
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 January 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.768101
 Tingyu Zhang1†
Tingyu Zhang1† Yuanni Liu2†
Yuanni Liu2† Ziruo Ge1
Ziruo Ge1 Di Tian1
Di Tian1 Ling Lin2
Ling Lin2 Zhenghua Zhao3
Zhenghua Zhao3 Yi Shen4
Yi Shen4 Xiaoli Yu4
Xiaoli Yu4 Yang Feng3
Yang Feng3 Chunqian Qiang2
Chunqian Qiang2 Jianping Duan5
Jianping Duan5 Yanli Ma5
Yanli Ma5 Tianli Fan5
Tianli Fan5 Yongxiang Zhao4
Yongxiang Zhao4 Zhihai Chen1*
Zhihai Chen1*Background: Triglyceride-glucose (TyG) index has been proposed as a reliable indicator for insulin resistance and proved to be closely associated with the severity and mortality risk of infectious diseases. It remains indistinct whether TyG index performs an important role in predicting in-hospital mortality in patients with severe fever with thrombocytopenia syndrome (SFTS).
Methods: The current study retrospectively recruited patients who were admitted for SFTS from January to December 2019 at five medical centers. TyG index was calculated in accordance with the description of previous study: Ln [fasting triglyceride (TG) (mg/dl) × fasting blood glucose (FBG) (mg/dl)/2]. The observational endpoint of the present study was defined as the in-hospital death.
Results: In total, 79 patients (64.9 ± 10.5 years, 39.2% female) who met the enrollment criteria were enrolled in the current study. During the hospitalization period, 17 (21.5%) patients died in the hospital. TyG index remained a significant and independent predictor for in-hospital death despite being fully adjusted for confounders, either being taken as a nominal [hazard ratio (HR) 5.923, 95% CI 1.208–29.036, P = 0.028] or continuous (HR 7.309, 95% CI 1.854–28.818, P = 0.004) variate. TyG index exhibited a moderate-to-high strength in predicting in-hospital death, with an area under the receiver operating characteristic curve (AUC) of 0.821 (95% CI 0.712–0.929, P < 0.001). The addition of TyG index displayed significant enhancement on the predictive value for in-hospital death beyond a baseline model, manifested as increased AUC (baseline model: 0.788, 95% CI 0.676–0.901 vs. + TyG index 0.866, 95% CI 0.783–0.950, P for comparison = 0.041), increased Harrell's C-index (baseline model: 0.762, 95% CI 0.645–0.880 vs. + TyG index 0.813, 95% CI 0.724–0.903, P for comparison = 0.035), significant continuous net reclassification improvement (NRI) (0.310, 95% CI 0.092–0.714, P = 0.013), and significant integrated discrimination improvement (0.111, 95% CI 0.008–0.254, P = 0.040).
Conclusion: Triglyceride-glucose index, a novel indicator simply calculated from fasting TG and FBG, is strongly and independently associated with the risk of in-hospital death in patients with SFTS.
Severe fever with thrombocytopenia syndrome (SFTS), caused by SFTS virus (SFTSV), has been recognized as a novel life-threatening infectious disease in recent years. SFTSV (New Bunyavirus) was isolated by Yu et al. and identified as a member of the genus phlebovirus in the Bunyaviridae family (1). SFTSV mainly infects humans through tick bites and may result in multiple organ dysfunction, particularly myocardial damage and kidney injury (2). Since it was first reported in China in 2009, the number of SFTS cases has increased rapidly, and related cases have been detected in neighboring countries, such as South Korea and Japan (1, 3, 4). Nowadays, SFTS has been involved in a wide range of regions throughout the world with a mortality rate of up to 30% (1), which makes it listed as an emerging infectious disease by the WHO in 2017 (5). For patients with severe situations, it may rapidly progress to multiple organ failure, then further lead to death within 1–2 weeks. Therefore, early risk stratification is of great clinical significance in identifying individuals with a high risk of poor outcomes and customizing specific strategies in line with the risk levels.
Triglyceride-glucose (TyG) index, which is simply calculated from fasting triglyceride (TG) and fasting blood glucose (FBG), has been proposed and considered as a reliable indicator for the estimation of insulin resistance levels (6). Certain previous studies have demonstrated that increased TyG index is significantly associated with an increased risk of chronic non-communicable diseases, consisting of diabetes mellitus, hypertension, non-alcoholic fatty liver disease, and cardiovascular disease (7–10). Furthermore, recent studies have indicated that TyG index plays important role in predicting the severity and mortality risk of infectious diseases, such as chronic hepatitis B and C (11, 12), and coronavirus disease-2019 (COVID-19) (13). It has been further revealed that the aggravation of acute or chronic infectious diseases caused by insulin resistance may be mainly mediated by multiple metabolic abnormalities and systemic inflammatory responses.
Currently, there is a relative lack of study targeting the association between TyG index and poor outcomes for patients with SFTS. The present study, therefore, was designed to investigate the value of TyG index in predicting the in-hospital mortality in patients who experienced SFTS, so as to provide useful information for the early identification of patients who were susceptible to adverse prognosis.
A total of 126 patients diagnosed with SFTS at five medical centers from China (Beijing Ditan Hospital, Yantai City Hospital for Infectious Disease, Tai'an City Central Hospital, Qingdao No. 6 People's Hospital, and Dandong Infectious Disease Hospital) between January and December 2019 were retrospectively screened. The diagnostic criteria for SFTS were as follows: (1) epidemiological history (working, living, or traveling in endemic areas during the epidemic season; contact history to SFTS patients; and recent tick bite history); (2) fever; (3) thrombocytopenia; and (4) positive serum nucleic acid test and/or IgM antibody for SFTSV (New Bunyavirus). Exclusion criteria included were as follows: (1) previous leukemia, idiopathic thrombocytopenic purpura, and other hematopathies; (2) previous acute and chronic viral hepatitis, alcoholic liver disease, and other hepatopathies; (3) previous autoimmune diseases; and (4) missing clinical data. Ultimately, 79 patients who met the enrollment criteria were included in the current analysis (Figure 1).
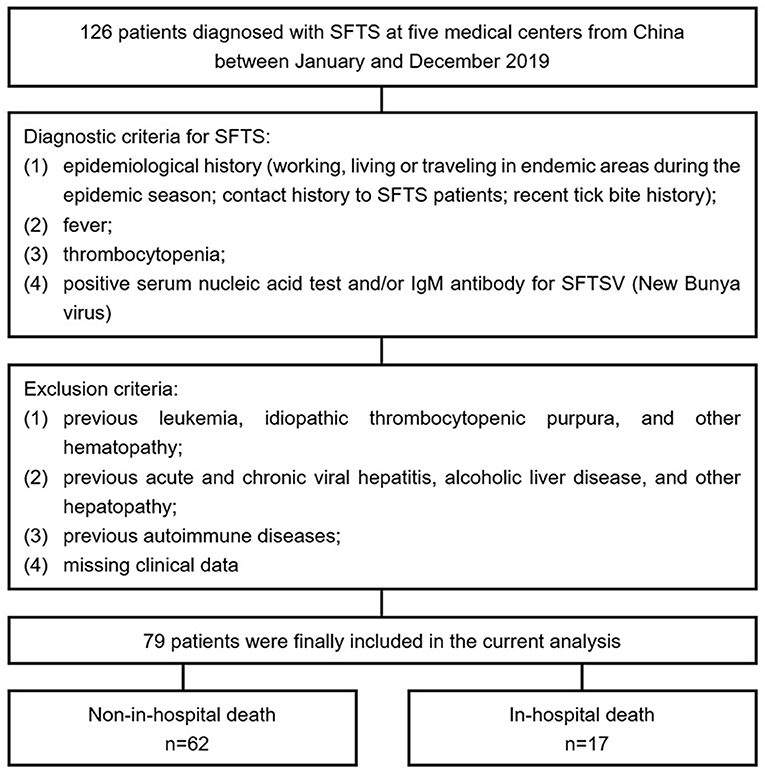
Figure 1. Flow diagram for the enrollment of study population. SFTS, severe fever with thrombocytopenia syndrome; SFTSV, SFTS virus.
The study protocol was endorsed by the Clinical Research Ethics Committee of Beijing Ditan Hospital, Capital Medical University. All subjects were informed and agreed to participate in the present study.
Demographic characteristics and clinical manifestations were acquired from a self-reported questionnaire and then entered into an established database by trained personnel who were blinded to the study protocol. The first laboratory examination during hospitalization, such as white blood cell count (WBC), neutrophil count (NEU), neutrophil percentage (NEU%), lymphocyte count (LYM), lymphocyte percentage (LYM%), platelet count (PLT), hemoglobin (Hb), T-lymphocyte (T-LYM), CD4 positive T-lymphocyte (CD4+ T-LYM), CD8 positive T-lymphocyte (CD8+ T-LYM), prothrombin time (PT), activated partial thromboplastin time (APTT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), creatinine, blood urea nitrogen (BUN), creatine kinase (CK), lactic dehydrogenase (LDH), and C-reactive protein (CRP), were used for the current analysis. TG and FBG were examined by using peripheral venous blood samples extracted at ≥8 h fasting state. The formula for calculation of TyG index was conformed with the former study: Ln [fasting TG (mg/dl) × FBG (mg/dl)/2] (14).
The observational endpoint of the present study was defined as the in-hospital death. The time of death was defined as the period from symptom onset to in-hospital death occurred.
All data analyses were conducted with SPSS 26.0 (IBM, Armonk, NY, USA), MedCalc 19.1 (Ostend, Belgium), and R Programming Language 3.6.3. A two-sided P < 0.05 was regarded as statistical significance.
Continuous parameters with normal or abnormal distribution were presented as mean with SD or median with interquartile range (IQR), comparisons of which were performed by T-test or Mann–Whitney U-test respectively. Nominal parameters were presented as numbers with percentages and compared by chi-square test (with or without continuity correction) or Fisher's exact test correspondingly.
Kaplan–Meier analysis was performed to evaluate the cumulative survival rates of groups with lower and higher median of TyG index. The differences between groups were examined by using the log-rank test. Univariate Cox regression analyses were performed to identify the potential risk predictors of in-hospital death. Then, three multivariate models, variates of which were selected based on the results of univariate analysis and clinical experience, were established to investigate the independent role of TyG index in predicting in-hospital death. Variates with underlying collinearity were not introduced into models simultaneously. The hazard ratio (HR) and 95% CI for in-hospital death were evaluated by regarding the TyG index as a nominal or continuous variate, respectively.
The predictive performance of TyG index for in-hospital death was further evaluated by receiver operating characteristic (ROC) analysis whereby the area under the ROC curves (AUCs) and the optimal cut-off value were determined. At the level of optimal cut-off value, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for predicting the in-hospital death were calculated, respectively. Additionally, to evaluate the incremental effects of TyG index on risk prediction based on the model of established risk factors, the AUC and the Harrell's C-index for respective models were assessed and compared by Z-test. In addition, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also calculated to evaluate the incremental ability of TyG index on risk reclassification and discrimination.
In total, 79 patients with SFTS who met the inclusion and exclusion criteria were ultimately included in this study, with a mean age of 64.9 ± 10.5 years, including 48 men (60.8%) and 31 women (39.2%). During the hospitalization period, 17 patients died in hospital, with a mortality rate of 21.5%.
Patients were divided into two groups based on the occurrence of in-hospital death. As manifested in Table 1, there were no significant discrepancies in demographic and medical characteristics between the non-in-hospital death and the in-hospital death group. Similarly, as for clinical manifestations, no significant differences emerged between the two groups (Table 2). In terms of laboratory examinations, patients who died in hospital showed higher levels of ALT, AST, creatine, BUN, FBG, CK, LDH, and TG, while lower levels of PLT, T-LYM, CD4+ T-LYM, and CD8+ T-LYM. The differences in WBC, NEU, NEU%, LYM, LYM%, Hb, PT, APTT, TBIL, and CRP were not significant (Table 3).
As shown in Figure 2A, the in-hospital mortality of SFTS patients with higher median of TyG index (median: 9.05) was significantly higher than that of patients with lower median [14 (35.0%) vs. 3 (7.7%), P = 0.007]. On the other hand, the level of TyG index in SFTS patients who died in hospital was significantly higher than that in patients who survived (9.74 ± 0.53 vs. 8.96 ± 0.70, P < 0.001) (Figure 2B).
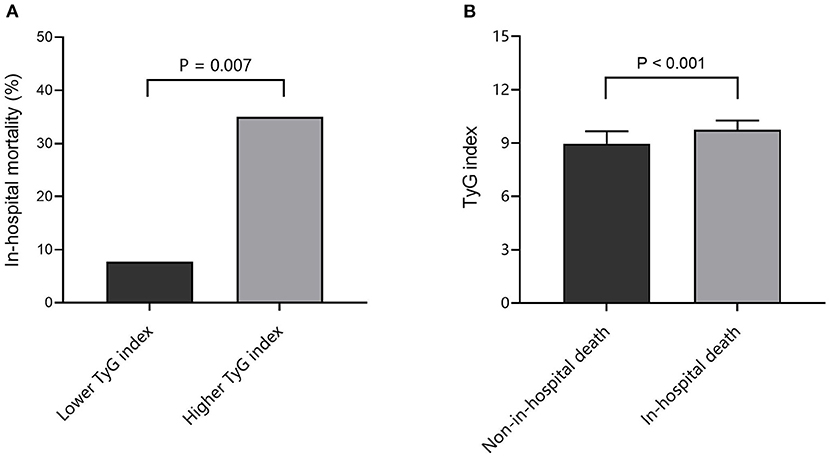
Figure 2. Association between TyG index and in-hospital death. (A) Comparison of in-hospital mortality at different TyG index levels; (B) Comparison of TyG index between non-in-hospital death and in-hospital death cases. TyG, triglyceride-glucose.
Kaplan–Meier survival analysis showed that the cumulative in-hospital survival rate of patients with SFTS with higher TyG index was significantly lower than that of patients with lower TyG index (Log-rank P = 0.004) (Figure 3). Moreover, univariate Cox regression analysis confirmed the significant predictive value of TyG index for in-hospital death, despite regarding TyG index as a nominal variable (unadjusted HR for taking lower median as reference 5.148, 95% CI 1.478–17.936, P = 0.010) or continuous variable (unadjusted HR for per 1-unit increase 4.572, 95% CI 2.064–10.127, P < 0.001).
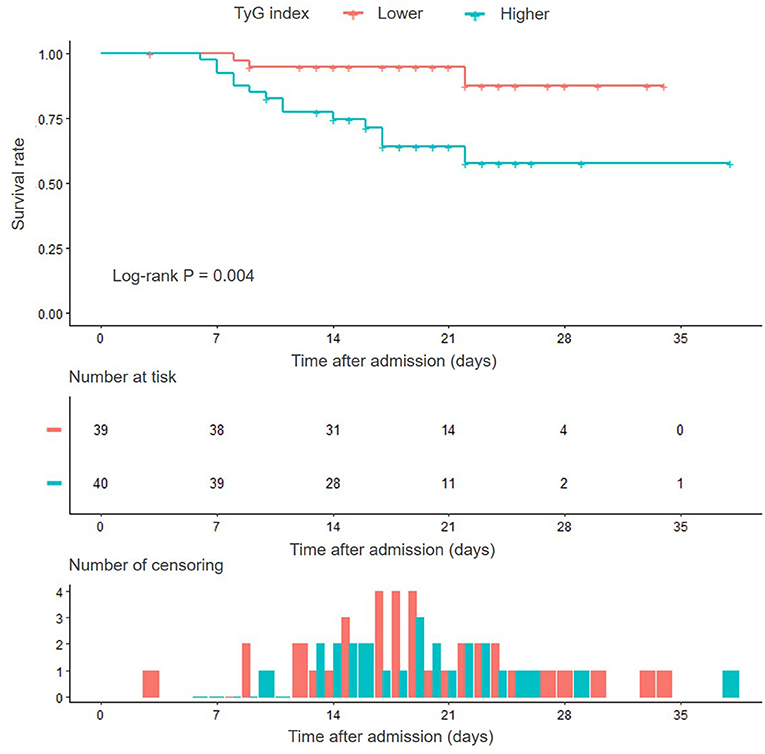
Figure 3. Kaplan–Meier survival curves according to the median of TyG index. TyG, triglyceride-glucose.
The results from univariate Cox regression analysis revealed that PLT, T-LYM, CD4+ T-LYM, CD8+ T-LYM, AST, FBG, CK, LDH, and TG were potential risk predictors of in-hospital death (Supplementary Table 1). Considering the results of univariate analysis and clinical experience, three multivariate models were established to investigate whether TyG index is an independent predictor of in-hospital death. With the adjustment of confounding factors, increased TyG index was independently and robustly associated with an increased risk of in-hospital death, either evaluating HR by taking the lower median of TyG index as reference (Model 1: HR 5.427, 95% CI 1.548–19.022, P = 0.008; Model 2: HR 6.288, 95% CI 1.746–22.651, P = 0.005; Model 3: HR 5.923, 95% CI 1.208–29.036, P = 0.028) or by examining 1-unit increase of TyG index (Model 1: HR 4.367, 95% CI 1.961–9.726, P < 0.001; Model 2: HR 6.782, 95% CI 2.336–19.686, P < 0.001; Model 3: HR 7.309, 95% CI 1.854–28.818, P = 0.004) (Table 4).
The predictive performance of TyG index for in-hospital death was further evaluated by ROC curve analysis. As shown in Figure 4A, TyG index displayed a moderate-to-high strength in predicting in-hospital death, with an AUC of 0.821 (95% CI 0.712–0.929, P < 0.001). The optimal cut-off value was 9.31, under which the sensitivity (95% CI), specificity (95% CI), PPV (95% CI), and NPV (95% CI) for predicting the in-hospital death were 82.4% (56.6–96.2%), 74.2% (61.5–84.5%), 46.7% (35.2–58.5%), and 93.9% (84.5–97.7%), respectively.
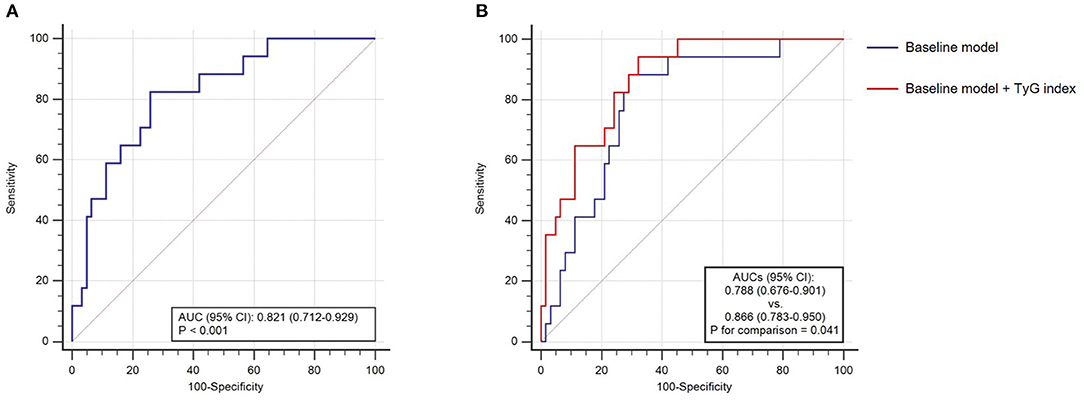
Figure 4. The receiver operating characteristics (ROC) curve analysis. (A) The predictive value of TyG index for in-hospital death; (B) the incremental effect of TyG index on the prediction of in-hospital death. AUC, area under the ROC curve; CI, confidence interval; and TyG, triglyceride-glucose.
After introducing TyG index into a baseline model, such as risk factors identified by former authoritative study (age, neurological manifestations, LDH, AST, BUN, and NEU%) (15), there was a significant incremental effect on the predictive performance for in-hospital death, manifested as an increased AUC (baseline model: 0.788, 95% CI 0.676–0.901 vs. baseline model + TyG index 0.866, 95% CI 0.783–0.950, P for comparison = 0.041) (Figure 4B). Moreover, the addition of TyG index to the baseline model contributed to an increased Harrell's C-index (baseline model: 0.762, 95% CI 0.645–0.880 vs. baseline model + TyG index 0.813, 95% CI 0.724–0.903, P for comparison = 0.035), significant continuous NRI (0.310, 95% CI 0.092–0.714, P = 0.013), and significant IDI (0.111, 95% CI 0.008–0.254, P = 0.040) (Table 5).
As we know, this is the first study to investigate the predictive value of TyG index for in-hospital death in patients with SFTS. The main findings are summarized as follows. (1) Patients with higher TyG index exhibited higher mortality rate, and in reverse, the TyG index in patients with in-hospital death was significantly higher than that of patients without; (2) Despite the adjustment for confounding risk factors, TyG index remained a significant and independent predictor for in-hospital death, either being taken as a nominal or continuous variate; (3) TyG index displayed a moderate-to-high strength in predicting in-hospital death; (4) After being introduced into a baseline model, TyG index showed an incremental effect on the prediction of in-hospital death.
In China, SFTS has been reported mainly in rural regions in the Eastern and Central provinces. SFTSV, which causes SFTS, is a novel phlebovirus in the Bunyaviridae family. The genome of SFTSV consists of three minus-strand RNA segments: large, medium, and small segments (16). SFTSV can invade multiple organs and propagate in the cytoplasm of host cells, such as the spleen, liver, and kidney (17). For critically ill patients with SFTS patients, their clinical symptoms usually exacerbate during the acute phase of the disease and quickly proceed to multiple organ dysfunction syndromes, disseminated intravascular coagulation, and ending in death ~1–2 weeks after the onset of the disease (18). No vaccines or effective drugs against SFTSV have yet been developed. Therefore, early identification of high-risk patients has important clinical significance for improving the prognosis of SFTS. In the early stages of SFTS, patients with severe conditions may have significantly increased markers compared with patients with mild conditions. Our study found that the TyG index in patients with in-hospital death was significantly higher than that of patients without, indicating that the TyG index may be an effective indicator for early identification of patients who are prone to poor outcomes.
As a novel surrogate marker of insulin resistance, TyG index has been extensively used to predict the risk and severity of several diseases. It has been demonstrated that TyG index is a significant predictor for the risk of chronic non-communicable diseases, such as type 2 diabetes mellitus (T2DM), hypertension, cardiovascular disease, and non-alcoholic fatty liver disease (7–10). As for infectious diseases, TyG index has been revealed to be closely associated with the severity of chronic hepatitis B and C, and directly correlated to the viral load of chronic hepatitis C (11, 12). A recent study from China, which investigated the association between TyG index and COVID-19, showed that an increased level of TyG index is closely related to a higher incidence of severe COVID-19. In addition, TyG index could predict the mortality risk of patients with COVID-19 (13). These studies suggest the great potential of TyG index to be served as a novel risk predictor for patients with infectious diseases. The present study, which first elucidated the predictive value of TyG index for in-hospital death in patients with SFTS, fills the gap of previous research in this field. The findings of this study provide a simple and accessible pathway for early identification of patients who are susceptible to develop adverse prognosis, which is of great clinical significance for customizing individualized intervention strategies that match the risk levels. Furthermore, a significant incremental effect on the prediction of in-hospital death emerged after introducing TyG index into a baseline model, such as risk factors identified by former authoritative study, indicating that TyG index may contribute more information to prognostic prediction on the basis of established risk factors.
Although the present study has revealed the close relationship between TyG index and in-hospital outcomes of patients with SFTS, the potential mechanisms involved in this association remained indistinct. Since it has been extensively demonstrated that TyG index is a significant surrogate marker of insulin resistance, we hypothesized that the reason for the association may be attributed to insulin resistance. Insulin resistance plays a vital role in the development of dyslipidemia and hyperglycemia, which makes them the two most important hallmarks of insulin resistance. The formula of TyG index is composed of fasting FBG and TG, while recent evidence has proved that FBG and TG play important role in the progress of SFTS.
Previous studies have shown that patients with SFTS with diabetes and/or increased FBG exhibit higher mortality (19, 20), indicating the important role of glucose metabolism in disease progression. Study has revealed that the plasma levels of glucagon, adrenaline, and glucocorticoid in patients with severe infections are significantly increased, which can further lead to disturbance of glucose metabolism characterized as significant decrease in insulin sensitivity and increase in blood glucose (21). The increased level of blood glucose resulting from severe infections can activate inflammatory responses that cause deterioration of the disease. It has been demonstrated that the increased level of blood glucose promotes a sharp increase in the concentrations of inflammatory cytokines, such as interleukin-6, tumor necrosis factor α, and interleukin-18, and this effect was more pronounced in subjects with abnormal glucose tolerance (22). Moreover, study has proved that the application of antioxidant glutathione can prevent the short-term rise of inflammatory cytokines induced by hyperglycemia, suggesting that hyperglycemia might affect the concentration of cytokines through the oxidative mechanism. On the other hand, studies have confirmed that lipid metabolism plays a pivotal role in viral lifecycle, such as replication, membrane homeostasis, endocytosis, and exocytosis (23, 24). A recent study has proved that the synthesis pathway of cholesterol, fatty acids, and TG regulated by site-1 protease plays an important role in SFTSV replication and reproduction (25), indicating that the excessive synthesis of lipid may be responsible for the increase in SFTSV replication. In vitro experiment confirmed that the replication of SFTSV in cells is significantly decreased after the treatment with lovastatin and fenofibrate, which inhibits the synthesis of cholesterol and TG, while distinctly increased after being introduced into an environment with a high level of TG, which may be an important explanation for the association of TG with adverse prognosis in patients with SFTS (25).
Former studies have revealed that T2DM may impair the immune system by suppressing complement components, dysregulating innate immunocytes (dendritic cell, macrophage, neutrophil, natural killer cell, etc.), and inhibiting adaptive immunocytes (B lymphocyte, T lymphocyte, etc.), which makes individuals more susceptible to the infection by microbes or to severe conditions after infection (26). As the most significant pathogenesis of T2DM, it can be speculated that insulin resistance plays an important role in these processes. On the other hand, infection with a variety of viruses, such as human immunodeficiency virus (27), hepatitis C virus (28), murine gamma herpesvirus 68 (29), may inversely cause or exacerbate insulin resistance, thus leading to a vicious cycle. Additionally, it has been demonstrated that insulin resistance is significantly associated with systemic inflammatory responses (30, 31), which determine the severity and outcomes of infectious diseases. In general, since the current study is hypothesis-generating, further research is required to explore the underlying pathophysiological process and mechanism mediating the association between TyG index and adverse prognosis of patients with SFTS.
Some limitations need to be acknowledged. First, as a retrospective, observational study with relatively small sample size, the statistical strength may be limited. Second, although the measurement of viral load and/or antibody titer can provide useful information, it was not available in the database. Third, the observational endpoint of the present study was defined as the in-hospital death, adverse events obtained from follow-up after discharge may offer more valuable information. Finally, situations, such as stress and fasting duration may have non-negligible effects on the estimation of TyG index.
Triglyceride-glucose index is strongly and independently associated with the risk of in-hospital death in patients with SFTS. TyG index, which is derived from the fasting TG and FBG, may provide valuable information on the basis of established risk factors in identifying patients at high risk of developing in-hospital adverse prognosis, thus can be served as an accessible and useful risk predictor in clinical practice. The findings require further large-scale and prospective studies to confirm.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Clinical Research Ethics Committee of Beijing Ditan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
TZ and ZC made substantial contributions to study design, data analysis, and manuscript writing. ZG and DT made substantial contributions to study design, intellectual direction, and manuscript revision. YL made substantial contributions to data collection, data follow-up, and manuscript revision. DT, LL, ZZ, YS, XY, YF, CQ, JD, YM, TF, and YZ made substantial contributions to data collection and follow-up. All authors have read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (82072295).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.768101/full#supplementary-material
SFTS, severe fever with thrombocytopenia syndrome; SFTSV, SFTS virus; TyG, Triglyceride-glucose; TG, triglyceride; FBG, fasting blood glucose; COVID-19, coronavirus disease-2019; WBC, white blood cell count; NEU, neutrophil count; NEU%, neutrophil percentage; LYM, lymphocyte count; LYM%, lymphocyte percentage; PLT platelet count, ; Hb, hemoglobin; T-LYM, T-lymphocyte; CD4+ T-LYM, CD4 positive T-lymphocyte; CD8+ T-LYM, CD8 positive T-lymphocyte; PT, prothrombin time; APTT, activated partial thromboplastin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; BUN, blood urea nitrogen; CK, creatine kinase; LDH, lactic dehydrogenase; CRP, C-reactive protein; HR, hazard ratio; CI, 95% confidence interval; ROC, receiver operating characteristic; AUC, area under the ROC curve; PPV, positive predictive value; NPV, negative predictive value; NRI, net reclassification improvement; IDI, integrated discrimination improvement; T2DM, type 2 diabetes mellitus.
1. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. (2011) 364:1523–32. doi: 10.1056/NEJMoa1010095
2. Wang M, Zuo J, Hu K. Identification of severe fever with thrombocytopenia syndrome virus in ticks collected from patients. Int J Infect Dis. (2014) 29:82–3. doi: 10.1016/j.ijid.2014.09.008
3. Oh WS, Heo ST, Kim SH, Choi WJ, Han MG, Kim JY. Plasma exchange and ribavirin for rapidly progressive severe fever with thrombocytopenia syndrome. Int J Infect Dis. (2014) 18:84–6. doi: 10.1016/j.ijid.2013.08.011
4. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. (2014) 209:816–27. doi: 10.1093/infdis/jit603
5. Mehand MS, Millett P, Al-Shorbaji F, Roth C, Kieny MP, Murgue B. World Health Organization methodology to prioritize emerging infectious diseases in need of research and development. Emerg Infect Dis. (2018) 24:e171427. doi: 10.3201/eid2409.171427
6. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. (2014) 13:146. doi: 10.1186/s12933-014-0146-3
7. Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. (2017) 16:30. doi: 10.1186/s12933-017-0514-x
8. Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. (2016) 34:1257–65. doi: 10.1097/HJH.0000000000000941
9. Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. (2017) 16:15. doi: 10.1186/s12944-017-0409-6
10. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. (2016) 46:189–97. doi: 10.1111/eci.12583
11. Sterling RK, King WC, Khalili M, Kleiner DE, Hinerman AS, Sulkowski M, et al. Performance of serum-based scores for identification of mild hepatic steatosis in HBV mono-infected and HBV-HIV co-infected adults. Dig Dis Sci. (2021). doi: 10.1007/s10620-021-06860-3 [Epub ahead of print].
12. Petta S, Di Marco V, Di Stefano R, Cabibi D, Cammà C, Marchesini G, et al. TyG index, HOMA score and viral load in patients with chronic hepatitis C due to genotype 1. J Viral Hepat. (2011) 18:e372–80. doi: 10.1111/j.1365-2893.2011.01439.x
13. Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, et al. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. (2020) 19:58. doi: 10.1186/s12933-020-01035-2
14. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
15. Li H, Lu QB, Xing B, Zhang SF, Liu K, Du J, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis. (2018) 18:1127–37. doi: 10.1016/S1473-3099(18)30293-7
16. Lei XY, Liu MM, Yu XJ. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes Infect. (2015) 17:149–54. doi: 10.1016/j.micinf.2014.12.002
17. Jin C, Liang M, Ning J, Gu W, Jiang H, Wu W, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci USA. (2012) 109:10053–8. doi: 10.1073/pnas.1120246109
18. Gai Z, Liang M, Zhang Y, Zhang S, Jin C, Wang SW, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis. (2012) 54:249–52. doi: 10.1093/cid/cir776
19. Zhang SF, Yang ZD, Huang ML, Wang ZB, Hu YY, Miao D, et al. Preexisting chronic conditions for fatal outcome among SFTS patients: An observational Cohort Study. PLoS Negl Trop Dis. (2019) 13:e0007434. doi: 10.1371/journal.pntd.0007434
20. Zhang Y, Zhang Y, Xu Y, Huang Y. The associations between fasting blood glucose levels and mortality of SFTS in patients. BMC Infect Dis. (2021) 21:761. doi: 10.1186/s12879-021-06463-3
21. Shamoon H, Hendler R, Sherwin RS. Altered responsiveness to cortisol, epinephrine, and glucagon in insulin-infused juvenile-onset diabetics. A mechanism for diabetic instability. Diabetes. (1980) 29:284–91. doi: 10.2337/diab.29.4.284
22. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. (2002) 106:2067–72. doi: 10.1161/01.CIR.0000034509.14906.AE
23. Lorizate M, Kräusslich HG. Role of lipids in virus replication. Cold Spring Harb Perspect Biol. (2011) 3:a004820. doi: 10.1101/cshperspect.a004820
24. Murillo A, Vera-Estrella R, Barkla BJ, Méndez E, Arias CF. Identification of host cell factors associated with astrovirus replication in caco-2 cells. J Virol. (2015) 89:10359–70. doi: 10.1128/JVI.01225-15
25. Urata S, Uno Y, Kurosaki Y, Yasuda J. The cholesterol, fatty acid and triglyceride synthesis pathways regulated by site 1 protease (S1P) are required for efficient replication of severe fever with thrombocytopenia syndrome virus. Biochem Biophys Res Commun. (2018) 503:631–6. doi: 10.1016/j.bbrc.2018.06.053
26. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. (2020) 11:1582. doi: 10.3389/fimmu.2020.01582
27. Geffner ME, Patel K, Jacobson DL, Wu J, Miller TL, Hazra R, et al. Changes in insulin sensitivity over time and associated factors in HIV-infected adolescents. AIDS. (2018) 32:613–22. doi: 10.1097/QAD.0000000000001731
28. Ding Y, Li G, Zhou Z, Deng T. Molecular mechanisms underlying hepatitis C virus infection-related diabetes. Metabolism. (2021) 121:154802. doi: 10.1016/j.metabol.2021.154802
29. Zhao L, Chen YX, Varghese Z, Huang AL, Tang RK, Jia B, et al. Murine gamma herpes virus 68 infection promotes fatty liver formation and hepatic insulin resistance in C57BL/6J mice. Hepatol Int. (2012) 6:520–30. doi: 10.1007/s12072-011-9283-x
30. Shoelson SE, Lee J. Goldfine A Inflammation B, insulin resistance. J Clin Invest. (2006) 116:1793–801. doi: 10.1172/JCI29069
Keywords: severe fever with thrombocytopenia syndrome, triglyceride-glucose index, in-hospital mortality, triglyceride, fasting blood glucose
Citation: Zhang T, Liu Y, Ge Z, Tian D, Lin L, Zhao Z, Shen Y, Yu X, Feng Y, Qiang C, Duan J, Ma Y, Fan T, Zhao Y and Chen Z (2022) Predictive Value of Triglyceride-Glucose Index for In-hospital Mortality in Patients With Severe Fever With Thrombocytopenia Syndrome: A Multi-Center Observational Study. Front. Med. 8:768101. doi: 10.3389/fmed.2021.768101
Received: 11 October 2021; Accepted: 25 November 2021;
Published: 04 January 2022.
Edited by:
Hamad Ali, Kuwait University, KuwaitCopyright © 2022 Zhang, Liu, Ge, Tian, Lin, Zhao, Shen, Yu, Feng, Qiang, Duan, Ma, Fan, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihai Chen, Y2hlbnpoaWhhaTAwMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.