- 1Department of Nuclear Medicine, Xiangya Hospital, Central South University, Changsha, China
- 2Key Laboratory of Biological Nanotechnology, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders (XIANGYA), Xiangya Hospital, Central South University, Changsha, China
Purpose: A meta-analysis was conducted to investigate the value of the volume parameters based on somatostatin receptor (SSTR)-positron emission tomography (PET) in predicting the prognosis in patients with neuroendocrine tumors (NETs).
Material: PUBMED, EMBASE, Cochrane library, and Web of Knowledge were searched from January 1990 to May 2021 for studies evaluating prognostic value of volume-based parameters of SSTR PET/CT in NETs. The terms used were “volume,” “positron emission tomography,” “neuroendocrine tumors,” and “somatostatin receptor.” Pooled hazard ratio (HR) values were calculated to assess the correlations between volumetric parameters, including total tumor volume (TTV) and total-lesion SSTR expression (TL-SSTR), with progression-free survival (PFS) and overall survival (OS). Heterogeneity and subgroup analysis were performed. Funnel plots, Begg's and Egger's test were used to assess possible underlying publication bias.
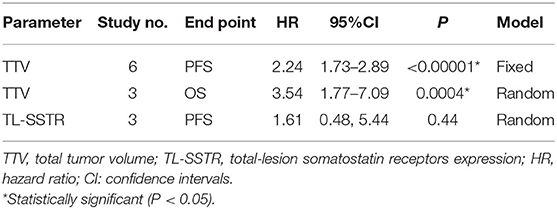
Results: Eight eligible studies involving 593 patients were included in the meta-analysis. In TTV, the pooled HRs of its prognostic value of PFS and OS were 2.24 (95% CI: 1.73–2.89; P < 0.00001) and 3.54 (95% CI, 1.77–7.09; P = 0.0004), respectively. In TL-SSTR, the pooled HR of the predictive value was 1.61 (95% CI, 0.48–5.44, P = 0.44) for PFS.
Conclusion: High TTV was associated with a worse prognosis for PFS and OS in with patients NETs. The TTV of SSTR PET is a potential objective prognosis predictor.
Advanced in Knowledge
The volume parameters based on SSTR PET can provide additional value for the prognosis of neuroendocrine tumors.
Introduction
Neuroendocrine tumors (NETs) are a group of highly heterogeneous neoplasm originating from neuroendocrine cells and it can occur in different organs. The emergence of diagnostic technologies increases early-stage NETs and the detection rate of metastases, raising its incidence and prevalence (1). However, in patients with the same tumor stage and grade, the outcome of disease and survival of NET patients vary greatly (2, 3). Therefore, identifying the prognostic markers is crucial for the management of patients with NETs. Some studies showed that morphological imaging is of limited value in predicting the survival, disease progression, and treatment effects of NETs (4, 5). Several widely-studied diagnostic biomarkers, especially chromogranin A (CgA), has been widely studied. Its plasma level is affected by many factors including the use of proton pump inhibitors (6, 7), but its prognostic utility is still controversial (8, 9).
Somatostatin receptors (SSTRs) are expressed in most NET cells, particularly type 2, and is an ideal target for imaging and therapy method (10). SSTR-mediated imaging is considered to be more accurate than SSTR immunostaining in determining individual prognosis (11). SSTR PET imaging is considered a better imaging method than SSTR scintigraphy using 111In-octreotide due to its higher spatial resolution, higher image quality, and higher lesion detection rate (12). 68Ga-DOTA-peptides can be used to reflect the expression of SSTR, especially in well-differentiated NETs (WD-NETs). High maximum standardized uptake value (SUVmax) is associated with a lower grade, better progression-free survival (PFS), and higher responsiveness to peptide receptor radionuclide therapy (PRRT) (13, 14). A meta-analysis by Lee and Kim (15) showed that the SUVmax of 68Ga-SSA is an important prognostic parameter for NETs patients. Low SUVmax is associated with the high risk of disease progression and mortality.
However, SUVmax reflects the value of a single voxel but does not represent the entire tumor. The volume parameters derived from PET in predicting the prognoses and monitoring the treatment can directly estimate systemic tumor burden, such as metabolic tumor volume (MTV) and total disease glycolysis (TLG), based on 2-deoxy-2-(18F) fluoro-D-glucose (18F-FDG) (16–19). However, well-differentiated NETs (WD-NETs) do not usually show high 18F-FDG uptake (20). SSTR-based PET/CT may be suitable for predicting the prognosis of WD-NETs patients. However, there are conflicting results regarding the prognostic value of volumetric parameters based on SSTR-PET in NETs (21, 22).
Therefore, we performed this meta-analysis to analyze the predictive value of volumetric parameters based on SSTR-PET for survival outcome in patients with NETs.
Materials and Methods
The preferred reporting items for systematic reviews and meta-analyses (PRISMA statement) guidelines were used to perform this meta-analysis (23).
Data Search and Study Selection
We performed a systematic search of PUBMED (to May 2021), EMBASE (to May 2021), Web of Science (to May 2021), and Cochrane (to May 2021) for English publications. The terms were as follows: (“neuroendocrine tumors” or “neuroendocrine tumor” or “tumor neuroendocrine” or “tumors neuroendocrine” or “neuroendocrine”) and (“PET”) or (“positron emission tomography”) and (“somatostatin receptor” or “SSTR”) and (“volume” or “volume-based parameters” or “tumor burden” or “tumor volume” or “volumetrical parameter”) and (“prognos*” or “predict*” or “Survival” or “outcome” or “PFS” or “OS” or “progress free survival” or “overall survival”). All searches were limited to human studies.
The inclusion criteria were studies using SSTR-based PET as an imaging tool, including volumetric parameters [total tumor volume (TTV) or total-lesion SSTR expression (TL-SSTR)] for whole body lesions and reported survival data. Reviews, abstracts, case reports, and editorial materials were excluded. Two authors independently searched and screened the eligible articles. A consensus resolved any discrepancies.
Data Extraction and Quality Assessment
Data were extracted from the enrolled studies independently by two reviewers and the following information was recorded: first author, publication year, country, patient number, tumor grade, tumor site, radiotracer used, treatment after PET/CT scans, reported survival, PET volumetric parameters, and cut-off values of volumetric parameters.
Two reviewers independently used the quality in prognostic studies (QUIPS) tool to evaluate the quality of the included studies (24). The tool assesses the risk of bias in six domains including study participation, study attrition, measurement of prognostic factors, measurement of outcome, study confounding, and statistical analysis and reporting. Consensus was reached through discussion.
Statistical Analysis
The primary outcome was PFS, including disease-free survival, recurrence-free survival, and event-free survival as the main outcome, and also the time interval from the date of starting therapy to the date of recurrence or metastasis. The secondary endpoint was overall survival (OS), defined as the time interval from the start of therapy to death from any cause. The effect of TTV or TL-SSTR on PFS and OS was measured by the effect size of the hazard ratio (HR). PFS or OS data were extracted using methods suggested in previous research (25). Univariate HR and 95% confidence intervals (CI) were extracted for each study, if provided by the author. If not, we used the Engauge Digitizer (http://markummitchell.github.io/engauge-digitizer/) to determine the survival rate according to the Kaplan–Meier curve to reconstruct HR estimate and its variance, assuming that patients were censored at a constant rate during the follow-up. Heterogeneity between studies was assessed by χ2 test and I2 statistics described by Higgins et al. (26). When I2 ≤ 50% and Cochran Q was P ≥ 0.1, a fixed effects model was used; when I2 > 50% or Cochran Q is P < 0.1, the random effect model was used. Subgroup analyses were performed according to the tumor grade and type of radiotracer. Further, funnel plots Begg's and Egger's test were performed to assess for any publication bias (27). Meanwhile, we performed the sensitivity analysis for prognosis by omitting each study to assess the influence of an individual study on the whole meta-analysis. P-values < 0.05 were considered statistically significant. Data from each study were analyzed using Review Manager (RevMan, Version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata Version 15.0 (College Station, TX).
Results
Study Characteristics
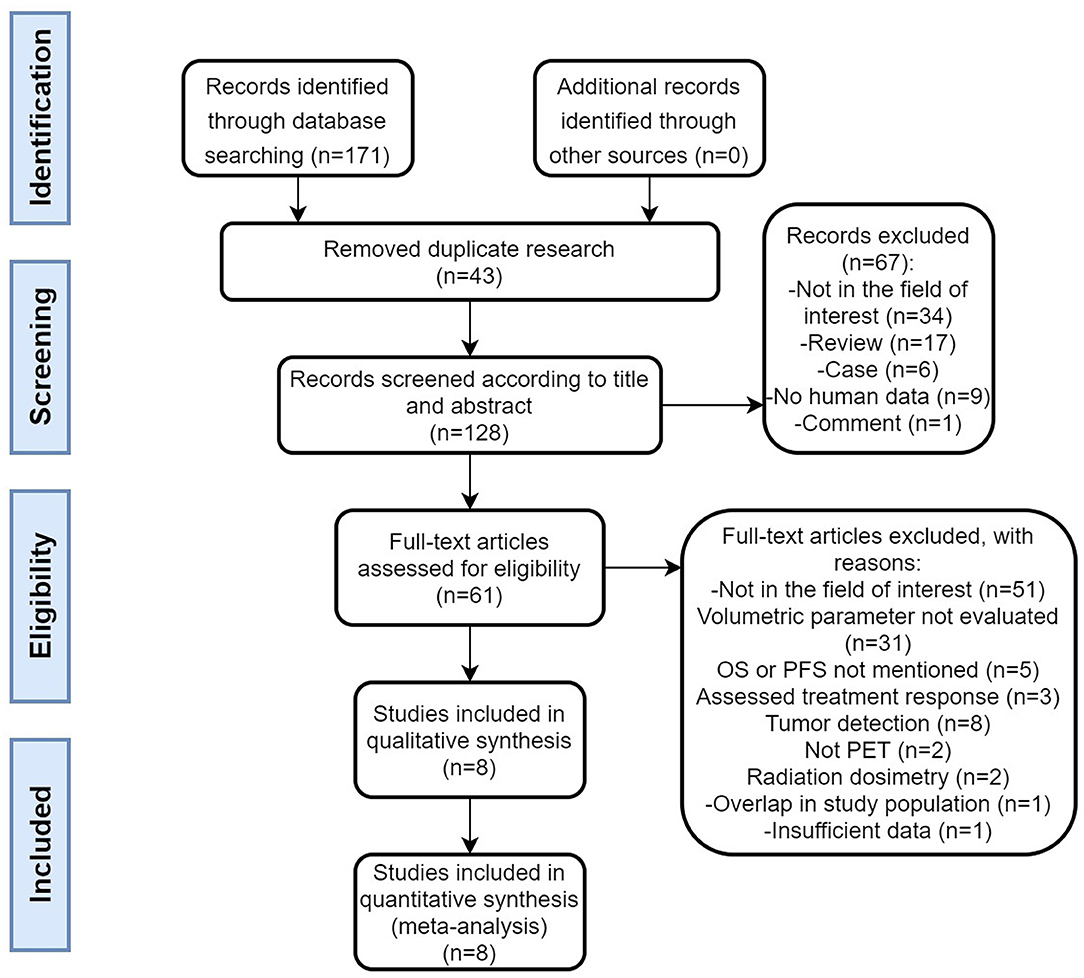
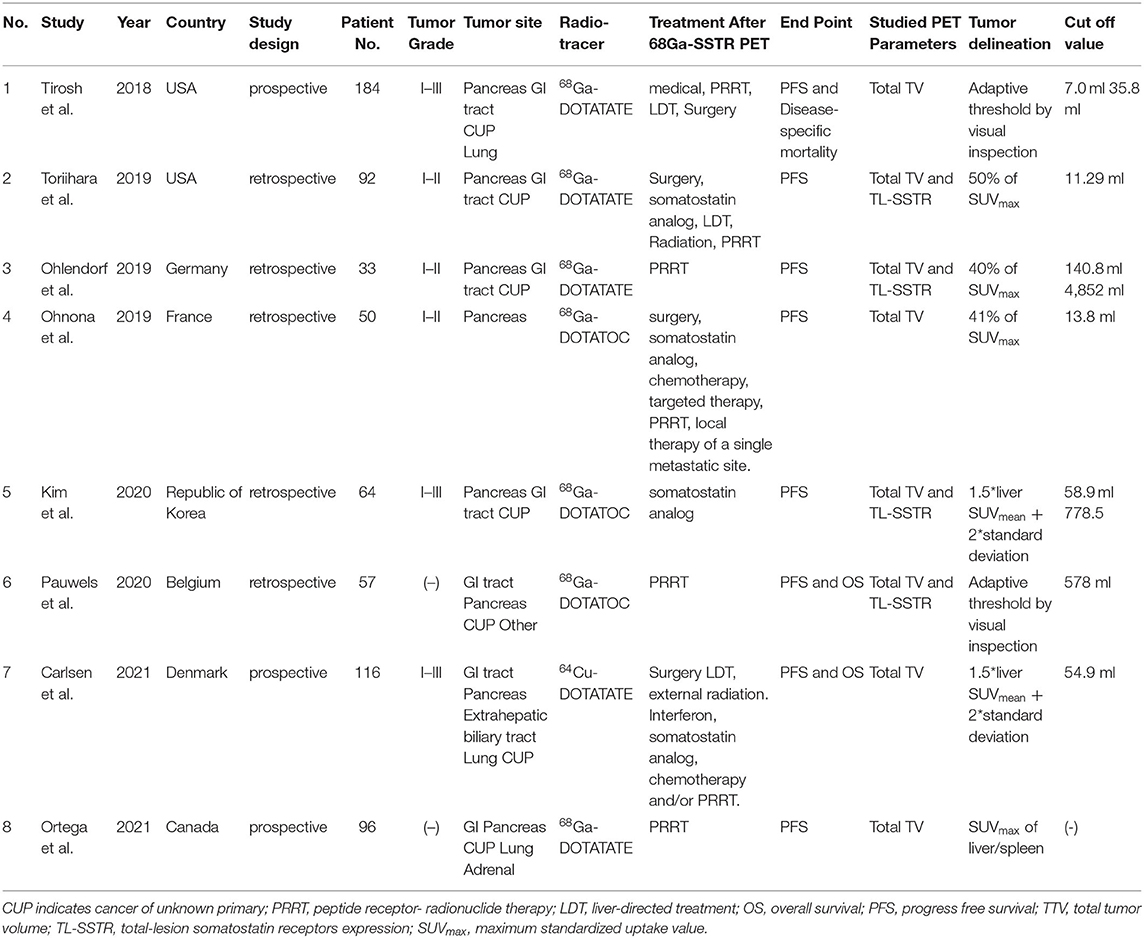
A flow chart of the data search and selection is presented in Figure 1. A total of eight studies involving 593 patients were included in our meta-analysis. Five studies (21, 22, 28–30) were retrospective and three studies (31–33) were a prospective design. According to the WHO grade, three researches (22, 29, 30) included well-differentiated NETs (grade 1 and/or 2). Three studies had heterogeneous populations containing all grades (21, 31, 33) and the remaining two studies did not clearly state the grade of the enrolled patients (28, 32). All the eight studies included pancreas origin NETs and seven studies enrolled gastric intestinal (GI) tract origin NETs, including the stomach or/and midgut or/and rectum (21, 22, 28, 29, 31–33). Seven studies had other site origin NETs such as the lung, extrahepatic biliary tract, adrenal, and cancer of unknown primary origin (21, 22, 28, 29, 31–33).
The characteristics of the included study are shown in Table 1. From them, four studies used 68Ga-DOTATATE (22, 29, 31, 32), three studies used 68Ga-DOTATOC (21, 28, 30), and one study (33) used 64Cu-DOTATATE for PET imaging. The parameters included TTV in eight studies and TL-SSTR in two studies (21, 29). Seven studies (21, 22, 29–33) analyzed the prognostic value of TTV regarding PFS, and three studies further evaluated the relationship between TTV and OS (or disease-specific mortality) (28, 31, 33). Four studies reported the relationship between PFS and TL-SSTR (21, 22, 28, 29). Six threshold methods were applied for the measurement of TTV and TL-SSTR of whole-body lesions (Table 1). Cutoff value of TTV ranged from 7 to 578 ml, and the cutoff value of TL-SSTR in PET in two studies were 778.5 and 4,852 ml, respectively.
Quality Assessment
According to the QUIPS tool quality assessment results, four studies (22, 30, 31, 33) had a moderate risk selection bias because they did not report whether the study population was consecutively selected, and two studies (21, 30) had high selection bias due to the relatively small number of cases enrolled in the group. All included studies showed a low risk of attrition bias. Regarding the measurement of prognostic factors, four studies (21, 29–31) showed a higher risk of bias due to the dependence on the cutoff value of the data, while two studies showed a moderate risk of bias because it was not mentioned whether blinded-manner was used in the measurement. For outcome measurement, seven studies (21, 22, 28–32) showed a moderate risk of bias because it was not clear whether the outcome measurement was performed without prognostic factors or the method used for the outcome measurement was unclear.
Regarding confounding bias, two studies (29, 31) showed high risk due to the lack of multivariate analysis. One study (28) showed moderate risk because grade was not considered. In terms of statistical analysis, two studies (22, 32) showed a higher risk of bias because the study included all variables that might be affected by multicollinearity into the multiple regression. In general, the results of the QUIPS tool indicated that the overall quality of the included studies was moderate (Supplementary Table 1).
Prognostic Value of TTV and TL-SSTR on PFS and OS
The effect of TTV on PFS was analyzed using seven studies. However, in one study (32), the study was omitted because HR could not be combined using continuous variables, while the other six studies were combined because all HR used binary variables. The combined HRs of 2.24 (95% CI: 1.73–2.89) was given a I2 of 0% using a fixed-model, showing a correlation between TTV and PFS (P < 0.00001) (Table 2; Figure 2). Also, we conducted sensitivity analysis (Supplementary Figure 1) to further estimate the impact on the combined HRs.
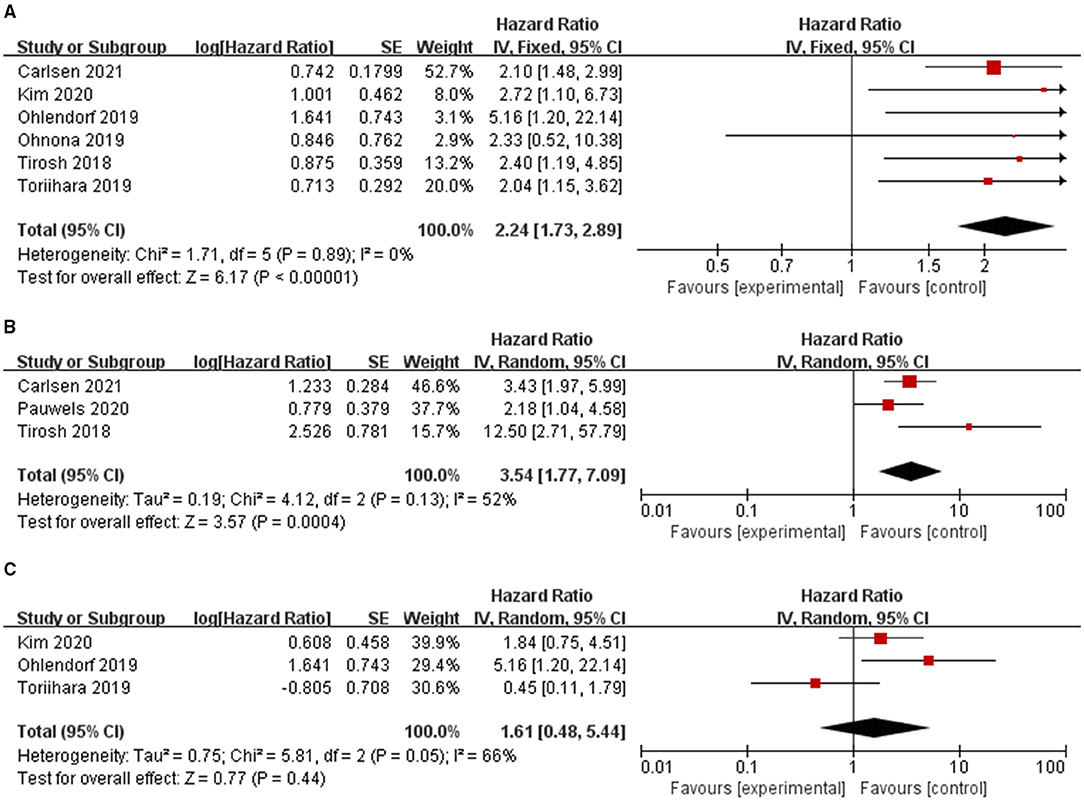
Figure 2. Forest plot results of the PFS (A) and OS (B) based on the total tumor volume and PFS based on the total tumor expressing SSTR (C).
The effect of TTV on OS was analyzed using three studies. The combined HR was 3.54 with statistical significance (95% CI, 1.77–7.09; P = 0.0004). Heterogeneity was moderate (χ2 = 4.12, P = 0.13; I2 = 52%). The combined HRs were found to be stable, suggesting no individual study significantly affected the results (Supplementary Figure 1).
The effect of TL-SSTR on PFS was analyzed using three studies (21, 22, 29). A random-effects model was used and the pooled HR was 1.61 (95% CI, 0.48–5.44, P = 0.05; I2 = 66%, Figure 2; Table 2) with significant heterogeneity. The results showed no statistically significant correlations with PFS and TL-SSTR (P = 0.44).
Subgroup Analysis
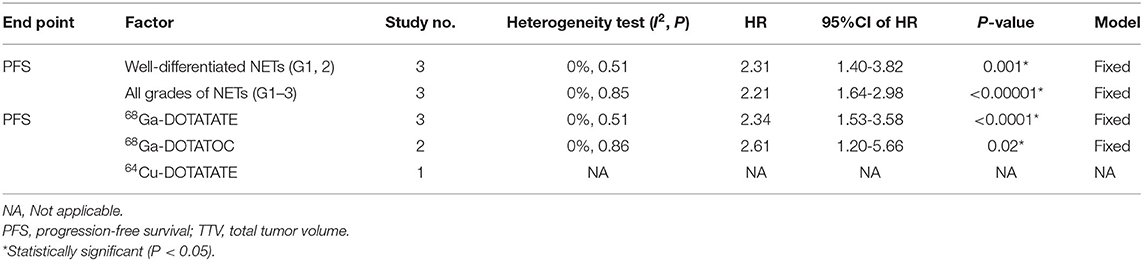
Subgroup analysis was performed to the tumor grade and type of radiotracer. Since the research on PFS based on TL-SSTR and the research on OS by TTV are relatively small, we only performed subgroup analysis on PFS based on TTV (Table 3). Among studies of TTV on PFS, no obvious heterogeneity was found between the studies on well-differentiated NETs (G1/2) (HR: 2.31, 95%CI: 1.40–3.82; P = 0.001) and studies on all grades of NETs (HR: 2.21, 95%CI: 1.64–2.98; P < 0.00001) (I2 = 0%, P = 0.88). Also there is no statistical difference between different imaging agents for predicting PFS (I2 = 0%, P = 0.88).
Publication Bias
Begg's and Egger's tests were used to assess publication bias. The funnel plot and P-value estimation indicated no publication bias for TTV on PFS and OS, as well as for TL-SSTR on PFS (Figure 3).
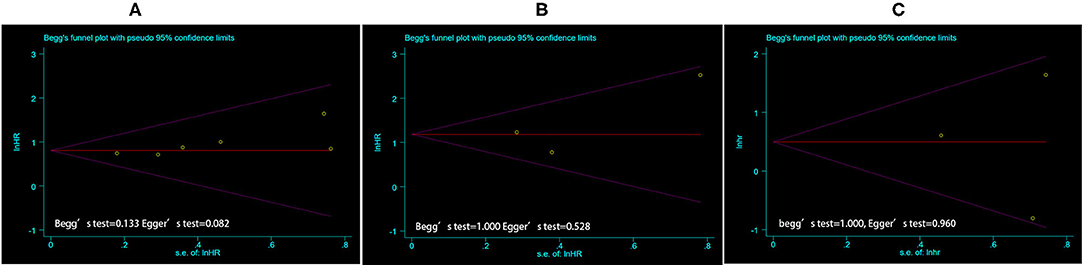
Figure 3. The funnel plot of publication bias estimates the results of PFS (A) and OS (B) based on TTV, and PFS based on TL-SSTR (C) in the meta-analysis. Egger's test and Begg's test were used for statistical analysis, where the P < 0.05 was considered as significant. PFS, Progress free survival; OS, overall survival; TTV, total tumor volume; TL-SSTR, total-lesion somatostatin receptors expression.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to evaluate the prognostic value of volume-based parameters of SSTR PET/CT in NETs. The volumetric parameter based on SSTR PET is useful in predicting PFS. Subgroup analysis reveals that tumor grade and radiotracers may not affect the prognosis.
18F-FDG is the most common PET imaging agent, which can non-invasively assess tumor glucose metabolism and proliferation (34, 35). 18F-FDG PET can be used not only for diagnosis and staging, but also for assessing the proliferative activity and malignancy of tumors. Studies have shown that 18F-FDG may also reflect the prognosis of many tumors, including NET (36–38). A meta-analysis based on 18F-FDG PET/CT showed that MTV as a volumetric parameter of 18F-FDG PET may be an independent prognostic factor for survival (39). However, none of the studies we included had 18F-FDG PET volume parameters for predictive evaluation of prognosis. Although it is not clear whether volumetric parameters based on SSTR PET have better prognostic value than volumetric parameters based on FDG (MTV and TLG) in this study, tumor volume and total tumor expressing SSTR based on SSTR-PET as prognostic biomarkers of NETs have unique advantages compared with MTV or TLG. On the one hand, SSTR2 was an independent prognostic marker in NETs (11), and tumor volume based on SSTR was also correlated with PFS and OS (40). On the other hand, these SSTR-based volume parameters can better reflect the SSTR situation in entire tumors. In the future, we expect to directly compare the ability of 18F-FDG and SSTR PET parameters to predict prognosis through prospective studies.
In this review, higher TTV based on SSTR-PET showed shorter PFS and OS. Although the study of Ortega et al. (32) did not include the meta-analysis, the study still suggests that higher TTV is associated with a worse prognosis. Of six studies (21, 22, 28, 30, 32, 33) in which multivariate analysis for PFS was performed, four out of (22, 30, 32, 33) six were prognostic markers for PFS. Two out of (30, 32) three studies showed that the TTV were prognostic markers for OS. However, TL-SSTR was not significantly related to the prognosis in our study. Only Ohlendorf et al. (29) showed TL-SSTR was associated with PFS. The author believed that the difference may come from the different methods of tumor burden measurement and the samples of enrolled patients.
Heterogeneity was detected in this meta-analysis. In pooled data, significant heterogeneity was found for TTV based on SSTR-PET in predicting PFS. After excluding the study of Ortega et al. (32), the results of the overall estimated values aggregated by PFS reduced heterogeneity (I2, from 87 to 0%) with a HR of 2.24 (95% CI: 1.73–2.89). This may be due to the different tumor volume threshold, which should be discussed in a prospective study. Further analysis found that tumor grade revealed that the TTV of SSTR-PET could predict PFS and OS of all grades of NETs. Since the NET grade depends on the biopsy site, and the heterogeneity of NETs is high, the volume parameter may be more conducive to predicting the prognosis, but it still needs further research to confirm. Additionally, we also performed subgroup analysis of radiotracer types. Subgroup analysis found that the use of single 68Ga-DOTANOC and 68Ga-DOTATATE showed prognostic value. As we all know, 68Ga-DOTANOC, which binds specifically to sst2, sst3, and sst5 (41), has ten-times lower sst2 affinity than the sst2-selective tracer 68Ga-DOTATATE (42). A study has shown that 68Ga-DOTANOC performed better in detecting liver metastasis and had a higher tumor-to-background ratio in liver lesions due to the broader SSTR-binding profile (43). However, another study showed that 68Ga-DOTATATE detected more liver lesions, mainly due to a higher lesion uptake (44). Therefore, whether different radiotracers have a significant impact on the prognosis of tumor burden remains to be further studied.
To the best of our knowledge, this is the first meta-analysis to evaluate the prognostic value of volumetric parameters in the SSTR-PET in NETs. However, due to limited literature, it is difficult to directly compare the HRs between SUVmax and the volume parameter. In our study, volumetric parameters based on SSTR-PET were independent prognostic markers in three studies (29–31) of eight. SUV was found to be an independent prognostic marker in only one study (28) of eight.
This study has several limitations. Firstly, there were only three studies involving OS, and few studies involving TL-SSTR. Secondly, there were significant differences in study design, image analysis, cutoff value, sample sizes, and patient selection among the studies included in the current meta-analysis, leading to publication bias. Thirdly, due to the limited studies we enrolled, we cannot evaluate the best cut-off value of tumor burden parameters for prognostic prediction under the same primary site, treatment, and course of disease. We look forward to further research in future large-sample prospective studies.
Conclusion
The TTV of SSTR-PET is a significant prognostic parameter in NETs patients. The high TTV is associated with an increased risk of disease progression and mortality, whether it is a well-differentiated NET group or a NET group of all grades. In the future, the TTV of SSTR-PET could be used as a potential predictor of prognosis in patients with NETs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JH was responsible for experimental design, experimental analysis and thesis writing. YY and NC were responsible for literature retrieval, data screening, and article revision. DC and SH were responsible for the guidance and review of the thesis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 91859207).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.771912/full#supplementary-material
References
1. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
2. Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. (2015) 121:589–97. doi: 10.1002/cncr.29099
3. Zandee WT, Herder WWde. The evolution of neuroendocrine tumor treatment reflected by ENETS guidelines. Neuroendocrinology. (2018) 106:357–65. doi: 10.1159/000486096
4. Schmid-Tannwald C, Schmid-Tannwald CM, Morelli JN, Neumann R, Haug AR, Jansen N, et al. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur J Nucl Med Mol Imaging. (2013) 40:897–907. doi: 10.1007/s00259-013-2371-5
5. Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, et al. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. (2016) 43:1784–91. doi: 10.1007/s00259-016-3357-x
6. Fisher AV, Lopez-Aguiar AG, Rendell VR, Pokrzywa C, Rocha FG, Kanji ZS. et al. Predictive value of chromogranin A and a pre-operative risk score to predict recurrence after resection of pancreatic neuroendocrine tumors. J Gastrointest Surg. (2019) 23:651–8. doi: 10.1007/s11605-018-04080-1
7. Lee L, Ito T, Jensen RT. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: recent advances and controversies. Expert Rev Anticancer Ther. (2019) 19:1029–50. doi: 10.1080/14737140.2019.1693893
8. Jilesen AP, Busch OR, van Gulik TM, Gouma DJ, Nieveen van Dijkum EJ. Standard pre- and postoperative determination of chromogranin a in resectable non-functioning pancreatic neuroendocrine tumors—diagnostic accuracy: NF-pNET and low tumor burden. Dig Surg. (2014) 31:407–14. doi: 10.1159/000370007
9. Di Giacinto P, Rota F, Rizza L, Campana D, Isidori A, Lania A. et al. From Laboratory to Clinical Aspects of Patients with Neuroendocrine Tumors. Int J Endocrinol. (2018) 2018:8126087. doi: 10.1155/2018/8126087
10. Binderup T, Knigge U, Mellon Mogensen A, Palnaes Hansen C, Kjaer A. Quantitative gene expression of somatostatin receptors and noradrenaline transporter underlying scintigraphic results in patients with neuroendocrine tumors. Neuroendocrinology. (2008) 87:223–32. doi: 10.1159/000113128
11. Brunner P, Jörg AC, Glatz K, Bubendorf L, Radojewski P, Umlauft M, et al. The prognostic and predictive value of sstr(2)-immunohistochemistry and sstr(2)-targeted imaging in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. (2017) 44:468–75. doi: 10.1007/s00259-016-3486-2
12. Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med. (2016) 57:872–8. doi: 10.2967/jnumed.115.165803
13. Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. (2010) 51:353–9. doi: 10.2967/jnumed.109.066662
14. Stefanova M, Kratochwil C, Mavriopoulou E, Afshar-Oromieh A, Mier W, Schwartz L, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. (2015) 17:313–8. doi: 10.1007/s11307-014-0795-3
15. Lee DY, Kim YI. Prognostic value of maximum standardized uptake value in 68Ga-somatostatin receptor positron emission tomography for neuroendocrine tumors: a systematic review and meta-analysis. Clin Nucl Med. (2019) 44:777–83. doi: 10.1097/RLU.0000000000002694
16. Tatsumi M, Isohashi K, Matsunaga K, Watabe T, Kato H, Kanakura Y. et al. Volumetric and texture analysis on FDG PET in evaluating and predicting treatment response and recurrence after chemotherapy in follicular lymphoma. Int J Clin Oncol. (2019) 24:1292–300. doi: 10.1007/s10147-019-01482-2
17. Nakamoto R, Zaba LC, Rosenberg J, Reddy SA, Nobashi TW, Davidzon G. et al. Prognostic value of volumetric PET parameters at early response evaluation in melanoma patients treated with immunotherapy. Eur J Nucl Med Mol Imaging. (2020) 47:2787–95. doi: 10.1007/s00259-020-04792-0
18. Werner RA, Bundschuh RA, Higuchi T, Javadi MS, Rowe SP, Zsótér N, et al. Volumetric and texture analysis of pretherapeutic (18)F-FDG PET can predict overall survival in medullary thyroid cancer patients treated with Vandetanib. Endocrine. (2019) 63:293–300. doi: 10.1007/s12020-018-1749-3
19. Mohamed E, Needham A, Psarelli E, Carroll M, Vinjamuri S, Sanghera B, et al. Prognostic value of (18)FDG PET/CT volumetric parameters in the survival prediction of patients with pancreatic cancer. Eur J Surg Oncol. (2020) 46:1532–8. doi: 10.1016/j.ejso.2020.02.002
20. Panagiotidis E, Alshammari A, Michopoulou S, Skoura E, Naik K, Maragkoudakis E, et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med. (2017) 58:91–6. doi: 10.2967/jnumed.116.178095
21. Kim YI, Yoo C, Oh SJ, Lee SJ, Kang J, Hwang HS, et al. Tumour-to-liver ratio determined by [68Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. EJNMMI Res. (2020) 10:1–1. doi: 10.1186/s13550-020-00651-z
22. Toriihara A, Baratto L, Nobashi T, Park S, Hatami N, Davidzon G, et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging. (2019) 46:2244–51. doi: 10.1007/s00259-019-04455-9
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
25. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
28. Pauwels E, Van Binnebeek S, Vandecaveye V, Baete K, Vanbilloen H, Koole M, et al. Inflammation-based index and (68)Ga-DOTATOC PET-derived uptake and volumetric parameters predict outcome in neuroendocrine tumor patients treated with (90)Y-DOTATOC. J Nucl Med. (2020) 61:1014–20. doi: 10.2967/jnumed.119.236935
29. Ohlendorf F, Henkenberens C, Brunkhorst T, Ross TL, Christiansen H, Bengel FM, et al. Volumetric 68Ga-DOTA-TATE PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with metastatic gastroenteropancreatic neuroendocrine tumors. Q J Nucl Med Mol Imaging. (2020) 17: 2815–34. doi: 10.1055/s-0039-1683655
30. Ohnona J, Nataf V, Gauthe M, Balogova S, Belissant Benesty O, Zhang-Yin J, et al. Prognostic value of functional tumor burden on 68Ga-DOTATOC PET/CT in patients with pancreatic neuro-endocrine tumors. Neoplasma. (2019) 66:140–8. doi: 10.4149/neo_2018_180328N209
31. Tirosh A, Papadakis GZ, Millo C, Hammoud D, Sadowski SM, Herscovitch P, et al. Prognostic utility of total (68)Ga-DOTATATE-avid tumor volume in patients with neuroendocrine tumors. Gastroenterology. (2018) 154:998–1008.e1. doi: 10.1053/j.gastro.2017.11.008
32. Ortega C, Wong RK, Schaefferkoetter J, Veit-Haibach P, Myrehaug S, Juergens R, et al. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. J Nucl Med. (2021). doi: 10.2967/jnumed.120.256727
33. Carlsen EA, Johnbeck CB, Loft M, Pfeifer A, Oturai P, Langer SW, et al. Semi-automatic tumor delineation for evaluation of 64Cu-DOTATATE PET/CT in patients with neuroendocrine neoplasms: prognostication based on lowest lesion uptake and total tumor volume. J Nucl Med. (2021) 62:1564–70. doi: 10.2967/jnumed.120.258392
34. Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin North Am. (2001) 39:883–917. doi: 10.1016/S0033-8389(05)70319-5
35. Hustinx R, Bénard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med. (2002) 32:35–46. doi: 10.1053/snuc.2002.29272
36. Xuan D, Wen W, Tian S, Piao M, Xu D, Liu L. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of 18F-FDG PET/CT in patients with renal carcinoma: a protocol for systematic review and meta analysis. Medicine. (2020) 99:e19988. doi: 10.1097/MD.0000000000019988
37. Chan DL, Bernard EJ, Schembri G, Roach PJ, Johnson M, Pavlakis N, et al. High metabolic tumour volume on 18-fluorodeoxyglucose positron emission tomography predicts poor survival from neuroendocrine neoplasms. Neuroendocrinology. (2020) 110:950–8. doi: 10.1159/000504673
38. Ito K, Schöder H, Teng R, Humm JL, Ni A, Wolchok JD, et al. Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. (2019) 46:930–9. doi: 10.1007/s00259-018-4211-0
39. Kim HS, Choi JY, Choi DW, Lim HY, Lee JH, Hong SP, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. (2014) 48:180–6. doi: 10.1007/s13139-013-0262-0
40. Lee L, Ito T, Jensen RT. Imaging of pancreatic neuroendocrine tumors: recent advances, current status, and controversies. Expert Rev Anticancer Ther. (2018) 18:837–60. doi: 10.1080/14737140.2018.1496822
41. Wild D, Mäcke HR, Waser B, Reubi JC, Ginj M, Rasch H, et al. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. (2005) 32:724. doi: 10.1007/s00259-004-1697-4
42. Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC. et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. (2007) 34:982–93. doi: 10.1007/s00259-006-0317-x
43. Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. (2013) 54:364–72. doi: 10.2967/jnumed.112.111724
Keywords: positron emission tomography/CT, neuroendocrine tumors, somatostatin receptors, prognosis, tumor volume
Citation: Hou J, Yang Y, Chen N, Chen D and Hu S (2021) Prognostic Value of Volume-Based Parameters Measured by SSTR PET/CT in Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Front. Med. 8:771912. doi: 10.3389/fmed.2021.771912
Received: 07 September 2021; Accepted: 19 October 2021;
Published: 26 November 2021.
Edited by:
Ronan ABGRAL, Centre Hospitalier Regional Universitaire (CHU) de Brest, FranceReviewed by:
Virginia Liberini, University of Turin, ItalyGiorgio Treglia, Ente Ospedaliero Cantonale (EOC), Switzerland
Copyright © 2021 Hou, Yang, Chen, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuo Hu, aHVzaHVvMjAxOEAxNjMuY29t
 Jiale Hou1
Jiale Hou1 Shuo Hu
Shuo Hu