
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 18 November 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.776955
This article is part of the Research Topic Burden of Diarrhoeal Diseases View all 11 articles
 Yuchong Zhao1†
Yuchong Zhao1† Yilei Yang1†
Yilei Yang1† Aruna1
Aruna1 Jun Xiao2
Jun Xiao2 Jun Song3
Jun Song3 Tizheng Huang4
Tizheng Huang4 Shuyu Li5
Shuyu Li5 Jiguang Kou6
Jiguang Kou6 Lu Huang7
Lu Huang7 Dexiong Ji8
Dexiong Ji8 Si Xiong1
Si Xiong1 Wang Peng1
Wang Peng1 Sanping Xu9*
Sanping Xu9* Bin Cheng1*
Bin Cheng1*Background: Whether probiotics helped the Helicobacter pylori (H. pylori) eradication was still highly controversial. The non-bacterial Saccharomyces boulardii (S. boulardii) has demonstrated its efficacy in the treatment of antibiotic-associated and infectious diarrhea. We aimed to evaluate the effects of S. boulardii combined with quadruple therapy for H. pylori eradication and associated side effects.
Methods: Three hundred and sixty H. pylori-infected patients were recruited in this multicenter, randomized controlled trial. The patients who underwent H. pylori eradication treatment were randomized in a ratio of 1:1 into two separate groups that received standard quadruple therapy (Group A) and quadruple therapy plus S. boulardii sachets (Group B) for 14 days. The everyday medication and side-effect records were collected for compliance and adverse effect analysis. All patients accepted 13C/14C-urea breath tests 4 weeks after the therapy completion.
Results: Saccharomyces boulardii and quadruple therapy-combined intervention significantly reduced the incidences of overall side effects (27.8 vs. 38.5%, p = 0.034) and diarrhea (11.2 vs. 21.2%, p = 0.012) in Group B compared with quadruple therapy alone in Group A, especially reduced the diarrhea duration (5.0 days vs. 7.7 days, p = 0.032) and incidence of severe diarrhea (4.7 vs. 10.1%, p = 0.040). Intention-to-treat (ITT) analysis and per-protocol (PP) analysis both indicated no statistical differences of eradication rate between Groups A and B (ITT: 82.7 vs. 85.8%, p = 0.426; PP: 89.7 vs. 94.2%, p = 0.146). The joint use of S. boulardii and quadruple therapy markedly improved the overall pre-eradication alimentary symptoms (hazard ratio (HR): 2.507, 95% CI: 1.449–4.338) recovery.
Conclusion: Saccharomyces boulardii ameliorated H. pylori eradication-induced antibiotic-associated side effects especially reduced the incidence of severe diarrhea and the duration of diarrhea. However, there was no significant effect of S. boulardii on the rate of H. pylori eradication.
Trial Registration: The protocol had retrospectively registered at ClinicalTrails.gov, Unique identifier: NCT03688828, date of registration: September 27, 2018; https://clinicaltrials.gov/show/NCT03688828
The prevalence of Helicobacter pylori (H. pylori) infection in the general population in China was approximately 60% (1, 2). The eradication of H. pylori has been demonstrated effective for alleviating various gastrointestinal (GI) diseases and reducing the risk of gastric cancer (3–6). Current Chinese guidelines recommended 14-day bismuth-containing quadruple therapy as a first-line regimen for H. pylori eradication (7). However, due to the increasing resistance to antibiotics and relatively high incidence of side effects, quadruple therapy was not as satisfying as before (8–10). Several previous studies indicated that the use of proton pump inhibitors (PPIs) and antibiotics led to dysbiosis and abundance changes of the gut microbiota (11–13). Though with a relatively low rate of severe side effects, the sporadic reports of H. pylori treatment-induced Clostridium difficile (C. difficile) infection and pseudomembranous colitis have been incessant over the past decades (14–18). Several novel regimens (e.g., high-dose PPI + amoxicillin dual therapy, and vonoprazan-containing therapy) were emerging to conquer the current difficulties, and the preliminary clinical data showed the effect of dual therapy was not inferior to quadruple therapy for H. pylori eradication whereas the efficacies of these regimens were restricted by poor compliance and availability (19–21). And the incidence of adverse events (AEs) was nearly equal between the novel treatment and quadruple therapy.
To prevent and treat the underlying AEs brought by H. pylori eradication, standard quadruple therapy with probiotic supplements, in particular, lactobacilli, bifidobacterial, and Saccharomyces boulardii (S. boulardii) were administrated (22). Non-pathogenic yeast S. boulardii was initially used to prevent the C. difficile infection and relapse, now it has demonstrated the efficacy of preventing and treating antibiotic-associated, infectious, and functional diarrhea (23, 24). The yeast nature of S. boulardii other than bacterial suggested the implications of joint use with antibiotics. Several reports claimed that S. boulardii protected intestinal epithelium against pathogen colonization and invasion through upregulating the secretion of sIgA into the luminal mucous, thus exerted the anti-H. pylori effect (25–27). However, current clinical studies to investigate the synergistic effect of S. boulardii on H. pylori infection were highly controversial, even some meta-analyses had contradictory results (28–33). And most of the current trials were about S. boulardii and triple therapy combination. Due to the high increasing resistance to antibiotics, triple therapy was no longer effective as used to be. Whether S. boulardii could improve the eradication rate of the highly effective 14-day quadruple therapy is still largely unknown.
Most clinical trials about S. boulardii focused on the synergetic effect on eradication, the evaluation of diarrhea prevention was usually the secondary aim. Antibiotic-associated diarrhea was the most common AE, ranged from 7.0 to 41.2% (33–38). Though most associated adverse effects were mild and tolerable, diarrhea was the main reason leading to eradication treatment discontinuation. To further investigate the diarrhea prevention and treatment effect of S. boulardii in H. pylori eradication therapy, we conducted this prospective multicenter randomized controlled trial. Meanwhile, we evaluated the potential synergistic effect of S. boulardii on H. pylori eradication and alimentary symptoms.
This study was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20180904) and registered on ClinicalTrials.gov (NCT03688828). Between October 2018 and September 2019 from 9 medical centers in China, 360 patients between the ages of 22 and 65 years were enrolled in this study after receiving endoscopic evaluations for various GI symptoms. Each of these patients had 13C/14C-urea breath test proof of H. pylori infection. The exclusion criteria included (1) previous attempts to eradicate H. pylori; (2) pregnant or lactation; (3) hypersensitivity to the drugs being used in the study; (4) previous treatment with PPIs, bismuth, H2 receptor antagonist, or antibiotics within 4 weeks of the study; and (5) treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or alcohol abuse during the study.
This was a randomized, parallel-group study. Three hundred and sixty H. pylori-infected patients were recruited in the study and randomly assigned by a computer program into two groups: standard quadruple therapy group (Group A) and quadruple therapy plus S. bouladii (Group B). Computer-generated randomization assignments were centralized using the block randomization method (block size of 8) by a data manager who was not involved in the data analysis or patient enrollment. Patients assigned to Group A received esomeprazole (AstraZeneca Pharmaceutical, Co. Ltd., Cambridge, United Kingdom) 20 mg two times a day (bid); amoxicillin (Baker Norton Pharmaceutical, Co., Ltd., Kunming, China) 1.0 g bid; clarithromycin (Abbott Laboratories Ltd., Shanghai, China) 500 mg bid; and Bismuth Potassium Citrate (Livzon Pharmaceutical Group, Inc., Zhuhai, China) 600 mg bid for 14 days. Patients assigned to Group B received the same quadruple therapy for 14 days, Additionally, S. boulardii sachets (Laboratories Biocodex, Inc., France) of 500 mg was given a bid to Group B for 14 days. Serious diarrhea patients (mushy stools or watery stools > two times a day) were additionally given montmorillonite powder 3 g tid. Considering the high resistance rate of metronidazole in the Center China population, we took amoxicillin and clarithromycin as our antibiotic choice (9, 39).
Patients were evaluated at five visits: screening (10–30 days before the baseline visit), baseline, 7 days after the treatment initiation, end of treatment/efficacy (14 days after the treatment initiation), and the second 13C/14C-urea breath test 4 weeks after the therapy completion and follow-up (44–94 days after treatment completion; Figure 1). 13C/14C-urea breath tests were applied to detect the H. pylori infection for the high sensitivity and specificity. Previous studies demonstrated that there were no statistical differences between the 13C and 14C-urea breath tests (40, 41). In this trial, 276 patients accepted the 13C-urea breath test and 84 patients received the 14C-urea breath test. The urea breath test technician was blinded to patient groups.
The primary outcome measure was the incidence of AEs. The investigator will record all AEs related to anti-H. pylori therapy, such as nausea, vomiting, taste abnormalities, abdominal pain, abdominal distension, diarrhea, and increased symptoms on the case report form. The incidence of adverse reactions will be assessed at three points: before treatment, during treatment (2 weeks), and after treatment (4–12 weeks). Patients returned their medication and side effects record form after the urea-test re-examination.
The secondary outcome measure was to investigate whether there was a statistical difference in the eradication rate between the two groups. Eradication rate = number of H. pylori eradicated cases after treatment/total cases ×100%. Non-ulcer patients will be tested 4 weeks after the end of the eradication treatment, and ulcer patients will be tested 2 weeks after the end of the total course of treatment. Eradication rates were determined by both ITT- and PP-based analyses. All enrolled patients were included in the ITT analysis, but the PP analysis excluded those patients who dropped out due to side effects, loss to follow-up, or poor compliance.
The effect of S. boulardii on H. pylori eradication rate and the incidence of AEs was also studied using binary logistic regression models, which included the following parameters: eradication rate, the overall incidence of AEs, and the incidence of antibiotic-induced diarrhea.
Based on a literature review of H. pylori eradication-induced antibiotic diarrhea (29, 35, 42), we expected a difference between quadruple therapy combined with S. boulardii and quadruple therapy alone on the incidence of diarrhea of 13.5 vs. 19.5%. The calculation yielded 179 for combined therapy and 179 for quadruple therapy, with a power of 80% and a two-sided significance level of 0.05 with an assumed 20% dropout rate. Each group should have 184 patients following the randomized block design of eight patients in a group. We calculated a final sample size of 368 patients (184 per group). The full analysis set should be as close as possible to the ITT set. The standards and population of the PP set will be finalized after data-blinding verification. The direct deletion method will be used to treat missing data.
In this study, the demographic and clinical characteristics of the patients will be summarized with mean and SD. The results of eradication and incidence of AEs are expressed in terms of the number of cases and percentage.
Qualitative variables were compared using the chi-squared test and Fisher's exact test, while Student's t-test and the Mann-Whitney U test were used for quantitative variables. The effect of S. boulardii plus sequential therapy on the eradication rate and the incidence of antibiotic-induced AEs were determined using binary logistic regression, and p-value < 0.05 with two-tail will be considered significant. All statistical analyses will be performed by blinded professional statisticians using SPSS V.26.0.
A total of 348 patients fulfilling the inclusion criteria were enrolled in this trial, with 179 patients in Group A and 169 patients in Group B for ITT analysis. Twenty-nine patients (8.33%) were excluded from PP analysis. Follow-up was incomplete in 10 patients (5.6%) and 8 patients (4.7%) in Groups A and B, respectively. Two patients in Group A discontinued treatment because of severe diarrhea while one patient in Group B discontinued for skin rash. Poor treatment compliance was reported in two (1.1%) patients and five (3.0%) patients in Groups A and B. Apart from this, there was one patient who dropped out from treatment in group B because of pregnancy (Figure 2). At baseline, there were no statistically significant differences in the baseline characteristics of patients included in the two study groups (Table 1).
Intention-to-treat analysis demonstrated that the eradication rates were 85.8% for Group B and 82.7% for Group A (hazard ratio, HR, = 1.038, p = 0.426, 95% CI = 0.948–1.136). PP analysis indicated that the eradication results were 89.7% for Group B and 94.2% for Group A (HR = 1.851, p = 0.146, 95% CI = 0.799–4.286; Table 2). Both ITT and PP analyses showed no statistical differences in the eradication rate between Groups A and B.
The follow-up analysis of the alimentary symptoms, from pre-treatment of 3 months after the eradication, indicated that the overall symptoms improvement rate of Group B (78.6 vs. 58.3%, p < 0.001) was significantly higher than that of Group A (Table 2). Further analysis showed that the abdominal distension recovery rate in Group B compared was markedly higher than in Group A (89.4 vs. 53.0%, p = 0.011).
The overall incidences of AEs in the experimental and control groups were 38.5 and 27.8%, respectively, representing a decrease of 10.7% in the experimental group (p = 0.034). The diarrhea rate of Group A was significantly higher than that of Group B (21.2 vs. 11.2%, p = 0.012). Meanwhile, the combination of S. boulardii and quadruple therapy decreased the duration of diarrhea (5.0 days vs. 7.7 days, p = 0.032) and incidence of severe diarrhea (10.1 vs. 4.7%, p = 0.040) in Group B compared with Group A (Figure 3). There were no statistical differences between Groups A and B in terms of vomiting, constipation, or allergy (Table 3). Results of the multivariate analysis further verified that the combination of S. boulardii and quadruple treatment reduced the overall incidence of AEs (odds ratio, OR: 0.378, 95% CI: 0.117–0.807) and incidence of diarrhea (OR: 0.359, 95% CI: 0.148–0.872) compared with quadruple therapy alone (Table 4). Two patients in Group A accepted intravenous (i.v.) treatment for severe diarrhea other than montmorillonite powder and discontinued the eradication therapy. However, no C. difficile was detected by fecal examination. And no patients in Group B discontinued the therapy due to diarrhea.
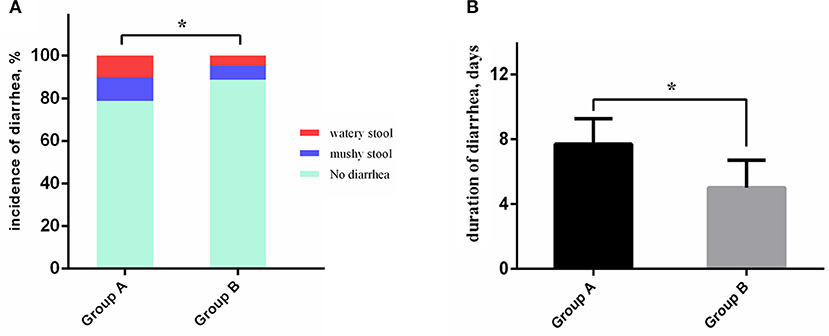
Figure 3. Antibiotic-associated diarrhea characteristics comparison between the two groups. (A) The severe diarrhea patients and overall diarrhea patients occupy a bigger proportion in Group A than in Group B. (B) The duration of diarrhea of patients in Group B was significantly shorter than in Group A. *P < 0.05.
The synergetic effect of S. boulardii on H. pylori eradication and associated side effects were analyzed in this study through the first multicenter randomized controlled trials of S. boulardii and quadruple therapy combination in China. We demonstrated that the administration of S. boulardii significantly decreased the incidence of eradication-associated AEs (OR: 0.378, 95% CI: 0.117–0.807), especially reduced the duration of diarrhea (5.0 days vs. 7.7 days, p = 0.032) and incidence of severe diarrhea (4.7 vs. 10.1%, p = 0.040). However, S. boulardii did not improve the eradication rate for bismuth quadruple therapy. However, the joint use of S. boulardii significantly improved gastritis/ulcer-associated symptoms (HR: 2.507, 95% CI: 1.449–4.338).
Unlike synergetic effects of H. pylori eradication, the effect of probiotics on AEs prevention and treatment was definite. Distinct from other bacterial probiotics, S. boulardii is a non-pathogenic fungus resistant to gastric acid and antibiotics, thus it could be used with eradication therapy simultaneously (24, 25). S. boulardii was used for the prevention of C. difficile infection originally (43). With sporadic reports of pseudomembranous colitis during H. pylori eradication, S. boulardii was gradually used as a supplement for the prevention and treatment of AEs. Acute severe diarrhea was the main reason for eradication therapy discontinuation thus leading to treatment failure. And some reports claimed that the diarrhea prevention effect of S. boulardii was only notable in children but not in adults (35, 44). Although various trials have already demonstrated the efficacy of S. boulardii to prevent and treat antibiotic-associated diarrhea (33, 35, 45, 46). Our study refined the diarrhea-associated data and verified that S. boulardii reduced the diarrhea duration and incidence of severe diarrhea correlated with eradication therapy. None of the diarrhea patients in the quadruple therapy plus S. boulardii group required further diarrhea treatment, whereas two patients in the quadruple therapy alone group accepted intravenous fluid infusion.
Probiotics might be effective for improving H. pylori eradication rates due to the decrease in the AEs and potential mucosal barrier protective effect (29, 36, 37, 47). Previous studies demonstrated the probiotics exerted the synergetic eradication effect through a similar mechanism including competitively inhibiting the H. pylori adhesion to gastric mucosa (48, 49) or producing antimicrobial molecules (29, 50). Recently, Yang et al. reported that the administration of S. boulardii could inhibit the H. pylori infection-induced gastric lymphoid follicle formation (26). Previous experiments mainly adopted the combination of S. boulardii and triple therapy. However, due to high resistance and decreasing efficacy, triple therapy was no longer the first-line recommendation for H. pylori eradication in China (7). The eradication rate for bismuth quadruple therapy has already reached 87.6–92.6% (51). In addition, the synergetic effect of S. boulardii on eradication was highly controversial for many years even with low-efficacy triple therapy. Some meta-analyses gave contradictory results, let alone some prospective and respective trials. Szayewska et al. reported in a meta-analysis that S. boulardii improved the eradication rate [Risk Ratio (RR): 1.11, 95% CI: 1.06–1.17; moderate-quality evidence] whereas Wang et al. found no better efficacy of any probiotic supplement for H. pylori eradication (24, 29). This is the first multicenter randomized controlled trial for S. boulardii and bismuth quadruple therapy in China. And our study showed that this probiotic supplement barely improved the eradication rate of quadruple therapy. Considering the eradication rate was still at a satisfying level compared with triple therapy, no improvement after the S. boulardii supplement became reasonable. It was a novel finding that joint administration of S. boulardii significantly improved the original dyspepsia symptoms.
Collectively, our study demonstrated that S. boulardii-ameliorated H. pylori eradication-induced antibiotic-associated diarrhea especially decreased the diarrhea duration and the incidence of severe diarrhea. Different from the combination with triple therapy, S. boulardii did not affect the quadruple therapy eradication rate of H. pylori, whereas improved the pre-treatment dyspepsia symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
BC and SXu designed the study. YZ and YY performed data analysis. SXi, YZ, A, JX, SL, JK, TH, LH, and DJ performed the acquisition of data. YZ and YY drafted the manuscript. SXi provided critical revision of the manuscript for important intellectual content. YZ, SXi, and YY performed technical support. BC and SXu performed study supervision. All authors have read and approved the manuscript.
This work was supported by the National Natural Science Foundation of China Grant Nos. 81802427 (to ZL), 81372352 (to BC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank Dr. Ping Han and Dr. Zhou Luan from the Tongji Hospital, Tongji Medical College, HUST, and Dr. Liangkai Chen and Prof. Ping Yin from the Department of Biostatistics, School of Public Health, Tongji Medical College for their efforts.
PPI, proton pump inhibitors; GI, gastrointestinal; AEs, adverse events; PP, Per-protocol; ITT, Intention-to-treat; H. pylori, Helicobacter pylori; S. boulardii, Saccharomyces boulardii; C. difficile, Clostridium difficile.
1. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
2. Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. (2016) 8:8. doi: 10.1186/s13099-016-0091-7
3. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. (2004) 291:187. doi: 10.1001/jama.291.2.187
4. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. (2015) 64:1353–67. doi: 10.1136/gutjnl-2015-309252
5. Fallone CA, Chiba N, Zanten SVV, Fischbach L, Gisbert JP, Hunt RH, et al. The toronto consensus for the treatment of helicobacter pylori infection in adults. Gastroenterology. (2016) 151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006
6. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of helicobacter pylori infection-the maastricht V/Florence consensus report. Gut. (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
7. Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth chinese national consensus report on the management of helicobacter pylori infection. Helicobacter. (2018) 23:e12475. doi: 10.1111/hel.12475
8. Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AHR, Goh KL, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2017) 2:707–15. doi: 10.1016/S2468-1253(17)30219-4
9. Hu Y, Zhu Y, Lu NH. Primary Antibiotic Resistance of Helicobacter pylori in China. Digest Dis Sci. (2017) 62:1–9. doi: 10.1007/s10620-017-4536-8
10. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. (2019) 157:44–53. doi: 10.1053/j.gastro.2019.04.011
11. Hsu PI, Pan CY, Kao JY, Tsay FW, Peng NJ, Kao SS, et al. Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter. (2018) 23:e12498. doi: 10.1111/hel.12498
12. Oh B, Kim BS, Kim JW, Kim JS, Koh SJ, Kim BG, et al. The effect of probiotics on gut microbiota during the helicobacter pylori eradication: randomized controlled trial. Helicobacter. (2016) 21:165–74. doi: 10.1111/hel.12270
13. Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. (2016) 65:749–56. doi: 10.1136/gutjnl-2015-310861
14. Nawaz A, Mohammed I, Ahsan K, Karakurum A, Hadjiyane C, Pellecchia C. Clostridium difficile colitis associated with treatment of Helicobacter pylori infection. Am J Gastroenterol. (1998) 93:1175–6. doi: 10.1111/j.1572-0241.1998.00358.x
15. Nei T, Hagiwara J, Takiguchi T, Yokobori S, Shiei K, Yokota H, et al. Fatal fulminant Clostridioides difficile colitis caused by Helicobacter pylori eradication therapy a case report. J Infect Chemother. (2020) 26:305–8. doi: 10.1016/j.jiac.2019.10.021
16. Trifan A, Girleanu I, Cojocariu C, Sfarti C, Singeap AM, Dorobat C, et al. Pseudomembranous colitis associated with a triple therapy for Helicobacter pylori eradication. World J Gastroenterol. (2013) 19:7476–9. doi: 10.3748/wjg.v19.i42.7476
17. Sato S, Chinda D, Yamai K, Satake R, Soma Y, Shimoyama T, et al. A case of severe pseudomembranous colitis diagnosed by colonoscopy after Helicobacter pylori eradication. Clin J Gastroenterol. (2014) 7:247–50. doi: 10.1007/s12328-014-0490-6
18. Prieto de. Paula JM, García Colodro J, Prieto Dehesa M, Franco Hidalgo S. Clostridium difficile infection associated with metronidazole-based treatment for Helicobacter pylori eradication. Gastroenterol Hepatol. (2019) 42:524. doi: 10.1016/j.gastre.2019.05.005
19. Gao W, Ye H, Deng X, Wang C, Xu Y, Li Y, et al. Rabeprazole-amoxicillin dual therapy as first-line treatment for H pylori eradication in special patients: a retrospective, real-life study. Helicobacter. (2020) 25:e12717. doi: 10.1111/hel.12717
20. Yu L, Luo L, Long X, Liang X, Ji Y, Graham DY, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: a randomized trial. Helicobacter. (2019) 24:e12596. doi: 10.1111/hel.12596
21. Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. (2020) 69:1019–26. doi: 10.1136/gutjnl-2019-319954
22. Currò D, Ianiro G, Pecere S, Bibbò S, Cammarota G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol. (2017) 174:1426–49. doi: 10.1111/bph.13632
23. Cárdenas PA, Garcés D, Prado-Vivar B, Flores N, Fornasini M, Cohen H, et al. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur J Clin Microbiol Infect Dis. (2020) 39:1365–72. doi: 10.1007/s10096-020-03854-3
24. Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. (2015) 41:1237–45. doi: 10.1111/apt.13214
25. Sakarya S, Gunay N. Saccharomyces boulardii expresses neuraminidase activity selective for α2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. (2014) 122:941–50. doi: 10.1111/apm.12237
26. Yang L, Tian ZB, Yu YN, Zhang CP, Li XY, Mao T, et al. Saccharomyces boulardii administration can inhibit the formation of gastric lymphoid follicles induced by Helicobacter suis infection. Pathogen Dis. (2017) 75:ftx006. doi: 10.1093/femspd/ftx006
27. Rodrigues AC, Cara DC, Fretez SH, Cunha FQ, Vieira EC, Nicoli JR, et al. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol. (2000) 89:404–14. doi: 10.1046/j.1365-2672.2000.01128.x
28. Chang YW, Park YM, Oh CH, Oh SJ, Cho JH, Kim JW, et al. Effects of probiotics or broccoli supplementation on Helicobacter pylori eradication with standard clarithromycin-based triple therapy. Korean J Intern Med. (2020) 35:574–81. doi: 10.3904/kjim.2019.139
29. Wang F, Feng J, Chen P, Liu X, Ma M, Zhou R, et al. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol. (2017) 41:466–75. doi: 10.1016/j.clinre.2017.04.004
30. Zojaji H, Ghobakhlou M, Rajabalinia H, Ataei E, Jahani SS, Moghimidehkordi B, et al. The efficacy and safety of adding the probiotic Saccharomyces boulardiito standard triple therapy for eradication of Hpylori: a randomized controlled trial. Gastroenterol Hepatol Bed Bench. (2013) 6:S99–S104.
31. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. (2010) 32:1069–79. doi: 10.1111/j.1365-2036.2010.04457.x
32. Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Pãdiatrica. (2009) 98:127–31. doi: 10.1111/j.1651-2227.2008.00977.x
33. Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. (2007) 12:309–16. doi: 10.1111/j.1523-5378.2007.00516.x
34. Kwon SB, Lee KL, Kim JS, Lee JK, Kim W, Jung YJ, et al. Antibiotics-associated diarrhea and other gastrointestinal abnormal responses regarding Helicobacter pylori eradication. Korean J Gastroenterol. (2010) 56:229–35. doi: 10.4166/kjg.2010.56.4.229
35. Szajewska H, Kołodziej M. Systematic review with meta-analysis: saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. (2015) 42:793–801. doi: 10.1111/apt.13344
36. Çekin AH, Sahintürk Y, Akbay Harmandar F, Uyar S, Yolcular BO, Çekin Y. Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turkish J Gastroenterol. (2017) 28:3–11. doi: 10.5152/tjg.2016.0278
37. de Vrese M, Kristen H, Rautenberg P, Laue C, Schrezenmeir J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J Dairy Res. (2011) 78:396–403. doi: 10.1017/S002202991100063X
38. Nyssen OP, Perez-Aisa A, Tepes B, Castro-Fernandez M, Kupcinskas J, Jonaitis L, et al. Adverse event profile during the treatment of helicobacter pylori: a real-world experience of 22,000 patients from the European Registry on H. pylori Management (Hp-EuReg). Am J Gastroenterol. (2021) 116:1220–9. doi: 10.14309/ajg.0000000000001246
39. Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multiregion prospective 7-year study. Clin Microbiol Infect. (2018) 24:780.e5–e8. doi: 10.1016/j.cmi.2017.11.010
40. Hart GC, Avison MP. 13C-urea versus 14C-urea breath test: is there still a need for 14C-urea? Nucl Med Commun. (1999) 20:495–6. doi: 10.1097/00006231-199905000-00141
41. Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Datab System Rev. (2018) 3:Cd012080. doi: 10.1002/14651858.CD012080.pub2
42. McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur Gastroenterol J. (2016) 4:546–61. doi: 10.1177/2050640615617358
43. Mcfarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, et al. A randomized placebo-controlled trial of saccharomyces boulardii in combination with standard antibiotics for clostridium difficile disease. JAMA. (1994) 271:1913–8. doi: 10.1001/jama.271.24.1913
44. Mcfarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. (2010) 16:2202–22. doi: 10.3748/wjg.v16.i18.2202
45. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. (2014) 134:e176–91. doi: 10.1542/peds.2013-3950
46. Surawicz CM, Elmer GW, Speelman P, Mcfarland LV, Chinn J, Van BG. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Zeitschrift Für Gastroenterol. (1989) 96:981–8. doi: 10.1016/0016-5085(89)91613-2
47. Song MJ, Dongil P, Jungho P, Hongjoo K, Yongkyun C, Chongil S, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Gastroenterology. (2010) 138:S335. doi: 10.1111/j.1523-5378.2010.00751.x
48. Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. (2002) 32:105–10. doi: 10.1111/j.1574-695X.2002.tb00541.x
49. Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol. (2015) 21:10644–53. doi: 10.3748/wjg.v21.i37.10644
50. Kim TS, Hur JW Yu MA, Cheigh CI, Kim KN, Hwang JK, et al. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot. (2003) 66:3–12. doi: 10.4315/0362-028X-66.1.3
Keywords: Helicobacter pylori, Saccharomyces boulardii, eradication, diarrhea, quadruple therapy
Citation: Zhao Y, Yang Y, Aruna, Xiao J, Song J, Huang T, Li S, Kou J, Huang L, Ji D, Xiong S, Peng W, Xu S and Cheng B (2021) Saccharomyces boulardii Combined With Quadruple Therapy for Helicobacter pylori Eradication Decreased the Duration and Severity of Diarrhea: A Multi-Center Prospective Randomized Controlled Trial. Front. Med. 8:776955. doi: 10.3389/fmed.2021.776955
Received: 14 September 2021; Accepted: 04 October 2021;
Published: 18 November 2021.
Edited by:
Marcello Dallio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Rinaldo Pellicano, Molinette Hospital, ItalyCopyright © 2021 Zhao, Yang, Aruna, Xiao, Song, Huang, Li, Kou, Huang, Ji, Xiong, Peng, Xu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Cheng, Yi5jaGVuZ0B0amgudGptdS5lZHUuY24=; Sanping Xu, eHVzYW5waW5naGFvQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.