleic acid (DNA) and other components (6) and can be transported to adjacent or distant organs and tissues through blood circulation (7). They can participate in various important physiological and pathological processes of the human body and affect disease development, which plays an important role in cell communication, migration, angiogenesis, immune response and tumour cell growth (8, 9).
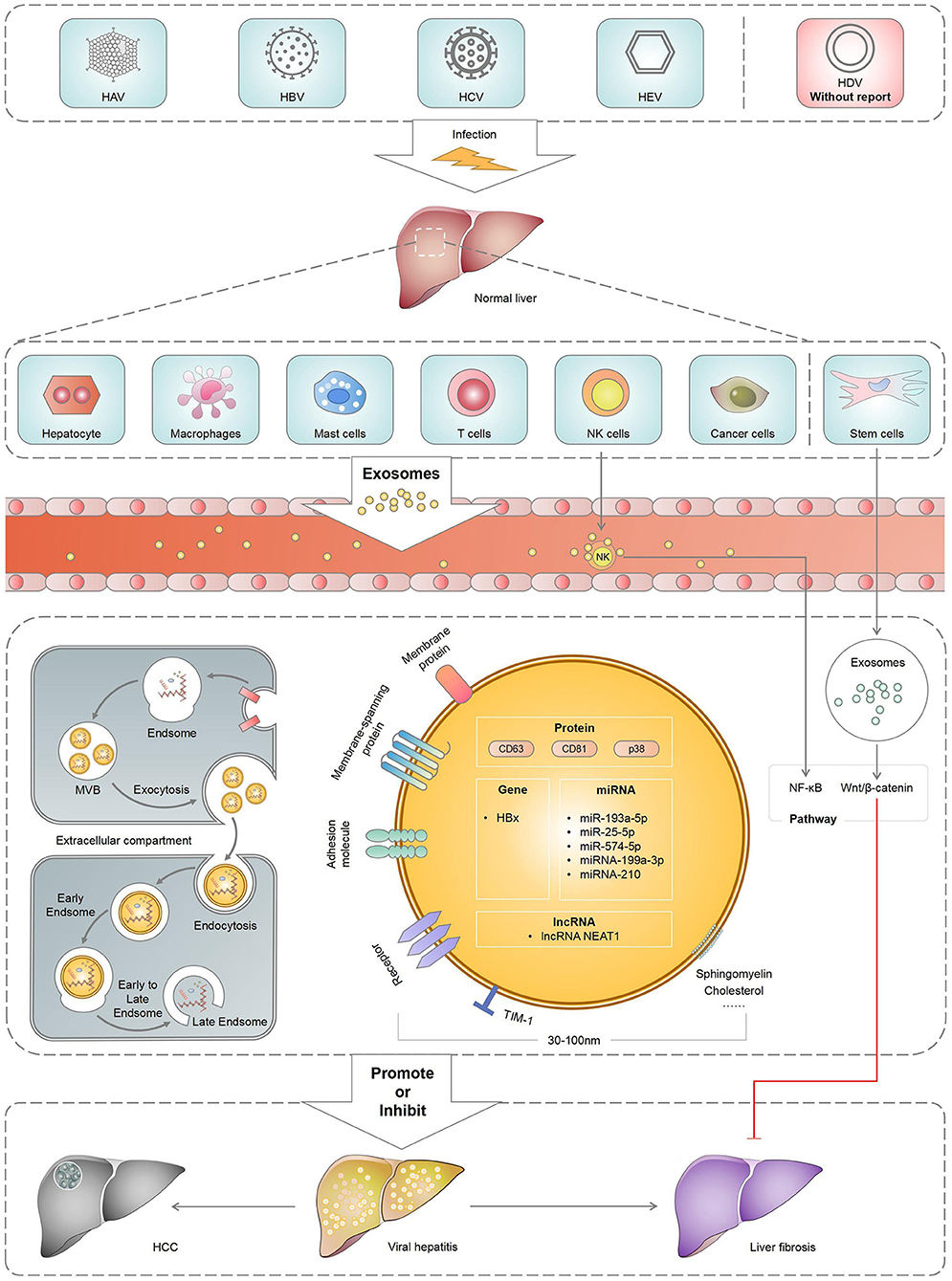
Viral hepatitis occurs worldwide, including the following five viruses as the main clinical manifestations: hepatitis A, B, C, D and E. By regulating host immune response and mediating hepatitis virus replication, exosomes could influence the pathogenesis of hepatitis virus. The exosomes released from cells infected with hepatitis virus can carry nucleic and protein components, which would help hepatitis virus participate in immune escape. Meanwhile, exosomes derived from immune cells help eliminate viruses and antiviral immune defence. Exosomes released or received by the liver cells can be used for cell-to-cell communication between healthy and damaged livers (10). Moreover, exosomes produced by hepatocytes infected with the hepatitis virus spread the infection and disrupt the innate immune response of chronic viral hepatitis (11). Similarly, they can also activate the body's immune response to hepatitis infection (12). As important carriers between cells, exosomes are involved in virus transmission, immune regulation, antiviral response and viral microenvironment and play an important role in the pathogenesis, prognosis and treatment of viral hepatitis and its associated liver diseases (Figure 1). Therefore, this review mainly summarised the role of exosomes in viral hepatitis and its associated liver diseases, aiming at providing new strategies for the clinical treatment of liver diseases.
The Role of Exosomes in Hepatitis A
Hepatitis A is caused by the hepatitis A virus (HAV) and is mainly transmitted through the faecal–oral route. HAV is a hepatophilic positive-chain RNA virus (13). Its worldwide spread is episodic and can cause acute liver disease but does not establish a persistent infection. Infected human cells can produce two types of HAV particles: non- and quasi-enveloped. Non-enveloped virus particles are stable in the faeces of infected people, whereas quasi-enveloped virus particles are present in the blood of infected individuals. The presence of quasi-envelopes protects the virus from immune response. Therefore, quasi-enveloped virus particles can spread to the liver (14).
Quasi-enveloped HAV (eHAV) was reportedly responsible for viral transmission and pDC activation (15). eHAV can be germinated from the endosomes of the HAV capsid into the MVB through the exosomes (16). HAV cell receptor 1 and cholesterol transporter NPC1 participate in the transport of exosomes from HAV-infected cells through mesh protein-mediated endogenous action, thereby promoting HAV infection (17). Furthermore, Costafreda et al. demonstrated that exosomes and HAV have similar fusion mechanisms independent of envelope glycoproteins (18). Jiang et al. confirmed that HAV structural protein pX could interact with apoptosis-associated gene 2-interacting protein X to promote virion and exogenous protein secretions through exosome-like vesicles (19).
Exosomes can protect virions from antibody-mediated neutralisation in HAV-infected cells. The presence of these exosomes can also prevent the detection of HAV by the host immune system and facilitate the spread of HAV in the liver. However, HAV virions coated with exosomes may limit replication after an eHAV infection, slowing the spread of HAV in the cells (15).
Nowadays, the diagnosis and therapeutic effects of exosomes in HAV infection have not been thoroughly explored. These results suggest that exosomes make a great difference in HAV transmission and protect HAV from the detection of the host immune system. Therefore, future studies should focus on the mechanism of action of exosomes in innate immunity and immune evasion to advance the exosomal diagnostic process and HAV infection treatment.
The Role of Exosomes in Hepatitis B
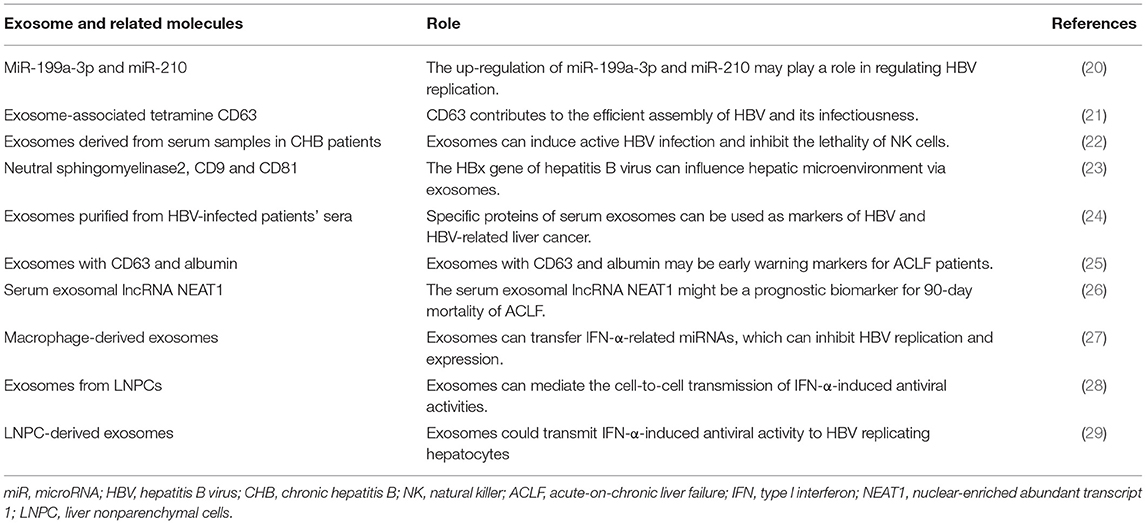
Several studies have reported on the role of exosomes in hepatitis B (Table 1). Hepatitis B virus (HBV) is a common liver-specific enveloped DNA virus that can cause chronic hepatitis B (CHB). CHB is a global epidemic infecting ~2 billion people, of which 240 million had chronic infection (30). Approximately 650,000 people die from HBV infection and liver diseases caused by HBV infection annually (31). In recent years, several studies have demonstrated that exosomes can play a role in and influence the replication, transmission, diagnosis and treatment of HBV by regulating HBV replication and transmission. A previous study showed that immune-related miRNAs could be involved in inflammatory and immune responses (32). Zhang et al. reported that miR-199a-3p and miR-210 effectively reduced the expression of hepatitis B surface antigens (HBsAg), thus inhibiting HBV replication (20). Ninomiya et al. reported that exosome-associated tetramine CD63 contributes to the efficient assembly of HBV and its infectivity (21).
Similarly, exosomes can regulate immune response, revealing the underlying mechanisms of immune escape. Yang et al. analysed the serum samples of patients with CHB and found that serum exosomes contained HBV components. These exosomes can induce active HBV infection in the primitive liver cells, inhibit the lethality of natural killer (NK) cells and destroy the body's immune response, thereby promoting HBV replication and transmission (22). Kapoor et al. also found that transcription and translation products of the HBx gene in HBV can be transported to the recipient cells through exosomes and promote HBV transmission by improving the liver microenvironment (23).
Exosomes are important in the predictive diagnosis of HBV infection. Zhao et al. compared the protein composition of Huh7 cell exosomes infected with HBx and that of the control group and confirmed the presence of liver cancer-related proteins, indicating that specific proteins of serum exosomes can be considered as HBV and HBV-related liver cancer markers (24). Jiao et al. also demonstrated that exosomes with albumin and vascular endothelial growth factor (VEGF) may be more accurate and specific biomarkers for assessing liver regeneration and prognosis in patients with acute-on-chronic liver failure (ACLF), whereas exosomes with CD63 and albumin may be early warning markers for patients with ACLF (25). The serum exosomal long-chain non-coding RNA nuclear-rich transcript 1 was reported to predict the 90-day mortality in patients with ACLF (26).
Exosomes also have antiviral activity. Kwon et al. demonstrated that type I interferon-alpha (IFN-α) can be an effective treatment for HBV infection (33). Interferon can inhibit the covalent closure of circular DNAs through HBsAg and HBV, which exhibit antiviral activity and effectively inhibit HBV replication (27). Li et al. also demonstrated that antiviral response induced by IFN-α can be transported from the liver non-parenchymal cells to HBV-infected cells through exosomes, leading to the storage of immune memory and exerting antiviral functions (28). Macrophage-derived exosomes transfer IFN-α-associated miRNA from the macrophages to HBV-infected hepatocytes through endocytosis and macropinocytosis and have antiviral activity against HBV replication and expression (29).
The Role of Exosomes in Hepatitis C
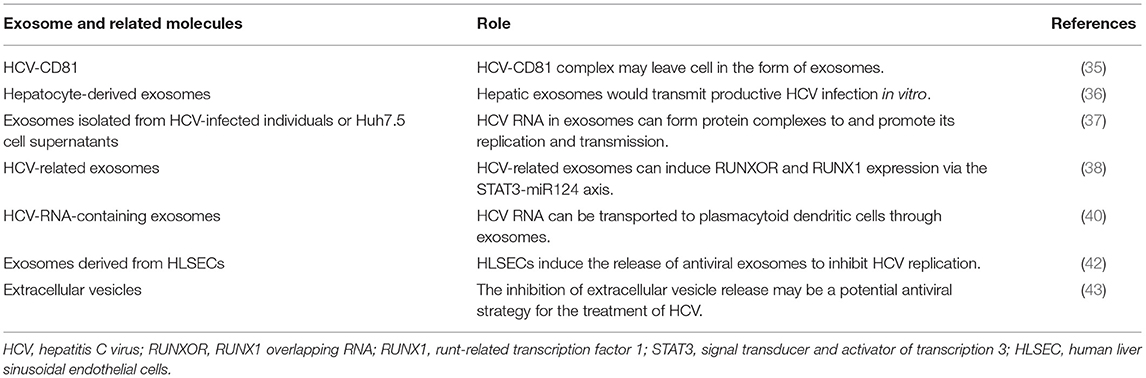
Hepatitis C virus (HCV) is a positive-chain RNA virus transmitted through the blood, affecting >71 million people worldwide (34). HCV infection is an important cause of end-stage liver disease. Therefore, the presence of exosomes is considered to play an important role in HCV replication and transmission. Masciopinto et al. reported the presence of HCV RNA after isolating exosomes from hepatocytes of patients with hepatitis C (35). Furthermore, Ramakrishnaiah et al. confirmed that HCV is transmitted by transporting exosomes between liver cells (36). HCV RNA in exosomes was also found to form protein complexes with Ago2, HSP90 and miR-122 to enhance stability and infectivity and promote replication and transmission (37).
HCV-related exosomes can play a role in the immune evasion process, and HCV can hijack exosomes released by the cells and evade the host immunity. HCV-related exosomes can also induce RUNXOR and RUNX1 expressions via the STAT3-miR124 axis, and RUNXOR and RUNX1 up-regulation may promote myeloid suppressor cell differentiation and host immune response inhibition, thereby evading host immunity (38). Ji et al. concluded that HCV can facilitate galectin-9 secretion in monocytes, which inhibit T-cell-mediated-specific immune response after interacting with T-cell Ig and mucin domain protein-3 (39).
The inhibitory effect of exosomes on viruses is considered a potential treatment for HCV infection. Exosomes can be the medium of HCV RNA transportation to pDCs (40). HCV RNAs were found to act on toll-like receptor 7 to activate pDC, thus promoting IFN synthesis and release and inhibiting HCV replication and transmission (41). Giugliano et al. found that human liver sinus endotho-othesotropic cells (HLSECs) can internalise HCV virus particles through intercellular contact, act on the conformation recognition receptor, which can up-regulate IFN gene expression, increase type I and III interferon levels, stimulate HLSECs to secrete exosomes and eventually inhibit HCV virus replication (42). Aydin et al. showed that blocking the release of extracellular vesicles and exosomes can significantly affect viral replication without affecting the host cell viability. Therefore, they suggested that inhibiting the extracellular vesicle release could be a potential antiviral strategy for the treatment of HCV and other emerging RNA viruses (43).
HCV-associated exosomes can affect virus replication and transmission and mediate immune evasion (Table 2). Further efforts are needed to explore the role of exosomes in HCV infection and to provide new ideas for the diagnosis and treatment of HCV infection.
The Role of Exosomes in Other Hepatitis Infections
To date, the role of exosomes in hepatitis D virus has not been systematically reported. However, as an intestinally transmitted and liver-obsessed virus, the effects of exosomes on hepatitis E virus (HEV) and its scoring model have been mostly investigated (44). Exosomes participate in the immune escape of HEV, enrich the cholesterol and phosphatidylserine levels, increase the HEV intake in the liver cells and promote HEV replication and transmission (45). Nagashima et al. found that HEV was transferred and released through MVB. The mechanisms underlying HEV infection in rats may be similar to those in humans (46). Primadharsini et al. confirmed that HEV in rats is released through MVB screening and that HEV release in rats requires a pathway associated with exosomes (47).
Non-enveloped HEV and eHEV enter the cells through different mechanisms. The main route of eHEV entry into the cells is through the mesh protein-mediated endophagus. Compared with non-enveloped HEV, eHEV binds to the cells much less efficiently and requires longer inoculation time to achieve its maximum infectiousness (48). Degradation of the eHEV membrane in the lysosomes to achieve membrane removal may greatly increase its infectiousness. Non-competitive neutral phospholipase inhibitors GW4869 or silent Rab27A/Hrs gene expression can inhibit the secretion of exosomes, resulting in a significant reduction in HEV release, providing a new treatment strategy for hepatitis E (46).
The Role of Exosomes in Hepatitis-Associated Hepatic Fibrosis
Hepatic fibrosis (HF) is caused by excessive production and accumulation of insoluble collagen and extracellular matrix components after sustaining chronic liver damage. Various chronic liver diseases can lead to HF and even liver cirrhosis. Activation of hepatic stellate cells (HSCs) is a primary event that results in HF development (49).
Exosomes can promote HF development. Exosomes from damaged liver cells are rich in cytochrome P450, and the reactive oxygen produced by cytochrome P450 2E1 (CYP2E1) can produce superoxide anion free radicals, hydrogen peroxide and strong oxidants, and increased CYP2E1 levels under various pathophysiological conditions can lead to hepatocellular apoptosis through the oxidative stress mechanism (50). Exosomes from damaged hepatocytes containing cytochrome P450 are speculated to be involved in the development of fatty degeneration by increasing the expression of fibrin and hepatocyte apoptosis (51). Hepatocyte lipotoxic fatty acid damage produces exosomes rich in miR17-92 clusters, which can be absorbed by HSCs, resulting in fibrotic activation (52). Exosomes released from the epithelial cells can activate fibroblasts to trigger fibrosis. Furthermore, exosomes produced by damaged epithelial cells are absorbed by adjacent fibroblasts, resulting in increased production of α-smooth muscle actin and type I collagen to drive HF (53). Exosomes from CCL4-processed hepatocytes include different types of self-RNA and toll-like receptor 3, which can increase IL-17 production in the liver γδT cells. Increased inflammatory cytokine levels were closely associated with HSC activation (54). T cells produced by IL-17 can regulate TGF-β1 in the Kupffer cells and directly activate HSCs (55).
The expression pattern of miRNAs in the serum rich in exosomes is a highly potential biomarker for diagnosing the grade and stage of liver diseases. Niu et al. analysed the serum exosomes of patients and rats with HF and found that exosome miR-155 can serve as a non-invasive biomarker for the diagnosis and progression of HF (56). Chen et al. also showed that miR-103-3p in the serum exosomes of patients with HF may be an HF biomarker (57). Exosomes can also be used for the treatment of HF. Exosomes from healthy humans may be beneficial to patients with HF, and the primary mechanism for repairing damaged liver may be the release of paracrine factors (58). Existing reports demonstrated that exosomes are the source of umbilical cord-filled, fat-filled and bone marrow interstitial stem cells for the possible treatment of HF (59–61).
HF formation is closely associated with HSC activation. Exosomes can regulate HSC activation and have an anti-fibrosis effect. Chen et al. found that serum exosomes from healthy donors have anti-fibrosis properties, partly owing to specific miR components with therapeutic effects on activated HSCs or damaged liver cells. Serum exosomes in healthy individuals have anti-fibrosis effects. MiR-34c, miR-151-3p, miR-483-5P, miR-532-5P and miR-687 expressions were higher in healthy mice than those in mice with fibrosis, and these miRNAs can inhibit the expression of fibrogenic genes in activated HSCs (62). Exosomes derived from the human bone mesenchymal stem cells were reported to reduce HF by inhibiting Wnt/β-catenin signalling to prevent HSC activation (61). Autophagy in HSCs was reported to reduce HF by inhibiting the release of fibrotic exosomes, indicating that exosomes can serve as potential new anti-fibrosis biological agents and have a positive therapeutic effect against fibrosis and important transformational significance for the treatment of fibrosis-related diseases (63).
Exosomes can promote and antagonise HF. Activated HSCs can also release fibrin-rich exosomes, suggesting that they are new biomarkers of potential pathological conditions and play a key role in the identification and treatment of HF-related diseases.
The Role of Exosomes in Hepatitis-Associated Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a highly life-threatening cancer and the leading cause of death in patients with cirrhosis. Its incidence in China accounts for 50% of global cases and deaths (64). Researchers have been focusing on the early diagnosis and treatment of HCC (65). Exosomes can functionally carry active proteins, RNA and other types of molecules that are associated with the cancer pathology (66). Therefore, investigating the role of exosomes may promote HCC diagnosis and treatment.
Exosomes can be involved in the occurrence, development and metastasis of HCC mainly through RNA transport and protein-mediated cellular communication. Kogure et al. found that exosomes in HCC cells contain varied miRNAs and can significantly promote the non-adhesive growth of liver cancer cell strains to promote tumour progression by regulating the transformational growth factor in their receptor cells to activate the kinase-1 (TAK1) signalling pathway (67). Li et al. reported that exosomes can transfer long-chain non-coding RNA FAL1 into HCC cells to promote cell growth, proliferation, migration and invasion (68). Exosomes derived from HCC cells (HepG2) were reported to be actively internalised by adipocytes, causing significant transcriptomic changes. Adipocytes treated by tumour exosomes could promote tumour growth, enhance angiogenesis and recruit more macrophages in a mouse model (69). Chen et al. also demonstrated that exosomes from highly metastatic MHCC97H cells can be ingested by the less metastatic HCC cells and subsequently promote malignant behaviours of the recipient cells. Exosomes derived from tumours may promote epithelial-to-mesenchymal transformation through signal transduction, further promoting HCC invasion and metastasis (70). Furthermore, Wei et al. found that Vps4A can regulate the secretion and ingestion of exosomes containing oncogenic and tumour suppressor miRNAs, and its down-regulated expression in HCC tissues can promote HCC development and metastasis (71). Recently, the loss of miR-320a was found to inhibit the miR-320a-PBX3-MAPK signalling pathway, induce epithelial–mesenchymal transformation and cyclin-dependent kinase-2 and MMP-2 expressions to promote the HCC development and metastasis (72).
The exchange of RNA and protein through exosomes not only plays a key role in the HCC pathogenesis and progression but also identifies specific and sensitive biomarkers for HCC recurrence and prognosis as potential non-invasive biomarkers and therapeutic targets. Examination of exosomes is conducive to promptly reflect the severity and possible progression of the disease and to control the development of the disease in the high-risk population. Serum exosomes hsa-circ-0028861 and hsa-circ-0070396 can serve as new biomarkers of HCC caused by HBV (73, 74). MiR-125b-5p and miR-223-3p can also be used as novel non-invasive biomarkers for HBV-positive HCC at an early CHB stage (75). Circulating exosome differentiation of antagonistic non-protein-coded RNAs is highly correlated with disease progression of HCV-associated HCC and may be a non-invasive prognostic biomarker for HCV-associated HCC (76).
Exosomes for the treatment of HCC are receiving increasing attention, including the adipose mesenchymal hepatocyte-, hepatocyte- and dendritic-cell-derived exosomes. Lou et al. transfected AMSC with miR-122, and the extracted adipose mesenchymal hepatocyte-derived exosomes changed the miR-122 target gene expression so that cancer cells could be sensitised to chemotherapeutic drugs. Intratumoral injection of exosomes could significantly improve the anti-tumour effects of sorafenib on HCC in vivo and enhance the chemotherapeutic sensitivity of HCC (77). Cheng et al. demonstrated that hepatocyte-derived exosomes could inhibit the HCC cell progression through the STAT3 pathway (78). A study that injected dendritic-cell-derived exosomes expressing AFP into the HCC mouse model found that DEXAFP was thought to induce a strong antigen-specific immune response, which significantly inhibited the HCC occurrence in mice (79). Currently, the application of exosomes in the treatment of HCC is limited to basic experiments, and further studies are required to explore the applications of exosomes in clinical practise.
Conclusion
Exosomes can carry proteins, miRNAs, lncRNAs and other molecules to mediate cellular information transduction, which plays a bidirectional role in viral hepatitis and its associated liver diseases. They can also encapsulate and transport the hepatitis virus, promote viral replication and transmission, mediate the antiviral response and serve as the target of immunotherapy. Furthermore, exosomes can reverse fibrosis and become the key mediators of fibrosis formation. Cell communication between exosomes can promote HCC development and metastasis. However, they can also inhibit the occurrence of HCC as an immunosuppressor. The mechanisms of exosome communication will enhance our understanding of liver pathophysiology, indicating their great potential as molecular biomarkers for the diagnosis and prognosis of liver diseases and as new therapeutic methods. Although studies on exosomes have made great progress in recent years, further efforts are required to use exosomes as biomarkers for the treatment of liver diseases in clinical practise.
Author Contributions
HZ and NJ had the idea for the article. YY and Z-hY performed the literature search and data analysis. HZ and CX drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DC declared a shared affiliation with several of the authors, HZ, YY, to the handling editor at the time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HAV, Hepatitis A virus; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HEV, Hepatitis E virus; HCC, Hepatocellular carcinoma; CHB, Chronic hepatitis B; pDCs, Plasmacytoid dendritic cells; lncRNAs, Long non-coding RNAs; miRNAs, micro ribonucleic acids; ACLF, acute-on-chronic liver failure; HSCs, hepatic stellate cells; HF, Hepatic fibrosis.
References
1. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. (1985) 101:942–8. doi: 10.1083/jcb.101.3.942
2. Bhat SP, Gangalum RK. Secretion of αB-Crystallin via exosomes: new clues to the function of human retinal pigment epithelium. Commun Integr Biol. (2011) 4:739–41. doi: 10.4161/cib.17610
3. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. (2013) 14:5338–66. doi: 10.3390/ijms14035338
4. Zhou H, Cheruvanky A, Hu X, et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. (2008) 74:613–21. doi: 10.1038/ki.2008.206
5. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. (2019) 177:428–45.e18. doi: 10.1016/j.cell.2019.02.029
6. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9:654–9. doi: 10.1038/ncb1596
7. Lee HD, Koo BH, Kim YH, Jeon OH, Kim DS. Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. FASEB J. (2012) 26:3084–95. doi: 10.1096/fj.11-201681
8. Isola AL, Chen S. Exosomes: the messengers of health and disease. Curr Neuropharmacol. (2017) 15:157–65. doi: 10.2174/1570159X14666160825160421
9. Wang L, Wu J, Song S, Chen H, Hu Y, Xu B, et al. Plasma exosome-derived sentrin SUMO-specific protease 1: a prognostic biomarker in patients with osteosarcoma. Front Oncol. (2021) 11:625109. doi: 10.3389/fonc.2021.625109
10. Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. (2013) 59:621–5. doi: 10.1016/j.jhep.2013.03.028
11. Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. (2015) 7:3204–25. doi: 10.3390/v7062770
12. Kouwaki T, Fukushima Y, Daito T, Sanada T, Yamamoto N, Mifsud EJ, et al. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front Immunol. (2016) 7:335. doi: 10.3389/fimmu.2016.00335
13. Wang X, Ren J, Gao Q, Hu Z, Sun Y, Li X, et al. Hepatitis a virus and the origins of picornaviruses. Nature. (2015) 517:85–88. doi: 10.1038/nature13806
14. Rivera-Serrano EE, González-López O, Das A, Lemon SM. Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses. Elife. (2019) 8:e43983. doi: 10.7554/eLife.43983.033
15. Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. (2013) 496:367–71. doi: 10.1038/nature12029
16. McKnight KL, Xie L, González-López O, Rivera-Serrano EE, Chen X, Lemon SM. Protein composition of the hepatitis a virus quasi-envelope. Proc Natl Acad Sci USA. (2017) 114:6587–92. doi: 10.1073/pnas.1619519114
17. Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. (1997) 277:228–31. doi: 10.1126/science.277.5323.228
18. Costafreda MI, Abbasi A, Lu H, Kaplan G. Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis a virus infection. Nat Microbiol. (2020) 5:1096–106. doi: 10.1038/s41564-020-0740-y
19. Jiang W, Ma P, Deng L, Liu Z, Wang X, Liu X, et al. Hepatitis a virus structural protein pX interacts with ALIX and promotes the secretion of virions and foreign proteins through exosome-like vesicles. J Extracell Vesicles. (2020) 9:1716513. doi: 10.1080/20013078.2020.1716513
20. Zhang GL, Li YX, Zheng SQ, Liu M, Li X, Tang H. Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210. Antiviral Res. (2010) 88:169–75. doi: 10.1016/j.antiviral.2010.08.008
21. Ninomiya M, Inoue J, Krueger EW, Chen J, Cao H, Masamune A, et al. The exosome-associated tetraspanin CD63 contributes to the efficient assembly and infectivity of the hepatitis B virus. Hepatol Commun. (2021) 5:1238–51. doi: 10.1002/hep4.1709
22. Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. (2017) 14:465–75. doi: 10.1038/cmi.2016.24
23. Kapoor NR, Chadha R, Kumar S, Choedon T, Reddy VS, Kumar V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. (2017) 240:166–74. doi: 10.1016/j.virusres.2017.08.009
24. Zhao X, Wu Y, Duan J, Ma Y, Shen Z, Wei L, et al. Quantitative proteomic analysis of exosome protein content changes induced by hepatitis B virus in Huh-7 cells using SILAC labeling and LC-MS/MS. J Proteome Res. (2014) 13:5391–402. doi: 10.1021/pr5008703
25. Jiao Y, Lu W, Xu P, Shi H, Chen D, Chen Y, et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol Int. (2021) 15:957–69. doi: 10.1007/s12072-021-10217-3
26. Gao S, Fan YC, Han LY, Wang K. Serum exosomal long noncoding RNA nuclear-enriched abundant transcript 1 predicts 90-day mortality in acute-on-chronic hepatitis B liver failure. Expert Rev Clin Immunol. (2021) 17:789–97. doi: 10.1080/1744666X.2021.1933442
27. Wu W, Wu D, Yan W, Wang Y, You J, Wan X, et al. Interferon-induced macrophage-derived exosomes mediate antiviral activity against hepatitis B virus through miR-574-5p. J Infect Dis. (2021) 223:686–98. doi: 10.1093/infdis/jiaa399
28. Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. (2013) 14:793–803. doi: 10.1038/ni.2647
29. Yao Z, Qiao Y, Li X, Chen J, Ding J, Bai L, et al. Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J Virol. (2018) 92:e01578–18. doi: 10.1128/JVI.01578-18
30. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. (2012) 30:2212–9. doi: 10.1016/j.vaccine.2011.12.116
31. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. Lancet. (2012) 380:2095–128. doi: 10.1016/S2468-1253(16)30181-9
32. van der Ree MH, Jansen L, Kruize Z, van Nuenen AC, van Dort KA, Takkenberg RB, et al. Plasma MicroRNA levels are associated with hepatitis B e antigen status and treatment response in chronic hepatitis B patients. J Infect Dis. (2017) 215:1421–9. doi: 10.1093/infdis/jix140
33. Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. (2011) 8:275–84. doi: 10.1038/nrgastro.2011.33
34. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. (2017) 2:161–76. doi: 10.1016/S0140-6736(12)61728-0
35. Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, Brunetto M, et al. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. (2004) 34:2834–42. doi: 10.1002/eji.200424887
36. Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci USA. (2013) 110:13109–13. doi: 10.1073/pnas.1221899110
37. Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. (2014) 10:e1004424. doi: 10.1371/journal.ppat.1004424
38. Thakuri BKC, Zhang J, Zhao J, Nguyen LN, Nguyen LNT, Schank M, et al. HCV-associated exosomes upregulate RUNXOR and RUNX1 expressions to promote MDSC expansion and suppressive functions through STAT3-miR124 axis. Cells. (2020) 9:2715. doi: 10.3390/cells9122715
39. Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, et al. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol. (2013) 43:458–67. doi: 10.1002/eji.201242768
40. Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. (2012) 12:558–70. doi: 10.1016/j.chom.2012.08.010
41. Zhang S, Kodys K, Babcock GJ, Szabo G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology. (2013) 58:940–9. doi: 10.1002/hep.25827
42. Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. (2015) 148:392–402.e13. doi: 10.1053/j.gastro.2014.10.040
43. Aydin Y, Koksal AR, Reddy V, Lin D, Osman H, Heidari Z, et al. Extracellular vesicle release promotes viral replication during persistent HCV infection. Cells. (2021) 10:984. doi: 10.3390/cells10050984
44. Wu J, Guo N, Zhang X, Xiong C, Liu J, Xu Y, et al. HEV-LFS: a novel scoring model for patients with hepatitis E virus-related liver failure. J Viral Hepat. (2019) 26:1334–43. doi: 10.1111/jvh.13174
45. Chapuy-Regaud S, Dubois M, Plisson-Chastang C, Bonnefois T, Lhomme S, Bertrand-Michel J, et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie. (2017) 141:70–9. doi: 10.1016/j.biochi.2017.05.003
46. Nagashima S, Jirintai S, Takahashi M, Kobayashi T, Tanggis, Nishizawa T, et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol. (2014) 95:2166–75. doi: 10.1099/vir.0.066910-0
47. Primadharsini PP, Nagashima S, Takahashi M, Kobayashi T, Nishiyama T, Nishizawa T, et al. Multivesicular body sorting and the exosomal pathway are required for the release of rat hepatitis E virus from infected cells. Virus Res. (2020) 278:197868. doi: 10.1016/j.virusres.2020.197868
48. Yin X, Ambardekar C, Lu Y, Feng Z. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J Virol. (2016) 90:4232–42. doi: 10.1128/JVI.02804-15
49. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. (2008) 134:1655–69. doi: 10.1053/j.gastro.2008.03.003
50. Cho EY, Yun CH, Chae HZ, Chae HJ, Ahn T. Anionic phospholipid-induced regulation of reactive oxygen species production by human cytochrome P450 2E1. FEBS Lett. (2008) 582:1771–6. doi: 10.1016/j.febslet.2008.04.048
51. Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. (2016) 64:2219–33. doi: 10.1002/hep.28814
52. Ban LA, Shackel NA, McLennan SV. Extracellular vesicles: a new frontier in biomarker discovery for non-alcoholic fatty liver disease. Int J Mol Sci. (2016) 17:376. doi: 10.3390/ijms17030376
53. Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. (2013) 24:385–92. doi: 10.1681/ASN.2012101031
54. Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. (2016) 64:616–31. doi: 10.1002/hep.28644
55. Ma HY, Xu J, Liu X, Zhu Y, Gao B, Karin M, et al. The role of IL-17 signaling in regulation of the liver-brain axis and intestinal permeability in alcoholic liver disease. Curr Pathobiol Rep. (2016) 4:27–35. doi: 10.1007/s40139-016-0097-3
56. Niu LJ, Zhang YM, Huang T, Sun XF, Luo SX. Exosomal microRNA-155 as a biomarker for hepatic fibrosis diagnosis and progression. Ann Transl Med. (2021) 9:137. doi: 10.21037/atm-20-7787
57. Chen L, Yao X, Yao H, Ji Q, Ding G, Liu X. Exosomal miR-103-3p from LPS-activated THP-1 macrophage contributes to the activation of hepatic stellate cells. FASEB J. (2020) 34:5178–92. doi: 10.1096/fj.201902307RRR
58. Fiore EJ, Mazzolini G, Aquino JB. Mesenchymal stem/stromal cells in liver fibrosis: recent findings, old/new caveats and future perspectives. Stem Cell Rev Rep. (2015) 11:586–97. doi: 10.1007/s12015-015-9585-9
59. Li T, Yan Y, Wang B, Qian H, Zhang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. (2013) 22:845–54. doi: 10.1089/scd.2012.0395
60. Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. (2017) 21:2491–502. doi: 10.1111/jcmm.13170
61. Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. (2019) 10:98. doi: 10.1186/s13287-019-1204-2
62. Chen L, Chen R, Kemper S, Cong M, You H, Brigstock DR. Therapeutic effects of serum extracellular vesicles in liver fibrosis. J Extracell Vesicles. (2018) 7:1461505. doi: 10.1080/20013078.2018.1461505
63. Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. (2020) 73:1144–54. doi: 10.1016/j.jhep.2020.04.044
64. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
65. Wang Y, Wu J, Xu J, Lin S. Clinical significance of high expression of stanniocalcin-2 in hepatocellular carcinoma. Biosci Rep. (2019) 39:BSR20182057. doi: 10.1042/BSR20182057
66. Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. Exosomes as divine messengers: are they the Hermes of modern molecular oncology?. Cell Death Differ. (2015) 22:34–45. doi: 10.1038/cdd.2014.130
67. Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. (2011) 54:1237–48. doi: 10.1002/hep.24504
68. Li B, Mao R, Liu C, Zhang W, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. (2018) 197:122–9. doi: 10.1016/j.lfs.2018.02.006
69. Wang S, Xu M, Li X, Su X, Xiao X, Keating A, et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. (2018) 11:82. doi: 10.1186/s13045-018-0625-1
70. Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. (2018) 9:513. doi: 10.1038/s41419-018-0534-9
71. Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. (2015) 61:1284–94. doi: 10.1002/hep.27660
72. Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. (2017) 397:33–42. doi: 10.1016/j.canlet.2017.03.004
73. Wang Y, Pei L, Yue Z, Jia M, Wang H, Cao LL. The potential of serum exosomal hsa_circ_0028861 as the novel diagnostic biomarker of HBV-derived hepatocellular cancer. Front Genet. (2021) 12:703205. doi: 10.3389/fgene.2021.703205
74. Lyu L, Yang W, Yao J, Wang H, Zhu J, Jin A, et al. The diagnostic value of plasma exosomal hsa_circ_0070396 for hepatocellular carcinoma. Biomark Med. (2021) 15:359–71. doi: 10.2217/bmm-2020-0476
75. Giray BG, Emekdas G, Tezcan S, Ulger M, Serin MS, Sezgin O, et al. Profiles of serum microRNAs; miR-125b-5p and miR223-3p serve as novel biomarkers for HBV-positive hepatocellular carcinoma. Mol Biol Rep. (2014) 41:4513–9. doi: 10.1007/s11033-014-3322-3
76. Wang SC, Li CY, Chang WT, Cheng WC, Yen CH, Tu WY, et al. Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. (2021) 41:956–68. doi: 10.1111/liv.14772
77. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. (2015) 8:122. doi: 10.1186/s13045-015-0220-7
78. Cheng Z, Lei Z, Yang P, Si A, Xiang D, Tang X, et al. Exosome-transmitted p120-catenin suppresses hepatocellular carcinoma progression via STAT3 pathways. Mol Carcinog. (2019) 58:1389–99. doi: 10.1002/mc.23022
Keywords: biomarkers, exosomes, fibrosis, hepatocellular carcinoma (HCC), therapeutic targets, viral hepatitis
Citation: Zhou H, Yan Z-h, Yuan Y, Xing C and Jiang N (2021) The Role of Exosomes in Viral Hepatitis and Its Associated Liver Diseases. Front. Med. 8:782485. doi: 10.3389/fmed.2021.782485
Received: 24 September 2021; Accepted: 29 October 2021;
Published: 22 November 2021.
Edited by:
Yijin Wang, Southern University of Science and Technology, ChinaReviewed by:
Dawei Cui, Zhejiang University School of Medicine, ChinaGuopan Liu, City University of Hong Kong, Hong Kong SAR, China
Guo-Ming Zhang, Shuyang People's Hospital, China
Copyright © 2021 Zhou, Yan, Yuan, Xing and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Jiang, bmFuajAxQGZveG1haWwuY29t; Chen Xing, Z2FiYmFpQDEyNi5jb20=
†These authors have contributed equally to this work
 Hao Zhou
Hao Zhou Zhi-han Yan2†
Zhi-han Yan2† Nan Jiang
Nan Jiang