- 1Department of Dermatology, Hunan Engineering Research Center of Skin Health and Disease, Hunan Key Laboratory of Skin Cancer and Psoriasis, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Dermatology, Soochow University Affiliated Children's Hospital, Suzhou, China
- 4Department of Dermatology, Dalian Dermatology Hospital, Dalian, China
Background: Psoriasis is a skin condition associated with increased risks of developing metabolic diseases, such as diabetes and hyperlipidaemia. Retinoid drugs, including acitretin, are commonly used to treat psoriasis due to its low cost and tolerable side effects.
Objective: This study aimed to explore the influence of acitretin on patients' metabolism levels, especially lipid and glucose.
Methods: In this retrospective study, a total of 685 psoriatic patients and 395 age/sex matched controls were enrolled. The demographic and biochemical indexes of each participant were recorded. Acitretin (30 mg/d) combined with the topical ointment calcipotriol was used to treat the psoriatic patients, and the glucose and lipid profiles of patients before and after acitretin treatment were analyzed.
Results: The blood glucose levels of 685 psoriasis patients were significantly higher than that of the control group (P < 0.001), while the blood lipid levels showed no difference between psoriatic patients and the matched controls. Triglyceride and low-density lipoprotein levels were significantly increased in 247 patients (P < 0.05) after 8 weeks of treatment with acitretin. Interestingly, there was a remarkable downward trend in body mass index (BMI) and blood glucose levels (P < 0.05) after acitretin treatment. Additionally, expression of both GLUT1 and GLUT4 in HaCaT and HepG2 cells were significantly increased when treated with acitretin. Compared to acitretin-free cells, the uptake of 2-NBDG was significantly higher in HaCaT and HepG2 cells after incubation with 5000 ng/mL acitretin for 36 h.
Conclusion: Acitretin plays a significant role of reducing the blood glucose level in psoriasis patients. The mechanism of lowering blood glucose may be through increasing glucose intake by cells, thereby reducing glucose levels in the peripheral blood.
Background
Psoriasis, an incurable disease caused by both genetic and environmental factors, affects about 2–3% of the world's population, resulting in serious economic burdens (1). Psoriasis mainly manifests as an abnormal proliferation and differentiation of keratinocytes, as well as an abnormal infiltration and imbalance of inflammatory cells (2). In addition to affecting the skin, multiple studies have shown a correlation between psoriasis and elevated blood sugar and blood lipid levels (3, 4). It has been reported that genes, such as GLUT1, GLUT2, GLUT4, GCK4, and INSIG1, are commonly associated with glucose uptake, transport, and metabolism. Studies have also revealed that psoriatic patients are at an increased risk for cardiovascular diseases, obesity, diabetes, hypertension, dyslipidaemia, and other comorbidities when compared with non-psoriatic individuals (2, 5–7). There are several classes of drugs used to treat psoriasis including retinol derivatives, methotrexate (MTX), and biologicals. As a non-immunosuppressant, retinol derivatives are widely used in Chinese patients with psoriasis.
Previous studies have shown that vitamin A plays an important role in human development and metabolism. After modification of its chemical structure, scientists have obtained numerous derivatives of vitamin A, known as retinoids. Previous studies have reported that retinoids can promote differentiation of keratinocytes and inhibit its proliferation, thus enhancing the skin tissue immunity, and alleviating inflammation (8–10). Acitretin, a retinoid drug, is widely used for the treatment of pustular psoriasis and moderate to severe plaque psoriasis in China (11–13). Compared to the parent drug etretinate, with a long half-life 120 days, acitretin has a shorter half-life (50–60 h), making it a potentially safer alternative (14, 15). Studies involving acitretin treatment revealed that with low dosage (<50 mg/d), ~23–52% of patients achieved a 75% improvement compared to the baseline psoriasis area and severity index (PASI) value (PASI75), and 66–85% of the patients achieved a PASI50 improvement (8, 14). In contrast, a higher dosage (~50–75 mg/d) of acitretin resulted in an increase in the efficacy and a decrease in the side effects (8). The most common adverse drug reaction (ADR) of acitretin is dyslipidaemia, which increases the risk of atherosclerosis in patients treated with retinoids over a long period of time (16). With the reported ADR of acitretin, and the correlation between psoriasis and metabolic changes, this study was designed to examine the glucose and lipid metabolic profile of Chinese patients with psoriasis after the treatment of acitretin.
Methods and Patients
Patients
The demographic and blood tests information of 685 patients with psoriasis, who visited the dermatology department, Xiangya Hospital of Central South University, from 2014 to 2020, were retrospectively analyzed. The study was approved by the Ethics Committee of Xiangya Hospital, with the clinical trial registration number ChiCTR-OCH-14004518 (Chinese Clinical Trial Registry online, https://www.chictr.org.cn/showproj.aspx?proj=5054). The study was conducted in accordance with the Declaration of Helsinki, and informed written consents were obtained from every patient. All the patients were diagnosed with plaque psoriasis by at least two clinicians. For the control group, 395 age- and gender-matched individuals with no prior clinical diagnosis of psoriasis, were recruited in the physical examination center of Xiangya Hospital. Blood tests, liver and kidney functions, blood glucose level, and other indicators were recorded in both controls and psoriatic patients before and after the treatment.
Treatment Regimens
Among the 685 psoriatic patients, 247 patients received acitretin 30 mg/d treatment in combination with the topical ointment calcipotriol for 8–16 weeks; Acitretin was obtained from Chongqing Huapont Pharmaceutical Co., Ltd., China; Calcipotriol was obtained from Bright Future Pharm, Hongkong.
Cell Culture and Acitretin Incubation
Both HepG2 and HaCaT cells were stored in our laboratory at −80°C, and were culturedat 37°C in a humidified 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM) (Hyclone Laboratories Inc., Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS; Biological Industries, Israel), 100 mg/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). Cell passage was carried out in a 1:3 proportion every 3–5 days. Acitretin 5000 ng/mL was then used to incubate HepG2 and HaCaT cells for 36 h.
Detecting the Uptake of 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-β-Dglucopyranose (2-NBDG)
2-NBDG (MKBio, Shanghai, China, CAS NO: 186689-07-6), a green, fluorescent deoxyglucose analog, was used to monitor the cell glucose uptake using flow cytometry or fluorescence microscopy, as an indicator of cellular viability (17). To detect the uptake of 2-NBDG, 1 × 105 cells/well were seeded in a 12-well plate, six of which were incubated with complete DMEM as the control, and the remaining six wells incubated with DMEM and acitretin 5000 ng/mL as the acitretin group. After 36 h, the cells were washed three times with 200 μL cold phosphate-buffered saline (PBS), treated with 0.5 mM 2-NBDG for 30 min, then washed thrice with cold PBS. This process was repeated three times.
The uptake of 2-NBDG by both HaCaT and HepG2 cells was observed by fluorescence microscopy (Nikon Eclipse TS2R, Japan). The cells were then resuspended with 200 μL PBS per well and assessed using the BD Aucrri C6 flow cytometer, as previously described (17).
RNA Extraction and Real Time PCR Analysis
The total RNA of the cells was extracted and 2 μg RNA was reverse transcribed into cDNA per manufacturer's instructions (Vazyme Biotech Co., Ltd, China). The real time PCR primers of human genes, GLUT1, GLUT2, GLUT4, GCK4, and INSIG1, are shown in Supplementary Table 1; Taq DNA polymerase (Continental Lab Products, San Diego, CA, USA) was used according to the manufacturer's instructions. Quantitative real time PCR was carried out on a Real Time PCR Detection System, Applied Biosystems 7500 (Applied Biosystems, Waltham, MA, USA). The PCR analyses were performed in 40 cycles with the following parameters: 94°C, 45 s; 60°C, 45 s.
Statistical Methods
The clinical data and laboratory parameters of all the enrolled participants were collated and analyzed. Continuous variables (e.g., age, BMI, and blood glucose and blood lipid levels) were expressed as the mean ± standard deviation (SD). An independent sample t-test was used to analyse whether the distribution of continuous variables had statistical differences between psoriatic patients and controls. Categorical variables were mainly expressed in terms of frequency (or percentage), and the Chi-squared test was used to compare the distribution between the different groups. A paired sample t-test was used to examine changes in BMI and blood glucose and lipid levels before and after treatment. Statistical significance was set at P < 0.05.
Results
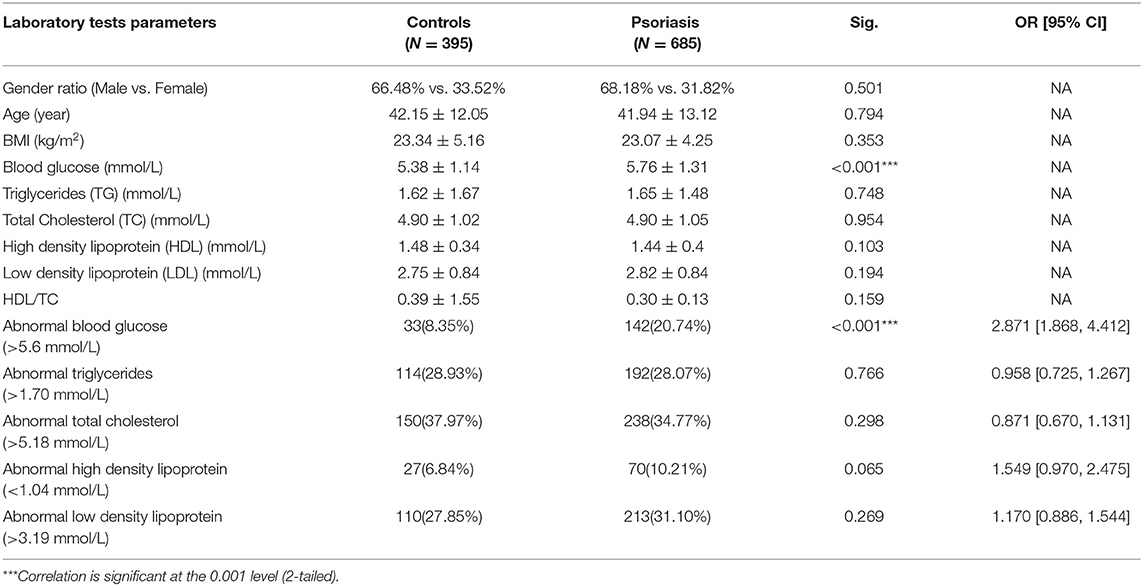
Blood Glucose and Lipid Levels in Psoriatic Patients and Controls
In this study, we enrolled 685 patients with psoriasis and 395 age/gender-matched controls. There were no observed differences in the BMI values between these two groups (23.34 ± 5.16 vs. 23.07 ± 4.25 kg/m2, P = 0.353). As mentioned above, studies have shown that fasting blood glucose and lipid levels are significantly higher in psoriatic patients than those without psoriasis. We confirmed that the 685 patients with psoriasis had higher blood glucose levels than the controls (5.76 ± 1.31 vs. 5.38 ± 1.14 mmol/L, P < 0.001). However, the blood lipid levels showed no difference between the psoriatic patients and the controls (P > 0.05), as shown in Table 1, and the other laboratory parameters of the two groups are also shown in Supplementary Table 2.
A high fasting glucose level (>5.6 mmol/L) in addition to dyslipidaemia values of triglyceride (>1.70 mmol/L), total cholesterol (>5.18 mmol/L), high density lipoprotein (HDL) (<1.04 mmol/L), and low-density lipoprotein (LDL) (>3.19 mmol/L) are indicative of hyperglycaemia, according to the clinical indexes. A comparison of the prevalence of hyperglycaemia and dyslipidaemia in both groups revealed that the occurrence of hyperglycaemia was higher among the psoriatic patients than the control group (20.74% compared with 8.35%, P <0.001). However, the lipid profile showed no difference between the cases and the controls (P > 0.05) (Table 1).
Blood Glucose and Lipid Levels in Patients With Psoriasis Pre- and Post-acitretin Treatment
Among the enrolled patients, 247 patients received acitretin therapy, and their BMI values and lipid profiles were recorded. We compared each patient's BMI value, blood lipid level, and other indicators pre- and post-acitretin treatment using a paired t-test and found that several parameters changed significantly. After receiving acitretin therapy for 8 weeks, the triglyceride and LDL levels increased (P < 0.05) while the HDL levels and BMI values significantly decreased (P < 0.05), as shown in Table 2. There was no observed significant difference in the patients' lipids levels at 12 weeks (N = 37) and 16 weeks (N = 10) (Supplementary Table 3). In this retrospective study, 95 of 247 patients with psoriasis who were treated with acitretin had their blood glucose levels checked before and after treatment. Interestingly, the result showed that the glucose level of the patients significantly decreased after acitretin treatment for 8 weeks (5.71 ± 0.91 vs. 5.55 ± 0.76 mmol/L, P = 0.0019) (Figure 1). For the fasting blood glucose level at 12 weeks was recorded in only 5 patients, therefore it was not suitable to explore the blood glucose change in such a small sample size.
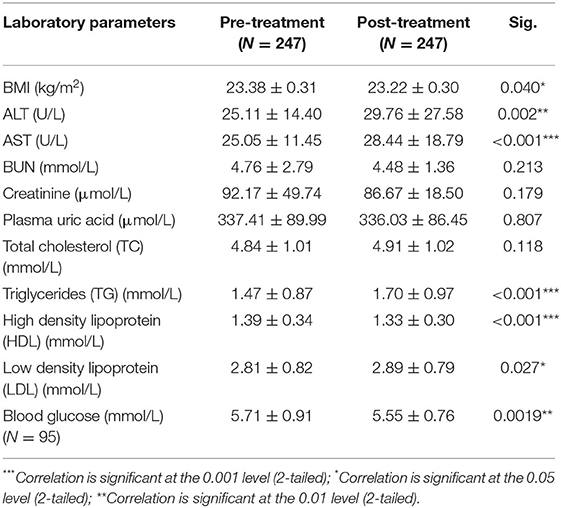
Table 2. Fasting blood glucose and lipids levels change in patients with psoriasis after acitretin treatment for 8 weeks.
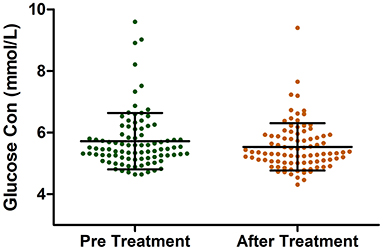
Figure 1. The Glucose Levels in patients with psoriasis pre and after acitretin treatment for 8 weeks.
Uptake of 2-NBDG Significantly Increased With Acitretin Incubation
Fluorescence microscopy and flow cytometry were used to observe the uptake of 2-NBDG. As shown in Figure 2, the HepG2 and HaCaT cells treated with acitretin (5000 ng/ml), had an increased uptake of 2-NBDG compared to the control cells (P < 0.05).
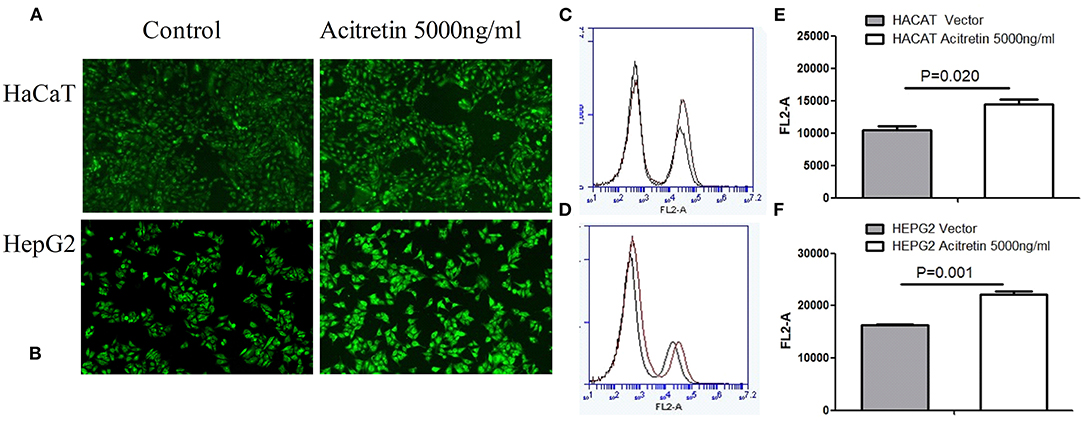
Figure 2. The uptake rate of 2-NBDG in HaCat cells and HepG2 cells when treated with acitretin. Legend: (A,B) The uptake of 2-NBDG by HaCaT and HepG2 cells was observed by fluorescence microscopy with or without acitretin incubation for 36h; (C,D) The uptake of 2-NBDG by HaCaT cells was measured by flow cytometry with or without acitretin incubation for 36h; (D,F) The uptake of 2-NBDG by HepG2 cells was measured by flow cytometry with or without acitretin incubation for 36h.
Increased Expression of Several Genes After Acitretin Incubation
The transcript-level expression of both GLUT1 and GLUT4, which are associated with glucose uptake, transport, and metabolism, were significantly increased in both HepG2 and HaCaT cells after acitretin incubation. However, expression of the related genes, GLUT2, GCK4, and INSIG1, was not significantly different as shown in Figure 3.
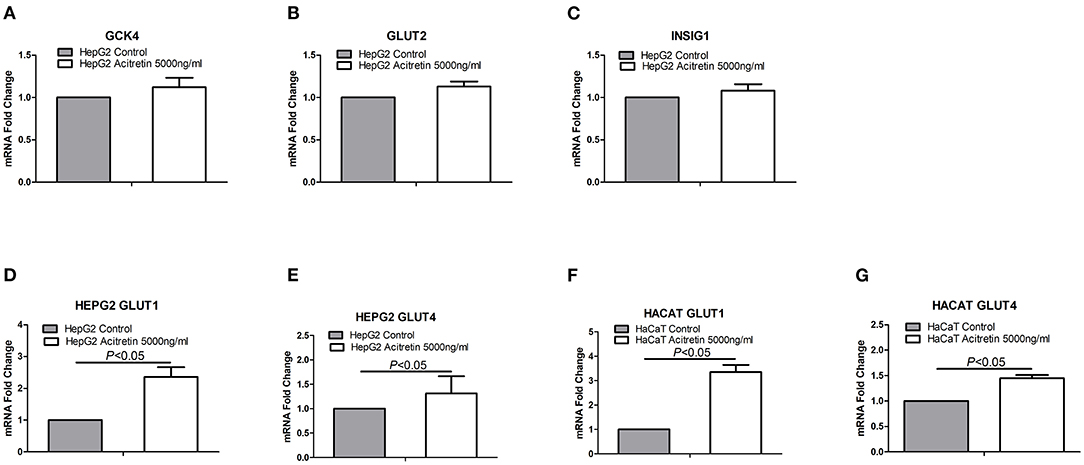
Figure 3. The expression of genes in HaCaT and HepG2 cells when treated with acitretin. Legend: (A–C) the transcript level expression of GCK4, GLUT2 and INSIG1 genes in HepG2 cells with or without the incubation of acitretin 5000ng/ml; (D,E) the GLUT1 and GLUT4 genes expression in HepG2 cells with or without acitretin incubation; (F,G) the GLUT1 and GLUT4 genes expression in HaCaT cells with or without acitretin incubation.
Discussion
In this study, we confirmed that patients with psoriasis had a higher blood glucose level than those without (P < 0.001). Therefore, reducing hyperglycaemia is an important issue for psoriatic patients. Interestingly, the study revealed that the blood glucose level significantly decreased after acitretin treatment. In addition to a remarkable decrease in fasting blood glucose levels, patients treated with acitretin also had a reduction in their BMI. This suggests that acitretin may be a potential therapeutic for psoriatic patients of high glucose level or high BMI value.
Acitretin is widely used in Chinese psoriatic patients due to its low cost and mild side-effects. Previous studies pointed out that both the basal serum glucose level (P < 0.001) and the body weight index (P < 0.05) decreased in ob/ob mice after all-trans retinoic acid (at-RA) was administered (18, 19). A possible rationale is that the retinol activation pathway is impaired in diabetes and obesity, and administering low-dose at-RA ameliorates adiposity, glucose intolerance, and insulin resistance (20). Corbetta et al. found that a low dosage of acitretin induced a mild, transient reduction of insulin sensitivity (21), which is consistent with our findings.
Several studies have shown that retinoids induce the secretion and sensitivity of insulin and gluconeogenesis, resulting in a reduction in the incidence of early obesity (22–28). This indicates that RA is an effective reference anti-diabetic agent (18, 26). While the detailed mechanisms by which RA affects glucose metabolism remain undiscovered, studies have pointed out that retinoids increase the expression of tyrosine protein phosphatase 1B (29) and enhance ATP synthesis in the presence of the PKCδ signalosome (30), or increase the ratio of serum retinol-binding protein 4 (26). Other studies have also indicated that retinoids potentially stimulate glucose uptake by activating p38 mitogen-activated protein kinase (31), retinoic acid receptors, retinoid X receptors, and PPAR gamma heterodimers (18, 32, 33). Additionally, retinoids restore the down-regulated expression of GLUT4 (34). GLUT4 is the main insulin-sensitive glucose transporter that facilitates tissue and cellular uptake of glucose (34). In this study, we found that acitretin upregulated GLUT4 and GLUT1 expressions in HaCaT and HepG2 cells. This contributed to the cellular uptake of peripheral blood glucose, resulting in a decrease in the fasting blood glucose levels, confirming the antihyperglycemic effect of acitretin in Chinese psoriasis patients. It has been shown that glucose transport in keratinocytes is mediated largely by the GLUT1 facilitative transporter. GLUT1 inactivation or inhibitor-assisted decrease resulted in proliferative hyperplasia in mouse models with a psoriasis-like disease (35, 36). From this point of view, the mechanism of acitretin on psoriasis is independent of the inhibition of the glucose metabolic pathway. However, this claim needs to be validated in a large population and using in vitro studies.
Additionally, from the results, there was no observable difference in the lipid profile between psoriatic patients and the control group. Hyperlipidaemia is the most commonly reported ADR to acitretin. Elevated serum triglyceride levels have been reported in ~1.7–50% of patients (37, 38), hypercholesterolaemia in 10–30% of patients, and decreased HDL levels have been found in ~40% of patients taking acitretin (8). Several other studies have also suggested that long-term retinoid therapy may increase the risk of atherosclerosis (16). Our results revealed a significant change in the patients' lipid profile (elevated cholesterol, triglyceride, and LDL levels, and decreased HDL levels), which is consistent with previous studies. However, this change is gradual and is considered (or will become) insignificant with treatment over time. This claim is consistent with reports from Chularojanamontri et al. (15), who stated that long-term and low-dose acitretin treatment in patients with psoriasis is unlikely to cause liver or lipids abnormalities. It is speculated that there is a homeostasis between glucose and lipids in the body, and retinoids is thought to regulate this homeostasis (18). The more peripheral glucose is absorbed by tissues after retinoids treatment, then the less glucose is converted to triglycerides.
There are a few limitations associated with this study. First, the results require further validation using a larger sample size and a multicentre study. Second, the changes in blood glucose levels were observed in patients with short-term treatment in this retrospective study; therefore, it is necessary to extend the duration of acitretin treatment. Finally, the results were obtained experimentally by clinical observation, which needs to be further elucidated through elaborate molecular research.
Conclusion
In this study, we confirmed that the blood glucose levels are elevated in Chinese psoriatic patients compared to those without psoriasis. We also demonstrated that acitretin exerts an apparent effect on lowering the blood glucose levels and the BMI values in Chinese patients with psoriasis. Additionally, it was observed that acitretin up-regulates the expression of GLUT1 and GLUT4 in HaCaT and HepG2 cells, potentially leading to a decrease in the peripheral blood glucose level. This indicates that acitretin may be used to control the blood glucose level in psoriatic patients, especially those with diabetes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of Xiangya Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WC and MC finished the data collection and operation. JS, CL, and WZ organized the paper. XC supported the funding. YK and HQ analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants received from the National Natural Science Foundation of China program (Nos. 81803118, 81903222, 81830096, and 81974479).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.764216/full#supplementary-material
References
1. Brezinski EA. Dhillon JS, Armstrong AW. Economic burden of psoriasis in the united states: a systematic review JAMA Dermatol. (2015) 151:651–8. doi: 10.1001/jamadermatol.2014.3593
3. Kimball AB, Szapary P, Mrowietz U, Reich K. Langley RG, You Y, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol. (2012) 67:76–85. doi: 10.1016/j.jaad.2011.06.035
4. Semenov YR, Herbosa CM, Rogers AT, Huang A, Kwatra SG, Cohen B, et al. Psoriasis and mortality in the US: data from the National Health and Nutrition Examination Survey. J Am Acad Dermatol. (2021) 85:396–403. doi: 10.1016/j.jaad.2019.08.011
5. Ni C, Chiu MW. Psoriasis and comorbidities: links and risks. Clin Cosmet Investig Dermatol. (2014) 7:119–32. doi: 10.2147/CCID.S44843
6. M.Rodríguez-Zúñiga JM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J. Am. Acad. Dermatol. (2017) 77:657–666 e8. doi: 10.1016/j.jaad.2017.04.1133
7. Fernández-Armenteros JM, Gómez-Arbonés X, Buti-Soler M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M, et al. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol. (2019) 33:128–35. doi: 10.1111/jdv.15159
8. Ormerod AD, Campalani E. Goodfield MJ. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. (2010) 162:952–63. doi: 10.1111/j.1365-2133.2010.09755.x
9. Beckenbach L, Baron JM, Merk HF, Löffler H, Amann PM. Retinoid treatment of skin diseases. Eur J Dermatol. (2015) 25:384–91. doi: 10.1684/ejd.2015.2544
10. Shapiro SS, Seiberg M. Cole CA. Vitamin A and its derivatives in experimental photocarcinogenesis: preventive effects and relevance to humans. J Drugs Dermatol. (2013) 12:458–63.
11. Qin X, Chen C, Zhang Y, Zhang L, Mei Y, Long X, et al. Acitretin modulates HaCaT cells proliferation through STAT1- and STAT3-dependent signaling. Saudi Pharm J. (2017) 25:620–4. doi: 10.1016/j.jsps.2017.04.034
12. Weninger W, Rendl M, Mildner M, Tschachler E. Retinoids downregulate vascular endothelial growth factor/vascular permeability factor production by normal human keratinocytes. J Invest Dermatol. (1998) 111:907–11. doi: 10.1046/j.1523-1747.1998.00393.x
13. Niu X, Cao W, Ma H, Feng J, Li X, Zhang X, et al. Acitretin exerted a greater influence on T-helper (Th)1 and Th17 than on Th2 cells in treatment of psoriasis vulgaris. J. Dermatol. (2012) 39:916–21. doi: 10.1111/j.1346-8138.2012.01637.x
14. Heath MS. Sahni DR, Curry ZA, Feldman SR. Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol. (2018) 14:919–27. doi: 10.1080/17425255.2018.1515198
15. Chularojanamontri L, Silpa-Archa N, Wongpraparut C, Limphoka P. Long-term safety drug survival of acitretin in psoriasis: a retrospective observational study. Int. J. Dermatol. (2019) 58:593–9. doi: 10.1111/ijd.14349
16. Vahlquist A. Retinoid-Induced Hyperlipidemia and the Risk of Atherosclerosis. Boca Raton, FL: CRC Press (2007). p. 249–60.
17. Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. (2011) 89:604–13. doi: 10.1093/cvr/cvq360
18. Manolescu DC, Sima A. Bhat PV. All-trans retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice. J Nutr. (2010) 140:311–6. doi: 10.3945/jn.109.115147
19. Shirai T, Shichi Y, Sato M, Tanioka Y, Furusho T, Ota T, et al. High dietary fat-induced obesity in Wistar rats and type 2 diabetes in nonobese Goto-Kakizaki rats differentially affect retinol binding protein 4 expression and vitamin A metabolism. Nutr Res. (2016) 36:262–70. doi: 10.1016/j.nutres.2015.11.018
20. Yang D, Vuckovic MG. Smullin CP, Kim M, Lo CP, Devericks E, et al. Modest Decreases in Endogenous All–Retinoic Acid Produced by a Mouse Heterozygote Provoke Major Abnormalities in Adipogenesis and Lipid Metabolism. Diabetes. (2018) 67:662–73. doi: 10.2337/db17-0946
21. Corbetta S, Angioni R, Cattaneo A, Beck-Peccoz P, Spada A. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. Eur J Endocrinol. (2006) 154:83–6. doi: 10.1530/eje.1.02057
22. Kuang J, Hou X, Zhang J, Chen Y, Su Z. Identification of insulin as a novel retinoic acid receptor-related orphan receptor α target gene. FEBS Lett. (2014) 588:1071–9. doi: 10.1016/j.febslet.2014.02.029
23. Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, et al. Retinoic acid-related orphan receptor c regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. (2019) 79:2604–18. doi: 10.1158/0008-5472.CAN-18-3842
24. Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. Retinoic acid-related orphan receptor gamma (RORgamma): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet. (2014) 10:e1004331. doi: 10.1371/journal.pgen.1004331
25. Brun PJ, Grijalva A, Rausch R, Watson E. Yuen JJ, Das BC, et al. Retinoic acid receptor signaling is required to maintain glucose-stimulated insulin secretion and beta-cell mass. FASEB J. (2015) 29:671–83. doi: 10.1096/fj.14-256743
26. Rhee EJ, Plutzky J. Retinoid metabolism and diabetes mellitus. Diabetes Metab J. (2012) 36:167–80. doi: 10.4093/dmj.2012.36.3.167
27. D.Leung TH, Rainczuk A, Nguyen T, Stephens A, Silke J, Fuller PJ, et al. Targeting XIAP and PPARγ in granulosa cell tumors alters metabolic signaling. J. Proteome Res. (2019) 18:1691–702. doi: 10.1021/acs.jproteome.8b00917
28. Zhao S, Li R, Li Y, Chen W, Zhang Y, Chen G. Roles of vitamin A status and retinoids in glucose and fatty acid metabolism. Biochem Cell Biol. (2012) 90:142–52. doi: 10.1139/o11-079
29. Jeyakumar SM, Vijaya Kumar P. Giridharan NV, Vajreswari A. Vitamin A improves insulin sensitivity by increasing insulin receptor phosphorylation through protein tyrosine phosphatase 1B regulation at early age in obese rats of WNIN/Ob strain. Diabetes Obes Metab. (2011) 13:955–8. doi: 10.1111/j.1463-1326.2011.01407.x
30. Shabrova E, Hoyos B, Vinogradov V, Kim YK, Wassef L, Leitges M, et al. Retinol as a cofactor for PKCdelta-mediated impairment of insulin sensitivity in a mouse model of diet-induced obesity. FASEB J. (2016) 30:1339–55. doi: 10.1096/fj.15-281543
31. Lee YM. Lee JO, Jung JH, Kim JH, Park SH, Park JM, et al. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J Biol Chem. (2008) 283:33969–74. doi: 10.1074/jbc.M804469200
32. Hong SE. Ahn IS, Jung HS, Rayner DV, Do MS. Effect of retinoic acid on leptin, glycerol, and glucose levels in mature rat adipocytes in vitro. J Med Food. (2004) 7:320–6. doi: 10.1089/1096620041938696
33. Kim K, Boo K, Yu YS, Oh SK, Kim H, Jeon Y, et al. RORα controls hepatic lipid homeostasis via negative regulation of PPARγ transcriptional network. Nat Commun. (2017) 8:162. doi: 10.1038/s41467-017-00215-1
34. Montessuit C, Papageorgiou I, Campos L, Lerch R. Retinoic acids increase expression of GLUT4 in dedifferentiated and hypertrophied cardiac myocytes. Basic Res Cardiol. (2006) 101:27–35. doi: 10.1007/s00395-005-0567-y
35. Zhang Z, Zi Z, Lee EE, Zhao J, Contreras DC, South AP, et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat Med. (2018) 24:617–27. doi: 10.1038/s41591-018-0003-0
36. Huang X, Chen J, Zeng W, Wu X, Chen M, Chen X. Membrane-enriched solute carrier family 2 member 1 (SLC2A1/GLUT1) in psoriatic keratinocytes confers sensitivity to 2-deoxy-D-glucose (2-DG) treatment. Exp Dermatol. (2019) 28:198–201. doi: 10.1111/exd.13850
37. Menter A, Korman NJ. Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis psoriatic arthritis: section 4 Guidelines of care for the management treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. (2009) 61:451–85. doi: 10.1016/j.jaad.2009.03.027
Keywords: psoriasis, acitretin, glucose, lipids, metabolism
Citation: Qian H, Kuang Y, Su J, Chen M, Chen X, Lv C, Chen W and Zhu W (2021) Reductive Effect of Acitretin on Blood Glucose Levels in Chinese Patients With Psoriasis. Front. Med. 8:764216. doi: 10.3389/fmed.2021.764216
Received: 25 August 2021; Accepted: 12 November 2021;
Published: 16 December 2021.
Edited by:
Maddalena Napolitano, University of Molise, ItalyReviewed by:
Reza Akbarzadeh, University Medical Center Hamburg-Eppendorf, GermanyIrina Khamaganova, Pirogov Russian National Research Medical University, Russia
Copyright © 2021 Qian, Kuang, Su, Chen, Chen, Lv, Chen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangqing Chen, bGFuY2hlbjIwMDhAMTYzLmNvbQ==; Wu Zhu, emh1d3U3MEBob3RtYWlsLmNvbQ==
 Hua Qian1,2,3
Hua Qian1,2,3 Xiang Chen
Xiang Chen Wangqing Chen
Wangqing Chen Wu Zhu
Wu Zhu