- 1Department of Cardiovascular Surgery, Kamagaya General Hospital, Kamagaya, Japan
- 2Department of Emergency Medicine, Kamagaya General Hospital, Kamagaya, Japan
- 3Department of Gastrointestinal Surgery, Kamagaya General Hospital, Kamagaya, Japan
Background: The coronavirus disease 2019 (COVID-19) pandemic remains a global healthcare crisis. Nevertheless, the majority of COVID-19 cases involve mild to moderate symptoms in the early stages. The lack of information relating to these cases necessitates further investigation.
Methods: Patients visiting the outpatient clinic at the Kamagaya General Hospital were screened by interview and body temperature check. After initial screening, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was suspected in 481 patients who then underwent blood tests and the loop-mediated isothermal amplification (LAMP) test for SARS-CoV-2. Clinical characteristics between positive and negative SARS-CoV-2 groups were compared. Further, the novel predictive value of routine blood test results for SARS-CoV-2 infection was evaluated using ROC analysis.
Results: A total of 15,560 patients visited our hospital during the study period. After exclusion and initial screening by interview, 481 patients underwent the LAMP test and routine blood tests. Of these patients, 69 (14.3%) were positive for SARS-CoV-2 and diagnosed with COVID-19 (positive group), and 412 (85.7%) were negative (negative group). The median period between the first onset of symptoms and visit to our hospital was 3.4 and 2.9 days in the negative and positive groups, respectively. Cough (p = 0.014), rhinorrhea (p = 0.039), and taste disorders (p < 0.001) were significantly more common in the positive group, while gastrointestinal symptoms in the negative group (p = 0.043). The white blood cell count (p < 0.001), neutrophil count (p < 0.001), and percentage of neutrophils (p < 0.001) were higher in the negative group. The percentage of monocytes (p < 0.001) and the levels of ferritin (p < 0.001) were higher in the positive group. As per the predictive values for COVID-19 using blood tests, the values for the area under the curve for the neutrophil-to-monocyte ratio (NMR), white blood cell-to-hemoglobin ratio (WHR), and the product of the two (NMWH) were 0.857, 0.837, and 0.887, respectively.
Conclusion: Symptoms in early stage COVID-19 patients were similar to those in previous reports. Some blood test results were not consistent with previous reports. NMR, WHR, and NMWH are novel diagnostic scores in early-stage mild-symptom COVID-19 patients in primary care settings.
Introduction
In December 2019, the novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported in Wuhan, China (1). The rapid spread of COVID-19 to other countries created a major public health crisis (2) and led the World Health Organization (WHO) to declare COVID-19 a global pandemic in May 2020 (3).
Despite the presence of extensive data concerning COVID-19 in hospitalized patients and those admitted to emergency departments (4–6), there is a paucity of information focusing on COVID-19 patients with mild symptoms in outpatient clinics. The majority of infected individuals present with mild to moderate symptoms, which hampers the early detection of the disease (7, 8) and drives the transmission of infection (9). In primary care settings, many detection tests cannot be performed, and the results cannot be obtained immediately (10–12). In such situations, it is useful to identify patients highly suspected of having COVID-19 from those who visit primary care clinics. Therefore, we aimed to report on the clinical features of early stage COVID-19 and to establish simple diagnostic values using routine blood tests to improve point-of-care diagnosis and treatment of COVID-19 in a primary care setting.
Materials and Methods
Patient Inclusion Criteria and Diagnosis of COVID-19
We performed this retrospective study to evaluate the clinical features of patients with COVID-19 who visited the Kamagaya General Hospital between January 1 and 31, 2021. All patients were interviewed (questioner list is shown in 2.1.1), and if they had any signs suggesting COVID-19 (fever above 37.5°C, cough, myalgia, headache, fatigue, rhinorrhea, sputum, taste disorder, gastrointestinal symptoms, and a history of exposure to COVID-19 patients) (13, 14), they underwent routine blood tests (Table 1) and a SARS-CoV-2 loop-mediated isothermal amplification (LAMP) test (Loopamp®, Eiken Chemical Co., Ltd. Tokyo). A positive COVID-19 diagnosis was made based on LAMP test results. Clinical features of the SARS-CoV-2-positive and -negative patient groups were compared. In addition, we attempted to establish simple predictive values for patients with mild COVID-19 symptoms using routine blood test results.
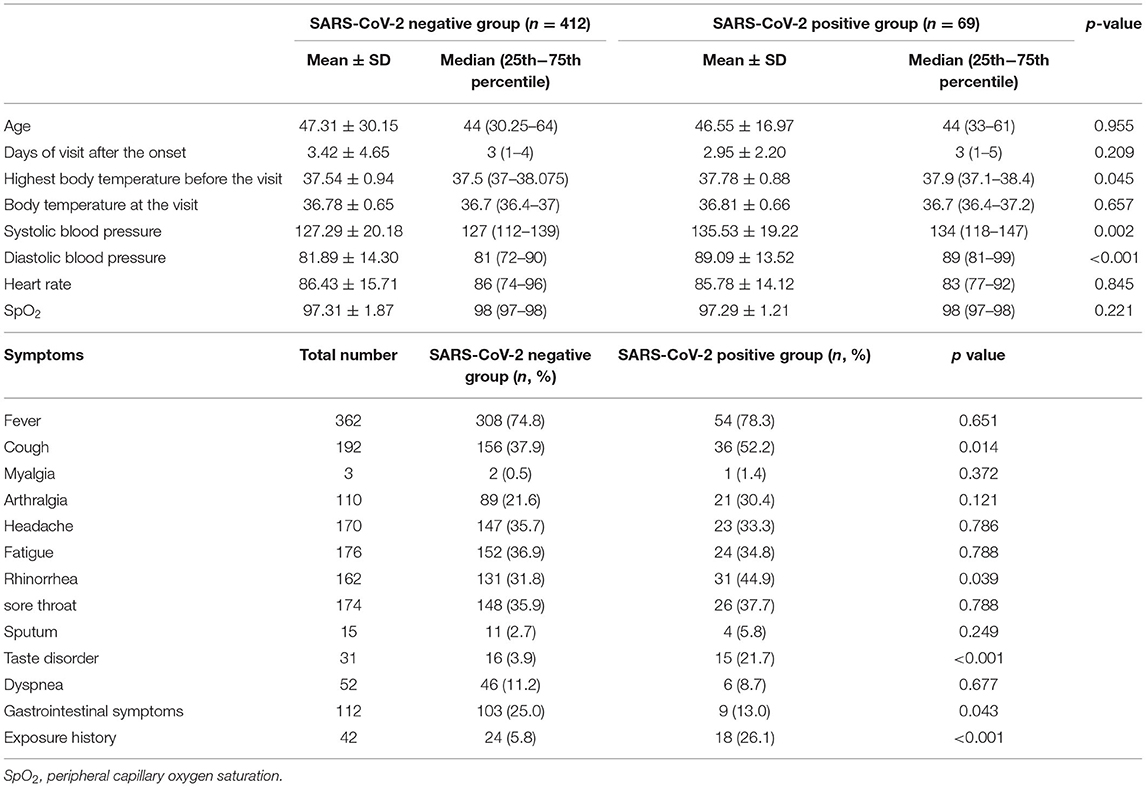
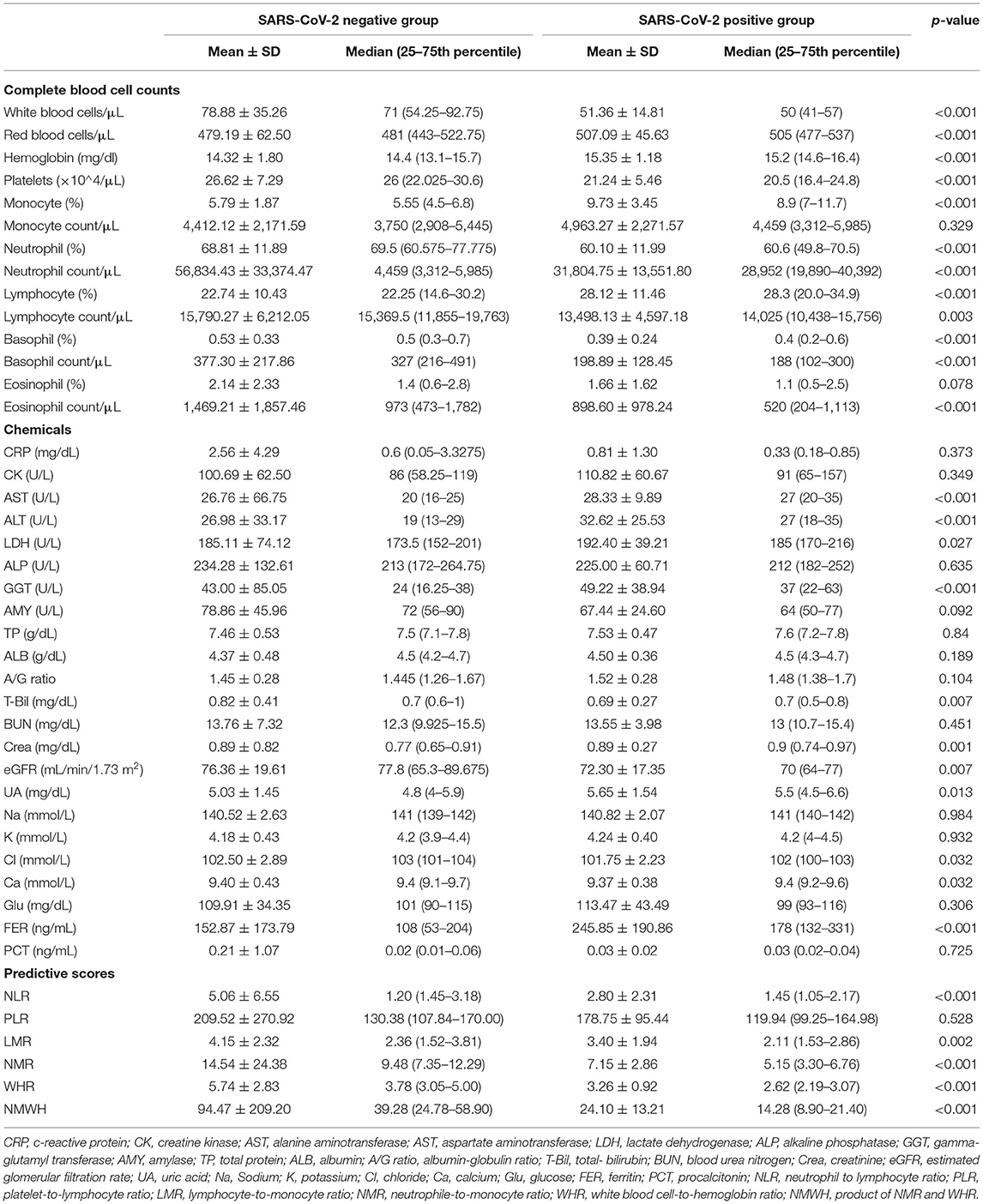
Table 1. Patient characteristics, vital signs, and symptoms upon presentation to the outpatient clinic.
Screening Questions
The screening questions were:
• What are your symptoms?
• Do you have any of the following symptoms: cough, aching muscles, headache, tiredness, runny nose, sputum, taste disorder, digestive problems?
• When did your first symptom start?
• Have you had contact with anyone with COVID-19 in the past 2 weeks?
• Do you know your highest temperature before this visit? (If yes:) What was it??
Statistical Analysis
All statistical analyses were performed using IBM SPSS for Macintosh, version 28 (IBM Corp., Armonk, NY, USA). Quantitative variables were expressed as mean ± standard deviation and median (interquartile range 2575%). Qualitative variables were expressed as frequencies and percentages in each group. Symptoms and blood test results of SARS-CoV-2-positive and -negative patients were analyzed for differences using the Mann-Whitney test or chi-square test. Receiver operating characteristic curve (ROC) analysis was performed to compare predictive values and optimal cut-off values to determine SARS-CoV-2 positivity. The level of statistical significance for all analyses was set at p < 0.05.
Ethical Approval and Patient Consent
This study was approved by the institutional review board (Approved number: TGE01745-64). We applied an opt-out method to obtain patient consent for participation and publication according to the guideline set by the institutional review board.
Results
A total of 15,560 patients visited our hospital during the study period. After excluding 1,140 patients who arrived for emergency medical treatment, the remaining 14,420 patients who visited our outpatient clinic were screened by interview. Based on initial screening, 481 patients underwent the LAMP test and diagnostic blood tests. Subsequently, 69 (14.3%) patients tested positive for SARS-CoV-2 and were diagnosed with COVID-19 (positive group), while 412 (85.7%) were negative for SARS-CoV-2 (negative group) (Figure 1).
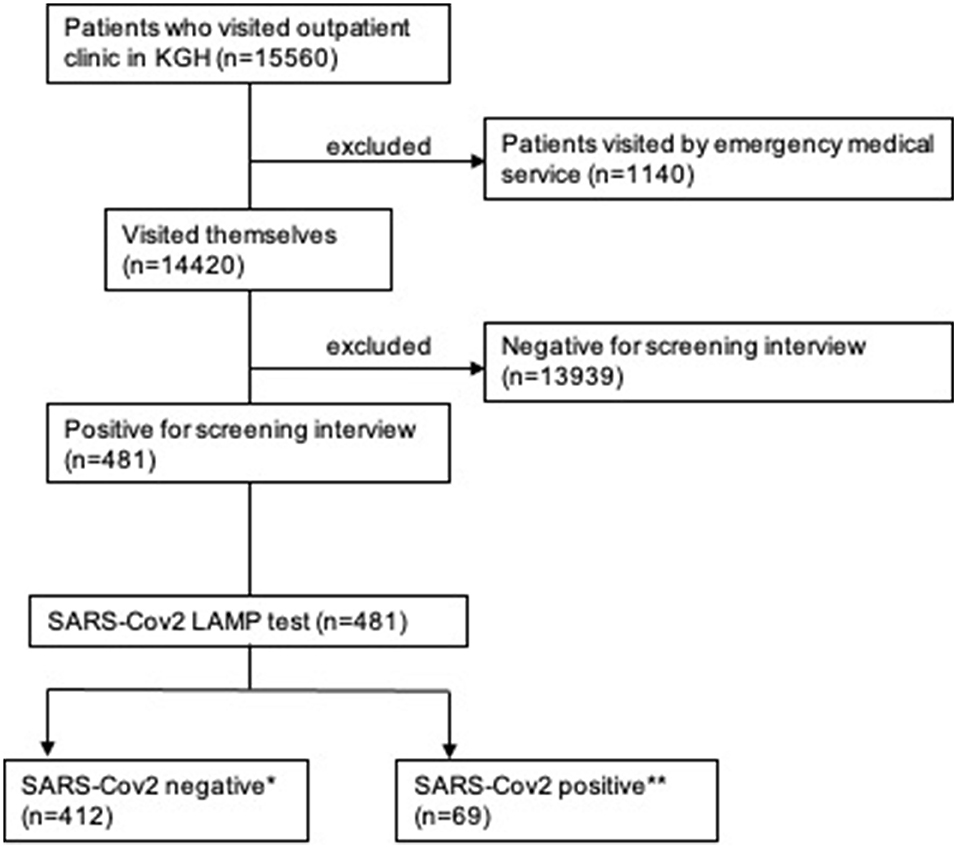
Figure 1. Flow diagram of the inclusion and exclusion criteria used in this study. *negative group, **positive group. KGH: Kamagaya General Hospital.
The mean and median values for the number of days from the first onset of symptoms to presentation to our outpatient clinic were 3.4 ± 4.6 and 3 (1–4) days in the negative group, and 2.9 ± 2.2 and 3 (1–5) days in the positive group, respectively. The mean and median ages were 47.2 ± 20.2 and 44 (30–64) years in the negative group and 46.5 ± 17.0 and 44 (33–61) years in the positive group, respectively. There were no significant differences between the two groups. Exposure history was observed more frequently in the positive group (18, 26.1%) than in the negative group (24, 5.8%). Body temperature, heart rate, and oxygen saturation (SpO2) were not significant. Blood pressure tended to be higher in the positive group than in the negative group. Cough, rhinorrhea, and taste disorders were observed more frequently in the positive group, but gastrointestinal symptoms were prevalent in the negative group (Table 1).
Routine blood test results and biochemical parameters were assessed (Table 2). White blood cell count and platelet count were higher in the negative group, while red blood cell count and hemoglobin levels were higher in the positive group. Neutrophil, eosinophil, and basophil counts and percentages were lower in the positive group. A low lymphocyte count but high lymphocyte percentage was noted in the positive group. Transaminase, gamma-glutamyl transferase, and estimated glomerular filtration rate was lower in the positive group than in the negative group. Conversely, creatinine, uric acid, and ferritin levels were higher in the positive group. Chloride and calcium ion levels were lower in the positive group, but sodium and potassium ion levels were not significantly different between the two groups. Total bilirubin, C-reactive protein, and procalcitonin levels were significantly higher in the negative group. All blood test results in the positive group were within normal ranges.
The area under the ROC curve (AUC) of neutrophil-to-monocyte ratio was 0.857 (95% CI, 0.814–0.900, p < 0.001). At a neutrophil-to-monocyte ratio cut-off point of ≤7.45, sensitivity and specificity were 90.0 and 56.5%, respectively. At an neutrophil-to-monocyte ratio cut-off point of ≤8.87, sensitivity and specificity were 80.1 and 73.9%, respectively. The AUC of white blood cell-to-hemoglobin ratio was 0.837 (95% CI, 0.793–0.881, p < 0.001). At a white blood cell-to-hemoglobin ratio cut-off point of ≤3.06, sensitivity and specificity were 90.3 and 52.2%, respectively. At a white blood cell-to-hemoglobin ratio cut-off point of ≤35.7, sensitivity and specificity were 80.1 and 78.3%, respectively (Figure 2A). The AUC of the product of neutrophil-to-monocyte ratio and white blood cell-to-hemoglobin ratio (NMWH) was 0.887 (95% CI, 0.853–0.921, p < 0.001). At a NMWH cut-off point of ≤25.0, sensitivity and specificity were 90.0 and 67.8%, respectively. At a NMWH cut-off point of ≤35.7, sensitivity and specificity were 80.1 and 78.3%, respectively (Table 3). The neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio were not effective diagnostic values in this study (Figure 2B).
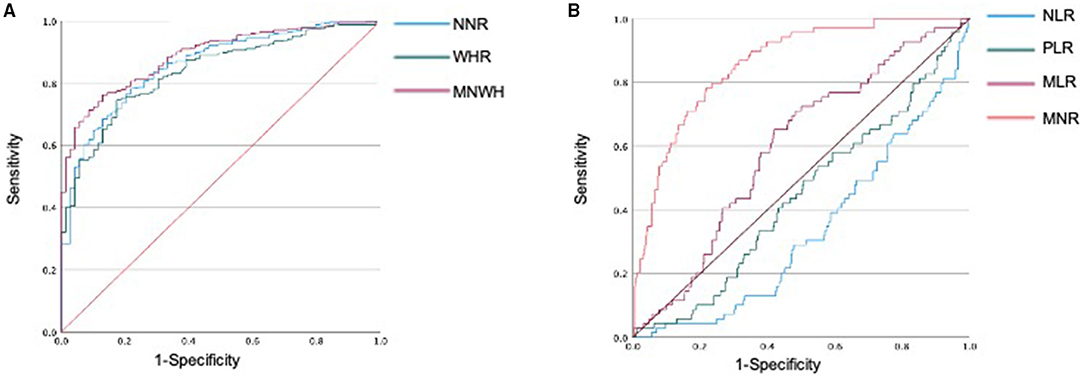
Figure 2. Receiver operating characteristic (ROC) analysis to evaluate the diagnostic value of hematologic markers for COVID-19. (A) Diagnostic values of neutrophils and lymphocytes in differentiating SARS-CoV-2 positive patients from negative patients. (B) Diagnostic values of NLR, MLR, and PLR in differentiating SARS-CoV-2-positive patients from SARS-CoV-2-negative patients. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; NMR, neutrophil-to-monocyte ratio; WHR, white blood cell-to-hemoglobin ratio; NMWH, product of NMR and WHR.
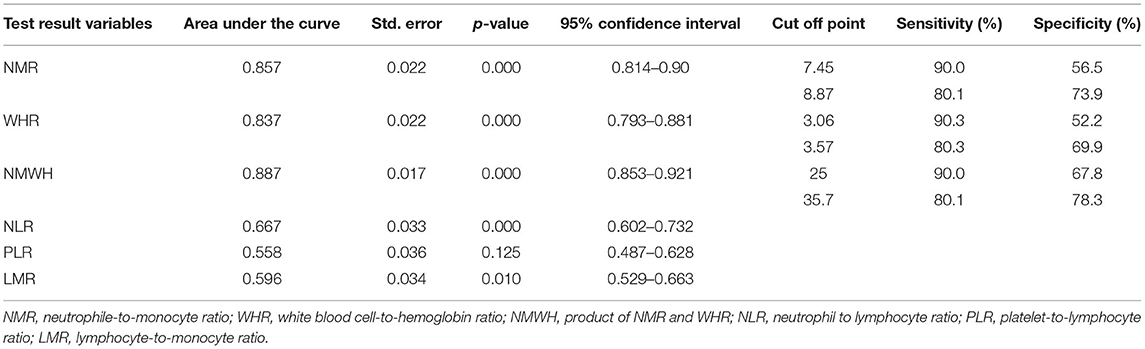
Table 3. Receiver operating characteristic (ROC) curve analysis of predictive scores and cut-off points.
Discussion
Diagnosis Technique
Currently, reverse transcription polymerase chain reaction (RT-PCR) is the gold standard for diagnosing COVID-19 (15, 16). However, it is expensive and time consuming (11). LAMP is a rapid, sensitive, cost-effective method (17, 18). In a meta-analysis by Anita et al., the LAMP test demonstrated a cumulative sensitivity of 95.5% (CI 97.5%, 90.8–97.9%) and cumulative specificity of 99.5% (CI 97.5%, 97.7–99.9%) for RT-PCR results (19). In our hospital, the LAMP test is used for diagnosing SARS-CoV-2 in the outpatient clinic. Though RT-PCR and LAMP are appropriate methods for detecting SARS-CoV-2 in the clinical setting, RT-PCR reportedly has a sensitivity of 70–90% (12, 20, 21). The possible reasons for the low efficiency of viral nucleic acid detection may include (1) subpar development of nucleic acid detection technology, (2) variations in detection rates of different manufacturers, (3) low viral load, or (4) improper specimen sampling. Further, Fang et al. reported that the sensitivity of RT-PCR for detecting SARS-CoV-2 was 71% (21). These findings support the idea of reduced reliance on RT-PCR or LAMP tests in the early stages of COVID-19 and show that there is potential for a better diagnostic method using blood tests.
Precise evaluation of the possibility of COVID-19 positivity among patients suggested of having early stage COVID-19 can improve primary care. We assume that it is highly useful if the evaluation is conducted through interviews and/or routine blood tests. This method can improve determining pre-test probability and enables us to identify patients strongly suggested for undergoing the RT-PCR or LAMP test.
Patient Background and Present Histories
Almost all patients with COVID-19 visited our hospital 3 days after the first onset of symptoms. In this study, the clinical features of the SARS-CoV-2-positive group represented mild symptoms and early stage COVID-19. We compared SARS-CoV-2-positive and -negative groups in order to identify useful features for predicting COVID-19 among suspected COVID-19 patients in primary care settings.
There are reports stating that SARS-CoV-2 positivity is associated with older age (22, 23), and other studies report that age is not associated with the disease (24). In this study, age was not significantly different between the SARS-CoV-2-positive and -negative groups. One of the reasons for the difference in results among reports was assumed to be the difference between the patients examined in this study and previous studies. This study concludes that in early stage COVID-19 patients with mild symptoms, age was not a factor.
SARS-CoV-2 may be transmitted via aerosols or fomites (1). The most common forms of transmission are droplets and physical contact (25). Therefore, exposure history is a vital factor. In our study, exposure history was higher in the SARS-CoV-2-positive group (18, 26.1% of patients). In a study conducted in China in February 2020, at a time when COVID-19 was endemic to the region, 27.8% of patients exhibited a history of exposure (26). Thus, exposure history is important for the detection of COVID-19 with mild symptoms.
Symptoms
Symptoms of the SARS-CoV-2 infection reportedly resemble those of the SARS-CoV infection (27, 28). While the mortality of COVID-19 is 1/10th that of SARS, SARS-CoV-2 has a much greater transmissibility rate; thus, it may be a greater threat to global health (29). The respiratory tract and lungs are the main targets of SARS-CoV-2 (30), and the symptoms of infection include mainly fever, cough, and fatigue/myalgia (31). In our study, about half of the positive group presented with cough (36, 52.2%, p = 0.014) and rhinorrhea (31, 44.9%, p = 0.039), which was significantly higher than that of the negative group. On the other hand, sputum production was not significantly different between both groups. It was revealed that early stage COVID-19 patients presented mainly with upper respiratory tract symptoms.
In previous reports, gastrointestinal (GI) symptoms were rarer than in other viral infections (4, 28, 31). A systematic review reported that the prevalence of diarrhea, nausea/vomiting, and abdominal pain in patients with COVID-19 were 9.1, 5.2, and 3.5%, respectively (32). In our study, all GI symptoms were observed in 13 and 25% of the positive and negative groups, respectively; this difference was significant (p = 0.043). We assume that the prevalence of symptoms in the early stages of COVID-19 is the same as noted in previous reports. Symptoms were observed more frequently in the negative group, possibly because the negative group included patients with diseases that mainly affected the GI system, such as cholecystitis or infective enteritis.
Further investigation after the pandemic revealed taste disorders in patients with COVID-19. Taste disorders can be an initial symptom or early manifestation of the disease (33, 34).
In an epidemiological survey of taste and olfactory disorders in COVID-19 patients, taste disorder was observed in 56.2% of the PCR-positive group, which was significantly higher than 13.9% of the negative group (35). In our study, the prevalence rate was lower than in other reports in both the positive (15, 21.7%) and negative groups (16, 3.9%, p < 0.001). However, the result that taste disorder was significantly more common in the positive group was consistent. We can conclude that taste disorder manifesting in early stage COVID-19 may have diagnostic utility. In general, the symptoms in the present study were consistent with those in the previous studies, although their magnitudes were different.
Blood Tests
Anemia has been reported to have developed with increasing severity of COVID-19 (36). Moreover, anemia may be associated with worse mortality rates (37). In the current study, hemoglobin [15.2 (14.6–16.4)] was within normal range in the positive group and lower in the negative group. Either the positive or the negative group has a tendency for anemia.
White blood cell count and differentiation were within the normal range in the positive group. Neutrophil count and percentage were lower in the positive group. Monocyte count did not show a significant difference, but the percentage was higher in the positive group. Lymphocyte count was lower, but lymphocyte percentage was higher in the positive group. The negative group had a tendency for leukocytosis, with increased neutrophils, whereas the positive group had almost normal test results. These results were not consistent with the previous reports. In many reports, lymphocytopenia has been observed in COVID-19 patients (38, 39). The lymphocyte count in this study did not show any significant abnormality. Other studies reported that there was no significant change in monocyte count in COVID-19 patients (40). This reported result matches with our study results. Eosinophil and basophil levels have reportedly been low in COVID-19 patients (41). Our study also showed the same result. However, the mechanism and magnitude of these parameters remain unclear. These differences between previous reports and the reports of this study may be because of the severity in patients. We focused on the patients with mild stages of COVID-19 in our study.
The levels of CRP (C-reactive protein) did not differ between the two groups. In the positive group, the CRP level was 0.33 (0.18–0.85), which was within the normal range. High CRP levels have reportedly been associated with severity and progression to pneumonia in COVID-19 patients (42, 43). In our study, the subjects with mild COVID-19 demonstrated normal CRP levels. In contrast to CRP levels, the ferritin levels were significantly higher in the positive than in the negative group (44). Ferritin has been reported to reflect systemic inflammation (45, 46). We assume that SARS-CoV-2 may cause inflammation in different ways than other infectious or inflammatory diseases in the negative group.
Transaminase and bilirubin levels were higher in the positive group than in the negative group, although the results were almost within the normal range. The elevation of liver enzymes in COVID-19 patients has been previously reported (47). In addition, coexisting liver failure is associated with increased mortality (48), and liver injury is associated with worse COVID-19 prognosis (32). Qi et al. reported that liver cholangiocytes were angiotensin-converting enzyme II (ACE2)-enriched cells (49); however, pathological findings indicated that SARS-CoV-2 was not observed in the liver of patients with COVID-19 (30). These reports indicate that the mechanism and importance of liver injury in COVID-19 patients is unclear.
Kidney injury has been reported in patients with COVID-19 and is associated with increased severity of symptoms and mortality (50). Chen et al. reported that ACE2, which is a receptor of SARS-CoV-2, is mainly expressed by different cell types in the human kidney. This indicates that SARS-CoV-2 mediates acute kidney injury (51). There are a few reports on renal function in the early stages of COVID-19. This study shows that the renal function tends to be lower in the positive group, although the magnitude is small. Thus, kidney injury may occur even in the early stages of COVID-19.
There are reports on the abnormality of ion levels in COVID-19 patients, but the significance of the abnormality is unclear. Dubey et al. reported that calcium levels were lower in patients with mild symptoms but higher in patients with moderate symptoms. They did not detect abnormalities in the levels of sodium and potassium ions (52). Our study shows that the chloride (p = 0.032) and calcium (p = 0.032) ion levels are slightly lower in the positive group than in the negative group. However, the implications of these findings are yet to be determined.
Prognostic Values
We attempted to explore new diagnostic values for mild COVID-19. In our study, neutrophil-to-monocyte ratio, white blood cell-to-hemoglobin ratio, and their product showed high AUC in the ROC analysis. Herein, neutrophil-to-monocyte ratio represents a lower neutrophil count and a higher monocyte percentage in the positive group than in the negative group. The white blood cell-to-hemoglobin ratio represents higher hemoglobin and lower white blood cell counts in the positive group than in the negative group. Moreover, NMWH may provide a more accurate score. There are a few reports regarding these scores pertaining to COVID-19 diagnosis. There are several other diagnostic scores for COVID-19, such as neutrophile-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio (22, 24, 26, 53–56). Prior to the COVID-19 pandemic, these scores were used in the evaluation of malignant tumors (57, 58). However, the scores were not significantly useful in our current study. One of the reasons may be the change in the differentiation of white blood cells in the course of COVID-19 (39). The neutrophil-to-monocyte ratio, white blood cell-to-hemoglobin ratio, and NMWH novel diagnostic scores are used for screening early-stage mildly symptomatic COVID-19 patients who visited the outpatient clinic, as well as for suspected COVID-19 cases. He et al. proposed diagnostic values for the SARS-CoV-2 PCR test using routine blood tests and machine learning (23). Their model showed an AUC of 0.854. The optimal cut-off value with the maximum Youden's index showed sensitivity and specificity of 0.761 and 0.808, respectively. Interestingly, our simple formula using routine blood tests also had a high AUC as theirs.
We assume that our prognostic scores can identify upper respiratory tract viral infections, which includes COVID-19, in the patients suspected of having COVID-19 after screening in the outpatient clinic. Peng et al. reported that the predictive values could distinguish COVID-19 patients from healthy participants; however, they could not distinguish COVID-19 patients from the patients with pneumonia caused by influenza (24). The same may be the case with our study.
Study Limitations
There were some limitations to the current study. The sensitivity of the method used in the study of early stage COVID-19 can vary between 70 and 90%. Further, this study was conducted retrospectively in a single facility. Additionally, almost all patients in the study were Japanese. Further larger studies in multiple different populations are required in order to check the generalizability of these results. Despite these limitations, we believe that our diagnostic values have the potential to increase pre-test probability or identify highly suspected COVID-19 patients in outpatient clinics, improving primary care medicine.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Tokushukai Group Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YK and TH were involved in the study conception and design. YK conducted all statistical analyses and wrote the manuscript. All authors were involved in patient treatment and contributed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The abstract of the study was presented in the 23rd annual meeting of Japanese Society of Hospital General Medicine.
References
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
2. Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. (2020) 27:taaa011. doi: 10.1093/jtm/taaa011
3. World Health Organization. Coronavirus Disease (COVID-19). COVID-19 Pandemic Spread to Other Countries, Developing Into a Major Public Health Crisis. WHO. Available online at: https://www.who.int/
4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
6. Kuisma M, Harve-Rytsälä H, Pirneskoski J, Boyd J, Lääperi M, Salo A, et al. Prehospital characteristics of COVID-19 patients in helsinki - experience of the first wave of the pandemic. Scand J Trauma Resusc Emerg Med. (2021) 29:95. doi: 10.1186/s13049-021-00915-0
7. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. (2020) 323:2512–4. doi: 10.1001/jama.2020.8391
8. Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Notomi T, et al. Loop-Mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology. (2020) 9:182. doi: 10.3390/biology9080182
9. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:411–5. doi: 10.15585/mmwr.mm6914e1
10. Amano Y, Kage H, Tanaka G, Gonoi W, Nakai Y, Kurokawa R, et al. Diagnostic prediction of COVID-19 based on clinical and radiological findings in a relatively low COVID-19 prevalence area. Respir Investig. (2021) 59:446–53. doi: 10.1016/j.resinv.2021.03.002
11. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection–challenges and implications. N Engl J Med. (2020) 383:e38. doi: 10.1056/NEJMp2015897
12. McGarry BE, SteelFisher GK, Grabowski DC, Barnett ML. COVID-19 test result turnaround time for residents and staff in US nursing homes. JAMA Intern Med. (2020) 181:556–9. doi: 10.1001/jamainternmed.2020.7330
13. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
14. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
15. Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. (2020) 6:591–605. doi: 10.1021/acscentsci.0c00501
16. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
17. Ganguli A, Mostafa A, Berger J, Aydin MY, Sun F, Ramirez SAS, et al. Rapid isothermal amplification and portable detection system for SARS-Cov-2. Proc Natl Acad Sci USA. (2020) 117:22727–35. doi: 10.1073/pnas.2014739117
18. Minami K, Masutani R, Suzuki Y, Kubota M, Osaka N, Nakanishi T, et al. Evaluation of SARS-CoV-2 RNA quantification by RT-LAMP compared to RT-qPCR. J Infect Chemother. (2021) 27:1068–71. doi: 10.1016/j.jiac.2021.05.004
19. Subali AD, Wiyono L. Reverse transcriptase loop mediated isothermal amplification (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis. Pathog Glob Health. (2021) 115:281–91. doi: 10.1080/20477724.2021.1933335
20. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. (2020) 323:1843–4. doi: 10.1001/jama.2020.3786
21. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. (2020) 296:E115–7. doi: 10.1148/radiol.2020200432
22. Kurstjens S, van der Horst A, Herpers R, Geerits MWL, Kluiters-de Hingh YCM, Göttgens EL, et al. Rapid identification of SARS-Cov-2-infected patients at the emergency department using routine testing. Clin Chem Lab Med. (2020) 58:1587–93. doi: 10.1515/cclm-2020-0593
23. Yang HS, Hou Y, Vasovic LV, Steel PAD, Chadburn A, Racine-Brzostek SE, et al. routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. Clin Chem. (2020) 66:1396–404. doi: 10.1093/clinchem/hvaa200
24. Peng J, Qi D, Yuan G, Deng X, Mei Y, Feng L, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): a multicenter, cross-sectional study. J Clin Lab Anal. (2020) 34:e23475. doi: 10.1002/jcla.23475
25. Xiong Y, Sun D, Liu Y, Fan Y, Zhao L, Li X, et al. Clinical and high-resolution ct features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. (2020) 55:332–9. doi: 10.1097/RLI.0000000000000674
26. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, D-NLR and PLR in COVID-19 patients. Int Immunopharmacol. (2020) 84:106504. doi: 10.1016/j.intimp.2020.106504
27. Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCov epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. (2020) 91:264–6. doi: 10.1016/j.ijid.2020.01.009
28. Wang FS, Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. (2020) 395:391–3. doi: 10.1016/S0140-6736(20)30300-7
29. Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. (2020) 20:e238–44. doi: 10.1016/S1473-3099(20)30484-9
30. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
31. Ghayda RA, Lee J, Lee JY, Kim DK, Lee KH, Hong SH, et al. Correlations of clinical and laboratory characteristics of COVID-19: a systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:5026. doi: 10.3390/ijerph17145026
32. Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with coronavirus disease 19: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. (2020) 44:653–61. doi: 10.1016/j.clinre.2020.04.012
33. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter european study. Eur Arch Otorhinolaryngol. (2020) 277:2251–61. doi: 10.1007/s00405-020-05965-1
34. Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez J, Natera-Villalba E, Gómez-Corral J. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. (2020) 27 1738–41. doi: 10.1111/ene.14273
35. Pérula de Torres LÁ, González-Lama J, Jiménez García C, Sánchez Montero R, Rider Garrido F, Ortega López Y, et al. Frequency and predictive validity of olfactory and taste dysfunction in patients with SARS-CoV-2 infection. Med Clin (Engl Ed). (2021) 156:595–601. doi: 10.1016/j.medcle.2020.12.024
36. Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. (2020) 95:E131–4. doi: 10.1002/ajh.25774
37. Liu Z, Sun R, Li J, Cheng W, Li L. Relations of anemia with the all-cause mortality and cardiovascular mortality in general population: a meta-analysis. Am J Med Sci. (2019) 358:191–9. doi: 10.1016/j.amjms.2019.05.016
38. Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. (2020) 14:3822–35. doi: 10.1021/acsnano.0c02624
39. Ding X, Yu Y, Lu B, Huo J, Chen M, Kang Y, et al. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin Chem Lab Med. (2020) 58:1365–71. doi: 10.1515/cclm-2020-0411
40. Jamal M, Bangash HI, Habiba M, Lei Y, Xie T, Sun J, et al. Immune dysregulation and system pathology in COVID-19. Virulence. (2021) 12:918–36. doi: 10.1080/21505594.2021.1898790
41. Yu H, Li D, Deng Z, Yang Z, Cai J, Jiang L, et al. Total Protein as a Biomarker for Predicting Coronavirus Disease-2019 Pneumonia. Available online at: https://ssrn.com/abstract=3551289
42. Jakob CEM, Mahajan UM, Oswald M, Stecher M, Schons M, Mayerle J, et al. Prediction of COVID-19 deterioration in high-risk patients at diagnosis: an early warning score for advanced COVID-19 developed by machine learning. Infection. (2021) 1–12. doi: 10.1007/s15010-021-01656-z
43. Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in COVID-19 patients. Clin Chim Acta. (2020) 509:135–8. doi: 10.1016/j.cca.2020.06.012
44. Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. (2020) 35:763–73. doi: 10.1007/s10654-020-00678-5
45. Kernan KF, Carcillo JA. Hyperferritinemia and Inflammation. Int Immunol. (2017) 29:401–9. doi: 10.1093/intimm/dxx031
46. Wessling-Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu Rev Nutr. (2018) 38:431–58. doi: 10.1146/annurev-nutr-082117-051749
47. Voiosu A, Roman A, Pop R, Boeriu A, Popp C, Zurac S, et al. Characteristics and outcomes of patients with COVID-19 and liver injury: a retrospective analysis and a multicenter experience. Rom J Intern Med. (2021). doi: 10.2478/rjim-2021-0027
48. Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. (2021) 74:567–77. doi: 10.1016/j.jhep.2020.09.024
49. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. (2020) 526:135–40. doi: 10.1016/j.bbrc.2020.03.044
50. Ke C, Xiao J, Wang Z, Yu C, Yang C, Hu Z. Characteristics of patients with kidney injury associated with COVID-19. Int Immunopharmacol. (2021) 96:107794. doi: 10.1016/j.intimp.2021.107794
51. Chen Z, Hu J, Liu L, Chen R, Wang M, Xiong M, et al. SARS-CoV-2 Causes acute kidney injury by directly infecting renal tubules. Front Cell Dev Biol. (2021) 9:664868. doi: 10.3389/fcell.2021.664868
52. Dubey DB, Mishra S, Reddy HD, Rizvi A, Ali W. Hematological and serum biochemistry parameters as a prognostic indicator of severally ill versus mild COVID-19 patients: a study from tertiary hospital in North India. Clin Epidemiol Glob Health. (2021) 12:100806. doi: 10.1016/j.cegh.2021.100806
53. Noor A, Akhtar F, Tashfeen S, Anwar N, Saleem B, Khan SA, et al. Neutrophil-to-lymphocyte ratio, derived neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio as risk factors in critically ill COVID-19 patients, a single centered study. J Ayub Med Coll Abbottabad. (2020) 32:S595–601.
54. Seyit M, Avci E, Nar R, Senol H, Yilmaz A, Ozen M, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. (2021) 45:569. doi: 10.1016/j.ajem.2020.12.069
55. Erdogan A, Can FE, Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR Ratio in COVID-19 patients. J Med Virol. (2021) 93:5555–9. doi: 10.1002/jmv.27097
56. Shang W, Dong J, Ren Y, Tian M, Li W, Hu J. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. (2020) 92:2188–92. doi: 10.1002/jmv.26031
57. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. doi: 10.1016/j.critrevonc.2013.03.010
Keywords: COVID-19, primary care, predictive value, predictive score, SARS-CoV-2, monocyte-to-neutrophile ratio
Citation: Kawatani Y, Nakayama K, Sawamura A, Fujikawa K, Nagai M and Hori T (2021) Clinical Features of Early Stage COVID-19 in a Primary Care Setting. Front. Med. 8:764884. doi: 10.3389/fmed.2021.764884
Received: 26 August 2021; Accepted: 02 November 2021;
Published: 23 November 2021.
Edited by:
Eric Matheson, Medical University of South Carolina, United StatesReviewed by:
Leah Stem, Medical University of South Carolina, United StatesJoshua Visserman, Medical University of South Carolina, United States
Copyright © 2021 Kawatani, Nakayama, Sawamura, Fujikawa, Nagai and Hori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takaki Hori, aGVhcnRAa2FtYWdheWEtaHAuanA=
 Yohei Kawatani
Yohei Kawatani Kei Nakayama2
Kei Nakayama2