
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 28 October 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.753257
This article is part of the Research TopicComputational Medicine in Visual Impairment and Its Related DisordersView all 7 articles
 Haishuang Lin1,2,3
Haishuang Lin1,2,3 Jing Sun4
Jing Sun4 Nathan Congdon5,6
Nathan Congdon5,6 Meiping Xu1
Meiping Xu1 Shanshan Liu4
Shanshan Liu4 Yuanbo Liang1,2,3
Yuanbo Liang1,2,3 Hailin Wang4
Hailin Wang4 Shaodan Zhang1,2,3,4*
Shaodan Zhang1,2,3,4*Purpose: To assess the potential of a health examination center-based screening model in improving service for uncorrected refractive error.
Methods: Individuals aged ≥18 years undergoing the routine physical examinations at a tertiary hospital in the northeast China were invited. Presenting visual acuity, noncycloplegic autorefraction, noncontact tonometry, fundus photography, and slit-lamp examination were performed. Refractive error was defined as having spherical equivalent ≤ -0.75 D or ≥ +1 D and uncorrected refractive error was considered as refractive error combined with presenting visual acuity < 6/12 in the better eye. Costs for the screening were assessed.
Results: A total of 5,284 participants (61 ± 14 years) were included. The overall prevalence of myopia and hyperopia was 38.7% (95% CI, 37.4–40.0%) and 23.5% (95% CI, 22.3–24.6%), respectively. The prevalence of uncorrected refractive error was 7.85% (95% CI, 7.13–8.58%). Women (p < 0.001 and p = 0.003), those with age ≥ 70 years (p < 0.001 and p = 0.003), and myopia (p < 0.001 and p < 0.001) were at higher risk of uncorrected refractive error and uncorrected refractive error-related visual impairment. Spectacle coverage rate was 70.6% (95% CI, 68.2–73.0%). The cost to identify a single case of refractive error and uncorrected refractive error was US$3.2 and US$25.2, respectively.
Conclusion: The prevalence of uncorrected refractive error is high in the urban Chinese adults. Health examination center-based refractive error screening is able to provide an efficient and low-cost model to improve the refractive services in China.
Uncorrected refractive error (URE), predominantly myopia, is the most common cause of moderate and severe visual impairment and the second leading cause of blindness globally, imposing a significant public health burden to the society (1, 2). Despite the relatively low cost of refractive correction, such as spectacles, the prevalence of URE remains high (3, 4). The main barrier keeping the affected adults from seeking refractive services is the absence of convenient and low-cost access to the healthcare delivery system (5).
China is one of the countries facing the greatest burden of refractive error (RE) (6). The prevalence of myopia in Chinese adults ranges from 21.1 to 62.9% (7–11). Meanwhile, China is experiencing the challenge of the high and growing prevalence of myopia among children and young adults, which is leading, in turn, to a growing burden of high myopia in adults (12). Refractive service is not well established and even absent in some underserved regions in China. Reorganizing eye care to the coexist established health services may be a way forward.
Health examination centers are well-established public health delivery system in China, which provide screening tests for early detection of specific diseases or risk factors among the population at large. People come to these centers for a general evaluation of their health status, either by the individual or organized by their employers. There are nearly 10,000 health examination centers across China covering a population of 700 million (13). These centers provide a unique platform for the screening of vision-threatening eye diseases. In this study, we report the efficacy of health examination center-based RE screening and referral among adults aged ≥18 years.
This single-center, cross-sectional study was approved by the Ethics Committee of the Fourth People's Hospital of Shenyang and was conducted according to the tenets of the Declaration of Helsinki. The Committee determined that informed consent was not required, as data were collected in deidentified fashion and used for the purposes of health service monitoring.
All the participants aged ≥18 years presenting to the health examination center of the Fourth People's Hospital of Shenyang from March 1 to April 30, 2017 were invited to attend. Three trained nonmedical staff conducted the ocular examinations including assessment of presenting visual acuity (PVA) (uncorrected if the participant did not own spectacles and with distance spectacles if worn), noncontact pneumotonometry (CT−1P Computerized Tonometer, Topcon Ltd., Tokyo, Japan), noncycloplegic autorefraction (ARK-510A, Nidek Co., Ltd., Tokyo, Japan), and nonmydriatic fundus photography (Canon CX-1, Tokyo, Japan). Only staffs achieving accuracy over 95% in PVA tests during the training phase were qualified for further screening. Fundus photographs were evaluated independently by two glaucoma specialists (SDZ and YBL). For autorefraction, three consecutive readings of sphere, cylinder, and axis of each eye were taken and the mean spherical equivalent (SE) (spherical power + * cylinder power) was used for analysis. Participants with PVA <6/12, intraocular pressure (IOP) ≥24 mm Hg, obvious lens opacity, or abnormalities on fundus photography in either eye were referred to the ophthalmology outpatient clinics.
Refractive error was categorized as follows by using the data from the better-seeing eye: myopia as SE ≤ −0.75 D, low myopia SE as ≤ −0.75 D to > −3 D, moderate myopia as SE ≤ −3 D to > −6 D, high myopia as SE ≤ −6 D, hyperopia as SE ≥ +1 D, and high hyperopia SE ≥ +3 D. URE was defined as RE with PVA < 6/12 in the better-seeing eye. URE-related visual impairment was defined as URE with PVA < 6/18 in the better-seeing eye (14). Participants with obvious macular or vascular abnormalities on fundus photography were excluded for the further URE-related analysis.
The need for spectacles was categorized as either “met” or “unmet.” “Met need” describes the number of the participants with RE as defined above achieving corrected visual acuity (VA) ≥ 6/12 in the better-seeing eye with current distance spectacles. “Unmet need” was defined as the number of the participants who did not achieve VA ≥ 6/12 with current spectacles or did not have any spectacles at all. Since the same criteria were used in this study, the “unmet need” for spectacles was equal to the URE. Spectacle coverage was defined as: met need/(met need + unmet need). Participants with unmet needs for spectacles were given a detailed explanation of their refractive condition and referred to an optometry clinic for treatment. Telephonic interviews were conducted to assess the adherence of the refraction correction in those with URE.
The costs of the screening were calculated by using a healthcare system perspective including personnel, equipment, and overhead costs. Personnel costs were calculated as the cost of each provider participating in a certain activity specifically dedicated to program-related screening based on the 2017 mean salary catalog of Liaoning province from the National Bureau of Statistics of China (15). Costs of the equipments including VA charts, tonometer, autorefractor, and fundus camera were calculated from list prices, assuming a lifespan of 5 years (16). Overhead costs including infrastructure, electricity, water, and internet links were also assessed. All the costs were given in US dollars at the average of 2017 exchange rate [1 US dollar = 6.75 Renminbi (RMB)] (15). An annual inflation rate of 2% was used to estimate the cost in 2020 based on the 2017–2020 Consumer Price Index of the National Bureau of Statistics of China (15). To account for real-world variability, sensitivity analyses were also performed as following. Overhead costs were allowed to vary from $0 (i.e., covered by the health examination center) to +20% of the base case value. Other costs were assumed to vary by ±20% of the base case value (17). The costs to identify a single case of RE and a case of URE were calculated.
Statistical analyses were performed by using the Statistical Package for the Social Sciences (SPSS) for Windows, version 11.0 (SPSS, Chicago, Illinois, USA). Continuous data with normal distribution were presented as mean ± SD. Data that did not follow a normal distribution were shown as median [interquartile range (IQR)]. Prevalence of RE for different ages and genders was compared by using the chi-squared test. The multivariate regression analysis was used to investigate the impact of gender, age, and RE on the rate of URE and URE-related visual impairment. p < 0.05 was considered as statistically significant.
In this study, a total of 5,522 eligible adults were initially enrolled with 5,284 adults (95.7%; 95% CI, 95.2–96.2%) completing all the examinations and fulfill the criteria for analysis. The mean age of these participants was 61 ± 14 years (range, 23–96 years) and 39.4% (n = 2,083; 95% CI, 38.1–40.7%) were the females. The distribution of SE was leptokurtic and asymmetric with a higher frequency of the negative (myopic) refractive powers (Figure 1). The median SE was −0.25 D (IQR −1.72, 0.88). The overall prevalence of the myopia and high myopia was 38.7% (n = 2,046; 95% CI, 37.4–40.0%) and 5.3% (n = 280; 95% CI, 4.69–6.90%), respectively with an age-standardized estimate of 49.5% (95% CI, 48.1–50.8%) and 7.14% (95% CI, 6.44–7.83%), respectively. Females had a significantly higher prevalence of myopia compared to the males [47.1% (95% CI, 45.0–49.3%) vs. 33.2% (95% CI, 31.6–34.9%); χ2 = 103, p < 0.001]. The prevalence of myopia decreased steadily with increasing age from a peak of 71.8% (95% CI, 68.0–75.6%) at age <40 years to 26.5% (95% CI, 23.9–29.0%) at age ≥ 70 years (χ2 = 588, p < 0.001). The overall prevalence of hyperopia was 23.5% (n = 1,240; 95% CI, 22.3–24.6%) with a male preponderance [males 25.6% (95% CI, 24.1–27.2%) vs. females 20.1% (95% CI, 18.4–21.8%), χ2 = 21.5, p < 0.001]. The prevalence of high hyperopia was 2.0% (n = 108; 95% CI, 1.66–2.43%), higher in the females (2.4%; 95% CI, 1.7–3.0%) compared to the males (1.8%; 95% CI, 1.4–2.3%, χ2 = 7.09, p = 0.006). The age-standardized prevalence of hyperopia and high hyperopia was 11.9% (95% CI, 11.0–12.8%) and 1.31% (95% CI, 1.00–1.61%), respectively. The prevalence of hyperopia demonstrated an age-related increase ranging from 2.6 to 11.4% (95% CI, 1.4–13.2%) in the participants <60 years to 31.6% (95% CI, 29.4–33.8%) of 60–69 years and 44.9% (95% CI, 42.1–47.8%) of ≥ 70 years (χ2 = 739, p < 0.001) (Table 1).
In total, 415 individuals (202 males and 213 females, mean age 63.3 ± 15.8 years) exhibited a SE > +1 D or < −0.75 D and presenting VA <6/12 in the better-seeing eye, presenting a prevalence of 7.85% (95% CI, 7.13–8.58%) for URE. Among these participants with URE, 360 (86.7%; 95% CI, 83.5–90.0%) were unaware of their refractive status and had never been diagnosed with RE, 24 (5.8%; 95% CI, 3.5–8.0%) were wearing inappropriate spectacles, and 31 (7.5%; 95% CI, 5.9–10.0%) had been diagnosed previously, but did not fill the prescription given to them for the glasses. URE-related visual impairment was observed in 3.0% (n = 157; 95% CI, 2.5–3.4%) participants. Women (χ2 = 14.67, p < 0.001 and χ2 = 7.962, p = 0.003), those with age of 70 years and older (χ2 = 27.21, p < 0.001 and χ2 = 15.84, p = 0.003), and myopia (χ2 = 56.87, p < 0.001 and χ2 = 50.81, p < 0.001) are at higher risk of both URE and URE-related visual impairment (Table 2).
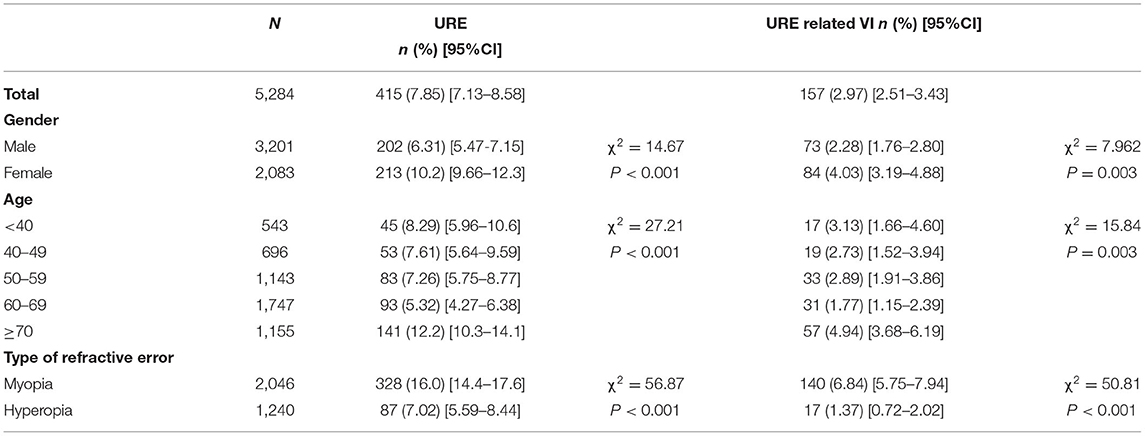
Table 2. The prevalence of uncorrected refractive error (URE) and URE-related visual impairment (VI) according to the age, gender, and type of RE.
Out of the 3,286 participants with RE (2,046 myopia and 1,240 hyperopia), 997 participants [526 males (52.8%; 95% CI, 49.7–55.9%) and 471 females (47.2%; 95% CI, 44.1–50.4%)] with mean age (53.3 ± 14.7 years) achieved presenting VA of 6/12 or above in the better eye with their current spectacles (met need). The unmet need was equal to the value of URE (n = 415) including 24 (5.8%; 95% CI, 3.5–8.0%) undercorrected and 391 (94.2%; 95% CI, 92.0–96.5%) uncorrected. Spectacle coverage rate was 70.6% [997/(997 + 415) ×100%] (95% CI, 68.2–73.0%) in the present RE population. Younger adults with age < 40 years had a peak of spectacle coverage 83.9% (95% CI, 79.6–88.3%), compared to those with age ≥ 70 years [48.5% (95% CI, 42.6–54.5%)] (χ2 = 103, p < 0.001). Participations with myopia [73.5% (95% CI, 71.0–75.9%)] had significantly higher spectacle coverage, compared to those with hyperopia [50.6% (95% CI, 43.1–58.0%)] (χ2 = 38.9, p < 0.001). demonstrated significantly higher spectacle coverage (Table 3). After the screening, 137 individuals with URE (33.0%; 95% CI, 28.5–37.6%) adhered to the referral suggestion and got their RE corrected with the spectacles.
The total cost of this 2-month program was estimated at US$10,449 (sensitivity analysis, varying from US$6,640 to US$12,539). Staff costs comprised US$4,892 (varying from US$3,914 to US$5,871) with equipment accounting for US$3,408 (varying from US$2,726 to US$4,089) and overhead costs US$2,149 (varying from US$0, if the health examination center covers the fees, to US$2,579). The cost to identify a single case of suspected RE and URE was US$3.2 (varying from US$2.0 to US$3.8) and US$25.2 (varying from US$16.0 to US$30.2), respectively. The cost for identifying per case of URE-related visual impairment was US$66.6 (varying from US$42.3 to US$79.9) (Table 4).
A most recent survey on the causes of vision loss in China revealed that URE remains the major leading cause of moderate/severe vision impairment and blindness in China in the overall population (19, 20). India and China account for approximately 50% of global vision impairment and blindness due to UREs (4, 21, 22). Improving the public awareness of visual health and the unmet demand for refractive care among the affected adults are still big challenges. In this study, we reported the outcome of integrating the RE screening into the general health examination, a well-established public health delivery system in China. It possesses the advantages of easy recruitment of screenees, reductions in demand for the human resources through scale, and the lower costs for equipment and travel, providing a potentially ideal opportunity for a large population to contact the available and routine eye care service by convenience.
Detected prevalence of RE and URE greatly depends on the screening method and criteria used. During health examination, an intensive population (usually 200–400) needs to be screened within 3 to 4 h in the morning. To guarantee the confluence of the entire procedure, a quick and noninvasive refractive assessment method is arbitrarily required. Visual correction by subjective refraction test or pinhole glasses is time-consuming and not suitable for the present protocol. It has been reported that SE of autorefraction were statistically similar compared to subjective refraction and were able to provide the reasonable and repeatable estimation of RE in the adults (23). In population with a high prevalence of RE, combining the uncorrected VA and noncycloplegic autorefraction in serial order, it achieved the adequate sensitivity and specificity for RE screening (24–26). So, we utilized a combination of autorefraction and presenting VA test for the diagnosis of URE in current screening. Considering the accuracy and specificity of the autorefraction test, a strict criterion was introduced (SE ≤ −0.75 D for myopia and ≥ +1 D for hyperopia) (27, 28). In this study, we invited all the individuals of 18 years and older coming to the centers for routine health examination without sampling. The prevalence of present URE may not be comparable with that derived from the population-based studies, especially when the different diagnostic criteria were used (29–36) (Table 5). Meanwhile, the impacts of URE in the Chinese adults were usually addressed when analyzing the cause of visual impairment (19, 20, 37–39). Studies directly reporting the prevalence, distribution, and service coverage for URE among the adults in China are very limited. Although with these differences, the URE in the present urban Chinese population is quite high (7.9%; 95% CI, 7.13–8.58%). Consistent with the previous studies, we observed higher URE in the older individuals (30, 35). Females demonstrated higher prevalence of URE (10.2%; 95% CI, 9.7–12.3%) and URE-related visual impairment (4.0%; 95% CI, 3.2–4.9%) compared to the males (6.3%; 95% CI, 5.5–7.2% and 2.3%; 95% CI, 1.8–2.8%, respectively). It is consistent with the previous findings that the older and female individuals persistently bear more burden of URE than their counterparts over the past few decades (4, 40). A gender-sensitive health policy may be helpful for managing the gender inequality in global vision loss caused by URE.
Spectacle coverage is another index of refractive service (5, 24, 41–43). The spectacle coverage rate in the present urban adults (≥18 years, 70.6%; ≥40 years, 67.3%; ≥60 years, 60.3%) was lower than that reported in the Australians (≥40 years, 82.2–93.5%), but higher than that among the semi-rural adults in Shanghai, China (≥60 years, 44.1%), India (≥15 years, 33.1%; ≥40 years, 53.6%), Colombia (≥15 years, 50.9%), Kenya (≥50 years, 25.5%), and Nigerian (≥40 years, 4.4%) (36, 44–49). Barriers to the spectacle use mainly include economic stability of the society and individuals, limited refractive care access, and poor health awareness (50).
Inconvenience associated with wearing glasses, the uncertainty of the perceived benefit, and lack of social desirability may also contribute to the low spectacle use (51). In a community-based screening program in Baltimore, 72% of the individuals with unmet need of spectacles did not obtain eyeglasses even with a very low price (52). Similarly, in this study, only one-third of the individuals with URE adhered to the referral suggestion and got their spectacles. Strategies to improve the public awareness of visual health are of great challenge (19). Incorporating educational content may help to improve the knowledge and awareness about URE, while introducing ready-made spectacles into vision screening may help to provide a direct experience of VA improvement and increase demand for and compliance with spectacles (53, 54). Recently, we are introducing a wavefront aberration-based subjective autorefraction into this screening model. Individuals with RE can obtain a refractive prescription immediately after the examination, which may help to increase the diagnostic accuracy and improve the adherence of spectacle use among those affected individuals. In addition, an additional advantage of the present model is that the health examinations at most of the centers are repeated annually, offering the potential to improve the compliance in the unresponded suspects over time.
The cost for RE screening varies significantly among different countries and settings, depending on the capacity for service, personnel involved, the type and amount of equipment utilized, and the model used. Being integrated into a coexist healthcare system, the demand for human resources, traffic fees, and other overhead costs in the present screening model were greatly reduced. Accordingly, the cost of present screening (US$3.2 per case of RE and US$25.2 per case of URE) was significantly lower than that among school children in rural China, which reported an average cost of US$37.53, US$52.19, and US$59.14 for per case of RE detected in the teacher, optometrist, and volunteer screening model, respectively (55). Noteworthy, the cost in this study not only includes fees for the screening of RE, but also for the detection of other suspected eye diseases including glaucoma, ocular hypertension, and retinal vascular diseases. In addition, the cost of present screening will be further decreased when it is applied to a larger population.
This study has some limitations. First, presbyopia, which is the most common cause of vision impairment in older adults, was not included in this study. Given the very high prevalence of presbyopia among working-age adults, low rates of correction in China and the modest cost of near-vision testing, it seems likely that such inclusion would have reduced the total program cost per beneficiary. Second, a threshold of VA <6/12 was used when calculating the prevalence of URE and the unmet need of the spectacles. However, individuals with RE and 12/12 ≥ PVA ≥ 6/12 may also benefit from the spectacles. Refractive correction among this population will further improve their quality of vision and, therefore, augment the efficiency of the present model. Third, without a corrected VA, we are not able to distinguish all the individuals that could benefit from a refractive correction. As discussed above, we are now introducing a wavefront aberration-based subjective autorefraction into this screening model to increase the diagnostic accuracy and to provide direct prescription of the spectacles to those affected individuals.
China bears a great burden of URE-related visual impairment in adults. The model of integrating RE screening into the general health examination is efficient, inexpensive, and practical. Unlike population-based or community-based screening that was initiated by the government or public health institutes, individuals come to the health examination centers for a routine physical examination actively. It facilitates greater contact with the healthcare system among the at-risk persons. Meanwhile, this integrated model greatly decreases the costs (traffic and infrastructure) of the screening. By scaling up this eye disease screening model more widely, we can better target the very large and growing cohort of individuals in China to improve the detection of URE and other ocular diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Fourth People's Hospital of Shenyang. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HL contributed to the formal analysis and the original draft writing. JS contributed to the investigation and formal analysis. NC contributed to the writing, review, and editing. MX contributed to the validation and data curation. SL contributed to the investigation and resources. YL contributed to the supervision. HW contributed to the project administration and funding acquisition. SZ contributed to the supervision, conceptualization, methodology, funding acquisition, writing, and the draft revising.
This study was funded by National Key Research and Development Program of China (2020YFC2008200), the Basic Scientific Research Program of Wenzhou (Y20190695), Key Innovation and Guidance Program of the Eye Hospital, Wenzhou Medical University (YNZD2201903), and the Shenyang Key Technology Research and Development Project (17-230-9-03). NC was supported by the Ulverscroft Foundation (UK) and was the Research Director for the Orbis International, a nongovernmental organization providing refractive error services in China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CL declared a shared affiliation with one of the authors, NC, to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank Kemi Feng for the statistical consultation.
1. Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision Loss Expert Group of the Global Burden of Disease S. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. (2017) 5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5
2. Tahhan N, Papas E, Fricke TR, Frick KD, Holden BA. Utility and uncorrected refractive error. Ophthalmology. (2013) 120:1736–44. doi: 10.1016/j.ophtha.2013.02.014
3. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123:1036–42. doi: 10.1016/j.ophtha.2016.01.006
4. Lou L, Yao C, Jin Y, Perez V, Ye J. Global patterns in health burden of uncorrected refractive error. Invest Ophthalmol Vis Sci. (2016) 57:6271–7. doi: 10.1167/iovs.16-20242
5. Ma Y, Congdon N, Shi Y, Hogg R, Medina A, Boswell M, et al. Effect of a local vision care center on eyeglasses use and school performance in rural china: a cluster randomized clinical trial. JAMA Ophthalmol. (2018) 136:731–7. doi: 10.1001/jamaophthalmol.2018.1329
7. Xu C, Pan C, Zhao C, Bi M, Ma Q, Cheng J, et al. Prevalence and risk factors for myopia in older adult east Chinese population. BMC Ophthalmol. (2017) 17:191. doi: 10.1186/s12886-017-0574-4
8. Xu L, Li J, Cui T, Hu A, Fan G, Zhang R, et al. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. (2005) 112:1676–83. doi: 10.1016/j.ophtha.2005.05.015
9. Liang YB, Wong TY, Sun LP, Tao QS, Wang JJ, Yang XH, et al. Refractive errors in a rural Chinese adult population the Handan eye study. Ophthalmology. (2009) 116:2119–27. doi: 10.1016/j.ophtha.2009.04.040
10. Li Z, Sun D, Cuj H, Zhang L, Lju P, Yang H, et al. Refractive error among the elderly in rural Southern Harbin, China. Ophthalmic Epidemiol. (2009) 16:388–94. doi: 10.3109/09286580903312285
11. He M, Huang W, Li Y, Zheng Y, Yin Q, Foster PJ. Refractive error and biometry in older Chinese adults: the Liwan eye study. Invest Ophthalmol Vis Sci. (2009) 50:5130–6. doi: 10.1167/iovs.09-3455
12. Wang SK, Guo Y, Liao C, Chen Y, Su G, Zhang G, et al. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without cycloplegia. JAMA Ophthalmol. (2018) 136:1017–24. doi: 10.1001/jamaophthalmol.2018.2658
13. IRIoC. B. Report on the health examination market survey and development trends in China (2014-2018). Available online at: http://www.askci.com/reports/201403/1111757286771.shtml (accessed July 14, 2021).
14. Organization. WH. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO version for 2016. Available online at: http://apps.who.int/classifications/icd10/browse/2016/en#/H53-H54 (accessed October 14, 2019).
15. The website of the National Bureau of Statistics of China. Available online at: http://data.stats.gov.cn (accessed October 14, 2019).
16. Jones SJ, Vernon SA, Cater L, Henry DJ. Costing a community based screening programme for the detection of glaucoma. Eye (Lond). (1990) 4:98–102. doi: 10.1038/eye.1990.11
17. Pizzi LT, Waisbourd M, Hark L, Sembhi H, Lee P, Crews JE, et al. Costs of a community-based glaucoma detection programme: analysis of the Philadelphia Glaucoma Detection and Treatment Project. Br J Ophthalmol. (2018) 102:225–32. doi: 10.1136/bjophthalmol-2016-310078
18. Cheng F, Shan L, Song W, Fan P, Yuan H. Distance- and near-visual impairment in rural Chinese adults in Kailu, Inner Mongolia. Acta Ophthalmol. (2016) 94:407–13. doi: 10.1111/aos.12808
19. Xu T, Wang B, Liu H, Wang H, Yin P, Dong W, et al. Prevalence and causes of vision loss in China from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Lancet Public Health. (2020) 5:e682–91. doi: 10.1016/S2468-2667(20)30254-1
20. Hu A, Gu SZ, Friedman DS, Cao K, Wang N. Six-Year Incidence and causes of low vision and blindness in a rural Chinese adult population: the handan eye study. Ophthalmic Epidemiol. (2021) 28:160–8. doi: 10.1080/09286586.2020.1795886
21. Naidoo KS, Leasher J, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, et al. Global vision impairment and blindness due to uncorrected refractive error, 1990-2010. Optom Vis Sci. (2016) 93:227–34. doi: 10.1097/OPX.0000000000000796
22. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. (2012) 96:614–8. doi: 10.1136/bjophthalmol-2011-300539
23. Paudel N, Adhikari S, Thakur A, Shrestha B, Loughman J. Clinical accuracy of the Nidek ARK-1 Autorefractor. Optom Vis Sci. (2019) 96:407–13. doi: 10.1097/OPX.0000000000001386
24. Guo Y, Liu LJ, Xu L, Lv YY, Tang P, Feng Y, et al. Visual impairment and spectacle use in schoolchildren in rural and urban regions in Beijing. Eur J Ophthalmol. (2014) 24:258–64. doi: 10.5301/ejo.5000348
25. Lai YH, Tseng HY, Hsu HT, Chang SJ, Wang HZ. Uncorrected visual acuity and noncycloplegic autorefraction predict significant refractive errors in Taiwanese preschool children. Ophthalmology. (2013) 120:271–6. doi: 10.1016/j.ophtha.2012.08.009
26. Wilson LB, Melia M, Kraker RT, VanderVeen DK, Hutchinson AK, Pineles SL, et al. Accuracy of autorefraction in children: a report by the American academy of ophthalmology. Ophthalmology. (2020) 127:1259–67. doi: 10.1016/j.ophtha.2020.03.004
27. Zadnik K, Sinnott LT, Cotter SA, Jones-Jordan LA, Kleinstein RN, Manny RE, et al. Prediction of juvenile-onset myopia. JAMA Ophthalmol. (2015) 133:683–9. doi: 10.1001/jamaophthalmol.2015.0471
28. Joseph S, Krishnan T, Ravindran RD, Maraini G, Camparini M, Chakravarthy U, et al. Prevalence and risk factors for myopia and other refractive errors in an adult population in southern India. Ophthalmic Physiol Opt. (2018) 38:346–58. doi: 10.1111/opo.12447
29. Brian G, Pearce MG, Ramke J. Refractive error and presbyopia among adults in Fiji. Ophthalmic Epidemiol. (2011) 18:75–82. doi: 10.3109/09286586.2010.551576
30. Nael V, Moreau G, Monferme S, Cougnard-Gregoire A, Scherlen AC, Arleo A, et al. Prevalence and associated factors of uncorrected refractive error in older adults in a population-based study in France. JAMA Ophthalmol. (2019) 137:3–11. doi: 10.1001/jamaophthalmol.2018.4229
31. Ferraz FH, Corrente JE, Opromolla P, Schellini SA. Influence of uncorrected refractive error and unmet refractive error on visual impairment in a Brazilian population. BMC Ophthalmol. (2014) 14:84. doi: 10.1186/1471-2415-14-84
32. Saw SM, Foster PJ, Gazzard G, Friedman D, Hee J, Seah S. Undercorrected refractive error in Singaporean Chinese adults: the Tanjong Pagar survey. Ophthalmology. (2004) 111:2168–74. doi: 10.1016/j.ophtha.2004.05.032
33. Fotouhi A, Hashemi H, Raissi B, Mohammad K. Uncorrected refractive errors and spectacle utilisation rate in Tehran: the unmet need. Br J Ophthalmol. (2006) 90:534–7. doi: 10.1136/bjo.2005.088344
34. Giloyan A, Khachadourian V, Petrosyan V, Harutyunyan T. Prevalence and determinants of uncorrected refractive error among a socially vulnerable older adult population living in Armenia. Public Health. (2021) 190:30–6. doi: 10.1016/j.puhe.2020.10.028
35. Sherwin JC, Khawaja AP, Broadway D, Luben R, Hayat S, Dalzell N, et al. Uncorrected refractive error in older British adults: the EPIC-Norfolk Eye Study. Br J Ophthalmol. (2012) 96:991–6. doi: 10.1136/bjophthalmol-2011-301430
36. Casas Luque L, Naidoo K, Chan VF, Silva JC, Naduvilath TJ, Peña F, et al. Prevalence of refractive error, presbyopia, and spectacle coverage in bogotá, colombia: a rapid assessment of refractive error. Optom Vis Sci. (2019) 96:579–86. doi: 10.1097/OPX.0000000000001409
37. Zhao J, Xu X, Ellwein LB, Guan H, He M, Liu P, et al. Causes of Visual Impairment and Blindness in the 2006 and 2014 Nine-Province Surveys in Rural China. Am J Ophthalmol. (2019) 197:80–7. doi: 10.1016/j.ajo.2018.09.011
38. Huang S, Zheng Y, Foster PJ, Huang W, He M, Liwan Eye S. Prevalence and causes of visual impairment in Chinese adults in urban southern China. Arch Ophthalmol. (2009) 127:1362–7. doi: 10.1001/archophthalmol.2009.138
39. Song W, Sun X, Shao Z, Zhou X, Kang Y, Sui H, et al. Prevalence and causes of visual impairment in a rural North-east China adult population: a population-based survey in Bin County, Harbin. Acta Ophthalmol. (2010) 88:669–74. doi: 10.1111/j.1755-3768.2009.01768.x
40. Lou L, Liu X, Tang X, Wang L, Ye J. Gender inequality in global burden of uncorrected refractive error. Am J Ophthalmol. (2019) 198:1–7. doi: 10.1016/j.ajo.2018.09.020
41. Wang X, Yi H, Lu L, Zhang L, Ma X, Jin L, et al. Population prevalence of need for spectacles and spectacle ownership among urban migrant children in eastern China. JAMA Ophthalmol. (2015) 133:1399–406. doi: 10.1001/jamaophthalmol.2015.3513
42. Ma X, Zhou Z, Yi H, Pang X, Shi Y, Chen Q, et al. Effect of providing free glasses on children's educational outcomes in China: cluster randomized controlled trial. BMJ. (2014) 349:g5740. doi: 10.1136/bmj.g5740
43. Congdon N, Li L, Zhang M, Yang A, Gao Y, Griffiths S, et al. Randomized, controlled trial of an educational intervention to promote spectacle use in rural China: the see well to learn well study. Ophthalmology. (2011) 118:2343–50. doi: 10.1016/j.ophtha.2011.06.016
44. Foreman J, Xie J, Keel S, Taylor HR, Dirani M. Treatment coverage rates for refractive error in the national eye health survey. PLoS ONE. (2017) 12:e0175353. doi: 10.1371/journal.pone.0175353
45. Zhu M, Tong X, Zhao R, He X, Zhao H, Liu M, et al. Visual impairment and spectacle coverage rate in Baoshan district, China: population-based study. BMC Public Health. (2013) 13:311. doi: 10.1186/1471-2458-13-311
46. Malhotra S, Kalaivani M, Rath R, Prasad M, Vashist P, Gupta N, et al. Use of spectacles for distance vision: coverage, unmet needs and barriers in a rural area of North India. BMC Ophthalmol. (2019) 19:252. doi: 10.1186/s12886-019-1262-3
47. Bastawrous A, Mathenge W, Foster A, Kuper H. Prevalence and predictors of refractive error and spectacle coverage in Nakuru, Kenya: a cross-sectional, population-based study. Int Ophthalmol. (2013) 33:541–8. doi: 10.1007/s10792-013-9742-6
48. Ezelum C, Razavi H, Sivasubramaniam S, Gilbert CE, Murthy GV, Entekume G, et al. Refractive error in Nigerian adults: prevalence, type, and spectacle coverage. Invest Ophthalmol Vis Sci. (2011) 52:5449–56. doi: 10.1167/iovs.10-6770
49. Marmamula S, Khanna RC, Kunuku E, Rao GN. Spectacles use in a rural population in the state of Telangana in South India. Indian J Ophthalmol. (2017) 65:509–15. doi: 10.4103/ijo.IJO_324_16
50. Jeganathan VSE, Robin AL, Woodward MA. Refractive error in underserved adults: causes and potential solutions. CurrOpinOphthalmol. (2017) 28:299–304. doi: 10.1097/ICU.0000000000000376
51. Ehrlich JR, Kourgialis N, Friedman DS. Impaired visual acuity and spectacle ownership of urban migrant children in eastern China. JAMA Ophthalmol. (2015) 133:1406–7. doi: 10.1001/jamaophthalmol.2015.3664
52. Quigley HA, Park CK, Tracey PA, Pollack IP. Community screening for eye disease by laypersons: the Hoffberger program. Am J Ophthalmol. (2002) 133:386–92. doi: 10.1016/S0002-9394(01)01380-0
53. Naidoo KS, Chinanayi FS, Ramson P, Mashige KP. Rapid assessment of refractive error in the eThekwini Municipality of KwaZulu-Natal, Durban, South Africa. Clin Exp Optom. (2016) 99:360–5. doi: 10.1111/cxo.12377
54. Zhu Z, Ellwein LB, Wang SK, Zhao J, He M. Meeting the need for corrective spectacles in visually impaired Chinese school children: the potential of ready-made spectacles. Br J Ophthalmol. (2019) 103:1106–11. doi: 10.1136/bjophthalmol-2018-312262
Keywords: uncorrected refractive error, spectacle coverage, opportunistic screening, health examination center, refractive service
Citation: Lin H, Sun J, Congdon N, Xu M, Liu S, Liang Y, Wang H and Zhang S (2021) Improving Access to Refractive Services in Adults: A Health Examination Center-Based Model. Front. Med. 8:753257. doi: 10.3389/fmed.2021.753257
Received: 04 August 2021; Accepted: 30 September 2021;
Published: 28 October 2021.
Edited by:
Wei Wang, Sun Yat-sen University, ChinaReviewed by:
Kourosh Sheibani, Basir Eye Health Research Center, IranCopyright © 2021 Lin, Sun, Congdon, Xu, Liu, Liang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaodan Zhang, c2hhb2Rhbl96aGFuZ193bXVAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.