
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 30 November 2021
Sec. Nephrology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.769490
 Hyung Woo Kim1†
Hyung Woo Kim1† Jong Hyun Jhee2†
Jong Hyun Jhee2† Young Su Joo1,3
Young Su Joo1,3 Ki Hwa Yang4
Ki Hwa Yang4 Jin Ju Jung5
Jin Ju Jung5 Ji Hyeon Shin6
Ji Hyeon Shin6 Seung Hyeok Han1
Seung Hyeok Han1 Tae-Hyun Yoo1
Tae-Hyun Yoo1 Shin-Wook Kang1,7
Shin-Wook Kang1,7 Jung Tak Park1*
Jung Tak Park1*Objective: Dementia is prevalent among elderly patients undergoing hemodialysis. However, the association between dialysis adequacy and the risk of dementia is uncertain.
Methods: A total of 10,567 patients aged >65 years undergoing maintenance hemodialysis who participated in a national hemodialysis quality assessment program were analyzed. The patients were classified into quartile groups based on single-pool Kt/V levels. The associations between single-pool Kt/V and the development of dementia, Alzheimer's disease (AD), and vascular dementia (VD) were examined.
Results: The mean age of the patients was 72.9 years, and 43.4% were female. The mean baseline single-pool Kt/V level was 1.6 ± 0.3. During a median follow-up of 45.6 (45.6–69.9) months, there were 27.6, 23.9, and 2.8 events/1,000 person-years of overall dementia, AD, and VD, respectively. The incidences of overall dementia, AD, and VD were lowest in the highest single-pool Kt/V quartile group. Compared with the lowest single-pool Kt/V quartile, the risks of incident overall dementia and AD were significantly lower in the highest quartile [sub-distribution hazard ratio (sHR): 0.69, 95% confidence interval (CI): 0.58–0.82 for overall dementia; sHR: 0.69, 95% CI: 0.57–0.84 for AD]. Inverse relationships were found between the risks of developing overall dementia and AD, and single-pool Kt/V. However, no significant relationship was observed between single-pool Kt/V levels and VD development.
Conclusions: Increased dialysis clearance was associated with a lower risk of developing dementia in elderly hemodialysis patients.
The number of elderly patients with end-stage kidney disease (ESKD) undergoing dialysis treatment is increasing worldwide (1, 2). Cognitive dysfunction, including dementia, is notably prevalent among elderly patients with ESKD (3–6). The 10-year risk of dementia after commencement of hemodialysis in patients aged 65 years is 20% and increases linearly with age (7). Cognitive dysfunction in patients with ESKD is closely related to adverse clinical outcomes, including functional impairment, frequent hospitalization, withdrawal from dialysis, and death, thus increasing the health care burden (8). Despite the high prevalence and clinical impact of cognitive dysfunction in patients with ESKD, the pathophysiology and related risk factors remain unclear.
The retention of molecular mediators in the circulation, which is potentially capable of brain damage, resulting from reduced kidney function has been proposed as one of the factors for kidney disease-related cognitive dysfunction (9, 10). Up to 9% of the uremic toxins accumulated in the circulation of kidney disease patients have been found to have neurological and central nervous system (CNS) effects (11, 12). Since the blood-brain barrier is less functional in advanced kidney disease patients, these toxins are capable of entering the CNS through diffusion and are suspected to have detrimental effects on the CNS, leading to cognitive dysfunction. Despite the plausible relationship between circulating uremic toxins and cognitive dysfunction in kidney disease patients, it is unknown whether increased clearance of these mediators through adequate dialysis affects cognitive function.
Therefore, in this study, the association between dialysis adequacy, measured by single-pool Kt/V (spKt/V), and the development of dementia was evaluated in elderly patients undergoing hemodialysis. This was done by assessing a national representative cohort of dialysis patients and claims data from a national health insurance database.
More than 98% of the Korean population is enrolled in a mandatory National Health Insurance Service (NHIS) program, while the remaining citizens who are in the lowest-income bracket receive government medical aid benefits. The Health Insurance Review and Assessment Service (HIRA) is a national organization that reviews and evaluates healthcare costs and quality of care. The HIRA periodically performs a nationwide obligatory quality assessment for hemodialysis patients to ensure quality healthcare since 2010. All hemodialysis patients aged 18 years or older who undergo hemodialysis at least twice a week (eight times per month) as outpatients at a single healthcare provider during the quality assessment target period in Korea are entitled to the periodic hemodialysis quality assessment. The assessment collects information on demographics, cause of ESKD, dialysis vintage, and laboratory measurements, including hemoglobin, albumin, calcium, phosphorus, iron, ferritin and total iron binding capacity, blood pressure, erythropoietin use, intravenous iron use, and dialysis adequacy indices. This study used the 4th and 5th periodic hemodialysis quality assessment data from 2013 to 2015. Comorbidities and outcome diagnosis information were retrieved from the NHIS claims database. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University Health System (4-2019-0901). The requirement for informed consent was waived because of the retrospective nature of the study. De-identification was performed and data usage was permitted by the National Health Information Data Request Review Committee of the NHIS.
Patients aged 65 years or older who were included in the 4th and 5th periodic hemodialysis quality assessment were initially screened. Patients with missing values of spKt/V, dialysis vintage <3 months, or a history of kidney transplantation were excluded. Patients who were diagnosed with dementia prior to the index date were also excluded. Furthermore, those who had dementia-related claims before the baseline were also excluded. A total of 10,677 patients on maintenance hemodialysis were included in the final analysis (Figure 1).
The assessment dates of the 4th and 5th periodic hemodialysis quality assessment were considered as baseline. Economic status was classified based on the relative household income-derived health insurance premiums during the index year. Dialysis-related information included cause of ESRD, dialysis vintage, and type of vascular access [arteriovenous fistula, arteriovenous graft (AVG), or central catheter]. Comorbidities were defined as a record of at least one medical claim per year for a specific diagnosis. Diagnosis was defined based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes (Supplementary Table 1). Blood samples were obtained immediately before a midweek dialysis session with overnight fasting. Evaluations were performed for hemoglobin; albumin; total calcium, and phosphorous; iron profile, including iron, ferritin, and total iron binding capacity.
Dialysis adequacy values that were measured at baseline were used for the main analysis. The baseline spKt/V was assessed using the dialysis adequacy index. The spKt/V was calculated according to second generation logarithmic estimates of spKt/V; Kt/V = –ln (R – 0.008 × t) + (4 – 3.5 × R) × UF/W, where R is the ratio of pre- to post-hemodialysis concentrations of BUN, t is the dialysis session duration (in hours), UF is the amount of ultrafiltration (L) during the given hemodialysis session, and W is the post-hemodialysis weight (kg) (13). The urea reduction ratio (URR) was also assessed and used as a supporting dialysis adequacy index in the sensitivity analysis. The URR was calculated by dividing the difference in pre- and post-hemodialysis BUN concentrations by the pre-hemodialysis BUN concentration and multiplying by 100 (%) (14). Study subjects were classified into quartiles according to baseline spKt/V or URR levels.
The primary outcome was defined as the occurrence of dementia, which was defined as a claim record for ICD-10 dementia codes and a concurrent prescription record for dementia-related treatment. The dementia ICD-10 codes included F00 or G30 for Alzheimer's disease (AD), F01 for vascular dementia (VD), and F02, F03, or G31 for dementia not otherwise specified. Prescriptions for medications including donepezil, rivastigmine, galantamine, and memantine were considered to be dementia related. The study patients were followed up from baseline to the date of incident dementia or until the end of the study period (June 30, 2019).
Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as absolute numbers with percentages. The Shapiro-Wilk test was used to determine the normality of the distribution of the parameters. Intergroup comparisons were performed using analysis of variance or Student's t-test for normally distributed continuous variables, while categorical variables were examined using the chi-square test or Fisher's exact test. Data that did not show a normal distribution were presented as medians with interquartile ranges and were compared using the Mann-Whitney U-test or Kruskal-Wallis test. To explore the association between dialysis adequacy and the development of dementia, sub-distribution hazard models were constructed. All-cause death was considered a competing risk (15). The sub-distribution hazard models were adjusted for age, sex, body mass index (BMI), pre-dialysis systolic blood pressure (SBP), economic status, cause of ESRD, dialysis vintage, vascular access type, Charlson Comorbidity Index (CCI; excluding diabetes and dementia), diabetes, hemoglobin, serum albumin, calcium, phosphorous, and use of intravenous iron or erythropoiesis-stimulating agent). To evaluate the relationship between the risk of incident dementia and spKt/V levels as a continuous variable, cubic spline analyses were conducted. A cubic spline was used, and each 1 percentile of the upper and lower spKt/V was eliminated to reduce distortion. Sensitivity analyses were performed to confirm these results. First, the association between spKt/V levels and the risk of dementia among subjects was assessed only for those who underwent hemodialysis at least three times per week. Second, the relationship between spKt/V levels and the risk of dementia was re-evaluated by censoring incident stroke history. Third, dialysis adequacy and the risk of dementia were further analyzed using the URR as a dialysis adequacy index. Fourth, evaluation considering kidney transplantation events as a competing risk was performed. Finally, analysis regarding spKt/V as a time varying variable was made to account for the effect of spKt/V change during follow-up in patients who underwent spKt/V measurements twice at a 2-year interval. All statistical analyses were performed using R (version 3.5.1; www.r-project.org; R Foundation for Statistical Computing, Vienna) and SAS Enterprise Guide, version 6.1 (SAS Institute). A P < 0.05 was considered as significant.
The baseline characteristics of the study subjects according to the spKt/V level quartiles are shown in Table 1. The mean age was 72.8 ± 5.7 years and 6,057 (56.7%) were male. The mean spKt/V level was 1.6 ± 0.3. Patients in the higher spKt/V quartile were older and included more female patients and medical aid beneficiaries. In addition, more patients used AVG and had longer dialysis vintages in the higher spKt/V quartiles. The BMI, SBP, diastolic blood pressure, number of patients using antihypertensive agents, and CCI were lower in the higher spKt/V quartiles. Laboratory results revealed higher serum calcium levels and lower phosphorous levels in the higher spKt/V quartiles.
During the median follow-up of 45.6 (45.6–69.9) months, there were 27.6, 23.9, and 2.8 events/1,000 person-years of overall dementia, AD, and VD, respectively. Among the groups stratified by spKt/V levels, the incidence of overall dementia and AD was lowest in the highest quartile. Compared with the lowest spKt/V quartile, the risks of developing overall dementia and AD were significantly lower in the highest quartile [subdistributed hazard ratio (sHR): 0.67, 95% confidence interval (CI): 0.57–0.80 for overall dementia; sHR: 0.68, 95% CI: 0.57–0.81 for AD]. However, no significant relationship was found with incident VD (sHR: 0.71, 95% CI: 0.44–1.16). This association remained significant even after making adjustments for confounding variables (sHR: 0.69, 95% CI: 0.58–0.82 for overall dementia; sHR: 0.69; 95% CI: 0.57–0.84 for AD). When spKt/V levels were treated as continuous variables, every increase of 0.1 in spKt/V was associated with a 3% risk reduction of incident overall dementia (sHR: 0.97, 95% CI: 0.95–0.99) and AD (sHR: 0.97, 95% CI: 0.94–0.99) after adjusting for confounding variables (Table 2). The hazard ratios of risk factors of dementia beside spKt/V are reported in Supplementary Table 2.
When the relationship between spKt/V and the adjusted hazard ratio for incident dementia was assessed using restricted cubic spline plots, the increase in spKt/V was associated with a linear decrease in overall dementia and AD risk. However, no clear relationship was found with VD (Figure 2).
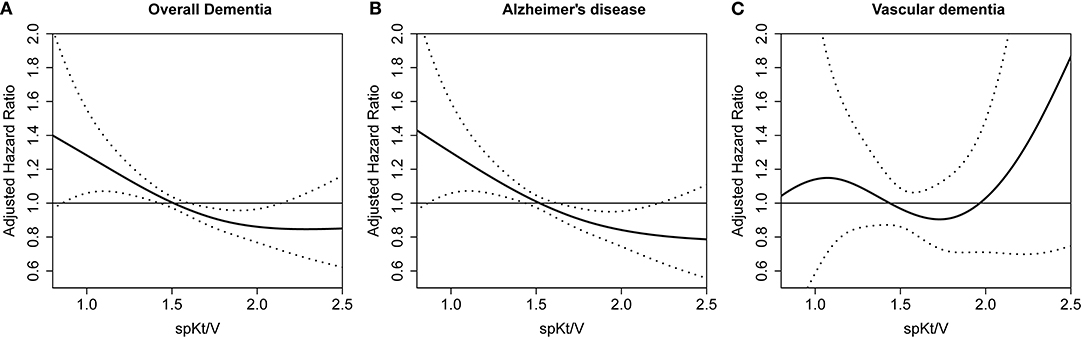
Figure 2. Restricted cubic spine plot for dementia occurrence according to spKt/V. (A) overall dementia (B) Alzheimer's disease (C) vascular dementia. spKt/V, single-pool Kt/V.
Subgroup analyses were performed in subjects stratified by age, sex, history of diabetes mellitus (no vs. yes), and dialysis vintage (median dialysis vintage of 2.89 years) (Figure 3). No significant interactions were found between the stratified variables and spKt/V for the incidence of overall dementia and AD, suggesting that the associations found in the main analysis are consistent regardless of age, sex, diabetes mellitus, and dialysis vintage.
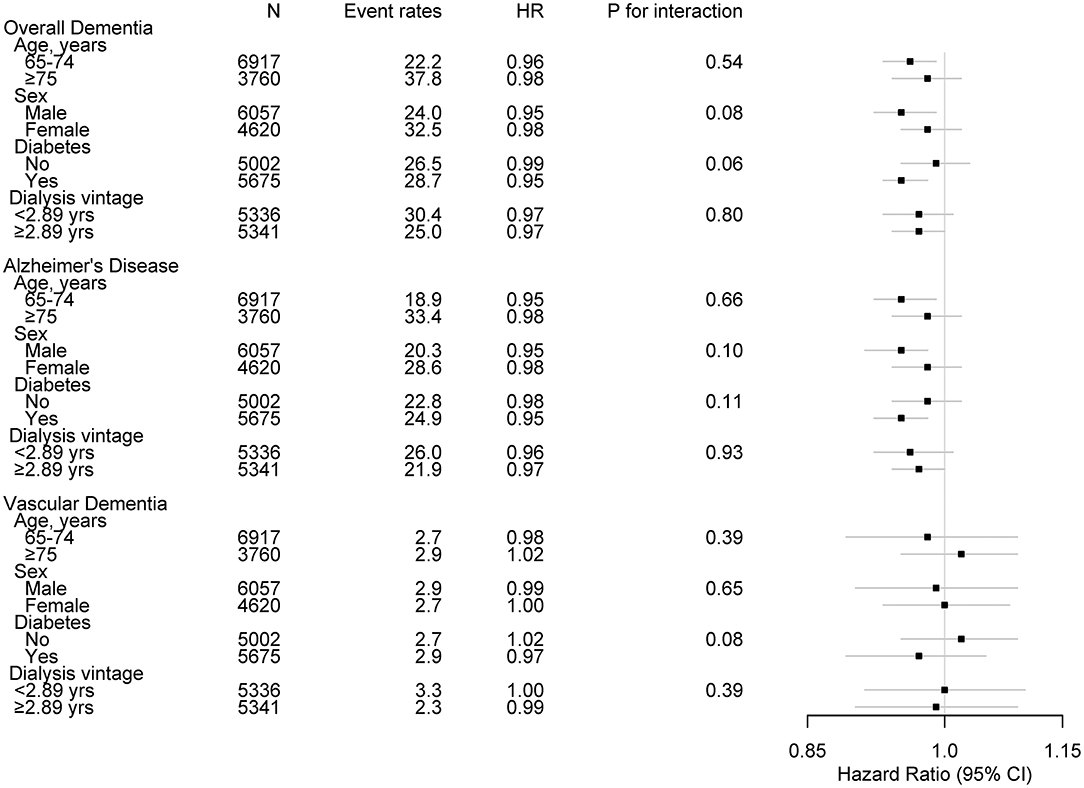
Figure 3. Subgroup associations of Kt/V with incident dementia. Event rates are per 1,000 person-years. HR was calculated with spKt/V levels treated as continuous variable for development of dementia. HR was adjusted for age, sex, BMI, pre-dialysis SBP, economic status, cause of ESKD, dialysis vintage, vascular access type, CCI (except for diabetes and dementia), diabetes, hemoglobin, serum albumin, calcium, phosphorous, and use of IV iron or ESA. HR, hazard ratio; spKt/V, single-pool Kt/V; BMI, body mass index; SBP, systolic blood pressure; ESKD, end-stage kidney disease; CCI, Charlson comorbidity index; IV, intravenous injection; ESA, erythropoiesis stimulating agent.
When subjects who underwent dialysis at least three times per week were assessed, the risk of overall dementia (adjusted sHR: 0.71, 95% CI: 0.59–0.86) and AD (adjusted sHR: 0.74, 95% CI: 0.60–0.90) had significantly reduced in the highest spKt/V quartile compared to that in the lowest quartile. No significant association was found with VD risk (adjusted sHR: 0.65, 95% CI: 0.38–1.12). A significant relationship was noted when considering spKt/V as a continuous variable (per 0.1 increase in spKt/V, adjusted sHR: 0.97, 95% CI: 0.94–0.99 for overall dementia; HR: 0.96, 95% CI: 0.94–0.99 for AD) (Supplementary Table 3). Additionally, when incident stroke events were censored, the risk of overall dementia (adjusted sHR: 0.68, 95% CI: 0.57–0.82) and AD (adjusted sHR: 0.68, 95% CI: 0.56–0.83) was clearly reduced in the highest spKt/V quartile compared with the lowest quartile. No significant relationship was noted with VD risk (adjusted sHR: 0.74, 95% CI: 0.42–1.30). A significant relationship was noted with spKt/V when considered as a continuous variable (per 0.1 increase in spKt/V, adjusted sHR: 0.97, 95% CI: 0.95–0.99 for overall dementia; sHR: 0.97, 95% CI: 0.94–0.99 for AD) (Supplementary Table 4). Moreover, a similar finding to the main analysis was obtained when dialysis adequacy was assessed using the URR. The risk of overall dementia (adjusted sHR: 0.80, 95% CI: 0.87–0.96) and AD (adjusted sHR: 0.80, 95% CI: 0.66–0.98) was significantly lower in the highest URR quartile than in the lowest quartile. No clear relationship was found with VD risk (adjusted sHR: 0.89, 95% CI: 0.52–1.53). With URR considered as a continuous variable, a significant risk decrease was noted with an increase in URR (per 10-fold increase in URR, adjusted sHR: 0.86; 95% CI: 0.78–0.95 for overall dementia; HR: 0.85, 95% CI: 0.76–0.94 for AD) (Supplementary Table 5). When both all-cause death and kidney transplantation events were considered as competing risks, the risk of overall dementia and Alzheimer's disease were lower with increasing values of spKt/V (overall dementia: adjusted sHR, 0.97; 95% CI 0.95–0.99, Alzheimer's disease: adjusted sHR, 0.97; 95% CI, 0.94–0.99) (Supplementary Table 6). Furthermore, analysis of 2,892 patients who underwent spKt/V measurements twice at 2-year intervals showed that risk of overall dementia and Alzheimer's disease was lower with higher spKt/V values, when spKt/V was regarded as a time varying variable (overall dementia: adjusted sHR, 0.94; 95% CI, 0.90–0.99; Alzheimer's disease: adjusted sHR, 0.94; 95% CI, 0.89–0.99) (Supplementary Table 7).
In this study of a nationwide hemodialysis quality assessment database, a significant relationship between dialysis adequacy and the risk of developing dementia was revealed. The risk of incident overall dementia and AD was significantly lower in patients in the group with the highest spKt/V values compared than in that with the lowest spKt/V values. In addition, inverse relationships were found between the risk of developing overall dementia and AD and spKt/V. The significance of these relationships was maintained even after adjustments were made for confounding factors. Moreover, a similar association with the risk of incident dementia was also found when dialysis adequacy was assessed using the URR. However, a clear relationship between dialysis adequacy and the risk of developing VD was not observed.
Incident dementia was diagnosed in more than 12% of the study patients during a median follow-up duration of 45.6 months. The development of dementia has been found to be prevalent among elderly patients undergoing dialysis. In the general population, the prevalence of dementia has been reported to be ~5%; this increases with age, reaching 7.4% at the age of 70 years (16, 17). In comparison, in the Dialysis Outcomes and Practice Patterns Study, which evaluated a cohort of 16,694 patients on hemodialysis, the overall prevalence was increased to 20% in elderly patients (8). Similarly, in an evaluation of the United States Renal Data System, which included 356,668 dialysis patients aged 66 years or older, the lifetime risk of developing dementia was over 20% (7). In addition, in that study, the risk of mortality increased two-fold in those who were diagnosed with dementia, showing that the development of dementia also has a considerable impact on patient outcome. Kidney disease is one of the strongest risk factors for the development of cognitive impairment and dementia (3). In a longitudinal observational study in the general population, kidney disease was a more powerful risk factor for developing dementia than genetic factors and was only exceeded by stroke and chronic anxiolytic use (18).
The results of this study show that the risk of incident dementia is lower among patients with increased dialysis clearance, suggesting that zealous removal of uremic toxins may reduce the risk of developing dementia among elderly dialysis patients. This possibility is supported by the results of several previous studies. In the Dialysis Outcomes and Practice Patterns Study, residual renal function was a significant factor in lowering the risk of dementia, suggesting that uremic toxin excretion through the kidneys may play a role in the development of dementia in these patients (8). In addition, in a recent cross-sectional analysis of Chinese dialysis patients, low spKt/V was found to be related to cognitive impairment (19). Nonetheless, a previous cross-sectional analysis evaluating patients enrolled in the Frequent Hemodialysis Network trials failed to reveal a close association between dialysis clearance of urea and cognitive function (20). The discrepancy found in these studies could be explained by several aspects, including whether AD and VD were distinguished as causes of dementia in the analysis. However, the amount of clearance delivered would have played a major role. In this study, the risk of developing dementia decreased in patients whose mean spKt/V was 1.94 ± 0.32, which surpasses the minimal clearance amount recommended by current guidelines (21). The findings of this study suggest that although the current minimal dialysis clearance requirements are sufficient to reduce overall mortality (22), they could be insufficient to reduce the risk of dementia. However, further prospective intervention trials are required to confirm these findings.
The risk of AD, but not VD, decreased in patients with higher dialysis clearance. One of the causes of AD in dialysis patients has been attributed to the effects of circulating uremic toxins (10, 11). Circulating tumor necrosis factor has been found to impair neuronal synaptic function and memory at increased concentrations (23–25). In addition, neuropeptide Y (NPY), which is normally produced in peripheral nerve endings, accumulates in the circulation of chronic kidney disease and ESKD patients (26). High levels of NPY in the cerebrospinal fluid have been reported to be related to cognitive impairment in patients with subarachnoid hemorrhage (27). Other uremic toxins commonly accumulated in ESKD patients, such as asymmetric dimethyl arginine, hippuric acid, and indoxyl sulfate, have been found to be closely related to cognitive impairment (11, 28–30). However, VD is a common consequence of reduced cerebral blood flow (31). Factors contributing to acute and variable hemodynamic changes in dialysis patients, such as vascular access and large ultrafiltration volumes, could rather be more responsible for the aggravation of VD in these patients (32–34). Nonetheless, the effects of these hemodynamic change-promoting factors were not evaluated in detail in this study; hence, further investigations to assess these factors are needed.
This study has several limitations. First, due to the observational nature of the study, the cause-effect relationship cannot be determined. Further prospective studies modifying dialysis clearance are needed to confirm the influence on the development of dementia. Second, dementia was defined based on claim records. Therefore, the possibility of misclassification could not be ruled out. However, a previous study validating the accuracy of this working definition in the NHIS database revealed that the positive predictive value was 94.7%, suggesting that the chances of outcome misclassification would not be high (35). Lastly, changes in dialysis clearance during the follow-up period and residual renal function were not taken into account. Multiple potential factors that would affect dialysis clearance and residual renal function have been included as covariates to assure an independent association with clearance. However, it is indubitable that the consideration of these aspects would further clarify the findings of this study.
In conclusion, this large-scale cohort study of elderly hemodialysis patients demonstrated that increased dialysis clearance is associated with a lower risk of developing dementia. Ascertaining adequate dialysis clearance may lower the incidence of dementia and accordingly improve patient outcomes in elderly hemodialysis patients. Nonetheless, further investigations are needed to confirm these findings.
The datasets presented in this article are not readily available because the original data is available after getting approval from HIRA. The datasets are available online at: https://opendata.hira.or.kr.
The studies involving human participants were reviewed and approved by Yonsei University College of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HWK, JHJ, and JTP designed the study. JHJ wrote the manuscript. HWK analyzed the data. JTP reviewed and edited the manuscript. YSJ, KHY, JJJ, JHS, SHH, T-HY, and S-WK researched data and contributed to discussion. JTP took responsibility for the integrity of the data. All authors critically revised the manuscript for key intellectual content and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This research was supported by a grant from the Joint Project on Quality Assessment Research, Republic of Korea. The epidemiologic data used in this study were obtained from Periodic Hemodialysis Quality Assessment by HIRA.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.769490/full#supplementary-material
1. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. (2020) 75(1, Supplement 1):S1–S64. doi: 10.1053/j.ajkd.2019.09.002
2. Jin D-C, Yun S-R, Lee SW, Han S-W, Kim W, Park J. Current characteristics of dialysis therapy in Korea: 2015 registry data focusing on elderly patients. Kidney Res Clin Pract. (2016) 35:204–11. doi: 10.1016/j.krcp.2016.09.006
3. Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. (2005) 16:2127–33. doi: 10.1681/asn.2005010005
4. Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. (2011) 79:14–22. doi: 10.1038/ki.2010.336
5. Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. (2008) 15:123–32. doi: 10.1053/j.ackd.2008.01.010
6. O'Lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. (2016) 67:925–35. doi: 10.1053/j.ajkd.2015.12.028
7. McAdams-DeMarco MA, Daubresse M, Bae S, Gross AL, Carlson MC, Segev DL. Dementia, Alzheimer's disease, and mortality after hemodialysis initiation. Clin J Am Soc Nephrol. (2018) 13:1339–47. doi: 10.2215/CJN.10150917
8. Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. (2006) 21:2543–8. doi: 10.1093/ndt/gfl275
9. Meyer TW, Hostetter TH. Approaches to uremia. J Am Soc Nephrol. (2014) 25:2151–8. doi: 10.1681/asn.2013121264
10. Depner TA. Uremic toxicity: urea and beyond. Semin Dial. (2001) 14:246–51. doi: 10.1046/j.1525-139x.2001.00072.x
11. Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, et al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. (2020) 16:452–69. doi: 10.1038/s41581-020-0266-9
12. Jhee JH, Lee E, Cha MU, Lee M, Kim H, Park S, et al. Prevalence of depression and suicidal ideation increases proportionally with renal function decline, beginning from early stages of chronic kidney disease. Medicine. (2017) 96:e8476. doi: 10.1097/md.0000000000008476
13. Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. (1993)4:1205–13.
14. Lowrie E, Lew N. The urea reduction ratio (URR). A simple method for evaluating hemodialysis treatment. Contemp Dial Nephrol. (1991) (12):11–20.
15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. (1999) 94:496–509. doi: 10.1080/01621459.1999.10474144
16. Ponjoan A, Garre-Olmo J, Blanch J, Fages E, Alves-Cabratosa L, Martí-Lluch R, et al. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol. (2019) 11:217–28. doi: 10.2147/clep.S186590
17. van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. (2005) 76 Suppl 5:v2–7. doi: 10.1136/jnnp.2005.082867
18. Lipnicki DM, Crawford J, Kochan NA, Trollor JN, Draper B, Reppermund S, et al. Risk factors for mild cognitive impairment, dementia and mortality: the Sydney Memory and Ageing Study. J Am Med Dir Assoc. (2017) 18:388–95. doi: 10.1016/j.jamda.2016.10.014
19. Luo Y, Murray AM, Guo YD, Tian R, Ye PP, Li X, et al. Cognitive impairment and associated risk factors in older adult hemodialysis patients: a cross-sectional survey. Sci Rep. (2020) 10:12542. doi: 10.1038/s41598-020-69482-1
20. Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. (2010) 5:1429–38. doi: 10.2215/cjn.01090210
21. Daugirdas JT, Depner TA, Inrig J, Mehrotra R, Rocco MV, Suri RS, et al. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. (2015) 66:884–930. doi: 10.1053/j.ajkd.2015.07.015
22. Bloembergen WE, Stannard DC, Port FK, Wolfe RA, Pugh JA, Jones CA, et al. Relationship of dose of hemodialysis and cause-specific mortality. Kidney Int. (1996) 50:557–65. doi: 10.1038/ki.1996.349
23. Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell. (2015) 163:1730–41. doi: 10.1016/j.cell.2015.11.023
24. Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. (2011) 25:181–213. doi: 10.1016/j.bbi.2010.10.015
25. Yang G, Parkhurst CN, Hayes S, Gan WB. Peripheral elevation of TNF-α leads to early synaptic abnormalities in the mouse somatosensory cortex in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. (2013) 110:10306–11. doi: 10.1073/pnas.1222895110
26. Zoccali C, D'Arrigo G, Leonardis D, Pizzini P, Postorino M, Tripepi G, et al. Neuropeptide Y and chronic kidney disease progression: a cohort study. Nephrol Dial Transplant. (2018) 33:1805–12. doi: 10.1093/ndt/gfx351
27. Bründl E, Proescholdt M, Schödel P, Bele S, Höhne J, Zeman F, et al. Excessive release of endogenous neuropeptide Y into cerebrospinal fluid after treatment of spontaneous subarachnoid haemorrhage and its possible impact on self-reported neuropsychological performance - results of a prospective clinical pilot study on good-grade patients. Neurol Res. (2018) 40:1001–13. doi: 10.1080/01616412.2018.1508547
28. Huang M, Wei R, Wang Y, Su T, Li P, Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol. (2018) 16:303–13. doi: 10.1016/j.redox.2018.03.010
29. Watson CP, Pazarentzos E, Fidanboylu M, Padilla B, Brown R, Thomas SA. The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood-brain barrier in vitro. Brain Res. (2016) 1648(Pt A):232–42. doi: 10.1016/j.brainres.2016.07.026
30. Yeh YC, Huang MF, Liang SS, Hwang SJ, Tsai JC, Liu TL, et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology. (2016) 53:148–52. doi: 10.1016/j.neuro.2016.01.006
31. Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. (2012) 43:2526–34. doi: 10.1161/strokeaha.112.655803
32. Yoshimitsu T, Hirakata H, Fujii K, Kanai H, Hirakata E, Higashi H, et al. Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin Nephrol. (2000) 53:445–51.
33. Wolfgram DF. Intradialytic cerebral hypoperfusion as mechanism for cognitive impairment in patients on hemodialysis. J Am Soc Nephrol. (2019) 30:2052–8. doi: 10.1681/asn.2019050461
34. Lee HS, Song YR, Kim JK, Joo N, Kim C, Kim HJ, et al. Outcomes of vascular access in hemodialysis patients: analysis based on the Korean National Health Insurance Database from 2008 to 2016. Kidney Res Clin Pract. (2019) 38:391–8. doi: 10.23876/j.krcp.19.015
Keywords: hemodialysis adequacy, dementia, hemodialysis, Alzheimer's disease, vascular dementia
Citation: Kim HW, Jhee JH, Joo YS, Yang KH, Jung JJ, Shin JH, Han SH, Yoo T-H, Kang S-W and Park JT (2021) Dialysis Adequacy and Risk of Dementia in Elderly Hemodialysis Patients. Front. Med. 8:769490. doi: 10.3389/fmed.2021.769490
Received: 27 September 2021; Accepted: 01 November 2021;
Published: 30 November 2021.
Edited by:
Min Chen, Peking University First Hospital, ChinaReviewed by:
Yong Kyun Kim, Catholic University of Korea, South KoreaCopyright © 2021 Kim, Jhee, Joo, Yang, Jung, Shin, Han, Yoo, Kang and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung Tak Park, anRwYXJrQHl1aHMuYWM=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.