- 1Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Clinical Microbiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Laboratory Medicine, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Departments of Clinical Laboratory, Shanxi Provincial People's Hospital, Affiliated of Shanxi Medical University, Taiyuan, China
- 5Emergency Department, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Bloodstream infections (BSIs) are recognized as important nosocomial infections. Klebsiella pneumoniae is one of the major causes of bacteremia. This retrospective study focused on drug susceptibility and molecular epidemiology of K. pneumoniae isolated from intensive care unit (ICU) patients with BSI in Shanghai, China.
Methods: Consecutive K. pneumoniae isolates were collected from ICU patients. Antibiotic susceptibility testing was conducted by the broth microdilution method. PCR was performed to detect antimicrobial resistance genes. We also completed multilocus sequence typing (MLST) and GoeBURST was used to analyze the result of MLST.
Results: A total of 78 K. pneumoniae isolates were enrolled. K. pneumoniae from ICU-BSIs were highly resistant to almost all common antibiotics. The most frequent resistance determinants responsible for extended-spectrum β-lactamase (ESBL) producers were blaCTX−M−14, blaCTX−M−15, and blaCTX−M−55. KPC was the only enzyme, which was detected by the carbapenemase producers. The most principal sequence types (STs) were ST11, ST15, and ST23.
Conclusion: This study presents for the first time the antibiotic resistance phenotype and molecular epidemiology of K. pneumoniae isolated from ICU patients with BSIs in Shanghai. ICU-BSI K. pneumoniae is characteristic of a high resistance rate. The occurrence of the KPC-2 enzyme may result from nosocomial clonal dissemination of ST11 K. pneumoniae.
Introduction
Bloodstream infection (BSI) is a leading cause of morbidity and mortality in both children and adults worldwide (1–3). Patients in intensive care units (ICUs) have a high risk to suffer from BSIs, due to the high severity of disease, the reduced host defenses, frequent use of invasive medical devices, and inadequate infection control procedures (4–6). BSIs occur in 5–15% of all patients within the first month of hospitalization in ICU (7). Meanwhile, the outcome of these infections is often poor, with an attributable mortality of up to 70% (8).
Klebsiella pneumoniae, an environmental or opportunistic pathogen, is frequently associated with HAIs and is recognized as the major source of BSIs caused by Gram-negative bacteria all over the world (6, 9, 10). According to European Center for Disease Prevention and Control, K. pneumonia ranked third in the main causative microorganisms of ICU-BSI (11). The SENTRY Antimicrobial Surveillance Program demonstrated that the prevalence of BSI K. pneumoniae increased consistently between 1997 and 2016 (12). Consistent with these results, K. pneumoniae is the second most common bacteremia pathogen in China, based on a retrospective survey involving 10 cities (13). Unfortunately, alongside the high frequency, K. pneumoniae can also develop antibiotic resistance, with resistance to β-lactams being most clinically significant (9). In China, K. pneumoniae was the most predominant pathogen in carbapenem-non-susceptible Enterobacteriaceae and the resistance rate of K. pneumoniae against carbapenem enhanced remarkably (from 3.0 to 25% in imipenem and from 2.9 to 26.3% in meropenem) (13, 14). The carbapenem-non-susceptible K. pneumoniae, likely to be associated with the production of carbapenemase, tend to have extensive drug-resistant (XDR, susceptibility limited to ≤ 2 categories) phenotypes, leading to limited treatment options, and poor outcomes (6, 15).
Appropriate and in-time antibiotic therapy, which depends on epidemiology characteristics and drug susceptibility profiles, is critically important to the outcome of BSIs (16). Although K. pneumoniae BSI in ICU patients is mortal, there are only some studies that focus on BSIs caused by K. pneumoniae and on sequence type (ST)11. Relatively few studies have attempted to conduct full-scale susceptibility surveillance and molecular epidemiology investigation of K. pneumoniae causing ICU-BSIs. In this study, we carried out the drug susceptibility testing, explored the distribution of antibiotic resistance genes, and analyzed the predominant STs of K. pneumoniae from ICU patients with BSIs in Shanghai. To our knowledge, this is the first study to investigate such molecular epidemiological data in mainland China. Such epidemiological data are useful to provide evidence for the empirical therapy and develop strategies to prevent these serious infections.
Materials and Methods
Setting and Study Design
This retrospective and cross-sectional study of K. pneumoniae BSI in ICU patients, aiming to analyze drug susceptibility and molecular epidemiology of this pathogen, was performed in Ruijin Hospital Affiliated with Shanghai Jiaotong University School of Medicine. It is a 3,000-bed comprehensive tertiary hospital located in Shanghai, a metropolitan region in China, with ~115,000 patient visits per year. Consecutive ICU patients with K. pneumoniae BSI were identified in the laboratory database of the Department of Clinical Microbiology from January 2016 to December 2019. Only the first positive blood culture of each patient was recorded and enrolled in follow-up experiments.
This study was approved by the Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine. The Review Board waived the request for informed consent because our study only put emphasis on bacteria and exerted no effect on patients.
Microbiology Identification and Storage
Identification of isolates from ICU patients with BSI was conducted on matrix-assisted laser desorption ionization time of flight mass spectrometer (bioMérieux, Marcy-l'Étoile, France) (17). K. pneumoniae strains were stored in LB broth (Sangon Biotech, Shanghai, China) with 30% glycerol (Sangon Biotech, Shanghai, China) at −80°C for further experiments.
Antibiotic Susceptibility Testing and Screening Test for Extended-Spectrum β-Lactamases and Carbapenemases
Drug susceptibility was examined by the broth microdilution method using Sensititre™ GNX2F (Thermo Fisher Scientific, Waltham, MA, USA). Nineteen various antibiotics were involved in the trial, including amikacin, aztreonam, cefepime, cefotaxime, ceftazidime, ciprofloxacin, colistin, doxycycline, ertapenem, gentamicin, imipenem, levofloxacin, meropenem, minocycline, piperacillin/tazobactam, ticarcillin-clavulanic acid, tigecycline, tobramycin, and trimethoprim/sulfamethoxazole. Pseudomonas aeruginosa ATCC 27853, K. pneumoniae ATCC 700603, and Escherichia coli ATCC 25922 were used as quality control in the antibiotics susceptibility assay. Results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) criteria (18). For tigecycline, the result was interpreted based on European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria.
Screening test for extended-spectrum β-lactamase (ESBL) production was accomplished with ceftazidime and cefotaxime according to CLSI 2019, whereas imipenem, ertapenem, or meropenem was used to screen for carbapenemase production (18). Screening tests for ESBL and carbapenemase production were performed by broth microdilution, and the synergy test (ceftazidime, cefotaxime, ceftazidime-clavulanate, and cefotaxime-clavulanate) was used as a confirmatory test for ESBL producers.
According to the consequences of susceptibility tests, K. pneumoniae isolates were classified into multidrug resistance (MDR, non-susceptibility to ≥1 agent in ≥3 antimicrobial categories), XDR, and pan-drug resistance (PDR, non-susceptibility to all agents in all antimicrobial categories).
DNA Extraction, Detection of Resistance Genes, and Confirmation Test for ESBLs and Carbapenemases
To obtain sample DNA from K. pneumoniae, bacteria were resuspended in distilled water and boiled at 100°C for 15 min in order to lyse the cells and release DNA into an aqueous phase. The sample DNA was then segregated from cell fragments through centrifugation at 3,000 × g for 15 min and K. pneumoniae DNA was dissolved in the supernatant, which would be used as the origin of template DNA in the PCR analysis. We detected the genotype of K. pneumoniae whose result was positive in the previous screening phase to clarify the causative genes that result in their ESBL and/or carbapenemase production. Seven genes associated with ESBLs were amplified using T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA), including blaTEM, blaSHV, CTX-M-1,−2,−8,-9,−25 group, and OXA-1,−2,−10 group, blaVEB, blaGES, blaPER, together with several carbapenemase genes, such as blaVIM, blaIPM, blaKPC, blaGIM, blaSPM, blaSIM, blaOXA−48, and blaNDM. After the PCR amplification, the target products were separated through electrophoresis in 1% agarose gel. All positive products were sequenced using the ABI3730xl DNAAnalyzer by Sangon Biotech (Shanghai, China). In addition, the genotypes were determined by comparing the sequencing results with the sequences in GenBank (http://www.ncbi.nlm.nih.gov/BLAST).
Multilocus Sequence Typing
Multilocus sequence typing (MLST) of K. pneumoniae was based on seven conserved housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB), which were amplified and sequenced in the study. After being aligned with the MLST database (http://bigsdb.web.pasteur.fr/klebsiella/primers_used.html), each housekeeping gene sequence was associated with a unique allele, and the combination of seven alleles determines the ST for each K. pneumoniae isolate. New alleles and STs discovered in our study were submitted to the curator of the database (a2xlYnNpZWxsYU1MU1RAcGFzdGV1ci5mcg==). GoeBURST was used for the MLST analysis, demonstrating the allelic relationship and prevalence of various STs. In this study, isolates were classified into the same group if six of the seven alleles were homologous.
Statistical Analysis
IBM SPSS 25.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Values of categorical variables were presented as a percentage of the group where they were derived. The chi-square test or Fisher's exact test was utilized to compare the classified variables, as appropriate. The p-value < 0.05 was considered to be statistically significant.
Results
Characteristics of Total Patient Population
From January 2016 to December 2019, a 48-month study period, a total of 78 consecutive K. pneumoniae isolates were consecutively collected from ICU patients with BSI. Among them, 18 isolates were obtained in 2016, 12 in 2017, 22 in 2018, and 26 in 2019. More men (54/78) than women (24/78) were enrolled in the study, and the age of the 78 patients ranged from 17 to 91 years, with a median age of 60 years.
Antimicrobial Susceptibility Tests
The rates of antibiotics resistance among 78 K. pneumoniae were displayed in Table 1. The highest resistance rates of K. pneumoniae isolated from ICU patients with BSI were ticarcillin-clavulanic acid (93.5%), aztreonam (93.5%), ciprofloxacin (92.3%), and levofloxacin (92.3%). In contrast, relatively low resistance appeared in colistin (11.5%) and tigecycline (23.0%). Among 78 K. pneumoniae isolates, 58 (74.4%) and 56 (71.7%) isolates were confirmed as ESBL-producers and carbapenemase-producers through gene detection, respectively. The ESBL-producing isolates exhibited statistically higher resistance to most antibiotics than non-ESBL-producing ones (p < 0.05), except for amikacin, doxycycline, minocycline, and tigecycline (p-value was 0.05, 0.170, 0.389, and 0.251, respectively). Similar to ESBL-producers, carbapenemase-producers exhibited statistically higher resistance to most antibiotics than non-carbapenemase-producing ones (p < 0.05), except for doxycycline, minocycline, trimethoprim-sulfamethoxazole, tigecycline, and colistin (p-value was 0.894, 0.777, 0.096, 0.394, and 0.289, respectively). The rate of XDR, MDR, and PDR K. pneumoniae isolates was 29.5, 61.5, and 2.6%, respectively.
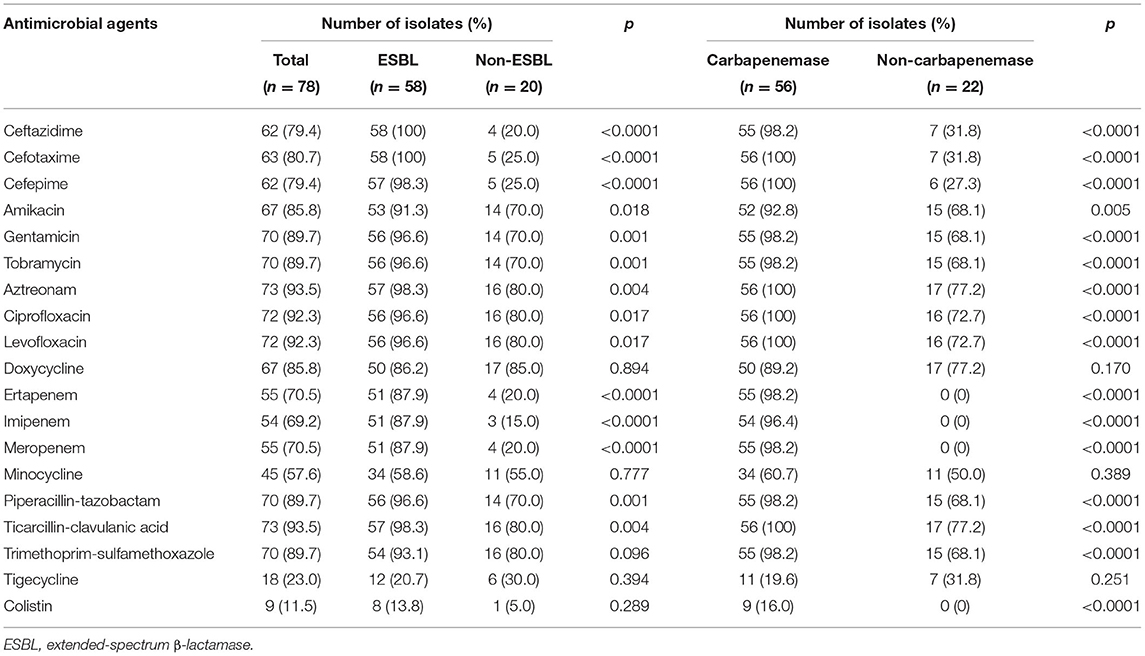
Table 1. Rates of antibiotics resistance among K. pneumoniae bloodstream infections (BSIs) in intensive care unit (ICU) patients.
Characterization of Resistance Genes
Among the 58 ESBL producers, the predominant enzyme was CTX-M (57/58, 98.3%), followed by TEM (43/58, 74.1%). blaCTX−M−14 (44/58, 75.9%) was the most frequently found genotype in ESBL-producers, together with blaCTX−M−15 (15/58, 25.9%) and blaCTX−M−55 (8/58, 13.8%), which were relatively less common. All TEM enzymes were encoded by blaTEM−1. No blaCTX−M (−2, −8, −25group), blaGES, blaVEB, blaOXA(−2, −10group), or blaPER genes were found. Although blaSHV was detected in 40 strains and blaOXA(−1group) in eight strains, the sequencing results demonstrated that these genes were blaSHV−1, blaSHV−11, and blaOXA−1, which belonged to β-lactamase genes rather than ESBL genes. It was also worth noting that 44 of 58 ESBL-producers harbored two or more ESBL genes.
The KPC enzyme is the only carbapenemase produced by isolates resistant to carbapenem, and all KPC enzymes were encoded by blaKPC−2, with no other carbapenemase genes detected in our study.
Multilocus Sequence Typing
Seventeen STs, including three new STs, were identified in 78 K. pneumoniae isolates, among which the most principal STs were ST11 (50/78, 64.1%), followed by ST15 (7/78, 9.0%) and ST23 (3/78, 3.8%). All STs were clustered into one non-overlapping group (Figure 1).
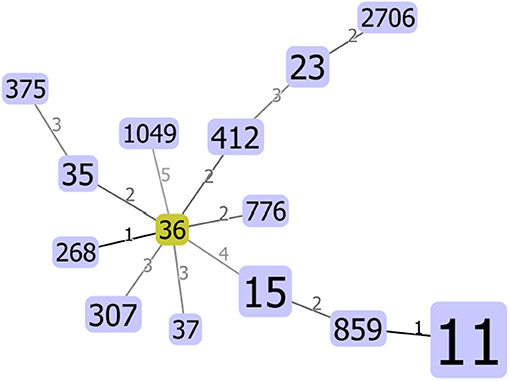
Figure 1. Minimum spanning tree (MST) constructed based on diversity of seven housekeeping genes of Klebsiella pneumoniae. The area of each circle corresponded to the prevalence of the sequence type (ST) in the multilocus sequence typing (MLST) data of this study.
Discussion
As a critical nosocomial pathogen, K. pneumoniae is one of the most common causative factors of BSIs, and the worldwide dissemination of antimicrobial-resistant K. pneumoniae, especially β-lactam and carbapenem-resistant K. pneumoniae, has attracted global attention due to the limited treatment options and high mortality (19–21). Alongside its high antimicrobial-resistant rate, K. pneumoniae is also increasingly associated with high virulence, which is called hypervirulent K. pneumoniae and can cause severe infections, including liver abscesses and bacteremia (22). Furthermore, K. pneumoniae is the most frequent pathogen responsible for ICU-BSIs, representing about 36.8% of all the ICU-BSIs in our hospital. Since ICU patients are predisposed to bacteremia, which can exert a negative impact on the prognosis (23), our study focused on ICU-BSIs caused by K. pneumoniae and intended to elucidate antibiotics susceptibility, resistance gene distribution, and STs of these K. pneumoniae so that clinicians can administer timely and appropriate antibiotics to improve the prognosis.
Consecutive ICU-BSI K. pneumoniae isolates in Shanghai from January 2016 to December 2019 were enrolled in this study. Comparing with the previous study, which investigated the molecular epidemiology of BSI K. pneumoniae from comprehensive source collected between 2012 and 2015, this recent research demonstrated that ICU-BSI K. pneumoniae isolates harbored extremely higher resistance rate to nearly all commonly used antibiotics, such as ceftazidime, cefepime, cefotaxime, amikacin, gentamicin, tobramycin, aztreonam, ciprofloxacin, levofloxacin, piperacillin-tazobactam, imipenem, meropenem, and trimethoprim-sulfamethoxazole (19). It is also worth noting that the proportion of MDR (23/78, 29.5%) and XDR (48/78, 61.5%) in this study is higher than that in other literature (24). Although ICU-BSI K. pneumoniae acquired great resistance to most antibiotics, it was relatively susceptible to tigecycline and colistin, which supported them as potential choices for empirical treatment of ICU-BSI caused by K. pneumoniae. However, in critically ill patients, frequent administration of colistin and tigecycline were considered as risk factors for colistin and tigecycline-resistant K. pneumoniae BSIs, respectively (25). Therefore, some new antibiotics have been developed, such as ceftazidime-avibactam, which can improve the prognosis of bacteremia associated with carbapenem-resistant K. pneumoniae deprived of metallo-β-lactamase (26–28) and can decrease the usage of tigecycline and colistin to slow down the evolution of antibiotic resistance.
In this study, the proportion of ESBL-producing K. pneumoniae isolated from ICU patients with BSI was 74.4%, much higher than our previous study (27.5%) (19), and also exceeded the proportion in Hong Kong (12%), Thailand (27.4%), and American (15.5%) (29–31). Despite the distinct ESBL-producing rate, the main type of ESBLs in K. pneumoniae was the CTX-M enzyme in our study, consistent with the global trend (32). Although blaCTX−M−15 was the dominant gene type in most regions worldwide, including India, Iran, and Lebanon (20, 33, 34), blaCTX−M−14 was the most prevalent ESBL gene found in our study, followed by blaCTX−M−15 and blaCTX−M−55, which indicated that the distribution of blaCTX−M genes may be geographically different. Similarly, the rate of carbapenemase-producer in ICU-BSI K. pneumoniae (71.8%) was higher than that in Taiwan (25%), Athens (60.7%), and Greece (62.3%) (34–36). All carbapenemase-producing ICU-BSI K. pneumoniae isolates harbored blaKPC-2, similar to the study conducted in central China (37). K. pneumoniae with KPC-2, comparing with NDM-1, was more resistant to amikacin and fosfomycin but more susceptible to trimethoprim/sulfamethoxazole, and there were fewer appropriate treatment choices for KPC-2-producing K. pneumoniae (38). According to the consequence of MLST, ST11 was the most predominant ST, and 90% of them possessed the blaKPC−2 gene, coinciding with other studies in China (6, 37). The similarity of antibiotics resistance pattern and the prevalence of ST11 suggested that the nosocomial clonal dissemination of KPC-2-producing ST11 K. pneumoniae happened in ICU patients (39), indicating that current prevention strategies against K. pneumoniae in ICU should be adjusted and improved (40). Moreover, ST11 was also dominant among hypervirulent carbapenemase-producing K. pneumoniae, which was frequently associated with severe infections (41, 42). Thus, we are likely to pay attention to the virulence factors of ICU-BSI K. pneumoniae and find out whether the convergence of carbapenemase production and hypervirulence will exert a negative effect on the outcomes of ICU patients with BSI in our following studies. It is noteworthy that two freshly new STs in our study harbored both ESBL and carbapenemase genes, suggesting the widespread of the antibiotic resistance genes.
Conclusion
In conclusion, this retrospective study focused on drug susceptibility and molecular epidemiology of K. pneumoniae from ICU patients with BSI in Shanghai. Our data demonstrated that ICU-BSI K. pneumoniae isolates were highly resistant to clinically common antibiotics, except for tigecycline and colistin. Thus, it would be relatively appropriate to select tigecycline and colistin for empirical treatment. MLST and genetic analysis showed that nosocomial clonal dissemination of KPC-2-producing ST11 had already appeared in ICUs. Meanwhile, blaCTX−M−14 and blaKPC−2 were confirmed as the most prevalent ESBL and carbapenemase genes, respectively. These alert us that rational administration of antibiotics and regular surveillance of the molecular epidemiology of pathogens were urgent to impede the dissemination of such highly antimicrobial-resistant and mortal pathogens.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by Ethics Committee of Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine. The Review Board waived request for informed consent because our study only put emphasis on bacteria and exerted no effect on patients.
Author Contributions
LH, QZ, and EC contributed to the conceptualization of the study. SX and TC were involved in data curation. TC, HW, and QC performed the formal analysis. EC contributed to funding acquisition. EC, ZY, and LH collected resources. TC involved in writing and original draft preparation. SX helped in writing, review, and editing. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the support of the Shanghai Jiao Tong University School of Medicine Multicenter Clinical Research Program (DLY201803).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to all the technicians of Clinical Microbiology in Ruijin Hospital for their support and assistance in bacteria collection and storage.
References
1. Yoon EJ, Choi MH, Park YS, Lee HS, Kim D, Lee H, et al. Impact of host-pathogen-treatment tripartite components on early mortality of patients with Escherichia coli bloodstream infection: Prospective observational study. EBioMedicine. (2018) 35:76–86. doi: 10.1016/j.ebiom.2018.08.029
2. Zhu S, Kang Y, Wang W, Cai L, Sun X, Zong Z. The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: an observational study based on electronic medical records. Crit Care. (2019) 23:52. doi: 10.1186/s13054-019-2353-5
3. Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. (2013) 173:2039–46. doi: 10.1001/jamainternmed.2013.9763
4. Parajuli NP, Acharya SP, Mishra SK, Parajuli K, Rijal BP, Pokhrel BM. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob Resist Infect Control. (2017) 6:67. doi: 10.1186/s13756-017-0222-z
5. Timsit JF, Ruppe E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. (2020) 46:266–84. doi: 10.1007/s00134-020-05950-6
6. Zheng SH, Cao SJ, Xu H, Feng D, Wan LP, Wang GJ, et al. Risk factors, outcomes and genotypes of carbapenem-nonsusceptible Klebsiella pneumoniae bloodstream infection: a three-year retrospective study in a large tertiary hospital in Northern China. Infect Dis. (2018) 50:443–51. doi: 10.1080/23744235.2017.1421772
7. Kallel H, Houcke S, Resiere D, Roy M, Mayence C, Mathien C, et al. Epidemiology and prognosis of intensive care unit-acquired bloodstream infection. Am J Trop Med Hyg. (2020) 19:877. doi: 10.4269/ajtmh.19-0877
8. Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. (2017) 74:131–41. doi: 10.1016/j.jinf.2016.11.001
9. Martin RM, Bachman MA. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. (2018) 8:4. doi: 10.3389/fcimb.2018.00004
10. Wyres KL, Holt KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. (2018) 45:131–9. doi: 10.1016/j.mib.2018.04.004
11. Frerot M, Lefebvre A, Aho S, Callier P, Astruc K, Aho Glele LS. What is epidemiology? changing definitions of epidemiology 1978–2017. PLoS ONE. (2018) 13:e0208442. doi: 10.1371/journal.pone.0208442
12. Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, et al. the microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial surveillance program. Antimicrob Agents Chemother. (2019) 63:19. doi: 10.1128/AAC.00355-19
13. Wang X, Zhao C, Li H, Chen H, Jin L, Wang Z, et al. Microbiological profiles of pathogens causing nosocomial bacteremia in 2011, 2013 and 2016. Sheng Wu Gong Cheng Xue Bao = Chinese J Biotechnol. (2018) 34:1205–17. doi: 10.13345/j.cjb.180192
14. Hu F, Guo Y, Yang Y, Zheng Y, Wu S, Jiang X, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. (2019) 38:2275–81. doi: 10.1007/s10096-019-03673-1
15. Piperaki ET, Syrogiannopoulos GA, Tzouvelekis LS, Daikos GL. Klebsiella pneumoniae: virulence, biofilm and antimicrobial resistance. Pediatr Infect Dis J. (2017) 36:1002–5. doi: 10.1097/INF.0000000000001675
16. Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, Russo A, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. (2020) 24:29. doi: 10.1186/s13054-020-2742-9
17. Martiny D, Busson L, Wybo L, EI Haj RA, Dediste A, Vandenberg O. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. (2012) 50:1313–25. doi: 10.1128/JCM.05971-11
18. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th Edn. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. (2019).
19. Xiao SZ, Wang S, Wu WM, Zhao SY, Gu FF Ni YX, et al. The resistance phenotype and molecular epidemiology of klebsiella pneumoniae in bloodstream infections in Shanghai, China, 2012-2015. Front Microbiol. (2017) 8:250. doi: 10.3389/fmicb.2017.00250
20. Chong Y, Shimoda S, Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol. (2018) 61:185–8. doi: 10.1016/j.meegid.2018.04.005
21. Wang B, Pan F, Wang C, Zhao W, Sun Y, Zhang T, et al. Molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int J Infect Dis. (2020) 93:311–9. doi: 10.1016/j.ijid.2020.02.009
22. Wang X, Xie Y, Li G, Liu J, Li X, Tian L, et al. Whole-Genome-Sequencing characterization of bloodstream infection-causing hypervirulent Klebsiella pneumoniae of capsular serotype K2 and ST374. Virulence. (2018) 9:510–21. doi: 10.1080/21505594.2017.1421894
23. Bassetti M, Righi E, Carnelutti A. Bloodstream infections in the intensive care unit. Virulence. (2016) 7:267–79. doi: 10.1080/21505594.2015.1134072
24. Del Prete R, Ronga L, Addati G, Magrone R, Abbasciano A, Decimo M, et al. Trends in Klebsiella pneumoniae strains isolated from the bloodstream in a teaching hospital in southern Italy. Infez Med. (2019) 27:17–25.
25. Papadimitriou-Olivgeris M, Bartzavali C, Spyropoulou A, Lambropoulou A, Sioulas N, Vamvakopoulou S, et al. Molecular epidemiology and risk factors for colistin- or tigecycline-resistant carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients during a 7-year period. Diagn Microbiol Infect Dis. (2018) 92:235–40. doi: 10.1016/j.diagmicrobio.2018.06.001
26. Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV. Ceftazidime-Avibactam is superior to other treatment regimens against carbapenem-resistant klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother. (2017) 61:17. doi: 10.1128/AAC.00883-17
27. Zhang W, Guo Y, Li J, Zhang Y, Yang Y, Dong D, et al. In vitro and in vivo bactericidal activity of ceftazidime-avibactam against Carbapenemase-producing Klebsiella pneumoniae. Antimicrob Resist Infect Control. (2018) 7:142. doi: 10.1186/s13756-018-0435-9
28. Mavroidi A, Katsiari M, Likousi S, Palla E, Roussou Z, Nikolaou C, et al. Changing characteristics and In Vitro susceptibility to Ceftazidime/avibactam of bloodstream extensively drug-resistant klebsiella pneumoniae from a Greek intensive care unit. Microb Drug Resist. (2020) 26:28–37. doi: 10.1089/mdr.2019.0090
29. Sawatwong P, Sapchookul P, Whistler T, Gregory CJ, Sangwichian O, Makprasert S, et al. High burden of extended-spectrum beta-lactamase-producing Escherichia Coli and klebsiella pneumoniae bacteremia in older adults: a seven-year study in two rural Thai Provinces. Am J Trop Med Hyg. (2019) 100:943–51. doi: 10.4269/ajtmh.18-0394
30. Abodakpi H, Chang KT, Sanchez Diaz AM, Canton R, Lasco TM, Chan K, et al. Prevalence of extended-spectrum beta-lactamase and carbapenemase-producing bloodstream isolates of Klebsiella pneumoniae in a tertiary care hospital. J Chemother. (2018) 30:115–9. doi: 10.1080/1120009X.2017.1399233
31. Man MY, Shum HP, Chan YH, Chan KC, Yan WW, Lee RA, et al. Clinical predictors and outcomes of Klebsiella pneumoniae bacteraemia in a regional hospital in Hong Kong. J Hosp Infect. (2017) 97:35–41. doi: 10.1016/j.jhin.2017.06.007
32. Calbo E, Garau J. The changing epidemiology of hospital outbreaks due to ESBL-producing Klebsiella pneumoniae: the CTX-M-15 type consolidation. Future Microbiol. (2015) 10:1063–75. doi: 10.2217/fmb.15.22
33. Obeid A, Maliha P, Abdallah S, Akl E, Deeb M, El Moussawi H, et al. ESBL-producing Escherichia coli and Klebsiella pneumoniae in two major Lebanese hospitals: molecular epidemiology and correlation with consumption. J Infect Dev Ctries. (2018) 12:16S. doi: 10.3855/jidc.10038
34. Veeraraghavan B, Shankar C, Karunasree S, Kumari S, Ravi R, Ralph R. Carbapenem resistant Klebsiella pneumoniae isolated from bloodstream infection: Indian experience. Pathog Glob Health. (2017) 111:240–6. doi: 10.1080/20477724.2017.1340128
35. Lin YT, Su CF, Chuang C, Lin JC, Lu PL, Huang CT, et al. Appropriate Treatment for Bloodstream Infections Due to Carbapenem-Resistant Klebsiella pneumoniae and Escherichia coli: A Nationwide Multicenter Study in Taiwan. Open Forum Infect Dis. (2019) 6:ofy336. doi: 10.1093/ofid/ofy336
36. Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. (2014) 58:2322–8. doi: 10.1128/AAC.02166-13
37. Li Y, Shen H, Zhu C, Yu Y. Carbapenem-Resistant Klebsiella pneumoniae infections among ICU admission patients in Central China: prevalence and prediction model. Biomed Res Int. (2019) 2019:9767313. doi: 10.1155/2019/9767313
38. Lin L, Xiao X, Wang X, Xia M, Liu S. In Vitro antimicrobial susceptibility differences between carbapenem-resistant KPC-2-Producing and NDM-1-Producing Klebsiella pneumoniae in a teaching hospital in Northeast China. Microb Drug Resist. (2020) 26:94–9. doi: 10.1089/mdr.2018.0398
39. Gu B, Bi R, Cao X, Qian H, Hu R, Ma P. Clonal dissemination of KPC-2-producing Klebsiella pneumoniae ST11 and ST48 clone among multiple departments in a tertiary teaching hospital in Jiangsu Province, China. Ann Transl Med. (2019) 7:716. doi: 10.21037/atm.2019.12.01
40. Liu J, Yu J, Chen F, Yu J, Simner P, Tamma P, et al. Emergence and establishment of KPC-2-producing ST11 Klebsiella pneumoniae in a general hospital in Shanghai, China. Eur J Clin Microbiol Infect Dis. (2018) 37:293–9. doi: 10.1007/s10096-017-3131-4
41. Karlsson M, Stanton RA, Ansari U, McAllister G, Chan MY, Sula E, et al. Identification of a carbapenemase-producing Hypervirulent Klebsiella pneumoniae isolate in the United States. Antimicrob Agents Chemother. (2019) 63:19. doi: 10.1128/AAC.00519-19
Keywords: bloodstream infection, Klebsiella pneumoniae, intensive care units, drug susceptibility, molecular epidemiology
Citation: Xiao S, Chen T, Wang H, Zeng Q, Chen Q, Yang Z, Han L and Chen E (2021) Drug Susceptibility and Molecular Epidemiology of Klebsiella pneumoniae Bloodstream Infection in ICU Patients in Shanghai, China. Front. Med. 8:754944. doi: 10.3389/fmed.2021.754944
Received: 07 August 2021; Accepted: 10 September 2021;
Published: 13 October 2021.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Diganta Dey, Ashoke Laboratory Clinical Testing Centre Private Limited, IndiaXiaoqiang Liu, Northwest A&F University, China
Copyright © 2021 Xiao, Chen, Wang, Zeng, Chen, Yang, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhong Han, aGFubGl6aG9uZzExMDdAMTYzLmNvbQ==; Erzhen Chen, Y2hlbmVyemhlbkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shuzhen Xiao1,2†
Shuzhen Xiao1,2† Lizhong Han
Lizhong Han