- 1Department of Clinical Laboratory, The First Affiliated Hospital, College of Medicine, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Infection Management, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, China
Objective: We aimed to investigate serum exosomal adenosylhomocysteinase (AHCY) expression in hepatitis B-induced liver cirrhosis (HBV-LC) patients and to determine the prognostic value of serum exosomal AHCY.
Methods: We collected serum samples from 100 patients with chronic hepatitis B (CHB) and from 114 HBV-LC patients to test serum exosomal AHCY expression using ELISA.
Results: Compared with the CHB and Grade A and B HBV-LC groups, the level of exosomal AHCY expression was significantly higher in the HBV-LC group [376.62 (291.50–448.02) vs. 248.12 (189.28–324.63), P > 0.001] and the Grade C HBV-LC group [408.70 (365.63–465.76) vs. 279.76 (215.16–336.07), P > 0.001], respectively. Serum exosomal AHCY expression and MELD score had a significant positive correlation (r = 0.844, P < 0.001). Survival curve analysis showed that patients with low exosomal AHCY expression had significantly longer survival than patients with high exosomal AHCY expression (P = 0.0038). The receiver operating characteristics (ROC) curve showed that the area under the curve (AUC) value for the mortality prediction ability of serum exosomal AHCY in HBV-LC patients was 0.921, which was higher than the values for the MELD score (AUC 0.815) and Child-Pugh classification (AUC 0.832), with a sensitivity and specificity of 93.41 and 76.00%, respectively.
Conclusions: The serum exosomal AHCY level is a novel potential prognostic biomarker in HBV-LC patients, which may be great significance for the prognosis of HBV-LC patients.
Introduction
Hepatitis B virus (HBV) is a pathogen that causes a generalized epidemic that constitutes a global problem (1). Worldwide, approximately 2 billion people have been infected by HBV, nearly 400 million people carry the HBV, and almost 20 million individuals have chronic hepatitis B (CHB). In hepatitis B-induced liver cirrhosis (HBV-LC) following chronic HBV infection, hepatocytes gradually become necrotic; moreover, this necrosis leads to the fibronodular proliferation of hepatocytes and hepatic tissues, and the normal liver lobules are replaced by pseudobullets (2, 3). In cirrhosis, the recurrent and continuous progression of hepatic fibrosis and inflammation can lead to hepatic dysfunction, ascites, esophagogastric varices and variceal bleeding, portal hypertension, acute kidney injury, and hepatic encephalopathy; with disease progression, cirrhotic patients can develop life-threatening hepatocellular carcinoma (HCC) (4, 5). In 2015 in China, there were 460,000 and 420,000 primary HCC cases and HCC-related deaths, respectively, which constituted more than 50% of the total global incidence and mortality of HCC; thus, HCC has become a public health problem that endangers human health (6, 7). The early diagnosis and treatment of cirrhosis are of great importance for the prognosis of patients with HCC. At present, imaging, histopathological examination, and serum index assessment are routinely used as the main diagnostic modalities to assess the stage of cirrhosis (8).
Originally identified from the supernatant of cultured sheep reticulocytes, exosomes are vesicles (diameter 30–150 nm; density 1.10–1.18 g/ml) (9) that contain various proteins, mRNA, and miRNA lipids; thus, exosomes can be used as carriers for information transfer and modulate various in vivo biological activities and thereby provide a novel route for cell communication (10, 11). Exosomes facilitate the study of the pathophysiology, diagnosis, treatment, and prognosis of many diseases because of their unique lipid bilayer membrane structure that protects their biological properties and helps to maintain their stability at extremely low temperatures and for long durations (12–14). The liver is one of the most important organs in the human body, and many liver cells can either secrete exosomes or are exosome-target cells, such as hepatocytes, bile duct epithelial cells, hepatic stellate cells (HSC), mononuclear macrophages, natural killer T lymphocytes, lymphocytes, etc. (15, 16). Furthermore, exosomes secreted by different cells have different functions. Exosomes are involved in the pathogenesis of HCC, viral hepatitis, liver fibrosis, and alcoholic and non-alcoholic fatty liver disease, and, increasingly, exosomal proteins and miRNAs have been identified as potential biomarkers of various diseases (17, 18).
S-adenosyl-L-homocysteine hydrolase (SAHase), a highly conserved enzyme, catalyzes the reversible hydrolysis of S-adenosyl-L-homocysteine (SAH) to homocysteine (Hcy) and adenosine (Ado) (19) and thereby regulates the intracellular adenosylhomocysteinase (AHCY) concentration, which is considered important for transmethylation reactions. In a mouse model of liver injury for screening a novel serum marker, Vazquez et al. found that the AHCY concentration significantly increased with the increasing severity of liver injury (20).
This study was performed to determine the expression of the prognostic assessment value of serum exosomal AHCY in HBV-LC patients.
Materials and Methods
Patients
We retrospectively collected serum samples from 100 CHB and 114 HBV-LC patients who were admitted to the First Hospital of Zhejiang University School of Medicine, the Second People's Hospital of Yancheng City, and the Fifth People's Hospital of Wuxi from August 2019 to August 2020, with a follow-up duration of 3 months.
The CHB case was defined as: there is a history of hepatitis B or HBsAg positive for more than 6 months, and HBsAg and/or HBV DNA are still positive. The diagnostic criteria for cirrhosis was as follows:
Diagnostic Basis of Compensated Cirrhosis (One Out of Four Required)
(1) Histologically consistent with the diagnosis of cirrhosis. (2) Endoscopy demonstrates esophagogastric varices or ectopic varices of digestive tract, except for non-cirrhotic portal hypertension. (3) Imaging examinations such as B-super, LSM, or CT indicate the characteristics of cirrhosis or portal hypertension. For example, splenomegas and portal veins ≥1.3 cm, LSM assays meet the diagnostic boundaries of cirrhosis for different etiology. (4) For those without histology, endoscopy, or imaging examination, the following inspection indicators indicate the presence of cirrhosis (two out of four required).
(1) PLT < 100×109/L, and there is no other reason to explain; (2) Serum albumin < 35g/L, excluding other causes such as malnutrition or kidney disease; (3) INR > 1.3 or PT extension (deactivation of thrombosis or anticoagulants for over 7 days); (4) AST/PLT Ratio Index (APRI): adult APRI score >2. Attention should be paid to the effects of factors such as antisense drugs on APRI.
Diagnostic Basis of Decompensated Cirrhosis
On the basis of cirrhosis, complications of portal hypertension and/or impaired liver function occur. (1) Have the diagnostic basis of cirrhosis; (2) Portal hypertension related complications occur, such as ascites, esophageal varicose vein rupture bleeding, sepsis, hepatoencephalopathy, liver and kidney syndrome, and so on.
Re-compensated Cirrhosis and Reversal of Cirrhosis
Clinical studies have shown that patients with decompensated HBV and HCV-related cirrhosis can significantly improve liver function through effective antiviral treatment, including improving liver compensatory function, reducing portal hypertension related complications, and ultimately avoiding liver transplantation, similar to compensated cirrhosis. Liver function re-compensation during antiviral therapy is more common in patients with HBV-associated cirrhosis than in patients with HCV-associated cirrhosis. At present, the definition of re-compensation for decompensated cirrhosis is still unclear and controversial. In short, patients with decompensated cirrhosis, due to effective control of etiology, effective treatment of complications or prevention, will no longer appear decompensated cirrhosis events (ascites, gastrointestinal bleeding, hepatic encephalopathy) in a longer period of time (at least 1 year), but still exist compensated cirrhosis clinical and laboratory characteristics, which is considered “re-compensated cirrhosis.”
We excluded patients with: (1) non-HBV-related cirrhosis; (2) a history of relevant drug treatment within the last month; and (3) severe heart, brain, kidney disease, thrombocytopenia, and so on.
The Child-Pugh grading system comprises five items (21)—ascites, albumin, total bilirubin, hepatic encephalopathy, and prothrombin time—that constitute a total score in the range of 5–15 points, based on which it is categorized as grades A, B, and C (total score: 5–8, 9–11, and 12–15 points, respectively).
The MELD score includes three objective indices (22): serum bilirubin concentration, serum creatinine concentration, and INR, as well as the etiology of liver cirrhosis. The MELD score is calculated as follows:
MELD score = 9.57 × In (serum creatinine) + 3.78 × In (serum bilirubin) + l1.2 × In (INR) + 6.43 × (The etiology of liver cirrhosis, wherein alcoholic and cholestatic etiologies were assigned 0 points, and the rest were scored 1 point).
The study protocol was approved by the Ethics Committee of the First Hospital of Zhejiang University School of Medicine (No. 2017003), and informed consent from each patient was obtained.
Serum Exosome Separation
Before the serum exosome separation, we washed the qEV separation column (qEV original 35 and 70 nm, Izon Christchurch, New Zealand) with at least 10 ml PBS (1×), which we then extracted from the top of the sieve plate with a pipette before removing the bottom sliding cap from the column. Then, we added 500 μl sample to the top of the sieve plate and immediately replaced the bottom sliding cap, and collecting 0.5 ml of the fraction. When the final sample is placed inside the top sieve plate of the column (at the same level), add 2.5 ml PBS (1×) to obtain a final void volume of 3 ml. The 1.5 ml liquid, which is the higher purity exosome solution, is retained after the void volume is collected in a 1.5-ml EP tube.
Transmission Electron Microscopy
For transmission electron microscopy (TEM), we diluted the exosome and filtered the 5 μl-sample by adding it drop by drop onto the copper net and then incubated it for 5 min at room temperature. Thereafter, we used blotting paper on one side to absorb the excess liquid, added a drop of 2% uranium peroxide acetate to the copper net, and re-incubated the net for 1 min at room temperature. Following this step, we used blotting paper on one side of the net to absorb the excess liquid, allowed the net to dry for approximately 20 min at room temperature, and then observed and photographed the shape of the exosome under the electron microscope.
Nanoparticle Tracking Analysis
For the nanoparticle tracking analysis (NTA), the frozen samples were thawed in a water bath at 25°C and placed on ice. The exosomes were diluted with PBS (1×) and used directly for the NTA (ZetaVIEW S/N 17-310) assay. The NTA software (ZetaView 8.04.02) was used to analyze the movement of particles and to calculate the number of exosomes.
Western Blotting
For the gel preparation, we used a 1.5-mm glass plate with a 15-well sample comb to prepare a 12% isolate gel and a 5% concentrate gel according to the molecular weight of the target protein. Electrophoresis was carried out at a steady pressure difference of 80 V until the loading buffer (indicator) entered the separation gel and then changed to a steady pressure of 120 V, which was maintained until the loading buffer reached the bottom of the gel to terminate electrophoresis. We selected a PVDF membrane that had a pore size of 0.22 μm and maintained a constant current of 200 mA for a transfer time of 90 min with 5% skimmed milk powder diluted in PBST, closed for 1 h. The PVDF membrane was washed three times, for 10 min each, using PBST and then placed in the hybridization cassette. The corresponding antibody was added, and the membrane was placed on a decolorization shaker overnight at 4°C. Then, the mix was shaken slowly to bring it to room temperature. The primary antibody [Annexin V (sc-393669, 1:1,000), CD9 (sc-13118, 1:2,000), Tsg101 (sc-7964, 1:2,000), and CD63 (sc-5275, 1:1,000); Santa Cruz Biotechnology, Inc., Texas, USA] was removed and the membrane was washed three times using PBST for 10 min each time; the secondary antibody was added into the hybridization cassette along with the membrane, which was then placed on a shaker and slowly shaken and incubated at room temperature for 1 h. Then, the secondary antibody was removed and the membrane was washed three times using PBST, for 10 min in each wash cycle. We added the appropriate amount of ECL luminescent solution and used program one in the digital imaging system to continuously photograph the membrane for a maximum duration of 1 min.
ELISA
For the ELISA < the residual cells were removed from the plasma samples and the cell fragments were diluted with 1 × PBS (1:500 diluted) and the exosomes were precipitated with 100 ml RIPA lysis buffer on ice for 30 min. The samples were diluted with PBS (1:3 diluted) after shaking and mixing. The ELISA plate that was previously coated with the AHCY antibody was taken out, and we added blank control solution to one well and solutions of gradient concentration to seven wells. The diluted exosome samples constituted a 100-μl solution. After incubation at 37°C for 60 min, the liquid in the well is discarded, and the plate is rotated and dried. Then, we added 100 μl Solution A to the plate, covered it with the film, incubated the plate in an oven at 37°C for 1 h, and then washed the plate three times. We added 100 μl Solution B to the plate, covered the plate with film, baked the plate in the oven at 37°C for half an hour, and then washed the plate five times. Next, we added 100 μl TMB substrate solution and colored it in the dark at 37°C for 20 min, and 50 μl of the termination reaction solution was added to the plate. The absorbance value was detected using the microplate reader at 450 nm wavelength.
Statistical Analysis
Data were statistically analyzed using SPSS 22.0, and the measurement data are expressed as (x ± s). One-way ANOVA and the SNK-q test for two-way comparison were used to determine the intergroup differences. Pearson linear correlation analysis and logistic one-way risk factor analysis were used to evaluate the predictive ability of plasma exosome-derived AHCY. We calculated the survival rate with the Kaplan Meier method. ROC curve analysis was used to assess the prognostic value of exosomal AHCY levels in HBV-LC patients. Differences were considered statistically significant at P < 0.05.
Results
Characteristics of Exosomes
The results of the TEM observation are shown in Figure 1A. Against a clear background, aggregates of exosomes were distributed while connected. The exosomes had a diameter of 100–200 nm and were shaped like a double disk-like vesicular structure that was completely covered with a lipid envelope. The results of exosome particle size detected by NTA are shown in Figure 1B. The median value of the overall particle size was approximately 100 nm, and particle size was mainly distributed between 50 and 200 nm. Western blotting showed the positive expression of the exosome marker proteins Annexin V, CD9, Tsg101, and CD63 in the exosome group (Figure 1C).
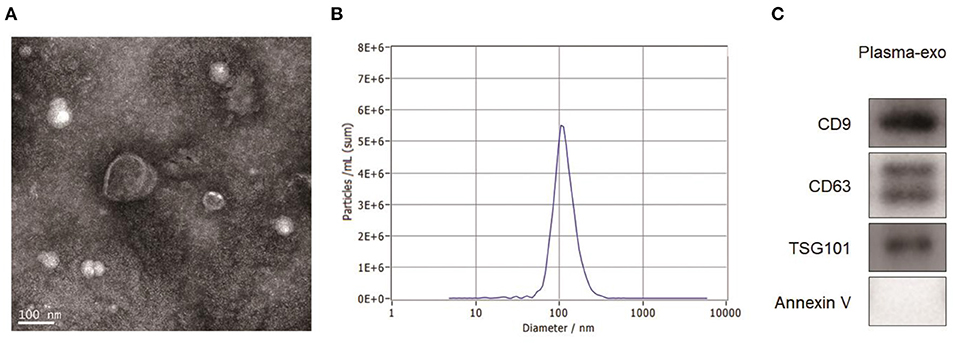
Figure 1. Exosome characterization. (A) TEM images showed that aggregates of exosomes were distributed while connected. The exosomes had a diameter of 100–200 nm and were shaped like a double disk-like vesicular structure that was completely covered with a lipid envelope. (B) The results of exosome particle size detected by NTA showed that the median value of the overall particle size was approximately 100 nm, and particle size was mainly distributed between 50 and 200 nm. (C) Western blotting showed the positive expression of the exosome marker proteins Annexin V, CD9, Tsg101, and CD63 in the exosome group.
Level of Exosomal AHCY Expression in HBV-LC Patients
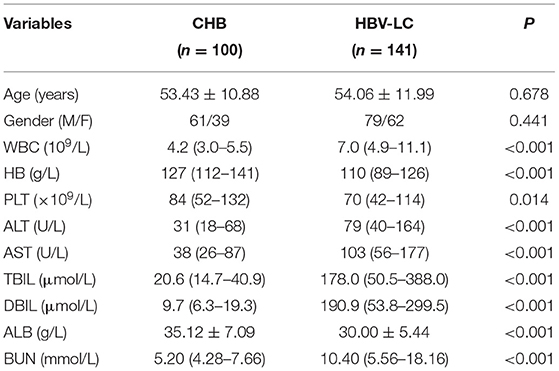
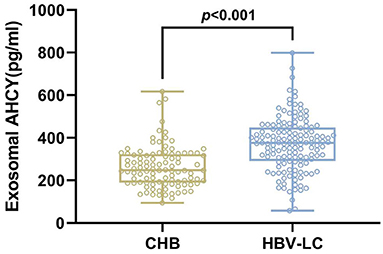
We evaluated the level of exosomal AHCY expression in HBV-LC patients (n = 141) and compared the expression levels with those in the CHB (n = 100) group. Table 1 shows the baseline characteristics of the CHB and HBV-LC groups. In the CHB group (age, mean ± SD: 53.43 ± 10.88 years), there were 61 male and 39 female participants; in the HBV-LC group (age, mean ± SD: 54.06 ± 11.99 years), there were 79 male and 62 female participants. The intergroup differences in age and sex were not statistically significant (P > 0.05). Compared with the CHB group, the level of exosomal AHCY expression was significantly higher in the HBV-LC group [376.62 (291.50–448.02) vs. 248.12 (189.28–324.63), P < 0.001; Figure 2].
Correlation Between the Serum Exosomal AHCY Expression and the Child-Pugh Class and MELD Score in the HBV-LC Group
Following the Child-Pugh classification, the HBV-LC patients were assigned to the Grade A, B, and C groups. Compared with the Grade A and B HBV-LC groups, the level of exosomal AHCY expression was significantly higher in the Grade C HBV-LC group [408.70 (365.63–465.76) vs. 279.76 (215.16–336.07), P < 0.001; Figure 3A]. In addition, we found a significant positive correlation between the serum exosomal AHCY level and the MELD score (r = 0.844, P < 0.001; Figure 3B).
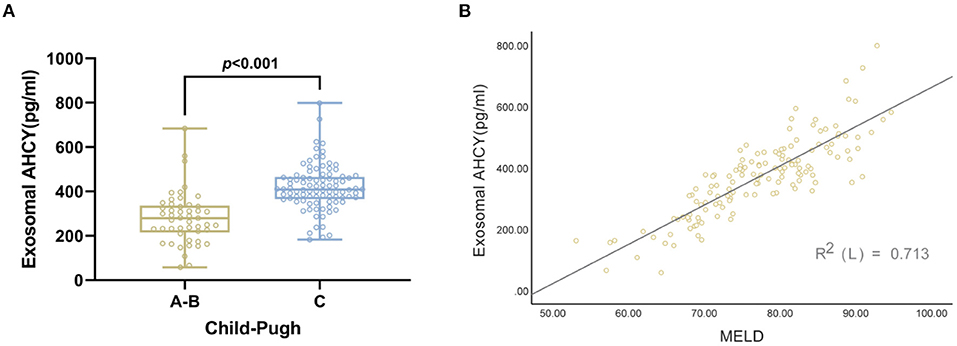
Figure 3. Correlation between serum exosomal AHCY level and child Pugh and MELD score in HBV-LC patients group. (A) The level of serum exosomal AHCY in HBV-LC patient with grade C group was significantly higher than that in HBV-LC patient with Grade A, B group [408.70 (365.63–465.76) vs. 279.76 (215.16–336.07), P > 0.001]; (B) there was a significant positive correlation between serum exosomal AHCY levels and MELD score (r = 0.844, P < 0.001).
Correlation Between the Serum Exosomal AHCY Level and Clinical Parameters in the HBV-LC Group
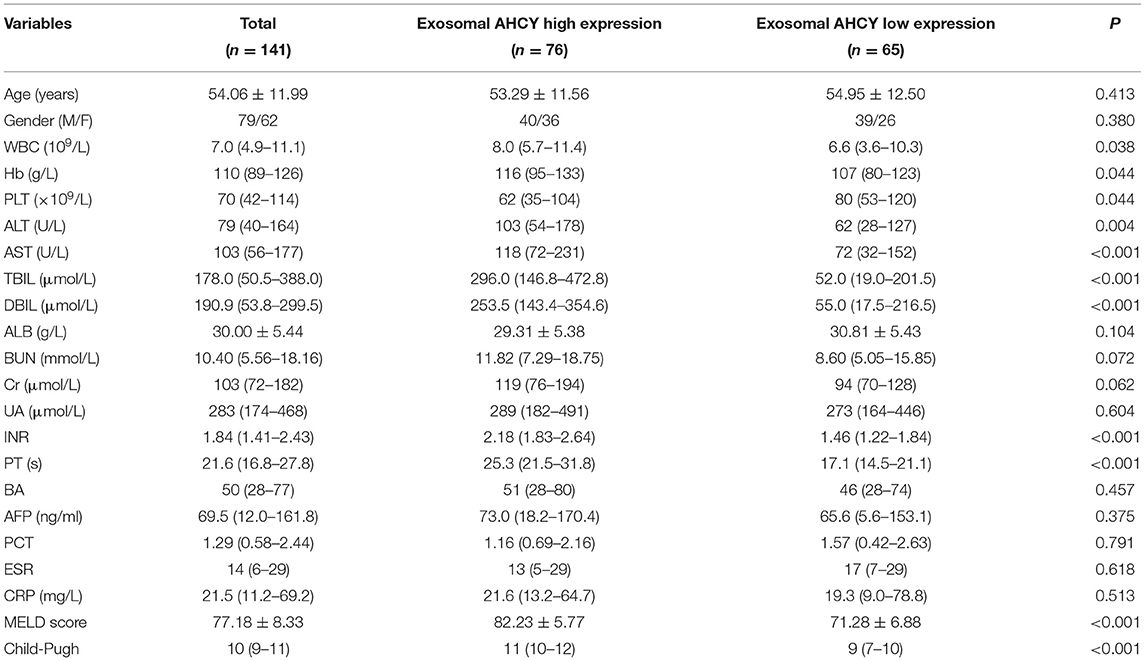
Using the mean exosomal AHCY expression level (370.21) in the HBV-LC group as a cutoff point, we assigned the HBV-LC patients into the high expression and low expression groups based on exosomal AHCY expression. Table 2 shows the baseline characteristics of the two subgroups. The levels of WBC, HB, ALT, AST, TBIL, DBIL; the INR and PT; and the MELD score and Child-Pugh grade of the patients in the high expression group were significantly higher than those in patients in the low expression group; however, the PLT count was significantly lower in patients in the high expression than in the low expression group. The survival curve analysis showed a significantly longer survival time for patients in the low expression group than for patients in the high expression group (P = 0.0038; Figure 4).
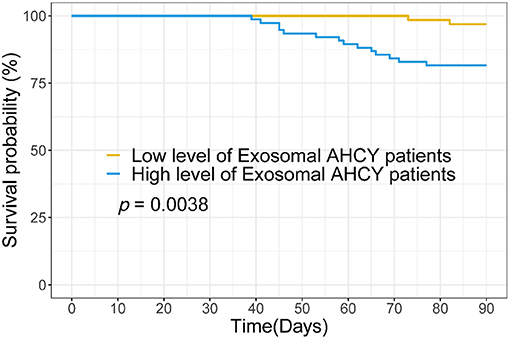
Figure 4. Survival curve analysis the relationship between serum exosomal AHCY level and survival time for HBV-LC patients.
Prognosis Predictive Ability Based on the Serum Exosomal AHCY Level in HBV-LC Patients
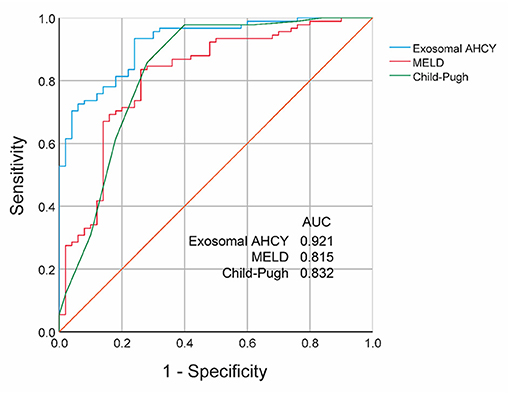
Finally, we evaluated the prognosis prediction ability based on the serum exosomal AHCY expression in HBV-LC patients. The receiver operating characteristics (ROC) curve showed an area under the curve (AUC) value of 0.921 for serum exosomal AHCY expression in predicting the mortality risk of HBV-LC patients, which was higher than the AUC of the MELD score (0.815) and the Child-Pugh classification (0.832) (Figure 5). The sensitivity and specificity of serum exosomal AHCY expression in prognosis prediction were 93.41 and 76.00%, respectively.
Discussion
Cirrhosis following an HBV infection is a chronic disease wherein the body, after HBV invasion, undergoes gradual liver cell necrosis that results in the fibronodular hyperplasia of liver tissue and the replacement of normal liver lobes by false lobes (23). In cirrhosis, repeated and continuously progressive liver fibrosis and inflammation induce loss of liver function, which results in decompensated liver function, ascites, esophageal gastric varices and variceal bleeding, portal hypertension, acute kidney injury, hepatic encephalopathy, and even HCC as well as several serious complications. In China, there were 460,000 primary liver cancer cases and 420,000 liver cancer-related deaths in 2015. The incidence of primary liver cancer in China in 2015 exceeded 50% of the total global incidence of primary liver cancer, which has emerged as a public health problem that endangers human health. Thus, the early diagnosis and treatment of cirrhosis are of great significance for the prognosis of patients.
In mammals, AHCY is the only enzyme that mediates the reversible catalysis of SAH to Ado and cysteine (24). The earliest manifestation of alcohol-related liver disease is steatosis, which is characterized by the accumulation of lipid droplets in liver cells. Arumugam et al. (25) demonstrated that many pathological changes, including steatosis, are associated with the alcohol-induced increase in SAH hepatocytes. A study that investigated the impaired Hcy metabolism in patients with alcohol-related liver disease from Taiwan (26) showed that impaired Hcy metabolism may disrupt antioxidant status. The role of alcohol-induced endoplasmic mesh stress in SREBP regulation and fatty liver, as well as the exact mechanism of beetroot protection, include decreased Hcy and SAH levels or increased S-adenosine methionine concentrations. Stender et al. (27) found that AHCY deficiency is associated with early-onset HCC. Vazquez et al. (20) screened novel serum markers in a mouse model of liver injury and demonstrated that AHCY expression significantly increased with the increase in the degree of liver injury. However, there are no studies of the role of exosomal AHCY in HBV-LC.
In this study, exosomes in the serum samples from patients with CHB and HBV-LC were separated, and TEM, NTA, and Western blotting were used to identify the characteristics of the exosomes. Transmission electron microscopy showed that exosomes had an interconnected aggregated distribution in a clear background, a diameter of 100–200 nm, and a complete lipid envelope, and were shaped as a double-disk vesicle. Nanoparticle tracking analysis showed an overall median exosome particle size of approximately 100 nm (range 50–200 nm). Western blotting revealed the positive expression of Annexin V, CD9, Tsg101, and CD63 in the exosome group. The exosomal AHCY levels of patients with HBV-LC were significantly higher than those of patients with CHB.
The Child-Pugh classification and the MELD score are important evaluation criteria in patients with severe liver disease. Therefore, we separately analyzed the correlation between the serum exosomal AHCY level and the abovementioned two assessment systems. The HBV-LC patients with Grade C cirrhosis had a significantly higher serum exosomal AHCY level than the HBV-LC patients with Grade A and B cirrhosis. In addition, we found a significant positive correlation between the serum exosomal AHCY level and the MELD score The above-described results indicated that the serum exosomal AHCY level has a good correlation with the two classic scoring models. Furthermore, we studied the correlation between the serum exosomal AHCY level and clinical parameters in the patients in the HBV-LC group and in the high expression and low expression subgroups that were based on exosomal AHCY expression. The levels of WBC, HB, ALT, AST, TBIL, and DBIL; the INR and PT; and the MELD score and Child-Pugh grade were significantly higher in patients with high expression of exosomal AHCY; however, the PLT count was significantly lower in patients with high exosomal AHCY expression than the PLT count in patients with low exosomal AHCY expression. Survival curve analysis showed that the survival time of patients with low exosomal AHCY expression was significantly longer. Finally, we evaluated the prognosis prediction ability of exosomal AHCY in HBV-LC patients. The ROC curve showed that the AUC value of serum exosomal AHCY for predicting the mortality risk of HBV-LC patients was higher than the AUC values of the MELD score and the Child-Pugh; moreover, exosomal AHCY expression showed good sensitivity and specificity in predicting the prognosis of HBV-LC patients.
This study has some limitations. First, the sample size of the study patients was not large; therefore, the predictive value of serum exosomal AHCY expression requires evaluation in multicenter, large studies. Second, this study lacked a control group for a controlled evaluation to verify whether the absence of exosomal AHCY expression is specific for the prognosis prediction in CHB or HBV-LC patients. Thirdly, our study did not involve the relevant mechanism research of AHCY involved in the development of disease.
In summary, our study showed that serum exosomal AHCY expression in HBV-LC patients is significantly upregulated and shows a good correlation with the Child-Pugh classification and the MELD score for prognosis prediction. HBV-LF patients with low exosomal AHCY expression had longer survival. The AUC value of serum exosomal AHCY in predicting the mortality risk of HBV-LC patients was higher than the AUCs of the MELD score and the Child-Pugh classification, which may facilitate the establishment of serum exosomal AHCY expression as a novel prognostic biomarker in HBV-LC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital of Zhejiang University School of Medicine (No. 2017003). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LT directed and supervised the study and revised the manuscript. CY and MW designed and performed most of the experiments. JY and HW participated in some experiments, analyzed the data, and completed the figures. LT and YW wrote the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.777452/full#supplementary-material
Abbreviations
HBV, hepatitis B virus; CHB, chronic hepatitis B; HBV-LC, hepatitis B-induced liver cirrhosis; HSC, hepatic stellate cells; AHCY, adenosylhomocysteinase; NTA, nanoparticle tracking analysis; TEM, transmission electron microscopy.
References
1. Tu T, Douglas MW. Hepatitis B virus infection: from diagnostics to treatments. Viruses. (2020) 12:1366. doi: 10.3390/v12121366
2. Jiang Y, Qin S, Wei X, Liu X, Guan J, Zhu H, et al. Highly activated TRAIL+ CD56bright NK cells are associated with the liver damage in HBV-LC patients. Immunol Lett. (2021) 232:9–19. doi: 10.1016/j.imlet.2020.12.008
3. Liu X, He L, Han J, Wang L, Li M, Jiang Y, et al. Association of neutrophil-lymphocyte ratio and T lymphocytes with the pathogenesis and progression of HBV-associated primary liver cancer. PLoS ONE. (2017) 12:e0170605. doi: 10.1371/journal.pone.0170605
4. Wu J, Yu J, Shi X, Li W, Song S, Zhao L, et al. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: a multicentre descriptive study. J Viral Hepat. (2021) 28:80–8. doi: 10.1111/jvh.13404
5. Ye F, Huang W, Xue Y, Tang E, Wang M, Shi F, et al. Serum levels of ITGBL1 as an early diagnostic biomarker for hepatocellular carcinoma with hepatitis B virus infection. J Hepatocell Carcinoma. (2021) 8:285–300. doi: 10.2147/JHC.S306966
6. Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. (2018) 67:945–52. doi: 10.1136/gutjnl-2017-314904
7. Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. (2018) 69:1066–73. doi: 10.1016/j.jhep.2018.07.018
8. Lee HW, Park SY, Lee M, Lee EJ, Lee J, Kim SU, et al. An optimized hepatocellular carcinoma prediction model for chronic hepatitis B with well-controlled viremia. Liver Int. (2020) 40:1736–43. doi: 10.1111/liv.14451
9. Ratajczak MZ, Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia. (2020) 34:3126–35. doi: 10.1038/s41375-020-01041-z
10. Wang L, Wu J, Song S, Chen H, Hu Y, Xu B, et al. Plasma exosome-derived sentrin SUMO-specific protease 1: a prognostic biomarker in patients with osteosarcoma. Front Oncol. (2021) 11:625109. doi: 10.3389/fonc.2021.625109
11. Kok VC Yu CC. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomedicine. (2020) 15:8019–36. doi: 10.2147/IJN.S272378
12. Ren Z, Qi Y, Sun S, Tao Y, Shi R. Mesenchymal stem cell-derived exosomes: hope for spinal cord injury repair. Stem Cells Dev. (2020) 29:1467–78. doi: 10.1089/scd.2020.0133
13. Samuel M, Gabrielsson S. Personalized medicine and back-allogeneic exosomes for cancer immunotherapy. J Intern Med. (2021) 289:138–46. doi: 10.1111/joim.12963
14. Zhang L, Ju Y, Chen S, Ren L. Recent progress on exosomes in RNA virus infection. Viruses. (2021) 13:256. doi: 10.3390/v13020256
15. Shen M, Shen Y, Fan X, Men R, Ye T, Yang L. Roles of macrophages and exosomes in liver diseases. Front Med (Lausanne). (2020) 7:583691. doi: 10.3389/fmed.2020.583691
16. Jun JH, Kim JY, Choi JH, Lim JY, Kim K, Kim GJ. Exosomes from placenta-derived mesenchymal stem cells are involved in liver regeneration in hepatic failure induced by bile duct ligation. Stem Cells Int. (2020) 2020:5485738. doi: 10.1155/2020/5485738
17. Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: a clinician's point of view. J Hepatol. (2020) 73:1507–25. doi: 10.1016/j.jhep.2020.07.014
18. Wang Y, Pei L, Yue Z, Jia M, Wang H, Cao LL. The potential of serum exosomal hsa_circ_0028861 as the novel diagnostic biomarker of HBV-derived hepatocellular cancer. Front Genet. (2021) 12:703205. doi: 10.3389/fgene.2021.703205
19. Brzezinski K. S-adenosyl-l-homocysteine hydrolase: a structural perspective on the enzyme with two Rossmann-fold domains. Biomolecules. (2020) 10:1682. doi: 10.3390/biom10121682
20. Vazquez JH, Clemens MM, Allard FD, Yee EU, Kennon-McGill S, Mackintosh SG, et al. Identification of serum biomarkers to distinguish hazardous and benign aminotransferase elevations. Toxicol Sci. (2020) 173:244–54. doi: 10.1093/toxsci/kfz222
21. Wranke A, Hardtke S, Heidrich B, Dalekos G, Yalçin K, Tabak F, et al. Ten-year follow-up of a randomized controlled clinical trial in chronic hepatitis delta. J Viral Hepat. (2020) 27:1359–68. doi: 10.1111/jvh.13366
22. Abdallah MA, Kuo YF, Asrani S, Wong RJ, Ahmed A, Kwo P, et al. Validating a novel score based on interaction between ACLF grade and MELD score to predict waitlist mortality. J Hepatol. (2021) 74:1355–61. doi: 10.1016/j.jhep.2020.12.003
23. Chen YP, Huang LW, Lin XY, Hu XM, Liang XE, Jiang RL. Alanine aminotransferase influencing performances of routine available tests detecting hepatitis B-related cirrhosis. J Viral Hepat. (2020) 27:826–36. doi: 10.1111/jvh.13293
24. Vizán P, Di Croce L, Aranda S. Functional and pathological roles of AHCY. Front Cell Dev Biol. (2021) 9:654344. doi: 10.3389/fcell.2021.654344
25. Arumugam MK, Talawar S, Listenberger L, Donohue TM Jr, Osna NA, Kharbanda KK. Role of elevated intracellular S-adenosylhomocysteine in the pathogenesis of alcohol-related liver disease. Cells. (2020) 9:1526. doi: 10.3390/cells9061526
26. Chien YW, Chen YL, Peng HC, Hu JT, Yang SS, Yang SC. Impaired homocysteine metabolism in patients with alcoholic liver disease in Taiwan. Alcohol. (2016) 54:33–7. doi: 10.1016/j.alcohol.2016.06.002
Keywords: adenosylhomocysteinase (AHCY), exosomes, hepatitis B-related cirrhosis (HBV-LC), prognosis predictor, chronic hepatitis B (CHB)
Citation: Tong L, Yan C, Wang M, Yang J, Wang H and Wang Y (2021) Prognostic Value of Serum Exosomal AHCY Expression in Hepatitis B-Induced Liver Cirrhosis. Front. Med. 8:777452. doi: 10.3389/fmed.2021.777452
Received: 15 September 2021; Accepted: 11 October 2021;
Published: 08 November 2021.
Edited by:
Yijin Wang, Southern University of Science and Technology, ChinaReviewed by:
Anquan Shang, Tongji University, ChinaYuzhu Dai, The 903th Hospital of the People's Liberation Army, China
Rongrong Ding, Fudan University, China
Copyright © 2021 Tong, Yan, Wang, Yang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, d2FuZ3k4MzAyMjRAc2luYS5jb20=; Hongmei Wang, cmVkbWVpMTk3MEAxNjMuY29t
 Ling Tong1
Ling Tong1 Cuilin Yan
Cuilin Yan Ying Wang
Ying Wang