
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 November 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.764653
 Zhe Zhang1,2,3,4,5†
Zhe Zhang1,2,3,4,5† Lingling Niu1,2,3,4,5†
Lingling Niu1,2,3,4,5† Jing Zhao1,2,3,4,5
Jing Zhao1,2,3,4,5 Huamao Miao1,2,3,4,5
Huamao Miao1,2,3,4,5 Zhuoyi Chen1,2,3,4,5
Zhuoyi Chen1,2,3,4,5 Yang Shen1,2,3,4,5
Yang Shen1,2,3,4,5 Xun Chen1,2,3,4,5
Xun Chen1,2,3,4,5 Yuhao Ye1,2,3,4,5
Yuhao Ye1,2,3,4,5 Xiaoying Wang1,2,3,4,5
Xiaoying Wang1,2,3,4,5 Xingtao Zhou1,2,3,4,5*
Xingtao Zhou1,2,3,4,5*Purpose: To compare the safety of the non-ophthalmic viscosurgical device (OVD) technique with that of the minimum OVD technique in EVO Implantable Collamer Lens (EVO-ICL) implantation.
Methods: A total of 180 eyes of 90 consecutive patients were enrolled in the study, of which 100 eyes of 50 patients were treated with non-OVD technique, with a 55% success rate. The remaining 80 eyes of 40 patients were treated with min-OVD technique, so they were classified into the min-OVD group. Preoperative and postoperative intraocular pressure (IOP) measurements were collected and analyzed at 1, 2, 3, and 24 h. Visual acuity, corneal endothelial cell density (ECD), and corneal densitometry 24 h postoperatively were evaluated.
Results: No significant difference was found in visual outcomes (P = 0.54) or ECD (P = 0.78) between the two groups. The operation time was significantly shorter in the non-OVD group (P < 0.0001). The IOP was significantly higher at 1 h (P < 0.0001), 2 h (P < 0.0001) and 3 h (P = 0.0045) postoperatively in the min-OVD group. The non-OVD group had significantly lower IOP than the min-OVD group at 1 h (P = 0.01) and 2 h (P = 0.013) postoperatively. The temporal corneal densitometry in the non-OVD group were significantly lower than those in the minimum group (P = 0.0063) 1 day after surgery.
Conclusion: The non-OVD technique is safe and efficient for ICL implantation. It can be a safer method of ICL implantation in that it completely eliminates ophthalmic viscoelastic devices related complications without causing additional complications in short term.
Myopia has become a worldwide public health issue (1, 2). Visian Implantable Collamer Lens (ICL) implantation has been reported to be a safe and effective way to correct myopia (3–6). Ophthalmic viscosurgical device (OVD) for ICL implantation is widely used to protect endothelial cells and maintain anterior chamber stability during surgery (7). Retained OVDs are the major cause of early (within 24 h postoperatively) acute IOP elevation (8–13). It has become well accepted that retained viscoelastic materials mechanically obstructs the trabecular outflow pathway and decreases the outflow facility (14–16), which is the main reason for the early postoperative intraocular pressure (IOP) elevation. IOP spikes of 30 mmHg or higher in the early period after surgery may be associated with corneal epithelial edema and pain and may increase the risk of retinal artery occlusion and anterior ischemic optic neuropathy (17–19). To prevent a postoperative IOP increase, complete removal of the OVD is of high importance.
The min-OVD technique has been applied by experienced surgeons in recent years (20, 21). The application of this technique can greatly reduce the occurrence of high IOP after surgery, and postoperative recovery is rapid. On the basis of the one-step min-OVD technique, we further invented the non-OVD technique. It can completely eliminate the use of the OVD and balanced salt solution (BSS) under the premise of maintaining the stability of the anterior chamber during the operation. With this technique, the risk of interference to the eyes during the operation will be minimal.
Due to the rapid postoperative recovery of the non-OVD technique, it is necessary to observe the early postoperative outcomes. Therefore, we designed a prospective study to observe the outcomes of ICL implantation without the use of OVD 1, 2, 3, and 24 h after surgery. The purpose of this prospective study was to evaluate the safety and efficacy of ICL implantation without the use of OVD.
This study was conducted at the Eye and ENT Hospital of Fudan University (Shanghai, China). All patients were fully informed of the details and potential risks of the procedure, and written informed consent was obtained from all patients. This study was approved by the Ethical Committee of the Eye and ENT Hospital of Fudan University, and all work was carried out in accordance with the Declaration of Helsinki.
A total of 90 continuous subjects (180 eyes) were recruited from August 2020 to October 2020 to participate in this study for applying non-OVD technique. If the patient was not successfully performed with OVD technique during surgery, we will apply min-OVD technique for patients and act as control group.
Participants were aged between 20 and 40 years, had stable refractive error (≤0.5 D change in refractive error in the past 2 years) and did not use contact lenses for 2 weeks. Exclusion criteria comprised eye disorders or systemic disease, IOP < 21 mmHg, anterior chamber depth (ACD) <2.8 mm and corneal endothelial cell density (ECD) <2,000 cells/mm2.
All patients underwent a standard ocular examination, including uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, slit lamp examination, corneal topography (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany), ACD (Oculus, Wetzlar, Germany), axial length (IOLMaster, Zeiss Humphrey, Carl Zeiss Meditec, Inc., Dublin, CA) and IOP (Canon, Japan). The ICL (EVO Visian ICL; STAAR Surgical AG) power was chosen using a modified vertex formula according to the manufacturer's suggestion. Lens size was calculated through the same protocol based on the white-to-white ratio and ACD. Data for UDVA, CDVA, the corneal densitometry value of the whole cornea and ECD were collected at 24 h postoperatively. IOP was monitored 1, 2, 3, and 24 h postoperatively.
All surgeries were performed by the same surgeon (XZ) using surface anesthesia with 0.4% oxybuprocaine. In the non-OVD group, no OVD or BSS was injected into the anterior chamber during the whole surgical procedure. ICL was implanted via a 3-mm temporal corneal incision. The anterior chamber depth is stable, indicating that a good self-closing incision has been made. ICL lens is implanted by an injector cartridge and unfolds smoothly, then, two distal haptics slowly reach the direction of the ciliary sulcus, then slowly push ICL, and gently press the two proximal haptics, and push the two proximal haptics under the iris. During the injection of ICL, the four haptics were directly implanted into the posterior chamber in one step, or all three haptics were implanted into the posterior chamber, and the proximal one haptic was not implanted into the posterior chamber. The min-OVD technique applied in the situation of one or two ICL haptics were inserted into the posterior chamber at one time. In the min-OVD group, the same procedure implemented as that in the non-OVD group at the beginning. After the ICL was inserted, a minimal amount of OVD was injected in front of the ICL to slightly adjust its position, and then a BSS was used to wash the OVD out of the anterior chamber (20, 22). The differences between the two methods are listed in Table 1. The operation time was calculated for each surgery (the time calculated from making the first corneal incision to closing the incision at the end). After the surgery, a topical antibiotic (0.5% levofloxacin, Cravit, Santen, Osaka, Japan) was administered four times per day for 7 days. A topical steroid (1.0% prednisolone acetate, Pred Forte; Allergan, Irvine, CA, USA) was used four times daily for 4 days, pranoprofen (Senju, Osaka, Japan) was used four times daily for 14 days and Natriumhyaluronat (Hycosan, Germany) was used four times daily for 3 months.
Corneal densitometry was measured from Scheimpflug images and was expressed in gray scale. The mean densitometry values on the diameters from 0–2 mm to 2–6 mm of the corneas' anterior layer (the first 120 μm of the complete corneal thickness), posterior layer (the last 60 μm of the complete corneal thickness) and central layer (the volume between the anterior layer and the posterior layer), the nasal and temporal total layer (the volume between the epithelium and endothelium of a cornea) were analyzed.
All statistical analyses were performed using SPSS version 23 (IBM Corp, USA). The data were presented as the mean ± standard deviation. Normality of data was assessed using the Shapiro-Wilk test, and data were normal in all cases. The baseline variables, were compared using Student's t-test. Comparison of sex was conducted using the chi-square test.
The IOP, ECD and corneal densitometry were also compared using repeated-measures ANOVA, with time being the intragroup factor and treatment modality being the intergroup factor. Tukey's multiple comparison tests were used for pairwise comparisons. A P < 0.05 was considered statistically significant.
According to the surgical procedures during the operation and the repeated watching of the surgical video postoperatively, we statistically concluded that the success rate of non-OVD technique in these patients was 55.6%. 100 eyes of 50 patients were treated with non-OVD technique. Eighty eyes of 40 patients were unsuccessful with non-OVD technique and were treated with the traditional surgery method (min-OVD technique). Baseline biometrics and comparisons among groups are shown in Table 2. No significant differences were found between the two groups in baseline biometrics. All surgeries were uneventful, and no intraoperative complications were observed. No postoperative adverse events were recorded in either group, except for the expected changes in IOP.
The mean efficacy indices (postoperative UDVA/preoperative CDVA) were 1.05 ± 0.15 and 1.08 ± 0.17 in the non-OVD group and min-OVD group, respectively (P = 0.52). The safety indices (postoperative CDVA/preoperative CDVA) were 1.15 ± 0.19 and 1.22 ± 0.23 in the non-OVD group and min-OVD group, respectively (P = 0.24). No eyes in either group lost one or more lines of CDVA. Figure 1 shows the visual outcomes of the two groups 1 day after surgery. The vault of non-OVD group and min-OVD group was 553.8 ± 195.9 μm and 511.4 ± 147 μm, respectively (P = 0.28).
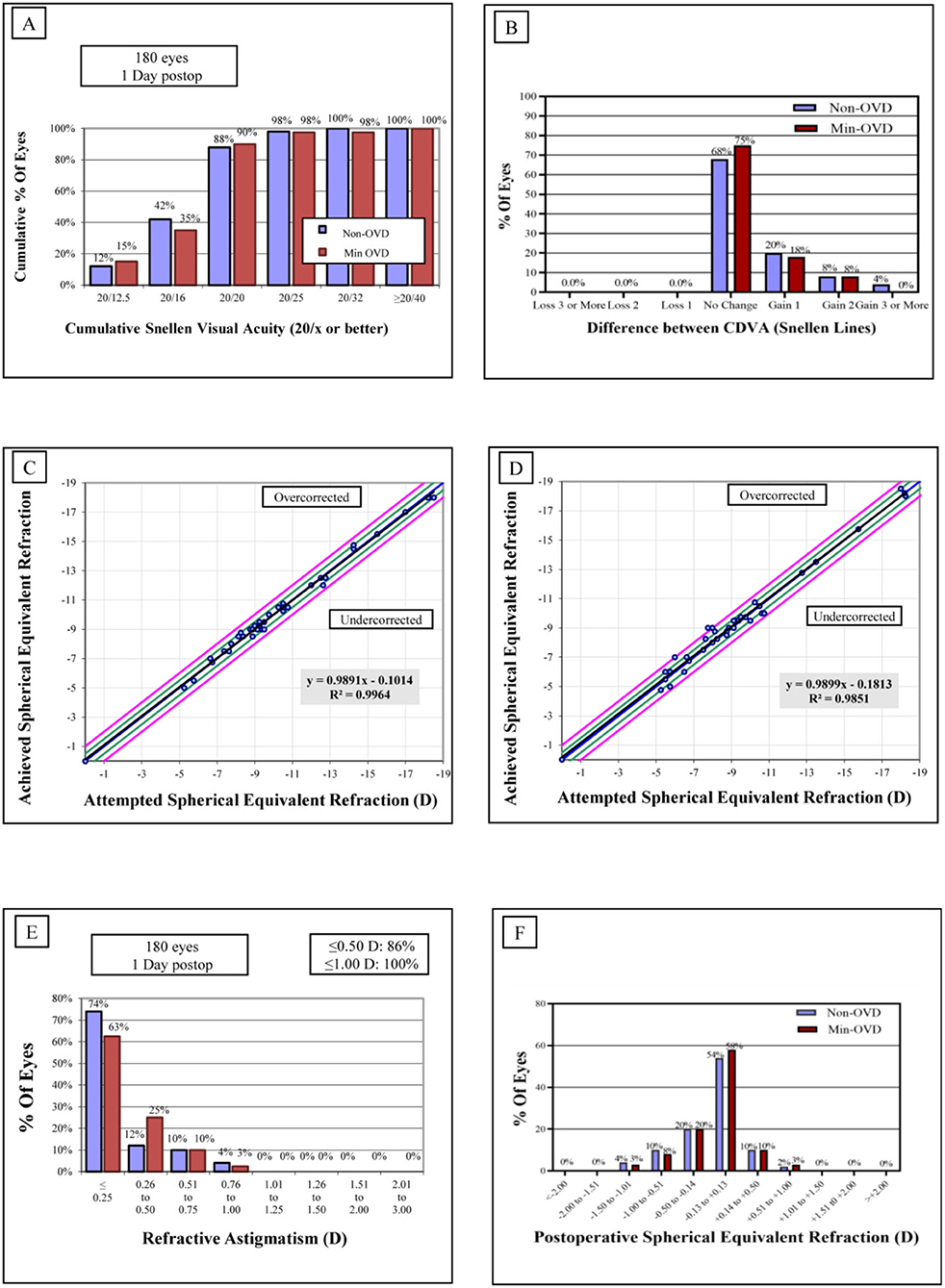
Figure 1. Refractive outcomes 1 day after implantable collamer lens (ICL). (A) Cumulative uncorrected distance visual acuity (UDVA). (B) Changes of corrected distance visual acuity (CDVA). (C,D) Attempted vs. achieved SE refraction after ICL in the non-OVD group (C) and min-OVD group (D). (E) Refractive astigmatism. (F) Spherical equivalent (SE) refraction.
In the non-OVD group, the operation time was 63.68 ± 27.35 s. The distribution of the operation time was as follows: 60 eyes (60%) took <1 mine and 40 eyes (40.0%) took <2 min. In the min-OVD group, the operation time was 144.8±33.27 s. The distribution of the operation time was as follows: 28 eyes (35%) took <1 min, 40 eyes (50.0%) took <2 min, and 12 eyes (15%) took <3 min. The operation time was significantly shorter in the non-OVD group (P < 0.0001).
The postoperative IOP was more stable in the non-OVD group than in the min-OVD group. The IOP was significantly higher than the preoperative value at 1 h (P < 0.0001), 2 h (P < 0.0001), and 3 h (P = 0.0045) postoperatively in the min-OVD group, while in the non-OVD group, there was no significant difference between preoperative intraocular pressure and postoperative intraocular pressure at each time point. The non-OVD group had significantly lower IOP than the min-OVD group at 1 h (P = 0.01) and 2 h (P = 0.013) postoperatively. There was no differences were found between the two groups after 24 h. The occurrence rate of paracentesis tap was significantly lower in the non-OVD group than in the min-OVD group (1% [1 of 100] vs. 5% [4 of 80], P < 0.01). Moreover, the use of IOP control drugs was significantly lower in the non-OVD group than in the min-OVD group (0 [0 of 100] vs. 3.8% [3 of 80], P < 0.01) (Figure 2).
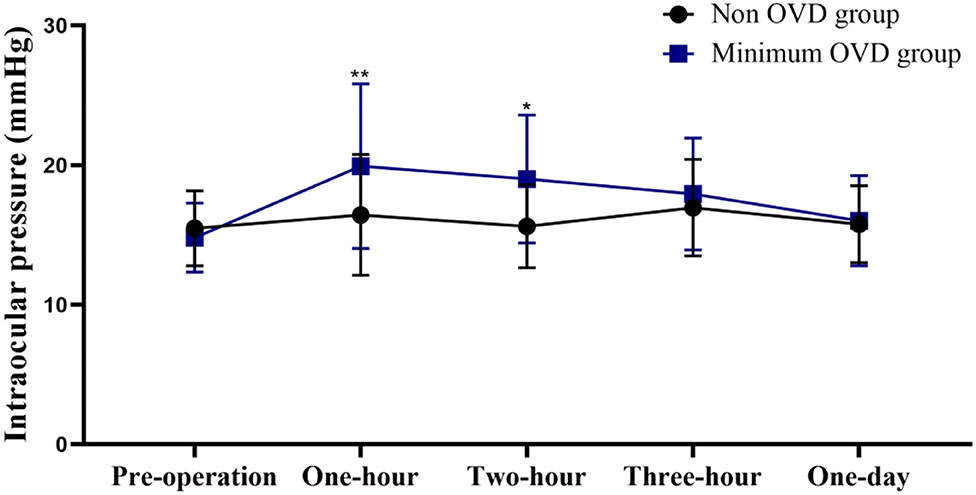
Figure 2. Intraocular pressure (IOP) of the non-OVD group and min-OVD group at each follow-up time point. Error bars represent standard deviation, *P < 0.05; **P < 0.01.
There was no significant difference between groups at each follow-up time point. In the min-OVD group, the ECD changed from 2,627 ± 202 cells/mm2 preoperatively to 2,571 ± 241 cells/mm2 at 3 h and to 2,586 ± 280 cells/mm2 1 day postoperatively (P > 0.05). In the non-OVD group, the ECD changed from 2,645 ± 204 cells/mm2 preoperatively to 2,596 ± 255 cells/mm2 at 3 h and to 2,619 ±182 cells/mm2 1 day postoperatively (P > 0.05). In addition, there was no significant difference in the coefficient of variation (CV) or hexagonal cell ratio (6A) between groups at each follow-up time point. In the min-OVD group, the CV changed from 42.04 ± 8.40 preoperatively to 40.06 ± 7.14 at 3 h and to 41.15±5.17 1 day postoperatively (P > 0.05). In the non-OVD group, the CV changed from 41.39 ± 5.90 preoperatively to 42.07 ± 5.95 at 3 h and to 40.91 ± 4.91 1 day postoperatively (P > 0.05). In the min-OVD group, the 6A changed from 40.75 ± 7.15 preoperatively to 40.12 ± 8.09 at 3 h and to 39.65 ± 6.69 1 day postoperatively (P > 0.05). In the non-OVD group, the 6A changed from 42.06 ± 6.86 preoperatively to 41.15 ± 9.16 at 3 h and to 40.97 ± 7.71 1 day postoperatively (P > 0.05).
Regarding the annular diameters of 0–2 and 2–6 mm, no significant changes were detected in the corneal densitometry values of the anterior layer (AL 0–2 mm and 2–6 mm), the central layer (CL 0–2 mm and 2–6 mm), the posterior layer (PL 0–2 mm and 2–6 mm) the total layer (TL 0–2 mm and 2–6 mm) or the nasal part within the first day between two groups. Temporal densitometry values assessed 1 day after the operation were significantly different between the two groups (P = 0.0063) and were significantly increased compared with the value obtained 1 day postoperatively (P < 0.0001) (Figure 3).
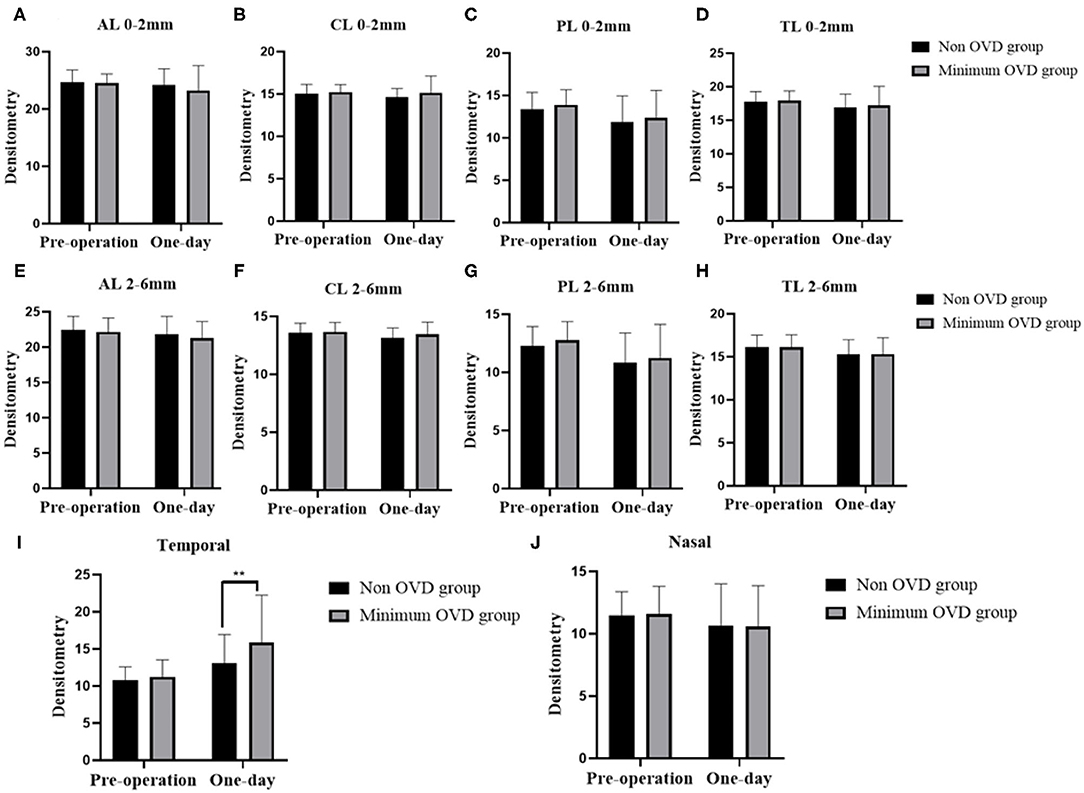
Figure 3. Densitometry of the cornea in the non-OVD group and min-OVD group at different time points postoperatively. Corneal densitometry values of the anterior layer (A), central layer (B), posterior layer (C) and total layer (D) from 0 to 2 mm. Corneal densitometry values of the anterior layer (E), central layer (F), posterior layer (G) and total layer (H) from 2 to 6 mm. Corneal densitometry values of temporal (I) and nosal (J) part. **P < 0.01, the error bar indicates the standard deviation.
In the current study, we developed a new technique, the non-OVD technique, for use in ICL implantation. The ICL was implanted into the posterior chamber with only one corneal incision, and no OVD or BSS was injected into the anterior chamber during the whole surgical procedure. Several min-OVD techniques have been reported to implant ICLs effectively and mastered by many surgeons, it has become a common surgical method for ICL implantation. Chen et al. inserted an ICL by making a two-step corneal incision and used BSS instead of OVD. In addition, OVD is still needed when adjusting the ICL position (21). Peng et al. (23) described a technique using BSS to load the ICL and maintain the anterior chamber. Therefore, we took min-OVD technology as the control group, compared our innovative non-OVD technique with it. Compared to previous studies, our technique resulted in less intraocular interference and fewer postoperative reactions.
In our study, IOP in the min-OVD group increased significantly within 3 h postoperatively and was significantly higher than that in the non-OVD group in the first 2 h after surgery. The IOP gradually decreased to the preoperative level, and no differences were found between the two groups after 24 h. The occurrence rate of paracentesis tap and IOP control drug usage were significantly lower in the non-OVD group. Thorough removal of viscoelastic substances is vital for avoidance of a postoperative IOP increase. However, complete removal of the OVD behind the IOL is known to be difficult. It has not been achieved with any technique. Therefore, it is beneficial to maintain intraocular pressure by not using OVD during surgery.
In traditional techniques, OVD is used to protect endothelial cells and maintain anterior chamber stability during surgery (24, 25). Therefore, we paid more attention to the effect of our new technique on the morphology and number of endothelial cells in our study. Our study revealed that the non-OVD technique did not cause any additional damage to endothelial cells. No significant differences were detected between the two groups in ECD during the first 24 h postoperatively in this study. A previous study also compared the changes in endothelial cells after ICL implantation or cataract surgery with and without OVD during a long follow-up period. The results consistently showed no significant reduction in endothelial cells at the 1-year follow-up (25, 26).
Specular microscopy was used to evaluate ECD by analyzing the central corneal image, which was taken through a central fixation area of the cornea; using this technique, peripheral endothelial damage may not be detected. Corneal densitometry assessments using a Pentacam HR rather than a specular microscope are able to detect various abnormalities in a wider zone (27–30). Corneal densitometry analysis may be employed to assess endothelial function in peripheral zones. The results of the current study demonstrated that the densitometry values of AL 0–2 mm, CL 0–2 mm, PL 0–2 mm, and TL 0–2 mm remained unchanged, which was consistent with the ECD results.
Our team's previous results showed that the values of AL 2–6 mm, CL 2–6 mm, PL 2–6 mm, and TL 2–6 mm measured at 1 day postoperatively, were unchanged compared with those measured before the surgery, indicating that the effects of ICL implantation on corneal densitometry may be insignificant and that the corneal histological structure is intact. However, the results of this study showed that temporal densitometry value (corneal incision location) was significantly different between the two groups 1 day after the operation. The densitometry values of the min-OVD group increased markedly. We hypothesize that this is due to the relatively long operation time and the injection of OVD and BSS through this area, which might affect corneal densitometry (31).
The present study also suggested that the non-OVD technique could reduce surgery duration, which might make the patient feel more comfortable during the surgery and improve patient satisfaction. Compared with the traditional technique, the non-OVD technique does not require time to inject and remove the OVD and reduces the cost of consumable material and the risk of intraoperative complications. Moreover, the non-OVD technique can increase the efficiency of the surgery, which is critical for carrying out this surgery extensively and serving more patients.
The key point of the non-OVD technique is that its effectiveness is based on good self-close corneal incision making, which ensures a stable anterior chamber depth. What's more, the ICL injection is another important part of the procedure because the lens has to be kept wet. In addition, the lens incarceration in the corneal incision and rotation in the anterior chamber should be avoided. When using the non-OVD technique, if any situation emerges that impacts the anterior chamber stability, surgeons should switch to the conventional method to avoid mechanical disturbance to the corneal endothelial cell, crystalline lens, or iris.
However, the limitation of this study was that the non-OVD technique requires a longer learning curve. The non-OVD technique is best learned after traditional surgical techniques are mastered, and the promotion of non-OVD technique requires further training of ophthalmologists. The second limitation was that the non-OVD technique effect on ocular biometrics should further monitor. We originally designed a long-term study to observe the changes of these indexes.
In conclusion, the non-OVD technique is safe and efficient for ICL implantation. It can be a safer method of ICL implantation in that it completely eliminates ophthalmic viscoelastic devices related complications without causing additional complications in short term.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the Eye and ENT Hospital of Fudan University. The patients/participants provided their written informed consent to participate in this study.
XZ and XW designed and directed the project. ZZ, LN, XC, YY, and ZC performed the experiments. YS and HM analyzed spectra. ZZ and LN wrote the article. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81770955), Joint Research Project of New Frontier Technology in Municipal Hospitals (SHDC12018103), and Project of Shanghai Science and Technology (grant no. 20410710100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.764653/full#supplementary-material
1. Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. (2019) 126:338–46. doi: 10.1016/j.ophtha.2018.10.029
2. Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of Myopia. Asia Pac J Ophthalmol (Phila). (2016) 5:386–93. doi: 10.1097/APO.0000000000000236
3. Lu Y, Yang N, Li X, Kong J. Four-year Follow-up of the changes in anterior segment after Phakic Collamer lens implantation. Am J Ophthalmol. (2017) 178:140–9. doi: 10.1016/j.ajo.2017.03.020
4. Moya T, Javaloy J, Montés-Micó R, Beltrán J, Muñoz G, Montalbán R. Implantable Collamer lens for myopia: assessment 12 years after implantation. J Refract Surg. (2015) 31:548–56. doi: 10.3928/1081597X-20150727-05
5. Rishi P, Attiku Y, Agarwal M, Narayanan R, Talwar D, Srinivasan B, et al. Retinal detachment after Phakic intraocular lens implantation: a 10-year multicenter study. Ophthalmology. (2019) 126:1198–200. doi: 10.1016/j.ophtha.2019.02.028
6. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Posterior chamber Phakic intraocular lens implantation for the correction of myopia and myopic astigmatism: a retrospective 10-year follow-up study. Am J Ophthalmol. (2019) 206:1–10. doi: 10.1016/j.ajo.2019.04.024
8. Li M, Yuan N, Chen X, Lu Y, Gong H, Qian L, et al. Impact of acute intraocular pressure elevation on the visual acuity of non-human primates. EBioMedicine. (2019) 44:554–62. doi: 10.1016/j.ebiom.2019.05.059
9. Almalki S, Abubaker A, Alsabaani NA, Edward DP. Causes of elevated intraocular pressure following implantation of phakic intraocular lenses for myopia. Int Ophthalmol. (2016) 36:259–65. doi: 10.1007/s10792-015-0112-4
10. Senthil S, Choudhari NS, Vaddavalli PK, Murthy S, Reddy JC, Garudadri CS. Etiology and management of raised intraocular pressure following posterior chamber Phakic intraocular lens implantation in myopic eyes. PLoS ONE. (2016) 11:e0165469. doi: 10.1371/journal.pone.0165469
11. Shimizu K, Kamiya K, Igarashi A, Shiratani T. Early clinical outcomes of implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for moderate to high myopia. Br J Ophthalmol. (2012) 96:409–12. doi: 10.1136/bjophthalmol-2011-300148
12. Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, et al. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. (2009) 116:2244–58. doi: 10.1016/j.ophtha.2009.08.018
13. Jimenez-Alfaro I, Benitez del Castillo JM, Garcia-Feijoo J, Gil de Bernabe JG, Serrano de La Iglesia JM. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. (2001) 108:90–9. doi: 10.1016/S0161-6420(00)00403-6
14. Berson FG, Patterson MM, Epstein DL. Obstruction of aqueous outflow by sodium hyaluronate in enucleated human eyes. Am J Ophthalmol. (1983) 95:668–72. doi: 10.1016/0002-9394(83)90388-4
15. Arshinoff S. Postoperative intraocular pressure spikes. J Cataract Refract Surg. (2004) 30:733–4. doi: 10.1016/j.jcrs.2004.02.059
16. Kocak-Altintas AG, Anayol MA, Cakmak HB, Simsek S. Effects of topical dorzolamide on IOP after phacoemulsification with different types of ophthalmic viscosurgical devices. Eur J Ophthalmol. (2007) 17:38–44. doi: 10.1177/112067210701700106
17. Unsal U, Baser G, Soyler M. Intraocular lens implantation without the use of ophthalmic viscosurgical device. Int Ophthalmol. (2017) 37:25–30. doi: 10.1007/s10792-016-0211-x
18. Fernandes P, Gonzalez-Meijome JM, Madrid-Costa D, Ferrer-Blasco T, Jorge J, Montes-Mico R. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. (2011) 27:765–76. doi: 10.3928/1081597X-20110617-01
19. Gonzalez-Lopez F, Bilbao-Calabuig R, Mompean B, de Rojas V, Luezas J, Djodeyre MR, et al. Intraocular pressure during the early postoperative period after 100 consecutive implantations of posterior chamber phakic intraocular lenses with a central hole. J Cataract Refract Surg. (2013) 39:1859–63. doi: 10.1016/j.jcrs.2013.06.020
20. Miao HM, Zhao F, Niu LL, Zhao J, Wang XY, Zhou XT. One-step viscoelastic agent technique for ICL V4c implantation for myopia. Int J Ophthalmol. (2021) 14:1359–64. doi: 10.18240/ijo.2021.09.10
21. Chen D, Cui G, Wang X, Li Y, Luo Y. Safety of the minimum ophthalmic viscosurgical device technique in Phakic implantable Collamer lens implantation. J Refract Surg. (2020) 36:42–8. doi: 10.3928/1081597X-20191119-02
22. Wei R, Li M, Zhang H, Aruma A, Miao H, Wang X, et al. Comparison of objective and subjective visual quality early after implantable collamer lens V4c (ICL V4c) and small incision lenticule extraction (SMILE) for high myopia correction. Acta Ophthalmol. (2020) 98:e943–50. doi: 10.1111/aos.14459
23. Peng M, Tang Q, Zhao L, Khan MA, Lin D. Safety of implantable Collamer lens implantation without ophthalmic viscosurgical device: a retrospective cohort study. Medicine (Baltimore). (2020) 99:e20691. doi: 10.1097/MD.0000000000020691
24. Bissen-Miyajima H. Ophthalmic viscosurgical devices. Curr Opin Ophthalmol. (2008) 19:50–4. doi: 10.1097/ICU.0b013e3282f14db0
25. Suzuki H, Igarashi T, Shiwa T, Takahashi H. Efficacy of ophthalmic viscosurgical devices in preventing temperature rise at the corneal endothelium during phacoemulsification. Curr Eye Res. (2016) 41:1548–52. doi: 10.3109/02713683.2015.1136420
26. Galvis V, Tello A, Gutierrez AJ. Human corneal endothelium regeneration: effect of ROCK inhibitor. Invest Ophthalmol Vis Sci. (2013) 54:4971–3. doi: 10.1167/iovs.13-12388
27. Chan TCY, Wong ES, Chan JCK, Wang Y, Yu M, Maeda N, et al. Corneal backward scattering and higher-order aberrations in children with vernal keratoconjunctivitis and normal topography. Acta Ophthalmol. (2018) 96:e327–33. doi: 10.1111/aos.13566
28. Alnawaiseh M, Zumhagen L, Wirths G, Eveslage M, Eter N, Rosentreter A. Corneal densitometry, central corneal thickness, and corneal central-to-peripheral thickness ratio in patients with fuchs endothelial dystrophy. Cornea. (2016) 35:358–62. doi: 10.1097/ICO.0000000000000711
29. Tekin K, Sekeroglu MA, Kiziltoprak H, Yilmazbas P. Corneal densitometry in healthy corneas and its correlation with endothelial morphometry. Cornea. (2017) 36:1336–42. doi: 10.1097/ICO.0000000000001363
30. Otri AM, Fares U, Al-Aqaba MA, Dua HS. Corneal densitometry as an indicator of corneal health. Ophthalmology. (2012) 119:501–8. doi: 10.1016/j.ophtha.2011.08.024
Keywords: ophthalmic viscosurgical device (OVD), intraocular pressure (IOP), implantable collamer lens (ICL), endothelial cell density (ECD), corneal densitometry
Citation: Zhang Z, Niu L, Zhao J, Miao H, Chen Z, Shen Y, Chen X, Ye Y, Wang X and Zhou X (2021) Safety of EVO ICL Implantation With an Ophthalmic Viscosurgical Device-Free Technique in the Early 24 h After Surgery. Front. Med. 8:764653. doi: 10.3389/fmed.2021.764653
Received: 25 August 2021; Accepted: 21 October 2021;
Published: 17 November 2021.
Edited by:
Michele Lanza, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Yuanbo Liang, Affiliated Eye Hospital of Wenzhou Medical University, ChinaCopyright © 2021 Zhang, Niu, Zhao, Miao, Chen, Shen, Chen, Ye, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingtao Zhou, ZG9jdHpob3V4aW5ndGFvQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.