- 1State Key Laboratory of Respiratory Diseases, National Clinical Research Center for Respiratory Diseases, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Guangdong Key Laboratory of Vascular Diseases, Guangzhou Medical University, Guangzhou, China
- 3Department of Pulmonary Medicine, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 4Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China
- 5School of Public Health, Guangzhou Medical University, Guangzhou, China
- 6Department of Clinical Medicine, Guangzhou Medical University, Guangzhou, China
Pulmonary hypertension (PH) is a common complication of chronic obstructive pulmonary disease (COPD) and induces increased mortality among COPD patients. However, there are no blood biomarkers to identify PH in COPD. Here, we investigated whether circulating angiogenic factors and cytokines could serve as (a) biomarker (s) for COPD-PH patients. Using Angiogenesis and Cytokine proteome profile array assay, we measured the level of 36 cytokines and 55 angiogenesis-associated proteins in plasma from four COPD patients with PH (COPD-PH) and four COPD patients without PH (COPD), respectively, tissue inhibitor of metalloproteinase 1 (TIMP-1) and thrombospondin 1(TSP-1) were significantly different between the two groups. Enzyme-linked immunosorbent assay (ELISA) was applied to measured TIMP-1 and TSP-1 in a validation cohort (COPD-PH, n = 28; COPD, n = 18), and TIMP-1 was the only factor that was significantly different between COPD-PH and COPD patients (P <0.01). Logistic regression analysis demonstrated that elevated TIMP-1 was an independent risk factor for COPD-PH [odds ratio (OR) = 1.258, 95% CI: 1.005–1.574, P <0.05). Next, we explored the expression level and function of TIMP-1 in human pulmonary arterial smooth muscle cells (hPASMCs) exposed to cigarette smoking extract (CSE, a major etiological factor of COPD). In cultured hPASMCs, CSE treatment increased both TIMP-1 protein level and cell proliferation, and exogenous TIMP-1 (25 ng/mL) treatment inhibited CSE-induced hPASMCs proliferation. Overall, our results indicated that TIMP-1 elevation could serve as a circulating biomarker to diagnose PH among COPD patients, and TIMP-1 elevation in COPD-PH could be adaptive.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible, progressive airflow obstruction. According to the World Health Organization, as of 2019, COPD was the third leading cause of death in both developing and developed countries (1–3). Pulmonary hypertension (PH) is a common complication of COPD and leads to cor pulmonale and even death (4, 5). The presence of PH in COPD (COPD-PH) patients leads to an increased risk of hospitalization, exacerbation (6), and mortality (7), and pulmonary arterial pressure (PAP) is negatively associated with life expectancy among COPD patients (8). Right heart catheterization (RHC), a gold standard of PH diagnosis, is invasive (9) and expensive (10), which makes it not feasible as a screening tool. Therefore, the development of screening tools is urgently demanding in the diagnosis of PH in COPD. Plasma biomarkers can be efficiently measured in an non-invasive manner, serving as an ideal screening tool for diseases. Deranged angiogenic signaling and inflammation contribute to the pathogenesis of PH (11–13), and altered circulating angiogenic factors and cytokines have been reported in pulmonary arterial hypertension patients (14–17). However, the changes in angiogenic factors or cytokines that are altered in PH among COPD patients are unknown. With an attempt to identify diagnostic blood biomarkers for PH in COPD, angiogenesis, and cytokines, proteome profile arrays were performed to screen the factors related to COPD-PH patients, and the identified biomarkers were further verified in a validation cohort.
Materials and Methods
Patients
All the COPD patients in this study were recruited based on the Global Initiative for COPD criteria. Patients with pulmonary arterial systolic pressure (PASP) ≥35 mmHg estimated by a designated Doppler Echocardiography (ECHO) were considered as COPD-PH. Among the COPD patients, 4 without PH (COPD group) and 4 with PH (COPD-PH group) were age- and gender-matched in the screening cohort; and 46 COPD patients were included in the validation cohort, 18 of them were without PH (COPD group), and 28 had PH (COPD-PH group). Subjects were recruited from the First Affiliated Hospital of Guangzhou Medical University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2018-hs-120). All participants signed written informed consent forms before their inclusion in the study.
Proteome Profiler Array Assay
Proteome Profiler assays were performed in the plasma from the screening cohort via Proteome Profiler Human Angiogenesis Array Kit (catalog #ARY007, R&D Systems, Inc., Minneapolis, MN, USA) and Proteome Profiler Human Cytokine Array Kit (catalog #ARY005B, R&D Systems) respectively according to the manufacturer's instructions. The array kits were designed to detect 55 angiogenesis-associated proteins and 36 cytokines in a single experiment by utilizing a sandwich immunoassay. Furthermore, pixel densities of the films were analyzed by Image J software (Image J software, 1.52a National Institutes of Health, Bethesda, MD).
Enzyme-Linked Immunosorbent Assay (ELISA)
Concentrations of Tissue Inhibitor of Metalloproteinase 1 (TIMP-1) and Thrombospondin 1 (TSP-1) in the plasma were measured by Human TIMP-1 Quantikine ELISA Kit (catalog #DTM100, R&D Systems) and Human Thrombospondin-1 Quantikine ELISA Kit (catalog #DTSP10, R&D Systems) according to the manufacturer's instructions. All assays were performed in duplicate. The detection limits were 0.08 ng/mL for TIMP-1 and 0.944 ng/mL for TSP-1, respectively. Results were obtained by first measuring absorbance at 450 nm and then calculating cytokine concentration based on a standard curve.
Preparation of Cigarette Smoking Extract (CSE)
According to a previously described protocol (18), CSE was freshly prepared from two Hongmei brand filtered cigarettes within 30 min before use. The acquired CSE suspension was yellowish with optical density (OD) 0.506 ± 0.008 at 405 nm. The CSE was adjusted to a pH value of 7.4 and filtered through a 0.22 μm filter, and the final product was considered a concentration of 100% for in vitro studies.
Cell Culture
Human Pulmonary Artery Smooth Muscle Cells (hPASMCs) and smooth muscle cell medium (SMCM) were purchased from Sciencell (CA, USA; catalog #3110 and #1101). hPASMCs were placed in a CO2 incubator (5%, Thermo, USA) with a humidified atmosphere containing 5% CO2-95% air at 37°C in complete SMCM containing 5% fetal bovine serum (FBS, Gibco; Grand Island, NY, USA).
For CSE treatment, after cells had grown to 50–60% confluence, the culture media was replaced with SMCM basal culture media supplemented with 0.3% FBS for 12–24 h, and then exposed to 0, 0.125, 0.25, 0.5, 1, 2, 4% of CSE stimuli for 24, 36, 48 h, respectively.
Cell Proliferation
Cell Counting Kit-8 assay (CCK-8, MCE company, New Jersey, USA) was used to detect cell proliferation following the manufacturer's recommendations. In brief, hPASMCs were seeded in 96-well plates at a density of 4 ×103 cells per well and cultured 24 h in SMCM complete medium, then changed as SMCM containing 0.3% FBS for 12 h to allow cell arrest. After that, the cells were subjected to 0, 0.125, 0.25, 0.5, 1, 2, 4% of CSE for 24, 36, 48 h for cell proliferation assay.
Western Blotting
Western blot was performed as previously described (19). Briefly, hPASMCs were washed in PBS and lysed in RIPA buffer supplemented with protease inhibitors on ice for 30 min. The supernatant was collected by centrifugation for 30 min with a speed of 12,000 rpm at 4°C. Protein concentrations were quantified by Bicinchoninic acid (BCA) Protein Assay Kit (catalog # 23227, Thermofisher Scientific) The protein samples were separated on 10% SDS-polyacrylamide gels and electrophoretically transferred onto PVDF membranes. Then the membrane was blocked in 3% non-fat milk in phosphate-buffered saline with Tween (PBST, 0.1% Tween 20) and incubated overnight at 4°C with anti-TIMP-1 primary antibody (1:500 dilution, catalog #A00561, Boster, Wuhan, China) and anti-β-actin antibody (1:2000 dilution, catalog #4695, Santa Cruz, CA, US), followed with secondary antibody incubation at room temperature for 2 h. Blots were developed using immobilon forte western horseradish peroxidase (HRP) substrate (WBLUF0100, Millipore) and then visualized using an enhanced chemiluminescence (ECL) reagent (KeyGen Biotechnology, Nanjing, China) and detected via ECL detection system (Tanon5200, ShangHai, China). The relative levels of immunoreactive proteins were quantified using the ImageJ software.
Statistical Analysis
Continuous variables were presented as mean ± SEM. In studies comparing only two experimental groups, data were analyzed with two-sample independent Student's t-test to determine the significance. The logistic regression was used for univariate and multivariate analyses to test each independent factor for PH prediction. A multivariable logistic regression analysis, using the Forward: LR method (20), as performed using a final model to control potential confounding factors, and the statistical significance was set at the alpha = 0.05 level. OR and 95% CIs were calculated as the summary statistics. Besides, forest plots were generated to demonstrate OR and 95% CIs of the potential baseline prognostic covariates of COPD-PH. The level of statistical significance was taken as P <0.05.
Results
Demographic Data and Clinical Characteristics of the Screening Cohort
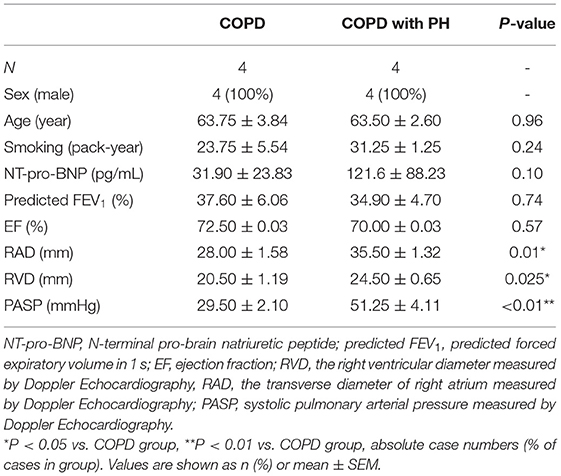
For the eight COPD patients (4 with PH and 4 without PH, respectively) in the screening cohort, their baseline clinical characteristics were described in Table 1. All the COPD patients in the screening cohort were assessed by Doppler Echocardiography (ECHO), and those with PASP ≥ 35 mmHg were verified with Right Heart Catheterization (RHC, meam PAP ≥ 20 mmHg) for PH diagnosis. In the screening cohort, participants were divided into two groups based on PASP determined by ECHO: a group with PASP <35 mm (n = 4, COPD group) and the other group with PASP ≥ 35 mm (n = 4, COPD-PH group). COPD-PH patients had a higher level of right atrium diameter (RAD) (COPD vs. COPD-PH, 28.00 ± 3.16 vs. 35.50 ± 2.64 mm, P <0.05), and right ventricular diameter (RVD) (COPD vs. COPD-PH, 20.50 ± 1.19 vs. 24.50 ± 0.65 mm, P <0.05) measured by ECHO than those without PH. There were no significant differences in age, sex, smoking, NT-pro-BNP, or predicted FEV1 (%) between the two groups (all P > 0.05).
We displayed two representative scanned images of COPD and COPD-PH patients in Figure 1A-a and Figure 1A-b, respectively. Using Proteome Profiler Human Angiogenesis Array Kit, as shown in Figure 1A, with a total of 55 angiogenesis-associated proteins, the following 42 proteins expressions were detected in the plasma of COPD and COPD-PH patients: ANG, Ang-1, Ang-2, Angiostatin, AR, Artemin, TF, CXCL16, DPPIV, EGF, PK1, CD105, Endostati, Endothelin-1, FGF acidic, GDNF, HB-EGF, HGF, IGFBP-1, IGFBP-2, IGFBP-3, IL-1β, Leptin, MCP-1, MMP-8, MMP-9, TSG-14, PD-ECGF, PDGF-AA, PDGF-AB, CXCL4, PlGF, Prolactin, PAI-1, TIMP-1, TIMP-4, TSP-1, TSP-2, uPA, Vasohibin, VEGF, and VEGF-C. Relative protein levels of these factors were showed in Figures 1B–E. Among these factors, the relative expression of Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) (COPD vs. COPD-PH, 0.52 ± 0.28 vs. 1.1 ± 0.26, P <0.05) Figure 1F, and Thrombospondin 1 (TSP-1) (COPD vs. COPD-PH, 0.19 ± 0.19 vs. 0.43 ± 0.11, P <0.05) Figure 1G, were significantly higher in COPD-PH patients. The rest of the factors did not show a significant difference between the two groups.
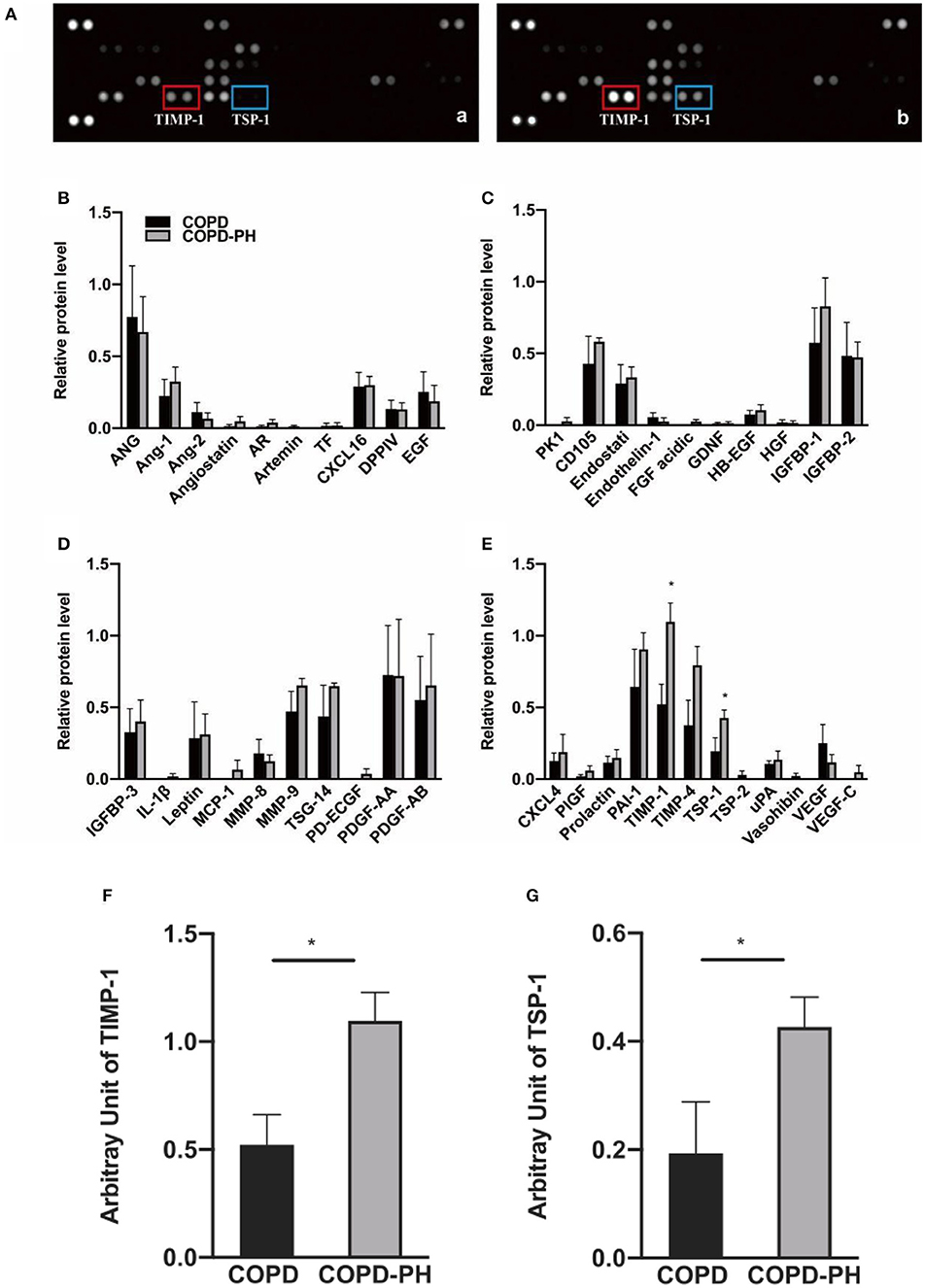
Figure 1. Angiogenesis array data. (A) Representative image of angiogenesis array of the plasma from COPD patients without PH [COPD, (a)] and with PH [COPD-PH, (b)] array blots. The levels of angiogenic factors are determined based on their blotting intensity in duplicates. (B–E) Relative protein level of for angiogenesis-associated protein determined by angiogenesis assay (Data were normalized to three pairs of reference spots). (F,G) Based on two-sample independent Student's t-test analysis, TIMP-1 and TSP-1 were significantly higher in COPD-PH than COPD group. Data were present as mean ± SEM (n = 4). *P <0.05 vs. COPD group.
For cytokine profiles, as shown in Supplementary Figure 2, with a total of 36 cytokines, the following 15 proteins expressions were detected in the plasma of COPD and COPD-PH patients: CCL5/RANTES, CD40 Ligand/TNFSF5, Complement Component C5/C5a, CXCL1/GROα, CXCL10/IP-10, CXCL11/I- TAC, CXCL12/SDF-1, G-CSF, ICAM-1/CD54, IL-1ra/IL-1F3, IL-13, IL-16, IL-18/IL-1F4, MIF, and Serpin E1/PAI-1. However, the difference did not reach statistical significance in the level among any of these cytokines between the two groups (P > 0.05).
Demographic Data and ELISA Results of the Validation Cohort
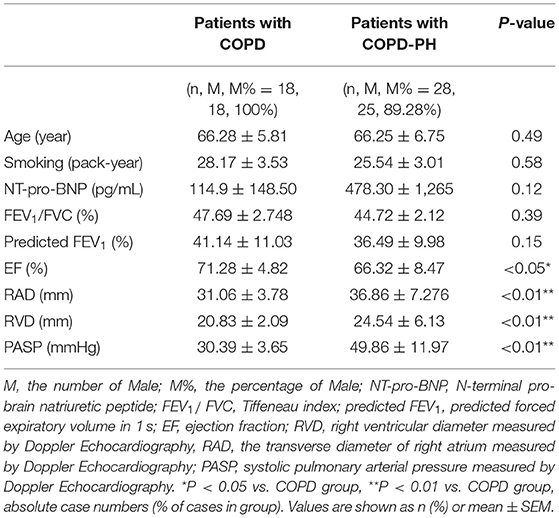
As shown in Table 2, there was no statistical significance in sex, age, and smoking status between the two groups (COPD, n = 18; COPD-PH, n = 28) in the validation cohort. Results of ECHO showed lower ejection fraction (EF) values (COPD vs. COPD-PH, 71.20 ± 4.824 (%) vs. 66.32 ± 8.47 (%), P <0.05), higher PASP (COPD vs. COPD-PH, 30.39 ± 3.65 vs. 49.86 ± 11.97 mmHg, P <0.01), RAD (COPD vs. COPD-PH, 31.06 ± 3.78 vs. 36.86 ± 7.276 mm) and RVD (COPD vs. COPD-PH, 20.83 ± 2.09 vs. 24.54 ± 6.13 mm) in COPD-PH group when compared to COPD group. Besides, there was no statistical significance in predicted FEV1 value or NT-pro-BNP between the two groups.
Results from ELISA of the plasma indicated that in the validation cohort, TIMP-1 level in COPD-PH patients was significantly higher (COPD vs. COPD-PH, 39.40 ± 3.493 vs. 44.48 ± 7.795 ng/mL, P <0.01, Figure 2A). However, the plasma level of TSP-1 is not statistically different between the two groups (COPD vs. COPD-PH, 99.34 ± 5.448 vs. 95.38 ± 12.01 ng/mL, P > 0.05, Figure 2B).
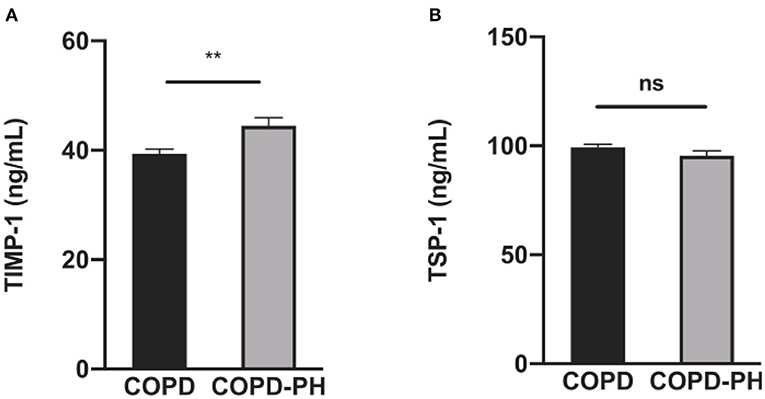
Figure 2. The results of TIMP-1 (A) and TSP-1 (B) between COPD and COPD-PH group in validation cohort by ELISA. The P-value of each factor was obtained from unpaired Student's t-test analysis. **P <0.01 vs. COPD group; ns, no significant statistical difference.
Plasma TIMP-1 Is a Biomarker of PH Among COPD Patients
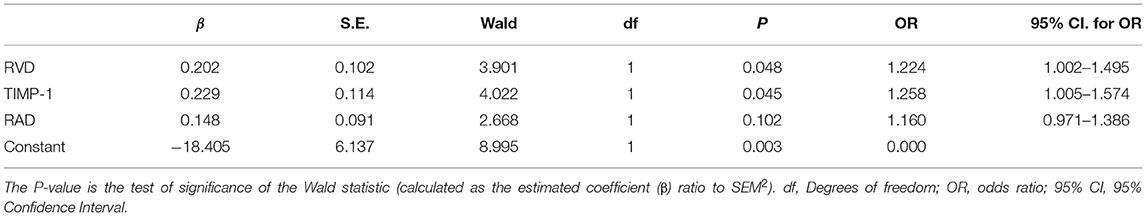
Multivariable analysis based on Logistic Regression Analysis was used to construct a predictive model considering the interaction between various confounding factors and estimate the probability of PH among COPD patients (Table 3 and Figure 3). The multivariable analysis considered age, smoking, sex, NT-pro-BNP level, RAD, and RVD as confounding factors in the verification cohort. Following adjustment for confounding factors, Logistic Regression Analysis demonstrated the elevation of TIMP-1 (OR, 1.258; 95% CI, 1.005–1.574; P <0.05) and RVD (OR, 1.224; 95% CI, 1.002–1.495; P <0.05) as independent predictive factors for the diagnosis of PH among COPD patients.
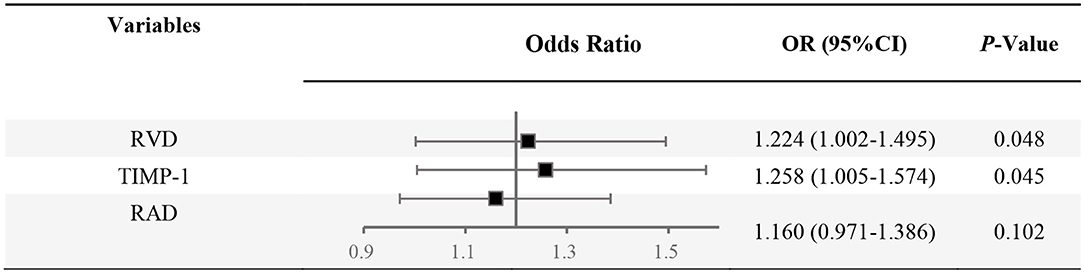
Figure 3. Forest plot of combined analysis on PH identification among COPD. OR, odds ratio; RVD, the right ventricular diameter measured by Doppler Echocardiography; RAD, the transverse diameter of right atrium measured by Doppler Echocardiography.
CSE Increases TIMP-1 and Cell Proliferation, and TIMP-1 Reduces CSE-Induced Cell Proliferation in hPASMCs
As shown in Supplementary Figure 3, hPASMCs proliferation peaked at 36 h under 0.5% cigarette smoke exposure (CSE) treatment among a series of CSE concentrations and time points. Under this condition of CSE treatment, TIMP-1 expression in hPASMCs was significantly upregulated as determined by western blot (P <0.01, Figure 4A), and hPASMCs proliferation was also increased significantly (P <0.01, Figure 4B). In addition, under CSE exposure, TIMP-1 (25 ng/mL) treatment reduced hPASMCs proliferation significantly (P <0.01, Figure 4B).
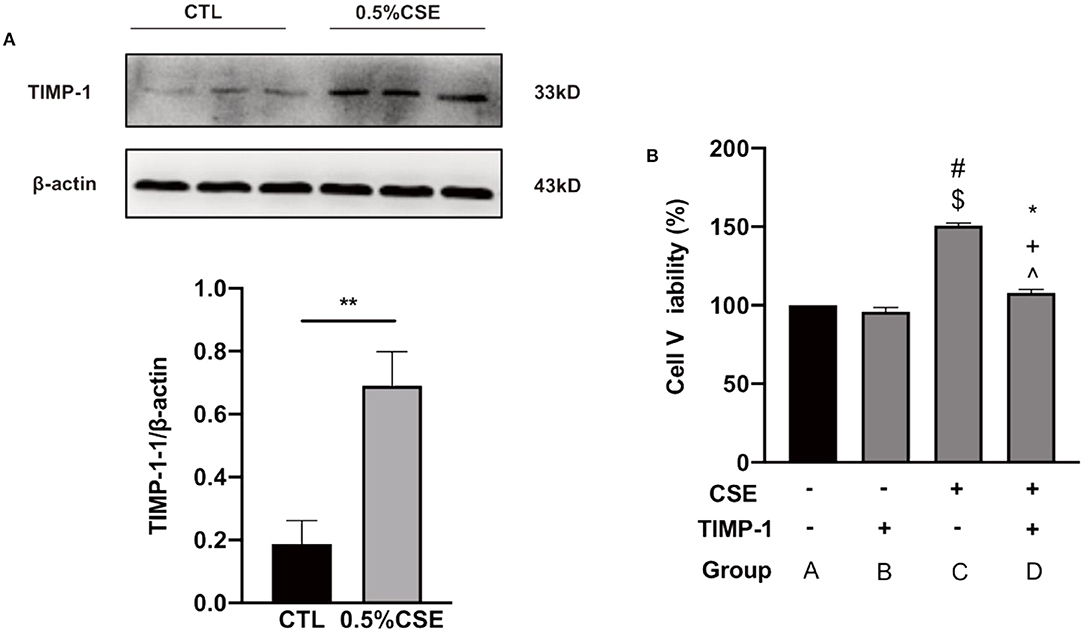
Figure 4. CSE increased TIMP-1 level in cultured hPASMCs, and TIMP-1 decreased hPASMCs proliferation under CSE. (A) Western Blotting indicates 0.5% CSE treatment for 36 h increased TIMP-1 expression in hPASMCs. (B) TIMP-1 attenuated CSE-stimulated hPASMCs proliferation. CSE, Cigarette Smoking Extract; hPASMCs, human Pulmonary Arterial Smooth Muscle Cell, **P <0.01 vs. Control group. TIMP-1, tissue inhibitor of metalloproteinase-1, CSE “+,” with the treatment of CSE; CSE “−,” without treatment of CSE; TIMP-1 “+,” with the treatment of TIMP-1; TIMP-1 “−,” without treatment of TIMP-1; #P <0.01 vs. GroupA, $P <0.01 vs. Group B, *P <0.05 vs. Group A, +P <0.05 vs. Group B, ∧P <0.01 vs. Group C, N = 6.
Discussion
PH is a common complication of COPD, especially in those with severe lung function impairment (4, 8), which may eventually progress to cor pulmonale and even death (21). However, diagnosis of PH among COPD patients is technically challenging. Screening from a total of 55 angiogenesis-associated proteins and 36 cytokines, our study indicated that plasma levels of TIMP-1 could serve as a diagnostic index for PH among COPD. The schematic workflow of the current study is shown in Figure 5.
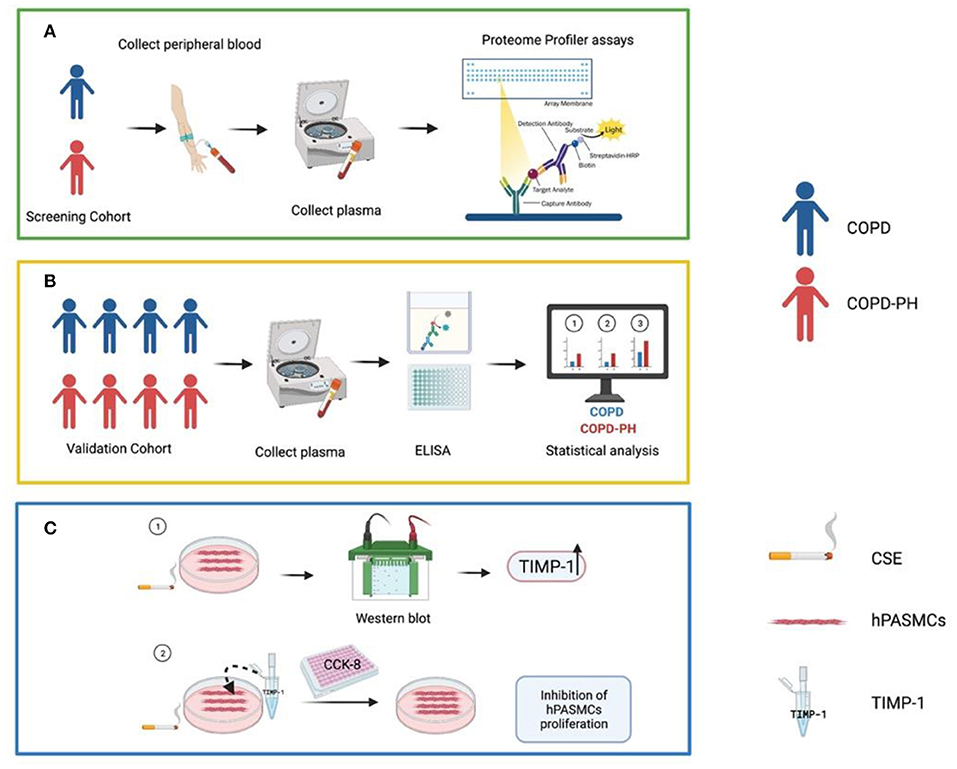
Figure 5. Summary figure of the schematic workflow of the current study. (A) Initial screening of angiogenic factors and cytokines. (B) ELISA verification in validation cohort and related statistical analysis. (C) Involvement of TIMP-1 in the hPASMCs mechanism. COPD, Chronic obstructive pulmonary disease; COPD-PH, Chronic obstructive pulmonary disease associated pulmonary hypertension; CSE, Cigarette smoke exposure; ELISA, Enzyme-Linked Immunosorbent Assay; hPASMCs, Human Pulmonary Artery Smooth Muscle Cells; TIMP-1, Tissue Inhibitor of Metalloproteinases-1. This figure was created using BioRender.
TIMP-1 is a crucial regulator of extracellular matrix degradation (22), and circulating TIMPs have been shown to be altered in several cardiovascular diseases (23). An early study shows that increased TIMP-1 levels is a risk factor of mortality among congestive heart failure patients (24). Recently, it has been demonstrated that PH patients with TIMP-1 elevation have an increased risk for death (25). In COPD patients, TIMP-1 levels are also increased (26, 27), and plasma TIMP-1 concentration is associated with the severity of COPD (27). Although elevated TIMP-1 concentrations have been demonstrated in PH or COPD in several previous studies, the changes of TIMP-1 in COPD patients with PH remain unknown. Here, we indicated that elevated TIMP-1 could be used to diagnose PH among COPD patients for the first time.
Cigarette smoke (CS) exposure is the most common etiological factor of COPD (4, 28–30) and also a risk factor for pulmonary vascular damages, which may lead to PH (31). In consistent, our study demonstrated that CSE treatment increased PASMCs proliferation, a characteristic pathological change of PH, and CSE also increased TIMP-1 in cultured PASMCs. TIMP-1 reduces pulmonary arterial pressure and attenuates pathological pulmonary vascular remodeling in monocrotaline-induced PH rat models. However, the mechanisms of PH development in COPD are different and could be more complicated. Additionally, the COPD-PH animal model has not been reported in previous studies to the best of our knowledge. Therefore, we tested the effects of TIMP-1 in cultured PASMs under CSE and found that TIMP-1 inhibits CSE-induced PASMCs proliferation, which may suggest that TIMP-1 elevation could be protective in COPD-PH patients.
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that play crucial roles in embryonic development, angiogenesis, arthritis, cardiovascular diseases, and cancer (32). MMPs are also involved in angiogenesis by mediating tissue remodeling and penetration of the extracellular matrix (33). The activity of MMPs is mainly inhibited by the presence of endogenous Tissue Inhibitor of Metalloproteinase (TIMPs) (34). Lepetit H's study showed that MMP-TIMPs imbalance (increased TIMP-1 and decreased MMP-3) contributed to smooth muscle cell proliferation in idiopathic pulmonary arterial hypertension (35). Among TIMPs, TIMP-1 preferentially inhibits MMP-9 (36). Zhu et al. demonstrated that MMP-2 and MMP-9 aggravate pulmonary arterial hypertension by increasing migration and proliferation in pulmonary endothelial cells (37), suggesting TIMP-1 may exert protective effects in PH. However, in our current study, elevated TIMP-1 was found in the plasma of COPD-PH patients compared to COPD patients without PH. However, there was no statistically significant difference in MMP-9 between the two groups, suggesting TIMP-1 is simply the biomarker of COPD-PH and did not exert protective effects via reducing MMP-9 in COPD-PH.
In another study, Zhang et al. screened 440 cytokines using the Human Cytokine Antibody Array among 20 COPD and 20 COPD-PH patients and found that MCP-4, CCL28, and CD40 were altered in the serum of COPD patients with PH (38). Together with our findings, it may also suggest multiple factors are involved in PH development in COPD patients. The mechanisms of PH development in COPD could be complicated; future studies are needed for in-depth knowledge of PH diagnosis and treatment among COPD patients.
Several limitations of our study merit description. Firstly, this is a single-center study. To maintain the freshness of blood, we attempted to choose the peripheral blood samples that were collected recently. Although the number of patients is limited, we found that TIMP-1 is a circulating marker of PH in COPD patients. Moreover, as there is no present COPD-PH animal model, we only investigated the role of TIMP-1 in the context of COPD in vitro through CSE- stimulated hPASMCs. Therefore, we believe it is fair to assume that the reported differences in the expression and/or activity of TIMP-1 were rather related to the manifestation of COPD-PH.
Conclusion
Plasma TIMP-1 levels may serve as a non-invasive index to diagnose PH among COPD patients; TIMP-1 inhibits CSE-induced hPASMCs proliferation, suggesting TIMP-1 elevation in COPD-PH patients could be adaptive.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (2018-hs-120). Informed consent was obtained from all subjects involved in the study. In addition, written informed consent has been obtained from the patients to publish this paper.
Author Contributions
WH, TW, and JW conceived and designed the study. CL provided study materials and patients. JL, FL, HL, WL, and BK analyzed and interpreted the data and wrote the paper. DW and HR provided their statistical assistance. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2016YFC1304102), the National Natural Science Foundation of China (81700426, 81970057, 81630004, and 81970046), Guangdong Outstanding Young Scientist Funding (2021B1515020006), Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020013 and ZNSA-202003), State Key Laboratory of Respiratory Disease (SKLRD-MS-201901), and the Science and Technology Program of Guangzhou, China (201904010329).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.774623/full#supplementary-material
Abbreviations
CCK-8, Cell Counting Kit-8 assay; CI, Cardiac Index; CO, Cardiac Output; COPD, Chronic obstructive pulmonary disease; COPD-PH, Chronic obstructive pulmonary disease associated pulmonary hypertension; CS, Cigarette Smoke; CSE, Cigarette smoke exposure; df, Degrees of freedom; ECHO, Doppler Echocardiography; EF, ejection fraction; FBS, fetal bovine serum; FEV1/FVC%, Forced expiratory volume in 1 s (FEV1)/Forced volume vital capacity (FVC) ratio; predicted FEV1, predicted forced expiratory volume in 1 s; hPASMCs, Human Pulmonary Artery Smooth Muscle Cells; mPAP, mean Pulmonary Arterial Pressure; PASP, Pulmonary Arterial Systolic Pressure; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; PH, Pulmonary hypertension; RAD, the transverse diameter of right atrium measured by Doppler Echocardiography; RHC, Right Heart Catheterization; RVD, the diameter of right ventricular measured by Doppler Echocardiography; SaO2, Oxygen saturation in the arterial blood; SMCM, smooth muscle cell medium; TIMP-1, Tissue Inhibitor of Metalloproteinases-1; TSP-1, Thrombospondin 1; 95% CI, 95% Confidence Interval.
References
1. GBDCoD Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
2. Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. (2009) 104:236–44. doi: 10.1161/CIRCRESAHA.108.182014
3. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. (2007) 370:741–50. doi: 10.1016/S0140-6736(07)61377-4
4. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. (2019) 53:1801914. doi: 10.1183/13993003.01914-2018
5. Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. (2008) 32:1371–85. doi: 10.1183/09031936.00015608
6. Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (1999) 159:158–64. doi: 10.1164/ajrccm.159.1.9803117
7. Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. (1972) 286:912–8. doi: 10.1056/NEJM197204272861703
8. Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2005) 172:189–94. doi: 10.1164/rccm.200401-006OC
9. Knight DS, Kotecha T, Martinez-Naharro A, Brown JT, Bertelli M, Fontana M, et al. Cardiovascular magnetic resonance-guided right heart catheterization in a conventional CMR environment - predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Reson. (2019) 21:57. doi: 10.1186/s12968-019-0569-9
10. Dennis A, Michaels AD, Arand P, Ventura D. Noninvasive diagnosis of pulmonary hypertension using heart sound analysis. Comput Biol Med. (2010) 40:758–64. doi: 10.1016/j.compbiomed.2010.07.003
11. Frump AL, Bonnet S, Jesus Perez VAde, Lahm T. Emerging role of angiogenesis in adaptive and maladaptive right ventricular remodeling in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L443–60. doi: 10.1152/ajplung.00374.2017
12. Khandagale A, Aberg M, Wikstrom G, Bergstrom Lind S, Shevchenko G, Bjorklund E, et al. Role of extracellular vesicles in pulmonary arterial hypertension: modulation of pulmonary endothelial function and angiogenesis. Arterioscler Thromb Vasc Biol. (2020) 40:2293–309. doi: 10.1161/ATVBAHA.120.314152
13. Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. (2014) 115:165–75. doi: 10.1161/CIRCRESAHA.113.301141
14. Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J. (2010) 31:2291–300. doi: 10.1093/eurheartj/ehq226
15. Tiede SL, Gall H, Dorr O, dos Santos Guilherme M, Troidl C, Liebetrau C, et al. New potential diagnostic biomarkers for pulmonary hypertension. Eur Respir J. (2015) 46:1390–6. doi: 10.1183/13993003.00187-2015
16. Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. (2009) 34:662–8. doi: 10.1183/09031936.00174908
17. Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. (2010) 122:920–7. doi: 10.1161/CIRCULATIONAHA.109.933762
18. Culpitt SV, Rogers DF, Shah P, Matos CDe, Russell RE, Donnelly LE, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2003) 167:24–31. doi: 10.1164/rccm.200204-298OC
19. Li K, He Z, Wang X, Pineda M, Chen R, Liu H, et al. Apigenin C-glycosides of Microcos paniculata protects lipopolysaccharide induced apoptosis and inflammation in acute lung injury through TLR4 signaling pathway. Free Radic Biol Med. (2018) 124:163–75. doi: 10.1016/j.freeradbiomed.2018.06.009
20. Nam JY, Choe AR, Sinn DH, Lee JH, Kim HY, Yu SJ, et al. A differential risk assessment and decision model for Transarterial chemoembolization in hepatocellular carcinoma based on hepatic function. BMC Cancer. (2020) 20:504. doi: 10.1186/s12885-020-06975-2
21. Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. (1995) 107:1193–8. doi: 10.1378/chest.107.5.1193
22. Grunwald B, Schoeps B, Kruger A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol. (2019) 29:6–19. doi: 10.1016/j.tcb.2018.08.006
23. Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, et al. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. (2004) 25:1509–16. doi: 10.1016/j.ehj.2004.05.029
24. Lieb W, Song RJ, Xanthakis V, Vasan RS. Association of circulating tissue inhibitor of metalloproteinases-1 and procollagen type III aminoterminal peptide levels with incident heart failure and chronic kidney disease. J Am Heart Assoc. (2019) 8:e011426. doi: 10.1161/JAHA.118.011426
25. Tiede SL, Wassenberg M, Christ K, Schermuly RT, Seeger W, Grimminger F, et al. Biomarkers of tissue remodeling predict survival in patients with pulmonary hypertension. Int J Cardiol. (2016) 223:821–6. doi: 10.1016/j.ijcard.2016.08.240
26. Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, et al. Matrix metalloproteinases and TIMP-1 production by peripheral blood granulocytes from COPD patients and asthmatics. Allergy. (2001) 56:145–51. doi: 10.1034/j.1398-9995.2001.056002145.x
27. Uysal P, Uzun H. Relationship between circulating serpina3g, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 and−2 with chronic obstructive pulmonary disease severity. Biomolecules. (2019) 9:62. doi: 10.3390/biom9020062
28. Milara J, Ortiz JL, Juan G, Guijarro R, Almudever P, Martorell M, et al. Cigarette smoke exposure up-regulates endothelin receptor B in human pulmonary artery endothelial cells: molecular and functional consequences. Br J Pharmacol. (2010) 161:1599–615. doi: 10.1111/j.1476-5381.2010.00979.x
29. Finks SW, Rumbak MJ, Self TH. Treating hypertension in chronic obstructive pulmonary disease. N Engl J Med. (2020) 382:353–63. doi: 10.1056/NEJMra1805377
30. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. (2009) 4:435–59. doi: 10.1146/annurev.pathol.4.110807.092145
31. Oakes JM, Xu J, Morris TM, Fried ND, Pearson CS, Lobell TD, et al. Effects of chronic nicotine inhalation on systemic and pulmonary blood pressure and right ventricular remodeling in mice. Hypertension. (2020) 75:1305–14. doi: 10.1161/HYPERTENSIONAHA.119.14608
32. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. (1993) 4:197–250. doi: 10.1177/10454411930040020401
33. van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. (2008) 78:203–12. doi: 10.1093/cvr/cvm102
34. Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. (2002) 21:2245–52. doi: 10.1038/sj.onc.1205291
35. Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, et al. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J. (2005) 25:834–42. doi: 10.1183/09031936.05.00072504
36. Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers. (2014) 6:240–96. doi: 10.3390/cancers6010240
37. Liu Y, Zhang H, Yan L, Du W, Zhang M, Chen H, et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J Mol Cell Cardiol. (2018) 121:36–50. doi: 10.1016/j.yjmcc.2018.06.006
Keywords: TIMP-1, pulmonary hypertension, chronic obstructive pulmonary disease, biomarker, diagnosis
Citation: He W, Liu C, Liao J, Liu F, Lei H, Wei D, Ruan H, Kunwar B, Lu W, Wang J and Wang T (2022) TIMP-1: A Circulating Biomarker for Pulmonary Hypertension Diagnosis Among Chronic Obstructive Pulmonary Disease Patients. Front. Med. 8:774623. doi: 10.3389/fmed.2021.774623
Received: 12 September 2021; Accepted: 18 November 2021;
Published: 25 February 2022.
Edited by:
Ji-Feng Li, Capital Medical University, ChinaReviewed by:
Sebastian Majewski, Medical University of Lodz, PolandEleni Papakonstantinou, Aristotle University of Thessaloniki, Greece
Liping Zhu, Huazhong University of Science and Technology, China
Copyright © 2022 He, Liu, Liao, Liu, Lei, Wei, Ruan, Kunwar, Lu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, aml3MDM3QGhlYWx0aC51Y3NkLmVkdQ==; Tao Wang, dGFvd2FuZ0BnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
 Wenjun He
Wenjun He Chunli Liu1,2†
Chunli Liu1,2† Danmei Wei
Danmei Wei Jian Wang
Jian Wang Tao Wang
Tao Wang