- Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetric and Gynecologic Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
Background: Abdominal aggressive fibromatosis (AF) can be confounded with abdominal wall endomentriosis (AWE) because they share considerable similarity. Because of the different patient prognoses and treatment strategies available, accurate pre-operative diagnosis is important.
Case Presentation: We here report two cases of abdominal masses presenting as periodic changes in tumor sizes, which occurred in correlation with the menstrual cycle. The clinical findings were highly suggestive of AWE. However, the final pathological findings revealed AF. The estrogen receptor and progesterone receptor expressions were negative in the two cases. The differences between the two diseases have been discussed in detail.
Conclusion: A diagnosis of AWE should be scrutinized closely if the patient does not complain of cyclic pain. Fine-needle aspiration cytology is a suitable tool for pre-operative evaluation.
Introduction
If a mass within or adjacent to a cesarean section scar is found in female patients of reproductive age, what is the first consideration? For many doctors, especially gynecologists, abdominal wall endometriosis (AWE) might be the first clinical diagnosis. However, other diseases, such as aggressive fibromatosis, can also occur in similar demographics and locations within the body. Aggressive fibromatosis (AF), also called desmoid-type fibromatosis (DF), is a benign monoclonal fibroblastic proliferation that arises in the deep soft tissues (1). AF is characterized by infiltrative growth and a tendency toward local recurrence but an inability to metastasize (1). AF can be further subdivided into extra-abdominal, abdominal, and intra-abdominal types (2). Both abdominal AF and AWE are inclined to occur in young women with a history of cesarean section and can appear as solitary masses with infiltrative margins, which can render pre-operative clinical diagnosis difficult (2–5). Although there are a few reports in the literature about misdiagnosis of AF as AWE, few of them have discussed the differences between the two diseases in detail. Here, we report two cases of abdominal AF that mimicked AWE and summarize differences between the two entities through literature review in order to guide proper pre-operative diagnosis and foster strong surgical outcome.
Case Presentation
Case One
A 35-year-old woman presented to our hospital with the complaint of a painless mass on the lower abdominal wall for 4 months. This gravida 1 para 1 patient had undergone a cesarean section operation 3 years earlier without any known post-operative complications. The mass was notably enlarged during the menstrual period. A 4-cm, round, solid, painless, fixed mass above the left side of cesarean section incision was detected upon physical examination. Ultrasound revealed a hypoechoic and avascular mass of 4.9 × 3.5 × 2.2-cm in diameter located in the subcutaneous muscle layer below the cesarean section incision. MRI revealed an abnormal signal of slightly hyperintense on T2WI and iso-intense on T1WI with enhancement, located in the left rectus abdominis with a maximum section of 3 × 2.6-cm (Figure 1).
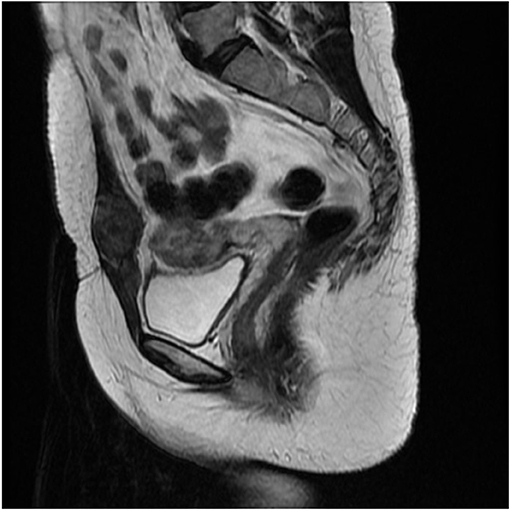
Figure 1. Magnetic resonance imaging (MRI) revealed a 3 × 2.6-cm soft tissue mass in the anterior abdominal wall.
With a pre-operative diagnosis of AWE, a wide surgical excision was then performed. The patient provided written informed consent. At surgery, a 4-cm mass was found in the extraperitoneal musculature, which was completely excised with a 1-cm tumor-free margin. Each layer opened was closed with suture and no mesh was needed. The tumor had no obvious capsule and when sectioned it was beige and firm and showed a swirling pattern. The pathological findings revealed AF. The post-operative course was uneventful. No recurrence occurred during the 2-year follow-up.
Case Two
A 39-year-old woman presented with an abdominal wall mass for 5 months. This gravida 4 para 2 patient had undergone cesarean section operations 12 and 3 years earlier. The abdominal wall mass increased during menstruation and decreased after menstruation. Physical examination revealed a 6 × 5-cm, fixed and firm abdominal mass on the right side of the scar. Ultrasound showed a 6.0 × 3.2 × 1.7-cm hypoechoic irregular mass in the muscular layer with a fuzzy boundary and hemogeneous internal echo. Color Doppler ultrasonography showed several strip blood flow signals inside with the peak systolic velocity (PSV) of 15.5 cm/s and resistance index (RI) of 0.77.
The clinical findings suggested the mass might be AWE. A wide local excision of the mass was performed. The patient provided written informed consent. At surgery, the lesion was found in the rectus abdominis under the fascia. The lesion was resected along the outer edge of the lesion and did not enter the abdominal cavity. The cut section showed areas of beige color. It was firm and solid. Histopathology confirmed desmoid-type fibromatosis. All margins were negative. Immunohistochemical findings were positive for SMA, β-catenin and CD34 (vessel), whereas desmin, Caldesmon and S-100 were negative. Fewer than 3% of cells were Ki67-positive. The patient is on follow-up with no clinical signs of recurrence after 4 years.
Discussion
Aggressive fibromatosis (AF) is a distinct rare entity with an incidence of five to six cases per 1 million of the population per annum and a peak age of 30–40 years (2). The exact etiology is not fully understood. Documented etiological factors are surgical trauma, genetic factors (e.g., familial adenomatous polyposis, FAP), hormonal influences, and pregnancy (1, 2). Abdominal AF can occur in any part of the abdominal wall, mainly in the lower abdomen. It usually presents as a painless, solitary, and fixed mass in the deep layer (2). Abdominal AF usually involves muscle or aponeurosis and presents as a single lesion but rarely can be multifocal (6). At MRI, the lesions appear as soft-tissue masses with heterogeneous internal signal on all sequences with avid enhancement, reflecting their proportionate cellular and fibrous contents (2, 6, 7). One hallmark feature is the presence of linear or sheet intra-lesional hypointense bands on T2-weighted images (6, 8). The fascial tail, described as the linear extension of the tumor along the fascial planes, is also a highly suggestive feature (7, 8). Macroscopically, the section of AF mass is beige, swirling, and firm, and it often infiltrates the adjacent muscles and aponeurosis (1). Histologically, AF is characterized by a fibromatous, benign proliferation of well-differentiated fibroblast and tentacle-like spiculated extensions with infiltrative growth (1). Approximately 85–90% of AF has nuclear positivity for ß-catenin, which is helpful in establishing the diagnosis (1).
Surgery is no longer the first-line treatment of AF because of the variable and unpredictable clinical course. Currently, a conservative wait-and-see policy for 1–2 years is the front-line approach to newly diagnosed patients, irrespective of clinical symptoms (1). In cases that progress, anti-hormonal therapy or surgical resection might be an option (1). Pharmacological options include anti-hormonal therapies such as tamoxifen, non-steroidal anti-inflammatory drugs (NSAIDs), and low-dose chemotherapy (1). It is necessary to remove the tumor with a margin of at least 2–3 cm to make sure a negative margin (9).
The clinical course of AF is variable and often unpredictable. Spontaneous regressions are observed in 20–30% of cases (1). The risk of progression during pregnancy is as high as 40–50% (1). The rate of local recurrence rate of abdominal AF after surgery ranges from <10 to 40% (1, 2).
The differences in clinical characteristics are summarized in Table 1. The two aforementioned cases were suspected for AWE because changes in size that correlate with the menstruation cycle are highly suggestive. However, the two patients did not complain of cyclic pain. A diagnosis of AWE should be scrutinized closely if the patient does not complain of cyclic pain. To our knowledge, this is the first report of periodic changes in size of abdominal AF.
In addition to cyclic pain, there are other clinical features for differentiation. The more common position for AWE is the adipose layer, while abdominal AF usually involves muscle or aponeurosis (6, 8). On MRI, the appearances of AF depend on the proportion of cellular and fibrous contents whereas those of AWE depend on lesion chronicity (2, 6, 7). The most common diagnosis of abdominal wall lesions exhibiting a high T1 signal that does not decrease with fat saturation signal is endometriosis (8). The section of the AWE mass is usually yellowish with areas of hemorrhage or chocolate-like micro-cysts (4), unlike AF. Thus, if the typical section is not found upon the removal of AWE, other diseases should be considered. A pathological examination of frozen samples should be made to make sure the proper resection range is used. Occurrence or progression during pregnancy or coexisting FAP are also suggestive of AF.
AF oncogenesis is associated with estrogen hormonal stimulus (2). To determine the causes of the periodic symptoms in our cases, the levels of expressions of estrogen receptors (ERs) and progesterone receptors (PRs) were here verified. The immunohistochemical testing showed negative results for both ER and PR in the two cases (Figure 2).
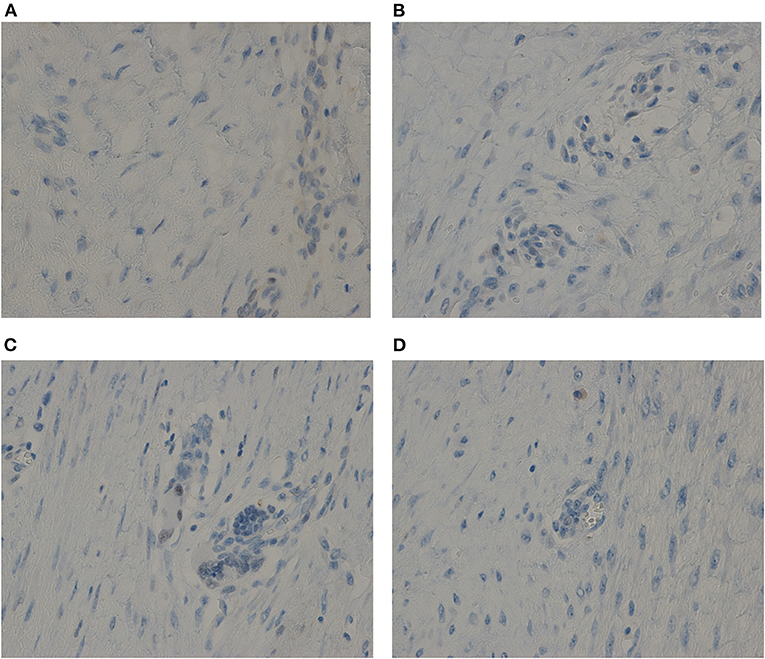
Figure 2. The immunohistochemical testing showed negative results for both ER and PR in the two cases. (A) For ER of Case one. (B) For PR of Case one. (C) For ER of Case two. (D) For PR of Case two. Anti-ER or Anti-PR antibody immunostaining, ×40.
Several differential diagnoses need to be considered, such as disseminated peritoneal leiomyomatosis (DPL), parasitic leiomyoma, abdominal wall metastase and soft tissue sarcomas. DPL is defined as the presence of multiple peritoneal/sub-peritoneal nodules of various sizes composed of bland smooth muscle cells. In one of the largest cohorts of DPL, 29% of DPL and 60% of malignant DPL had abdominal wall involvement (11). Parasitic leiomyoma is a rare complication of power morcellation following laparoscopic myomectomy or hysterectomy, in which the fragment of myoma may be trapped somewhere along the trocar tract in the abdominal wall (12). Abdominal wall metastasis comprise tumors that reach the abdominal wall by implantation, direct invasion and metastasis. Imaging appearances are usually non-specific, often resembling other sites of primary disease (8). Implantation cancers have been reported to occur in 1.18% of patients with gynecological malignancies after laparoscopy (13). Soft tissue sarcomas usually occur later in life, with a median age at presentation of ~50 years. Fixation to underlying structures is suggestive of a soft-tissue sarcoma (14). Therefore, patients with abdominal wall masses should be referred to specialist centers benefiting from multidisciplinary teams experienced in the management of soft tissue tumors.
Conclusion
AF and AWE are important conditions that should be considered in the differential diagnosis of masses located at cesarean section scars. Cyclic pain is an important differential point. Imaging is a helpful tool for diagnosis but its value is limited. In cases without typical manifestation, fine-needle aspiration cytology can help to prevent mistakes. Furthermore, a multidisciplinary team should be recommended.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HS, HL, QF, and JL diagnosed the patients. YW did the immunohistochemical test. XC wrote the manuscript. All authors revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the patients for agreeing and providing their case history.
References
1. Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. (2017) 28:2399–408. doi: 10.1093/annonc/mdx323
2. Couto Netto SD, Teixeira F, Menegozzo CAM, Leão-Filho HM, Albertini A, Ferreira FO, et al. Sporadic abdominal wall desmoid type fibromatosis: treatment paradigm after thirty two years. BMC Surg. (2018) 18:37. doi: 10.1186/s12893-018-0367-6
3. Grigore M, Socolov D, Pavaleanu I, Scripcariu I, Grigore AM, Micu R, et al. Abdominal wall endometriosis: an update in clinical, imagistic features, and management options. Med Ultrason. (2017) 19:430–7. doi: 10.11152/mu-1248
4. Cărăuleanu A, Popovici RM, Costea CF, Mogoş RA, Scripcariu DV, Florea ID, et al. Abdominal wall endometriosis versus desmoid tumor-a challenging differential diagnosis. Rom J Morphol Embryol. (2020) 61:45–50. doi: 10.47162/RJME.61.1.05
5. Rindos NB, Mansuria S. Diagnosis and management of abdominal wall endometriosis: a systematic review and clinical recommendations. Obstet Gynecol Surv. (2017) 72:116–22. doi: 10.1097/OGX.0000000000000399
6. Fiore M, MacNeill A, Gronchi A, Colombo C. Desmoid-type fibromatosis: evolving treatment standards. Surg Oncol Clin N Am. (2016) 25:803–26. doi: 10.1016/j.soc.2016.05.010
7. Khanna M, Ramanathan S, Kambal AS, Al-Berawi M, Yadav S, Kumar D. Multi-parametric (mp) MRI for the diagnosis of abdominal wall desmoid tumors. Eur J Radiol. (2017) 92:103–10. doi: 10.1016/j.ejrad.2017.04.010
8. Bashir U, Moskovic E, Strauss D, Hayes A, Thway K, Pope R, et al. Soft-tissue masses in the abdominal wall. Clin Radiol. (2014) 69:e422–31. doi: 10.1016/j.crad.2014.06.006
9. Liu X, Zong S, Cui Y, Yue Y. Misdiagnosis of aggressive fibromatosis of the abdominal wall: a case report and literature review. Medicine (Baltimore). (2018) 97:e9925. doi: 10.1097/MD.0000000000009925
10. Khan Z, Zanfagnin V, El-Nashar SA, Famuyide A O, Daftary GS, Hopkins MR. Risk factors, clinical presentation, and outcomes for abdominal wall endometriosis. Minim Invasive Gynecol. (2017) 24:478–84. doi: 10.1016/j.jmig.2017.01.005
11. Rosati A, Vargiu V, Angelico G, Zannoni GF, Ciccarone F, Scambia G, et al. Disseminated peritoneal leiomyomatosis and malignant transformation: a case series in a single referral center. Eur J Obstet Gynecol Reprod Biol. (2021) 262:21–7. doi: 10.1016/j.ejogrb.2021.05.006
12. Chan ACK, Chiu JHF, Chan YHY. Parasitic leiomyoma in the anterior abdominal wall. ANZ J Surg. (2020) 90:E52–3. doi: 10.1111/ans.15220
13. Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. Radiographics. (2011) 31:393–408. doi: 10.1148/rg.312105080
Keywords: abdominal wall mass, aggressive fibromatosis, differential diagnosis, endometriosis, periodic symptoms
Citation: Chen X, Wang Y, Liu H, Shi H, Fan Q and Lang J (2021) Case Report: Two Cases of Abdominal Aggressive Fibromatosis That Mimicked Abdominal Wall Endometriosis and Review of Literature. Front. Med. 8:774235. doi: 10.3389/fmed.2021.774235
Received: 11 September 2021; Accepted: 05 November 2021;
Published: 02 December 2021.
Edited by:
Andrea Rosati, Agostino Gemelli University Polyclinic, ItalyReviewed by:
Salim Alfred Bassil, Al-Arz Hospital, LebanonSvend Lindenberg, Copenhagen Fertility Center, Denmark
Copyright © 2021 Chen, Wang, Liu, Shi, Fan and Lang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyuan Liu, bGl1aGFpeXVhbnB1bWNAMTI2LmNvbQ==
 Xin Chen
Xin Chen Haiyuan Liu
Haiyuan Liu