- 1Department of Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Department of Nursing, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Critical Care Medicine, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, China
- 4Shanghai Key Lab of Pulmonary Inflammation and Injury, Fudan University, Shanghai, China
Background: Enteral nutrition (EN) is recommended within the first 24–48 h for patients with hemodynamic stability, following admission to an intensive care unit (ICU). However, for patients with approximate stable hemodynamics requiring mechanical circulatory support and vasoactive drugs, the application of early EN remains controversial. We sought to evaluate the tolerance of early EN in patients with cardiogenic shock who required vasoactive drugs and mechanical circulatory support after cardiac surgery.
Methods: This single-center, prospective observational study included patients with cardiogenic shock, requiring vasoactive drugs and mechanical circulatory support after cardiac surgery, undergoing EN. The primary endpoint was EN tolerance and secondary endpoints were mortality, length of mechanical ventilation, and length of ICU stay.
Results: From February 2019 to December 2020, 59 patients were enrolled, of which 25 (42.37%) developed intolerance within 3 days of starting EN. Patients in the EN intolerant group had a longer median length of mechanical ventilation (380 vs. 128 h, p = 0.006), a longer median ICU stay (20 vs. 11.5 days, p = 0.03), and a higher proportion of bloodstream infections (44 vs. 14.71%, p = 0.018). The median EN calorie levels for all patients in the first 3 days of EN were 4.00, 4.13, and 4.28 kcal/kg/day, respectively. Median protein intake levels of EN in the first 3 days were 0.18, 0.17, and 0.17 g/kg/day, respectively. No significant difference was observed in the median dose of vasoactive drugs between the groups (0.035 vs. 0.05 μg/kg/min, p = 0.306).
Conclusions: Patients with cardiogenic shock after cardiac surgery had a high proportion of early EN intolerance, and patients with EN intolerance had a worse prognosis, but no significant correlation was identified between EN tolerance and the dose of vasoactive drugs.
Introduction
According to the guidelines from the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN), enteral nutrition (EN) is recommended for patients admitted to an intensive care unit (ICU) once hemodynamics are stable (1). Similarly, the European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines also recommend that if oral intake is not possible, early EN (within 48 h) in critically ill adult patients should be performed/initiated rather than delaying it (2). Early EN nourishes the intestinal mucosa, maintains intestinal integrity, maintains intestinal microbial diversity, and improves immunity and metabolic function (3). Therefore, when compared with delayed EN, early EN reduces infectious complications (4–20).
Although early EN is recommended for most critically ill patients, the European Society of Intensive Care Medicine (ESICM) guidelines advocate seven scenarios that require delayed EN, including uncontrolled shock and failure to achieve hemodynamic and tissue perfusion targets (14). Some studies have demonstrated that vasoactive drugs aggravate visceral vasoconstriction and intestinal metabolic disorders caused by shock, and this may lead to intestinal ischemia (21). Similarly, early EN was speculated to cause abdominal distension, diarrhea, vomiting, aspiration, and possibly death (22–25). However, a recent study reported that intestinal ischemia is rare in patients receiving vasoactive drugs during EN; the incidence is 0.3–3.8% (21). However, while previous studies have focused on septic shock patients, data on EN safety in patients with cardiogenic shock are limited, and critically, conclusions are inconsistent (26–31). Furthermore, a consensus has not been reached on safe vasoactive drug doses during early EN.
Patients with cardiac surgery frequently present with circulatory failure for various reasons (32–35). Vasoactive drugs and mechanical circulatory support are thus required to achieve hemodynamic targets. In this study, we investigated the tolerance of early EN in patients taking vasoactive drugs and undergoing mechanical circulatory support after cardiac surgery to determine the effects of different vasoactive drug doses on the safety of early EN administration.
Materials and Methods
Study Design
This was a single-center prospective observational study. From February 2019 to December 2020, patients were continuously enrolled from a cardiac surgery ICU of a tertiary hospital. The ICU has 40 beds and accommodates various cardiac surgery perioperative patients. The study was approved by the ethics committee of Zhongshan Hospital, Fudan University (Approval No. B2019-075R), and patients or family members provided informed consent prior to study commencement.
Participant Selection
Inclusion criteria were as follows: (1) age ≥ 18 years, (2) patients with cardiogenic shock receiving vasoactive drugs and mechanical circulatory support, including extracorporeal membrane oxygenation (ECMO) or intraaortic balloon pump (IABP), (3) mean arterial pressure ≥ 65 mmHg, (4) starting EN within 48 h after hemodynamic stability, and estimated EN duration ≥ 72 h.
Exclusion criteria included were as follows: (1) discontinued vasoactive drugs and mechanical circulatory support within 1 h after EN commencement, (2) situations where ESICM guidelines recommended EN should be delayed, i.e., uncontrolled hypoxemia and acidosis, uncontrolled gastric intestinal bleeding, overt bowel ischemia, bowel obstruction, abdominal compartment syndrome, and gastric aspirate volume > 500 ml/6 h. Cardiogenic shock was defined as a state of critical end-organ hypoperfusion due to reduced cardiac output (36).
Data Collection and Outcome Definitions
According to the EN tolerance of patients, they were divided into EN tolerant and EN intolerant groups. EN intolerance was defined as gastric residual volume (GRV) > 250 ml on any day or any kind of EN complication (vomiting, abdominal distension, diarrhea, intestinal ischemia, and aspiration) within 3 days of EN (10, 12). Aspiration was defined as digestive fluid or EN solution in the respiratory tract by bronchoscope. Continuous gastrointestinal decompression was performed 1 h after the end of EN, and the amount of gastrointestinal decompression was defined as the GRV.
Patient baseline data were collected within 24 h after ICU admission, including patient characteristics, such as age, gender, height, weight, body mass index (BMI), types of mechanical circulatory support, comorbidity, previous cardiac surgery, left ventricular ejection fraction (LVEF) before surgery, acute physiology and chronic health evaluation (APACHE) II scores, surgery time, cardiopulmonary bypass time, and laboratory data such as liver function, renal function, cardiac biomarker, and serum lactate indices. Nutrition-related data were collected for 3 consecutive days after the start of EN and included daily GRV, EN volume, protein levels, calories provided by EN, calories provided by parenteral nutrition (PN), and calories provided by propofol. These data were derived from the in-house electronic medical record system and nurse-record sheets.
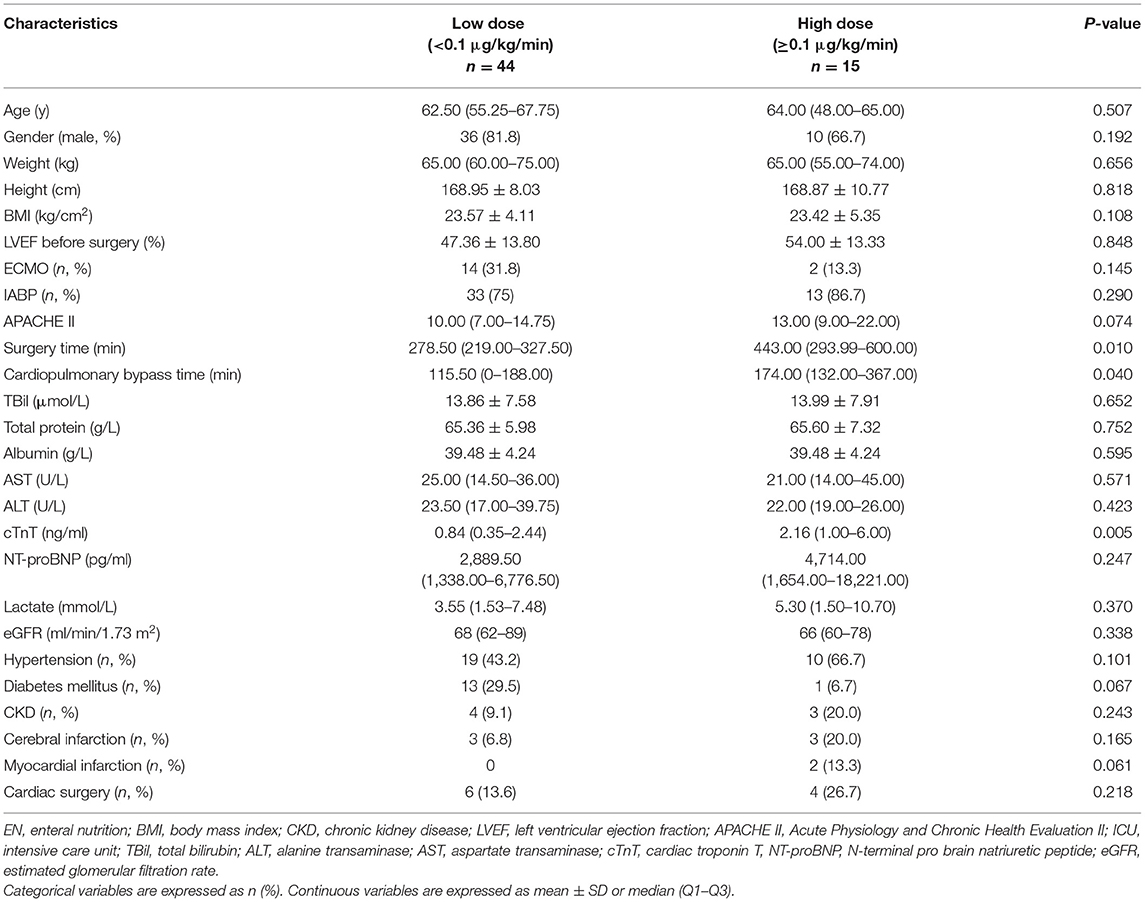
Information on vasoactive drug types and doses in the first 3 days of EN were collected. The following formula was used to calculate the equivalent dose of norepinephrine, where equivalent dose of norepinephrine = norepinephrine (μg/min) + dopamine (μg/min) ÷ 2 + epinephrine (μg/min) + phenylephrine (μg/min) ÷ 10 + vasopressin (U/h) × 8.33 (26). Based on previous practices, according to vasoactive drug doses, patients were divided into low- and high-dose groups: the average equivalent dose of norepinephrine <0.1 μg/kg/min during EN was defined as the low-dose group, whereas ≥0.1 μg/kg/min was defined as the high-dose group (27).
The primary study endpoint was patient EN intolerance. Secondary endpoints were the length of mechanical ventilation, length of ICU stay, length of stay in the hospital, in-hospital mortality, the incidence of infection, and the site of infection (e.g., pulmonary, wound, urinary system, and bloodstream infection). Intolerance signs were also recorded, including GRV > 250 ml and EN-related adverse events (e.g., vomiting, abdominal distension, diarrhea, intestinal ischemia, and aspiration).
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics version 20. After using the KS test to evaluate data normality, normally distributed data were represented by the mean (±SD), and non-normally distributed data were represented by the median [interquartile range (IQR)]. The χ2 -test was used for categorical variables and the t-test or Mann–Whitney U-test for continuous variables. A p < 0.05 value was considered statistically significant.
Results
Baseline Patient Characteristics
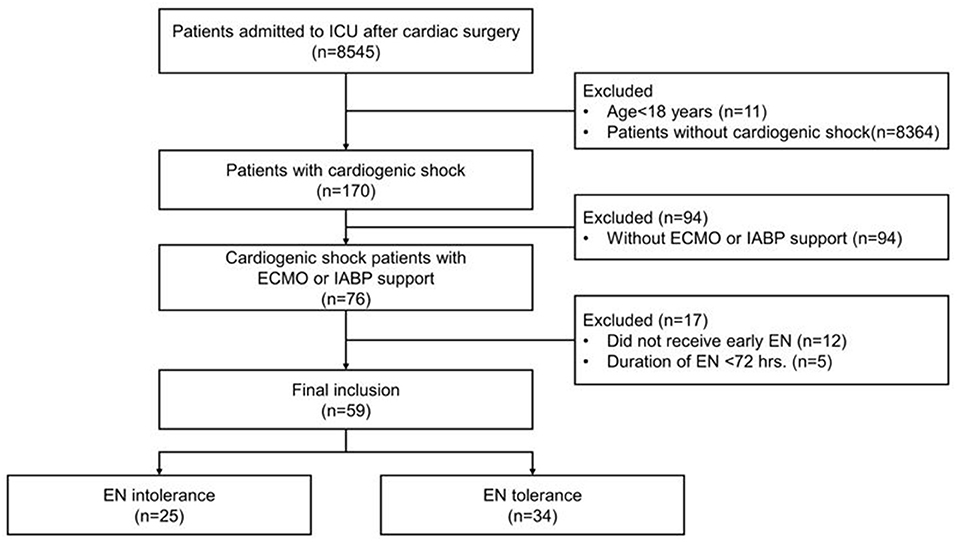
From February 2019 to December 2020, 8,545 patients, after cardiac surgery, were admitted to the cardiac surgery ICU. In total, 170 adult patients were diagnosed with cardiogenic shock with an ICU stay >72 h. Of these, 94 patients were excluded because they did not receive mechanical circulatory support, 12 were excluded because they did not receive early EN, and five were excluded because the EN duration was <72 h. Therefore, 59 patients were finally enrolled and divided into two groups; 25 in the EN intolerant group and 34 in EN tolerant group (Figure 1).
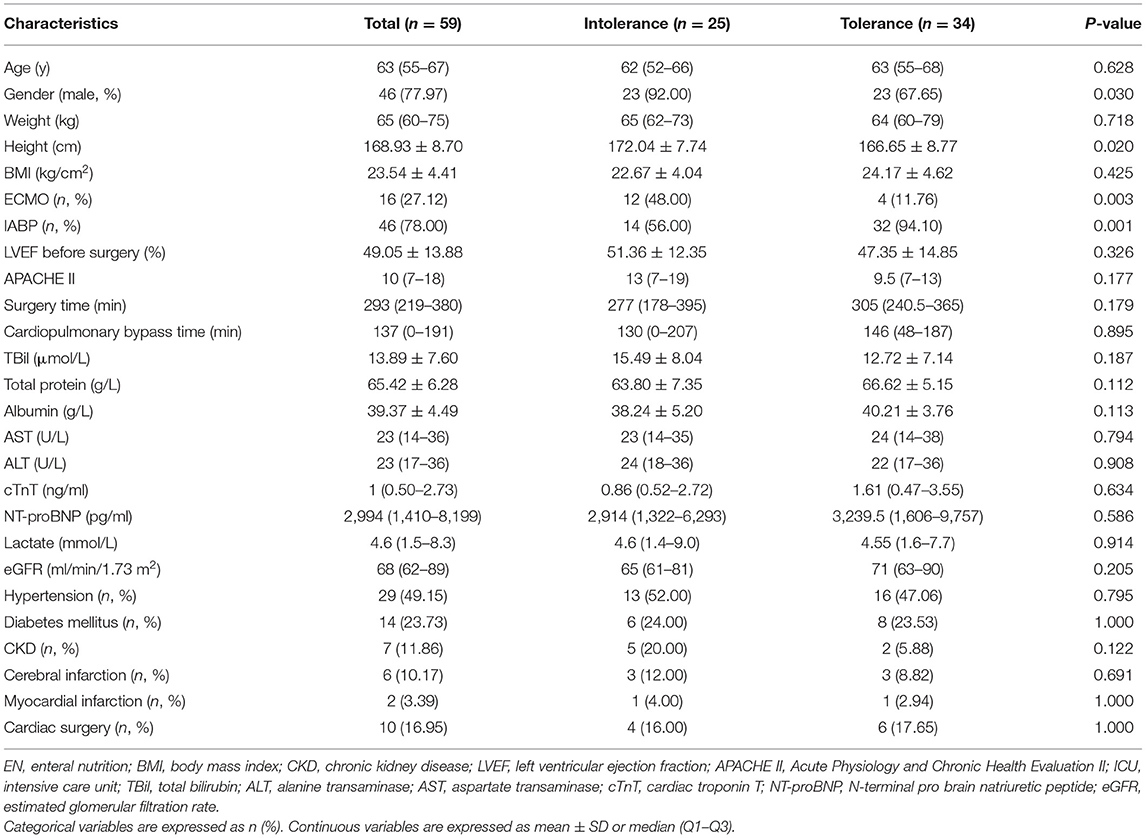
Patient baseline data are shown in Table 1. The median age was 63 years old, the majority were male (77.97%), and the average BMI was 23.5 kg/cm2. Sixteen patients received ECMO, 46 received IABP, and 10 had received cardiac surgery prior to this admission. The median APACHE II score was 10, the average LVEF before surgery was 49%, the median surgery time was 293 min, the median cardiopulmonary bypass time was 137 min, the median cardiac troponin T (cTnT) level was 1 ng/mL, the median N-terminal probrain natriuretic peptide (NT-proBNP) level was 2,994 pg/mL, and the median lactate level was 4.6 mmol/L. The proportion of men in the EN intolerant group was significantly higher (92 vs. 68%, p = 0.03). In the EN intolerant group, the proportion of patients receiving ECMO was higher (48 vs. 11.76%, p = 0.003). We observed no statistical differences between groups in terms of age, BMI, comorbidity, pre-operative cardiac functions, APACHE II scores, surgery time, cardiopulmonary bypass time, and indices for liver function, renal function, cardiac biomarkers, and serum lactate levels.
Primary and Secondary Outcomes
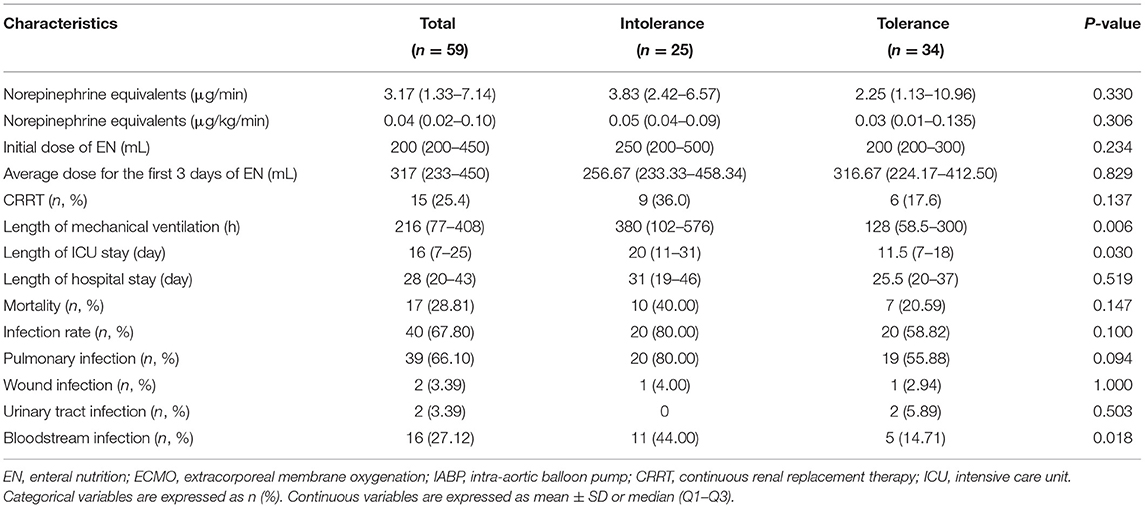
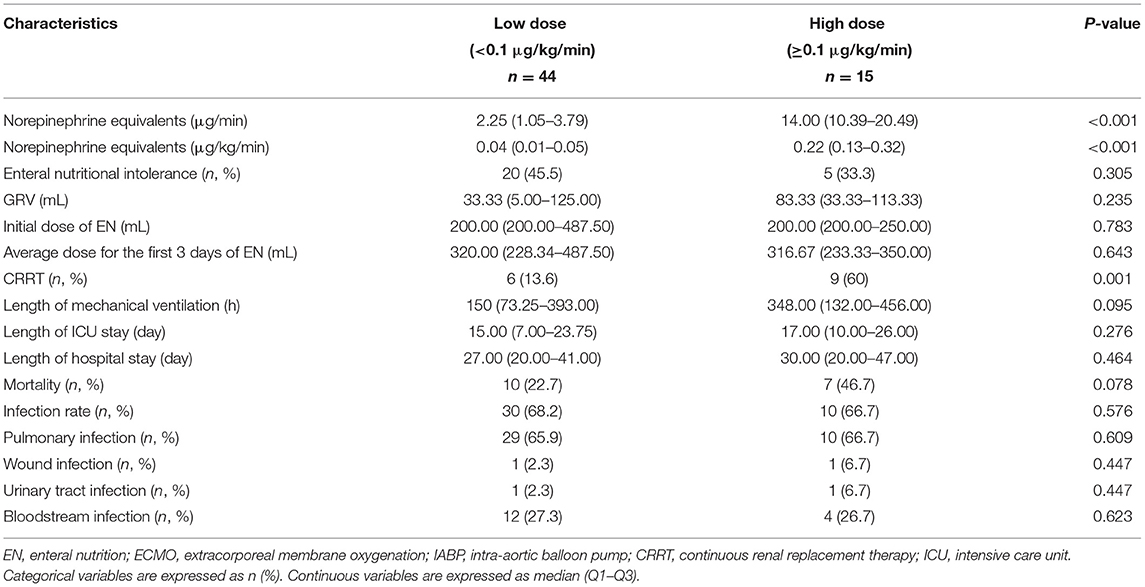
As indicated, 25 (42.37%) patients were EN intolerant. As shown in Table 2, the median dose of norepinephrine equivalent in EN tolerant and intolerant groups was not statistically different (0.035 vs. 0.05 μg/kg/min, p = 0.306). The median length of mechanical ventilation in the EN intolerant group was significantly longer (380 vs. 128 h, p = 0.006). In addition, the median length of ICU stay for the EN intolerant group was longer than the EN tolerant group (20 vs. 11.5 days, p = 0.03), but no significant difference in the length of hospital stay was observed between the groups (31 vs. 25.5 days, p = 0.519). Although the mortality rate between groups was not statistically different, the mortality rate of the EN intolerant group was almost twice that of the EN tolerant group (40 vs. 20.59%, p = 0.147). Moreover, the proportion of patients receiving CRRT was slightly higher in the EN intolerant group (36 vs. 17.65%, p = 0.137).
We observed no statistical difference in the dose of enteral feeding on the first 3 days between the groups. In terms of post-operative infections, the bloodstream infection rate in the EN intolerant group was higher than the EN tolerant group (44 vs. 14.71%, p = 0.018). Pulmonary infection was more common in the EN intolerant group, although no statistical differences were identified between the groups (80 vs. 55.88%, p = 0.094).
Among EN intolerant patients, nine (36%) had GRV > 250 mL, seven (28%) experienced aspiration, six (24%) had diarrhea, three (12%) experienced vomiting, and five (20%) had abdominal distension. No bowel ischemia occurred in this study (Table 3).
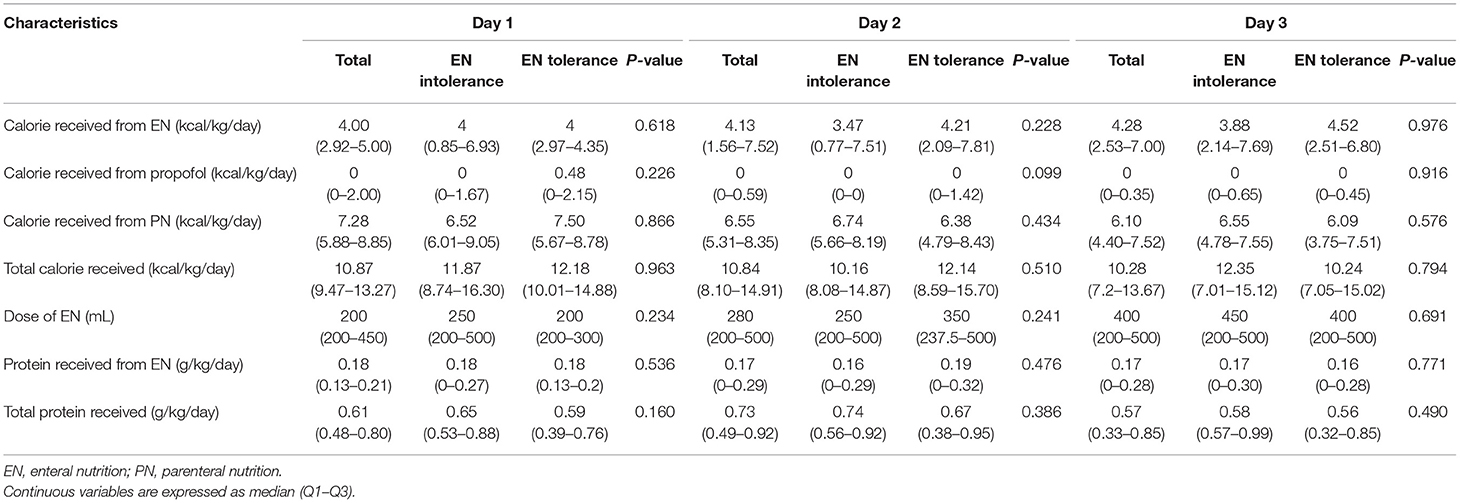
The calorie and protein intake of all patients in the first 3 days are shown (Table 4). The median calorie levels of EN in the first 3 days were 4.00, 4.13, and 4.28 kcal/kg/day, respectively, with an upward trend each day. The median calorie levels of PN in the first 3 days were 7.28, 6.55, and 6.10 kcal/kg/day, respectively, indicating a daily downward trend. The median total calories in the first 3 days were 10.87, 10.84, and 10.28 kcal/kg/day, respectively, which were basically the same. The median EN volume for patients in the first 3 days was 200, 280, and 400 mL, respectively, indicating an increasing trend. The median protein intake of EN in the first 3 days was 0.18, 0.17, and 0.17, respectively, whereas the median total protein intake was 0.61, 0.73, and 0.57 g/kg/day, respectively (Table 4). However, no significant differences were observed in either calorie or protein intake between the groups.
According to vasoactive drug doses, the average equivalent dose of norepinephrine <0.1 μg/kg/min was defined as the low-dose group, whereas norepinephrine ≥0.1 μg/kg/min was defined as the high-dose group. Surgery time (278.5 vs. 443.0 min, p = 0.01) and cardiopulmonary bypass time (115.5 vs. 174.0 min, p = 0.04) were significantly shorter in the low-dose group. Levels of cTnT after surgery in the low-dose group were lower (0.84 vs. 2.16 ng/mL, p = 0.005), and NT-proBNP levels in the low-dose group exhibited a lower trend (2,889.5 vs. 4,714 pg/mL, p = 0.247). Serum lactate levels after surgery in both the groups were not significantly different (3.55 vs. 5.30 mmol/L, p = 0.37).
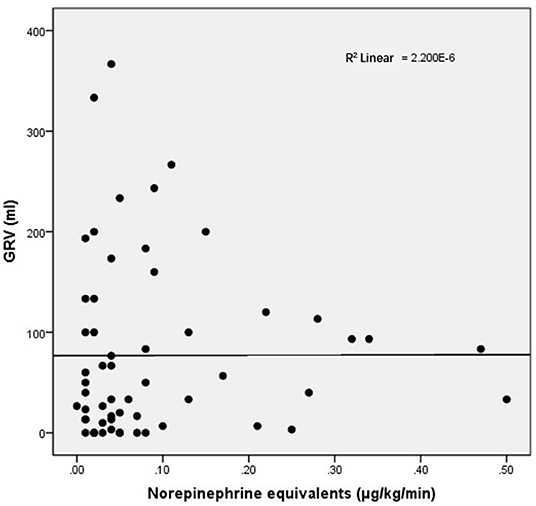
For other baseline data, no significant differences were recorded between the groups (Table 5). Clinical outcomes are shown in Table 6. The CRRT rate in the high-dose group was higher (60 vs. 13.6%, p = 0.001). The length of mechanical ventilation tended to be prolonged in the high-dose group (348 vs. 150 h, p = 0.095), and the mortality rate also tended to increase in the high-dose group (46.7 vs. 22.7%, p = 0.078). No significant difference in GRV was observed between high- and low-dose groups (83.33 vs. 33.33 mL, p = 0.235). As shown in Figure 2, the equivalent dose of norepinephrine and GRV was discretely distributed in the scatter plot, and R2 was 2.200 × 10−6, which indicated no correlation between the equivalent dose of norepinephrine and GRV. No significant difference was observed in the EN intolerance rates between high- and low-dose groups (33.3 vs. 45.5%, p = 0.305).
Discussion
In our study, 25/59 patients (42.37%) with cardiogenic shock after cardiac surgery were intolerant to EN, similar to previous studies (26–28). Merchan et al. reported for patients with sepsis receiving vasoactive drugs that 62/120 were EN tolerant, indicating an intolerance rate of 48.33% (26). However, in another study, 259 patients with vasoactive drug support had an EN intolerance rate of 25.1% (27). For patients undergoing mechanical ventilation, a previous study with 1,888 ICU patients showed an EN intolerance rate of 30.5% (28). Thus, differences in EN intolerance rates between these studies may have arisen due to different patient populations and non-uniform definitions of EN intolerance. In patients who received mechanical ventilation >72 h after cardiovascular surgery, an EN intolerance rate of 43.68% was determined (28), similar to our data. This result suggested that patients with cardiogenic shock had a higher rate of EN intolerance during vasoactive drug and mechanical circulatory support. Therefore, caution should be exercised when these patients commence EN.
It was previously demonstrated that patients with EN intolerance had higher mortality, shorter length of mechanical ventilation free time, longer ICU stays, reduced calorie intake, and poorer outcomes (26, 28). Our study confirmed these findings. Patients with EN intolerance had significantly longer mechanical ventilation time, longer ICU stays, and a higher incidence of bloodstream infections. Although no statistical differences in mortality were observed between groups, mortality in the EN intolerant group was approximately twice that of the EN tolerant group. Our research demonstrated that 28% of EN intolerant patients experienced aspiration issues, which may be partially explained by the high incidence of pulmonary infections in these patients. Unfortunately, there was no statistical difference in mortality due to the small sample size.
In previous studies, the caloric compliance rate of EN in some patients with shock was between 40 and 89.8% (28, 29), among which EN intolerant patients received approximately 10.9–12 kcal/kg/d (26, 27). Our ICU had previously adopted a trophic nutrition strategy for patients with cardiogenic shock. Both calorie and protein intake levels were lower than previous studies. This may have been due to the high rate of EN intolerance in this study, and also the requirement for patients with cardiogenic shock to be fluid restricted. Hence, calorie and protein nutrition from EN and PN were both low. Furthermore, based on our previous findings, the implementation of a soybean-based intravenous fat emulsion restriction diet in cardiac surgical patients was associated with a reduced post-operative nosocomial infection rate (37). It also reduced the length of ICU/hospital stay, hospital costs, mechanical ventilation time, and a lower incidence of cholestasis. Therefore, our cardiovascular center implemented a soybean-based intravenous fat emulsion restriction diet for cardiac surgical patients. This factor was the cause of relatively low calorie and protein levels.
The relationship between vasoactive drug doses and tolerance and prognosis of early EN is inconsistent in the literature. Mancl et al. reported that in patients with septic shock, the incidence of EN intolerance was positively correlated with vasoactive drug doses (27). Therefore, many studies have sought to determine safe vasoactive drug doses when implementing early EN. Ohbe et al. compared differences in clinical outcomes for early (<48 h) or late (≥48 h) EN in shock patients on mechanical ventilation taking vasoactive drugs. Patients were divided into three groups based on the norepinephrine equivalent: low (<0.1 μg/kg/min), medium (0.1–0.3 μg/kg/min), and high (>0.3 μg/kg/min) doses. The 28-day mortality rate was significantly lower in the early EN than in the late EN groups in low- and medium-dose groups. In the high-dose group, the 28-day mortality rate did not differ significantly between the early EN and the late EN groups. Additionally, no significant difference was observed in the non-obstructive mesenteric ischemia rate (0.2 vs. 0.3%) between the early and the late EN groups (28). Thus, when supported by low and medium vasoactive drug doses, early EN was considered safe and it improved patient outcomes. Another study revealed it was safe to commence early EN in mechanically ventilated septic shock patients, with norepinephrine usage <0.14 μg/kg/min (26). However, early EN is not always safe for patients taking vasoactive drugs and those who are on mechanical ventilation support. Reignier et al., compared patient outcomes in those receiving EN and PN who were under mechanical ventilation and vasoactive drug support. Although no differences in mortality were determined, intestinal ischemia was significantly increased in patients on EN. It should be noted these patients received a very high vasopressor dose (average 0.53 μg/kg/min) (21). This observation suggested that when high-dose vasoactive drugs are used, EN should be administered with caution.
In our study, based on the vasoactive drug dose, the EN intolerance rate was 45.5% in the low-dose group and 33.3% in the high-dose group. Furthermore, no significant correlation between the average GRV and vasoactive drug dose was observed. This result was inconsistent with previous studies (28) and maybe related to our small sample size. Previous studies reported it was unsafe to commence EN when high-dose vasoactive drugs were used, and the rate of EN tolerance was negatively correlated with the dose of vasoactive drugs. The reported safe dosage is <0.14–0.32 μg/kg/min (26, 31). In our study, the overall vasoactive drug dose was relatively low, and most patients were within safe doses, as reported previously. This possibly explained the non-significant correlation between the average GRV and the vasoactive drug dose. In addition, serum lactate levels in low- and high-dose groups exhibited no statistical differences, suggesting that the timing of EN initiation cannot be based only on the vasoactive drug dose and serum lactate levels. Thus, the optimal timing for EN initiation requires further investigation.
Our study had some limitations. First, the sample size was small, with only 59 patients; thus, some bias may have been introduced. Second, the overall dose of vasoactive drugs was low, and most were within safe doses as indicated by previous studies; however, this factor may have restricted analyses of the relationships between vasoactive drug doses and the rate of EN intolerance. Third, patient calorie and protein intakes were low, which may have been related to fluid restriction and the high EN intolerant rate generated by cardiogenic shock.
Conclusions
Patients with cardiogenic shock, taking vasoactive drugs, and undergoing mechanical circulatory support had a high proportion of early EN intolerance, which was associated with adverse prognoses. However, no significant correlations were identified between EN tolerance and vasoactive drug doses.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
W-jL, JZ, G-wT, and ZL: conception and design. G-wT and ZL: administrative support. JZ, J-cL, and J-fM: collection and assembly of data. W-jL and JZ: data analysis and interpretation. All authors: manuscript writing and final approval of manuscript.
Funding
This study was supported by the Construction Program of key but weak disciplines of Shanghai Health Commission (2019ZB0105), Natural Science Foundation of Shanghai (20ZR1411100 and 21ZR1412900), Science and Technology Commission of Shanghai Municipality (20DZ2261200), National Natural Science Foundation of China (82070085), Clinical Research Project of Zhongshan Hospital (2020ZSLC38 and 2020ZSLC27), Smart Medical Care of Zhongshan Hospital (2020ZHZS01), Project for the elite backbone of Zhongshan Hospital (2021ZSGG06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (ASP EN). J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863
2. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037
3. McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: the gut in stress. Clin Nutr. (2018) 37:19–36. doi: 10.1016/j.clnu.2017.07.015
4. Jolliet P, Pichard C, Biolo G, Chiolero R, Grimble G, Leverve X, et al. Enteral nutrition in intensive care patients: a practical approach. J Clin Nutr. (1999) 18:47–56. doi: 10.1016/S0261-5614(99)80049-1
5. Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA. Early versus delayed feeding with an immune-enhancing diet in patients with severe head injuries. J Parenter Enteral Nutr. (2000) 24:145–9. doi: 10.1177/0148607100024003145
6. Peck MD, Kessler M, Cairns BA, Chang YH, Ivanova A, Schooler W. Early enteral nutrition does not decrease hypermetabolism associated with burn injury. J Trauma. (2004) 57:1143–8; discussion 1148–9. doi: 10.1097/01.ta.0000145826.84657.38
7. Nguyen NQ, Fraser RJ, Bryant LK, Burgstad C, Chapman MJ, Bellon M, et al. The impact of delaying enteral feeding on gastric emptying, plasma cholecystokinin, and peptide YY concentrations in critically ill patients. Crit Care Med. (2008) 36:1469–74. doi: 10.1097/CCM.0b013e31816fc457
8. Moses V, Mahendri NV, John G, Peter JV, Ganesh A. Early hypocaloric enteral nutritional supplementation in acute organophosphate poisoning-a prospective randomized trial. Clin Toxicol. (2009) 47:419–24. doi: 10.1080/15563650902936664
9. Chourdakis M, Kraus MM, Tzellos T, Sardeli C, Peftoulidou M, Vassilakos D, et al. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury: an open-labeled randomized trial. J Parenter Enteral Nutr. (2012) 36:108–16. doi: 10.1177/0148607110397878
10. Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. (2001) 17:91–4. doi: 10.1016/s0899-9007(00)00508-6
11. Malhotra A, Mathur AK, Gupta S. Early enteral nutrition after surgical treatment of gut perforations: a prospective randomized study. J Postgrad Med. (2004) 50:102–6. Available online at: https://www.jpgmonline.com/text.asp?2004/50/2/102/8246
12. Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. (2005) 29:1023–7; discussion 1027–8. doi: 10.1007/s00268-005-7491-z
13. Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. (2011) 30:560–6. doi: 10.1016/j.clnu.2011.02.006
14. Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. (2017) 43:380–98. doi: 10.1007/s00134-016-4665-0
15. Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early enteral nutrition provided within 24 hours of ICU admission: a meta-analysis of randomized controlled trials. Crit Care Med. (2018) 46:1049–56. doi: 10.1097/CCM.0000000000003152
16. Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, et al. Dutch pancreatitis study group early versus on demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. (2014) 371:1983–93. doi: 10.1056/NEJMoa1404393
17. Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. (2014) 371:1673–84. doi: 10.1056/NEJMoa1409860
18. Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol. (2013) 19:917–22. doi: 10.3748/wjg.v19.i6.917
19. Boelens PG, Heesakkers FFBM, Luyer MDP, van Barneveld KWY, de Hingh IHJT, Nieuwenhuijzen GAP, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled study. Ann Surg. (2014) 259:649–55. doi: 10.1097/SLA.0000000000000288
20. Aiko S, Yoshizumi Y, Sugiura Y, Matsuyama T, Naito Y, Matsuzari J, et al. Beneficial effects of immediate enteral nutrition after esophageal cancer surgery. Surg Today. (2001) 31:971–8. doi: 10.1007/s005950170005
21. Park WM, Bower TC. Contemporary management of acute mesenteric ischemia: factors associated with survival. J Vasc Surg. (2002) 35:445–52. doi: 10.1067/mva.2002.120373
22. Gaddy MC, Max MH, Schwab CW, Kauder D. Small bowel ischemia: a consequence of feeding jejunostomy? South Med J. (1986) 79:180–2. doi: 10.1097/00007611-198602000-00011
23. Brenner DW, Schellhammer PF. Mortality associated with feeding catheter jejunostomy after radical cystectomy. Urology. (1987) 30:337–40. doi: 10.1016/0090-4295(87)90296-2
24. Schunn CD, Daly JM. Small bowel necrosis associated with postoperative jejunal tube feeding. J Am Coll Surg. (1995) 180:410–6.
25. Kowal-Vern A, McGill V, Gamelli RL. Ischemic necrotic bowel disease in thermal injury. Arch Surg. (1997) 132:440–3. doi: 10.1001/archsurg.1997.01430280114020
26. Merchan C, Altshuler D, Aberle C, Papadopoulos J, Schwartz D. Tolerability of enteral nutrition in mechanically ventilated patients with septic shock who require vasopressors. J Intensive Care Med. (2017) 32:540–6. doi: 10.1177/0885066616656799
27. Mancl EE, Muzevich KM. Tolerability and Safety of Enteral Nutrition in Critically Ill Patients Receiving Intravenous Vasopressor Therapy. Journal of Parenteral and Enteral Nutrition. (2013) 37:641–51. doi: 10.1177/0148607112470460
28. Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Dukes G, Williamson R, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. J Parenter Enteral Nutr. (2015) 39:441–8. doi: 10.1177/0148607114526450
29. Bear DE, Smith E, Barret NA. Nutrition support in adult patients receiving extracorporeal membrane oxygenation. Nutr Clin Pract. (2018) 33:738–46. doi: 10.1002/ncp.10211
30. Ohbe H, Jo T, Matsui H, Fushimi K, Yasunaga H. Differences in effect of early enteral nutrition on mortality among ventilated adults with shock requiring low-, medium-, and high-dose noradrenaline: a propensity-matched analysis. Clin Nutr. (2020) 39:460–7. doi: 10.1016/j.clnu.2019.02.020
31. Patel JJ, Rice T, Heyland DK. Safety and outcomes of early enteral nutrition in circulatory shock. J Parenter Enteral Nutr. (2020) 44:779–84. doi: 10.1002/jpen.1793
32. Hernandez AF, Grab JD, Gammie JS, O'Brien SM, Hammill BG, Rogers JG, et al. A decade of short-term outcomes in post-cardiac surgery ventricular assist device implantation. Circulation. (2007) 116:606–12. doi: 10.1161/CIRCULATIONAHA.106.666289
33. DeRose JJ, Umana JP, Argenziano M, Catanese KA, Levin HR, Sun BC, et al. Improved results for postcardiotomy cardiogenic shock with the use of implantable left ventricular assist devices. Ann Thorac Surg. (1997) 64:1757–62; discussion 1762–3. doi: 10.1016/S0003-4975(97)01107-7
34. Muehrcke DD, McCarthy PM, Stewart RW, Foster RC, Ogella DA, Borsh JA, et al. Extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. Ann Thorac Surg. (1996) 61:684–91. doi: 10.1016/0003-4975(95)01042-4
35. Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol. (2016) 13:481–92. doi: 10.1038/nrcardio.2016.96
36. Levy B, Bastien O, Bendjelid K, Cariou A, Chouihed T, Combes A, et al. Experts' recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. (2015) 5:17. doi: 10.1186/s13613-015-0063-y
Keywords: enteral nutrition, cardiogenic shock, mechanical circulatory support, vasoactive drugs, tolerance
Citation: Liu W-j, Zhong J, Luo J-c, Zheng J-l, Ma J-f, Ju M-j, Su Y, Liu K, Tu G-w and Luo Z (2021) Early Enteral Nutrition Tolerance in Patients With Cardiogenic Shock Requiring Mechanical Circulatory Support. Front. Med. 8:765424. doi: 10.3389/fmed.2021.765424
Received: 27 August 2021; Accepted: 04 October 2021;
Published: 06 December 2021.
Edited by:
Xiaotong Hou, Capital Medical University, ChinaReviewed by:
Jianneng Pan, University of Chinese Academy of Sciences, ChinaJun Gu, Shanghai Ninth People's Hospital, China
Copyright © 2021 Liu, Zhong, Luo, Zheng, Ma, Ju, Su, Liu, Tu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-wei Tu, dHUuZ3Vvd2VpQHpzLWhvc3BpdGFsLnNoLmNu; Zhe Luo, ZG9jdG9ybHpAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Wen-jun Liu1†
Wen-jun Liu1† Jing-chao Luo
Jing-chao Luo Min-jie Ju
Min-jie Ju Ying Su
Ying Su Guo-wei Tu
Guo-wei Tu