
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 15 October 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.745803
Purposes: Acute kidney injury (AKI) is a common complication in critically ill patients and is usually associated with poor outcomes. Serum osmolality has been validated in predicting critically ill patient mortality. However, data about the association between serum osmolality and AKI is still lacking in ICU. Therefore, the purpose of the present study was to investigate the association between early serum osmolality and the development of AKI in critically ill patients.
Methods: The present study was a retrospective cohort analysis based on the medical information mart for intensive care III (MIMIC-III) database. 20,160 patients were involved in this study and divided into six subgroups according to causes for ICU admission. The primary outcome was the incidence of AKI after ICU admission. The association between early serum osmolality and AKI was explored using univariate and multivariate logistic regression analyses.
Results: The normal range of serum osmolality was 285–300 mmol/L. High serum osmolality was defined as serum osmolality >300 mmol/L and low serum osmolality was defined as serum osmolality <285 mmol/L. Multivariate logistic regression indicated that high serum osmolality was independently associated with increased development of AKI with OR = 1.198 (95% CL = 1.199–1.479, P < 0.001) and low serum osmolality was also independently associated with increased development of AKI with OR = 1.332 (95% CL = 1.199–1.479, P < 0.001), compared with normal serum osmolality, respectively.
Conclusions: In critically ill patients, early high serum osmolality and low serum osmolality were both independently associated with an increased risk of development of AKI.
Acute kidney injury (AKI) is a common clinical syndrome characterized by a quick decrease in renal function within a short time, with an obvious accumulation of creatinine and urea or a decrease in urinary output (1, 2). The primary potential pathogenesis of AKI may be renal cell injury due to unstable hemodynamics, systemic inflammation, or sepsis (3). AKI is frequent in hospitalized patients and especially common in critically care ill patients (4–6). Previous studies reported that the development of AKI was commonly associated with patient poor outcomes including prolonged length of hospital stay and increased mortality (7, 8). Therefore, the number of investigations and researches on AKI in critically ill patients was continuously increasing and relevant results were discovered. Such as trauma, increased blood lactate, shock, old age, red blood cell distribution width (RDW), and increased procalcitonin were investigated to be independent risk factors for the development of AKI (9–12).
Serum osmolality is the serum concentration of ions and particles dissolved in body fluid to reflect the body fluid balance and renal function, and is strongly affected by the concentration of Na+, K+, glucose, and urea (13–15). Therefore, many studies about serum osmolality affecting patient outcomes were conducted. Shen and his colleagues reported that the association between serum osmolality and mortality presented a U-shaped curve in critically ill patients (high osmolality and low osmolality were both associated with increased mortality) (16). Masanari and his (her) team found that elevated serum osmolality was an independent risk factor for developing chronic kidney disease (17). Elevated serum osmolality on intensive care unit (ICU) admission was also associated with an increased risk of critically ill patient mortality (18). In summary, serum osmolality is a useful and valuable indicator to predict or reflect patient outcomes in hospitalized patients and critically ill patients.
Even though several studies about serum osmolality were conducted and proved that abnormal serum osmolality was an independent risk factor for poor outcomes, data on the relationship between early serum osmolality on ICU admission and the development of AKI in critically ill patients has not been explored and validated. Therefore, the present study was designed and conducted to investigate the association between early serum osmolality on ICU admission and AKI in critically ill patients, and subgroup analyses were performed according to causes for ICU admission.
Medical information mart for intensive care III (MIMIC-III, version 1.4) database is a single-center and big database consisting of related information about 61,532 ICU stays (53,432 stays for adult patients and 8,100 for neonatal patients) at a large tertiary hospital from 2001 to 2012. The MIMIC-III database includes patient demographics, clinical measurements, clinical laboratory tests, interventions, pharmacotherapy, medical data, survival data, and more (19). The researchers who completed and passed the required training course could acquire access to this database. The consent for original data acquisition was obtained and the institutional review boards of the Massachusetts Institute of Technology approved the establishment of the database. Thus, patient informed consent and ethics approval were inapplicable for the present study. Data collected and presented in this study were extracted by the author Yang and Cheng who completed and passed the required training course.
We conducted a retrospective cohort study based on the MIMIC-III database. Patients older than 18 years old were involved in the present study. Patients were excluded if they had been diagnosed with AKI on ICU admission and patients without sufficient data to calculate serum osmolarity were also excluded.
Patient medical information was extracted and collected using PostgreSQL tools version 13.0. The data were collected including demographics and characteristics, signs and symptoms, comorbidities, causes for admission, laboratory findings, treatment, and outcomes. Except for patient outcomes, the rest of the variables were collected on patient ICU admission. The primary outcome in the present study was the incidence of AKI after enrolled patients on ICU admission. AKI was diagnosed according to “KDIGO Clinical Practice Guidelines”: increase in serum creatinine by ≥0.3 mg/dl (or ≥ 26.5 μmol/l) in 48 h, increase in serum creatinine to 1.5 times over baseline in 7 days, and patient urine output ≤ 0.5 ml/kg/h for 6 h. Other secondary outcomes included length of ICU stay, length of hospital stay, ICU mortality, hospital mortality, and use of vasopressor (20).
Serum osmolality was calculated by the following equation: (2 × Na+ + K+) + (glucose/18) + (urea/2.8) (15). The values of Na+, K+, glucose, and urea measured at the same time were involved in serum osmolality calculation. In the MIMIC III database, many results of serum osmolality were calculated by the above equation in each critically ill patient within the corresponding ICU record, and the first serum osmolality in this ICU record was selected to reflex the early serum osmolality on each patient ICU admission. The normal range of serum osmolality in the present investigation was defined as 285–300 mmol/L according to a previous study reported in “The New England Journal of Medicine” (21). Therefore, high serum osmolality was defined as serum osmolality >300 mmol/L, low serum osmolality was defined as serum osmolality <285 mmol/L, and a normal range of 285–300 mmol/L was used as the reference group. Subgroup analyses were also conducted based on causes for ICU admission, including sepsis, respiratory diseases, cardiac diseases, cerebral diseases, hepatic diseases, and cancer.
Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) according to their distribution. Independent t-test was used for normal distribution or Mann–Whitney U test was used for skewed distribution to compare differences. Categorical variables were expressed as numbers and percentages, and compared by Chi-square or Fisher's exact probability test as appropriate. Univariate and multivariate logistic regression analyses (variables presenting P < 0.05 in univariate analysis were included in multivariate logistic regression) were used to identify the associations between high or low serum osmolality and the development of AKI. The Lowess smoothing was also used to explore the curve relationship between serum osmolality and the incidence of AKI, and crude relationships between serum osmolality and AKI in six subgroups divided by causes for ICU admission. P < 0.05 was considered statistically significant in comparing differences between two groups or logistic regression models. All statistical analyses in the present study were performed using SPSS version 22.0, STATA version 16.0, and R software version 4.0.4.
There were 20,160 critically ill patients involved in this study. The involved patients were divided into six subgroups based on causes for ICU admission, such as sepsis, respiratory diseases, cardiac diseases, cerebral diseases, hepatic diseases, and cancer (shown in Figure 1). 7,619 patients developed AKI after ICU admission and 12,541 patients did not develop AKI in this ICU treatment stage. All patients' median age was 67.3 years old and the median age of patients diagnosed with AKI was significantly older than patients without AKI (68.8 vs. 54.0, P < 0.001). The median SOFA score in the AKI group was significantly higher than the non-AKI group (5.0 vs. 3.0, P < 0.001). Proportions of high serum osmolality and low serum osmolality in the AKI group were both higher than the non-AKI group (39.0 vs. 30.2% and 10.8 vs. 9.3%, P < 0.001). Other secondary outcomes including length of ICU stay, length of hospital stay, ICU mortality, and hospital mortality were all longer or higher in patients with AKI (P < 0.001). Other comparisons of demographics and clinical characteristics between the non-AKI group and the AKI group were shown in Table 1.
Table 2 showed the results of univariate and multivariate logistic analyses. High serum osmolality (>300 mmol/L) was an independent risk factor for the development of AKI (OR = 1.332, 95% CL = 1.199–1.479, P < 0.001) and low serum osmolality (<285 mmol/L) was also an independent risk factor for the development of AKI (OR = 1.198, 95% CL = 1.119–1.283, P < 0.001), compared with normal serum osmolality (285–300 mmol/L). Figure 2 using the Lowess smoothing demonstrated the non-line relationship between serum osmolality and the incidence of AKI. A U-curve relationship between serum osmolality and the incidence of AKI was found in Figures 2A,B. High serum osmolality or low serum osmolality were both associated with increased risk for AKI. This U-curve relationship (Figures 2A,B) was consistent with multivariate logistic analysis. We also explored the crude relationship between serum osmolality and the incidence of AKI in six subgroups using Lowess smoothing. The approximately U-curve relationships between serum osmolality and the incidence of AKI were also found in six subgroups shown in Figures 2C–H. The U-curve relationship between serum osmolality and SOFA score was presented in Figure 3. We further conducted subgroup analyses based on causes for ICU admission to determine whether serum osmolality also affected the development of AKI in different diseases. Figure 4 showed that abnormal serum osmolality was independently associated with the development of AKI in sepsis, respiratory diseases, cardiac diseases, and cerebral diseases after adjustment for related covariates (P < 0.05).
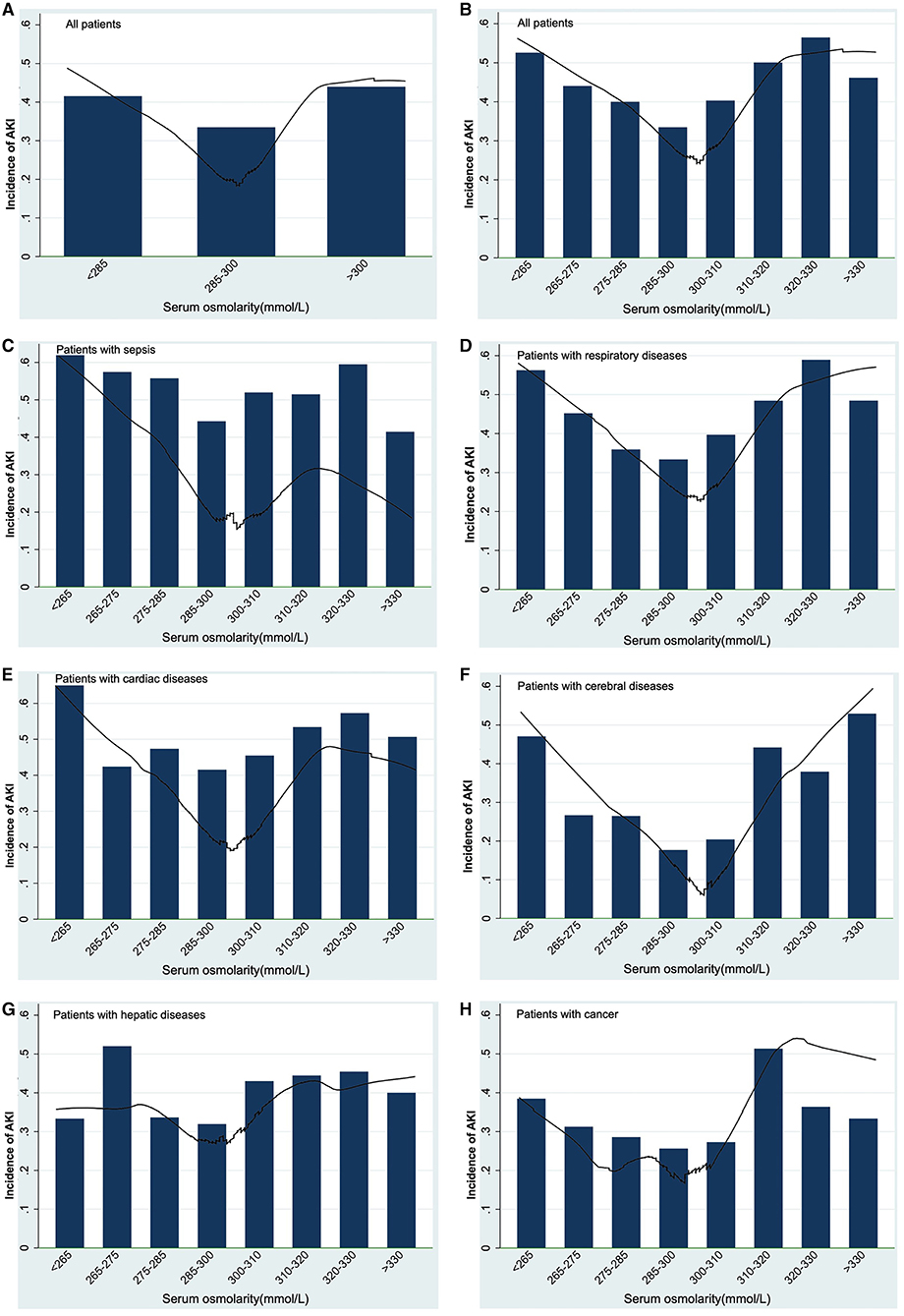
Figure 2. Association between serum osmolality and the development of AKI using Lowess smoothing: (A,B), all patients; (C) patients with sepsis; (D) patients with respiratory diseases; (E) patients with cardiac diseases; (F) patients with cerebral diseases; (G) patients with hepatic diseases; (H) patients with cancer. Non-liner relationships (U-shaped curves) were exhibited in the present figure.
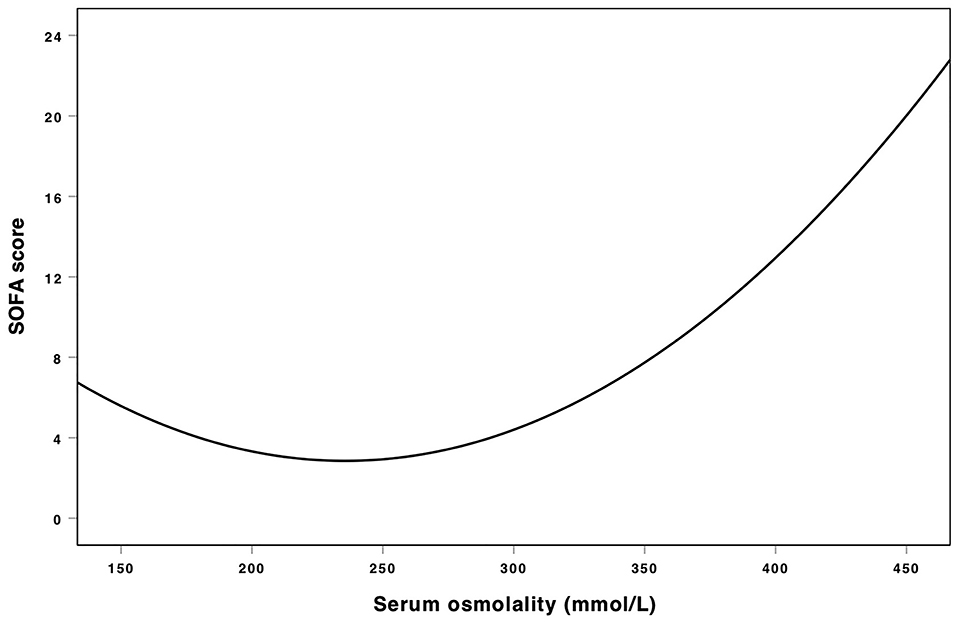
Figure 3. Association between serum osmolality and SOFA score. Non-liner relationships (U-shaped curves) were exhibited in the present figure.
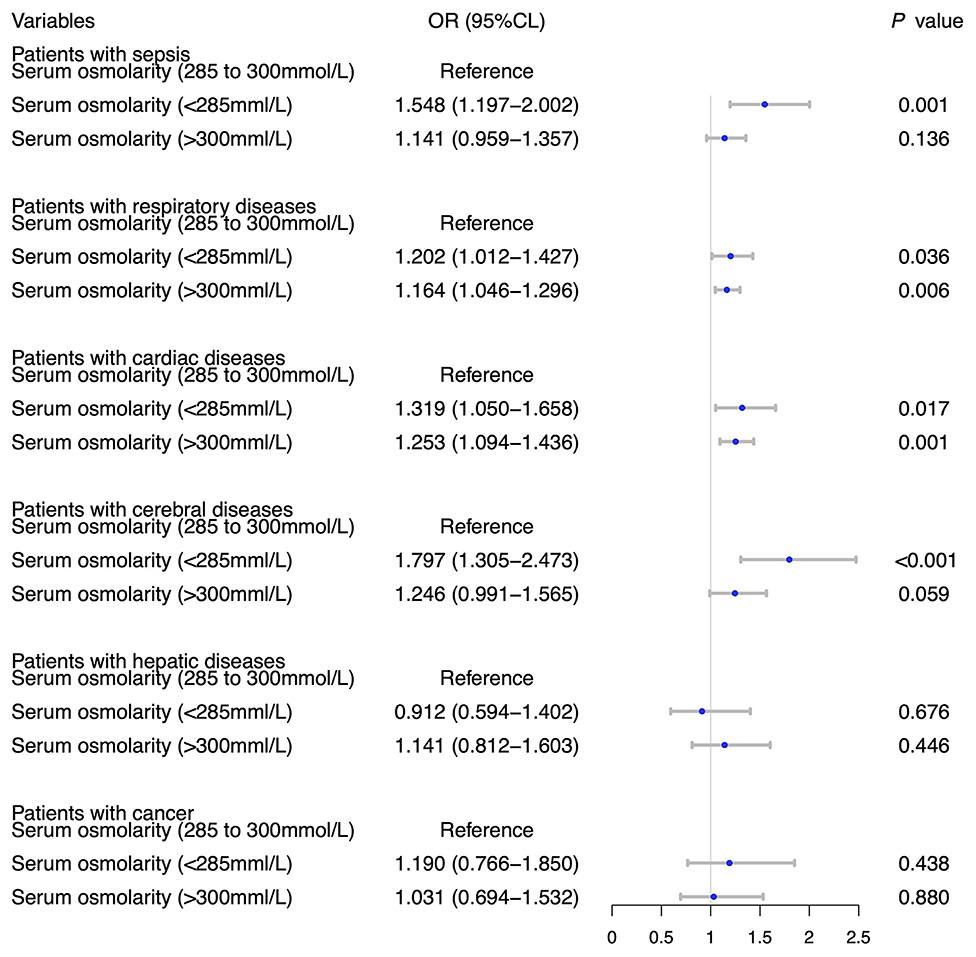
Figure 4. Subgroup analyses on association between serum osmolality and the development of AKI based on causes for ICU admission with adjustment for age, BMI, MAP, temperature, hypertension, diabetes, SOFA, WBC count, platelet count, hemoglobin, use of albumin, and use of norepinephrine.
The crude associations between serum osmolality and secondary outcomes were explored without adjustment for related covariates (shown in Table 3). ICU length of stay and hospital length of stay in the groups of high or low serum osmolality were both longer than the group of normal serum osmolality (P1 <0.001 and P2 <0.001). ICU mortality and hospital mortality were both higher in the groups of high and low serum osmolality (P1 <0.001 and P2 <0.001). The proportion of use of vasopressor was also higher in the group of high serum osmolality (P2 <0.001).
The incidence of AKI in critically ill patients in the present study was 37.8%, similar to the incidences reported in previous studies (22–24). We investigated the association between serum osmolality and the development of AKI in this study. The results of this study demonstrated that high serum osmolality and low serum osmolality were both independently associated with an increased risk of AKI in critically ill patients, compared with normal serum osmolality. The U-curve relationships between serum osmolality and AKI, either or SOFA score were also found. Further subgroup analyses based on causes for ICU admission showed that high or low serum osmolality were independently associated with an increased incidence of AKI in critically ill patients with sepsis, respiratory diseases, cardiac diseases, and cerebral diseases. These subgroup analyses could explain why abnormal serum osmolality was an independent risk factor for AKI in critically ill patients to some extent. The univariate analysis without adjustment for covariates showed that high osmolality or low serum osmolality were potentially associated with other outcomes. Therefore, patient serum osmolality could be considered as a potentially useful and valuable risk factor or predictor for AKI, even other patient outcomes.
In the present study, serum osmolalities >300 mmol/L or <285 mmol/L were defined as high or low serum osmolality, and abnormal serum osmolality was independently associated with an increased risk of developing AKI. Serum osmolality was a typical indicator to represent body fluid balance and renal function, and was usually calculated by the concentration of Na+, K+, glucose, and urea (25, 26). Thus, the level of these ions and particles above mentioned may reflect and affect patient renal function. Sodium imbalance was generally associated with patient poor outcomes including the development of AKI, and previous studies had proved this finding (27, 28). Relevant studies also found that early treatment for hyperkalemia could reduce potassium level and AKI, and potassium level was closely related to patient renal function (29–32). As well as sodium and potassium, the normal glucose level is important for normal physiological functions, such as reducing microvascular and macrovascular complications and infection (33). However, abnormity of glucose metabolism is frequent in critically ill patients and affects patient outcomes (34–36). Previous studies have investigated that abnormal glucose metabolism was associated with the development of AKI, and this finding may be related to increased release of inflammatory cytokines or increased inflammatory response (37–39). Therefore, appropriate control of glucose level is pivotal to maintain normal renal function and prevent AKI. Urea is generated via urea cycle enzymes mainly in the liver and subsequently eliminated in terms of urine via the kidney (40). A previous study demonstrated that the blood urea nitrogen was a useful biomarker and usually used to evaluate patient renal function (41). If renal function is injured and deficient, the capacity of excretion could be reduced and blood urea nitrogen level may elevate. Urea (blood urea nitrogen) is a potential predictor for AKI. However, with the development of research, the use of urea alone may be not a very reliable marker for AKI due to many factors affecting the excretion of urea (such as tissue breakdown, protein ingestion, and age) (41–43). Therefore, the exploration of a new comprehensive indicator for the development of AKI is important. Because imbalances of sodium, potassium, glucose, or urea were common in critically ill patients and associated with renal function, serum osmolality calculated by these particles may be a potential risk factor for developing AKI in critically ill patients. Clinicians could evaluate patient renal function preliminarily using serum osmolality on patient ICU admission and prevent developing AKI by timely appropriate treatment.
Because the causes for ICU admission in critically ill patients were complicated, we conducted subgroup analyses based on causes for ICU admission to explore the exact association between serum osmolality and AKI in different diseases. In critically ill patients with sepsis, respiratory diseases, cardiac diseases, and cerebral diseases, high osmolality or low serum osmolality were risk factors for AKI. Therefore, we should pay more attention to the abnormal changes of serum osmolality in sepsis, respiratory diseases, cardiac diseases, and cerebral diseases. The relationships between serum osmolality and other outcomes were also explored using univariate analyses. We found that high osmolality or low serum osmolality not only affected the development of AKI but also might affect patient ICU/hospital length of stay and ICU/hospital mortality, consistent with previous studies (16, 44). However, these explored results were from univariate analyses without adjustment for related covariates and lacked the power of persuasion.
Because the present study was based on the MIMIC-III database with a large sample, we could adjust for more covariates, conduct subgroup analyses, and get more information. However, limitations also existed in this study. Firstly, the present study was a retrospective study and further prospective investigation was necessary. Secondly, serum osmolality in this study was calculated by related particles and not measured directly. The association and difference in serum osmolality between calculation and direct measure were further needed exploration.
In critically ill patients, early high serum osmolality and low serum osmolality were both independently associated with an increased risk of the development of AKI compared with normal serum osmolality. When patients on ICU admission and under treatment, clinicians should pay more attention to the change of serum osmolality in critically ill patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The consent for original data acquisition was obtained and the institutional review boards of the Massachusetts Institute of Technology approved the establishment of the database. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JY: study design, data extraction, data analyses, and manuscript writing. YC: data extraction and data analyses. RW: data extraction. BW: manuscript revising. All authors contributed to the article and approved the submitted version.
This work was supported by the Project of Establishment of Early Warning and First-aid Screening System for Critically ill Patients in Hospitals Based on Artificial Intelligence (2020YFS0093) and the Project of Establishment of Treatment System Emergency and Critical Care with Cardiopulmonary Resuscitation as the Core Based on Cloud Platform (2019YFS0533).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The present study data was based on the MIMIC-III database. We would like to thank all staff and patients involved in the construction of the MIMIC-III database.
AKI, acute kidney injury; MIMIC-III, medical information mart for intensive care III; RDW, red blood cell distribution width; ICU, intensive care unit; SD, standard deviation; IQR, interquartile range.
1. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. (2004) 8:R204–12. doi: 10.1186/cc2872
2. Mercado MG, Smith DK, Guard EL. Acute kidney injury: diagnosis and management. Am Fam Physician. (2019) 100:687–94.
3. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. (2012) 380:756–66. doi: 10.1016/S0140-6736(11)61454-2
4. Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. (2006) 1:43–51. doi: 10.2215/CJN.00220605
5. Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. (2007) 72:208–12. doi: 10.1038/sj.ki.5002297
6. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. (2017) 376:11–20. doi: 10.1056/NEJMoa1611391
7. Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. (2008) 30:581–9. doi: 10.1080/08860220802134649
8. Gomes E, Antunes R, Dias C, Araújo R, Costa-Pereira A. Acute kidney injury in severe trauma assessed by RIFLE criteria: a common feature without implications on mortality? Scand J Trauma Resusc Emerg Med. (2010) 18:1. doi: 10.1186/1757-7241-18-1
9. Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care. (2018) 22:344. doi: 10.1186/s13054-018-2265-9
10. Wang RR, He M, Ou XF, Xie XQ, Kang Y. The predictive value of RDW in AKI and mortality in patients with traumatic brain injury. J Clin Lab Anal. (2020) 34:e23373. doi: 10.1002/jcla.23373
11. Chun K, Chung W, Kim AJ, Kim H, Ro H, Chang JH, et al. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci Rep. (2019) 9:4777. doi: 10.1038/s41598-019-41291-1
12. Wang R, He M, Ou XF, Xie XQ, Kang Y. Serum procalcitonin level predicts acute kidney injury after traumatic brain injury. World Neurosurg. (2020) 141:e112–e7. doi: 10.1016/j.wneu.2020.04.245
13. Najem O, Shah MM, De Jesus O. Serum Osmolality. Treasure Island, FL: StatPearls Publishing LLC (2021).
14. Langhoff E, Ladefoged J. Sodium activity, sodium concentration, and osmolality in plasma in acute and chronic renal failure. Clin Chem. (1985) 31:1811–4. doi: 10.1093/clinchem/31.11.1811
15. Heavens KR, Kenefick RW, Caruso EM, Spitz MG, Cheuvront SN. Validation of equations used to predict plasma osmolality in a healthy adult cohort. Am J Clin Nutr. (2014) 100:1252–6. doi: 10.3945/ajcn.114.091009
16. Shen Y, Cheng X, Ying M, Chang HT, Zhang W. Association between serum osmolarity and mortality in patients who are critically ill: a retrospective cohort study. BMJ Open. (2017) 7:e015729. doi: 10.1136/bmjopen-2016-015729
17. Kuwabara M, Hisatome I, Roncal-Jimenez CA, Niwa K, Andres-Hernando A, Jensen T, et al. Increased serum sodium and serum osmolarity are independent risk factors for developing chronic kidney disease; 5 year cohort study. PLoS ONE. (2017) 12:e0169137. doi: 10.1371/journal.pone.0169137
18. Holtfreter B, Bandt C, Kuhn SO, Grunwald U, Lehmann C, Schütt C, et al. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol Scand. (2006) 50:970–7. doi: 10.1111/j.1399-6576.2006.01096.x
19. Johnson AEW, Pollard TJ, Shen L, Lehman L-WH, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
20. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. (2012) 120:c179–84. doi: 10.1159/000339789
21. Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. (1984) 310:102–5. doi: 10.1056/NEJM198401123100207
22. Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. (2010) 77:527–35. doi: 10.1038/ki.2009.502
23. Xu X, Nie S, Liu Z, Chen C, Xu G, Zha Y, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. (2015) 10:1510–8. doi: 10.2215/CJN.02140215
24. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. (2006) 34:344–53. doi: 10.1097/01.CCM.0000194725.48928.3A
25. Aoki R, Yokoyama U, Ichikawa Y, Taguri M, Kumagaya S, Ishiwata R, et al. Decreased serum osmolality promotes ductus arteriosus constriction. Cardiovasc Res. (2014) 104:326–36. doi: 10.1093/cvr/cvu199
26. Ebonwu EO, Nagel SE, Repsold L, Pillay TS. Critical evaluation of equations for serum osmolality: proposals for effective clinical utility. Clin Chim Acta. (2020) 510:79–87. doi: 10.1016/j.cca.2020.06.043
27. Lombardi G, Ferraro PM, Naticchia A, Gambaro G. Serum sodium variability and acute kidney injury: a retrospective observational cohort study on a hospitalized population. Intern Emerg Med. (2021) 16:617–24. doi: 10.1007/s11739-020-02462-5
28. Kumar AB, Shi Y, Shotwell MS, Richards J, Ehrenfeld JM. Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: a retrospective analysis. Neurocrit Care. (2015) 22:184–91. doi: 10.1007/s12028-014-0067-8
29. Smith S, Behrens B, McCully B, Murphy J, Bommiasamy A, Goodman A, et al. Aggressive treatment of acute kidney injury and hyperkalemia improves survival in a combat relevant trauma model in swine. Am J Surg. (2020) 219:860–4. doi: 10.1016/j.amjsurg.2020.02.058
30. Bianchi S, Aucella F, De Nicola L, Genovesi S, Paoletti E, Regolisti G. Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol. (2019) 32:499–516. doi: 10.1007/s40620-019-00617-y
31. Ravioli S, Pluess E, Funk GC, Walter P, Schwarz C, Exadaktylos AK, et al. Dyskalemias in patients with acute kidney injury presenting to the emergency department are common and independent predictors of adverse outcome. Int J Clin Pract. (2021) 75:e13653. doi: 10.1111/ijcp.13653
32. Gilligan S, Raphael KL. Hyperkalemia and hypokalemia in CKD: prevalence, risk factors, and clinical outcomes. Adv Chronic Kidney Dis. (2017) 24:315–8. doi: 10.1053/j.ackd.2017.06.004
33. Butler SO, Btaiche IF, Alaniz C. Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy. (2005) 25:963–76. doi: 10.1592/phco.2005.25.7.963
34. Mesotten D, Preiser JC, Kosiborod M. Glucose management in critically ill adults and children. Lancet Diabetes Endocrinol. (2015) 3:723–33. doi: 10.1016/S2213-8587(15)00223-5
35. Lv S, Ross P, Tori K. The optimal blood glucose level for critically ill adult patients. Nurs Crit Care. (2017) 22:312–9. doi: 10.1111/nicc.12285
36. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. (2003) 78:1471–8. doi: 10.4065/78.12.1471
37. Yoo S, Lee HJ, Lee H, Ryu HG. Association between perioperative hyperglycemia or glucose variability and postoperative acute kidney injury after liver transplantation: a retrospective observational study. Anesth Analg. (2017) 124:35–41. doi: 10.1213/ANE.0000000000001632
38. Stolker JM, McCullough PA, Rao S, Inzucchi SE, Spertus JA, Maddox TM, et al. Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol. (2010) 55:1433–40. doi: 10.1016/j.jacc.2009.09.072
39. Lee S, Nam S, Bae J, Cho YJ, Jeon Y, Nam K. Intraoperative hyperglycemia in patients with an elevated preoperative C-reactive protein level may increase the risk of acute kidney injury after cardiac surgery. J Anesth. (2021) 35:10–9. doi: 10.1007/s00540-020-02849-w
41. Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ. (2006) 333:733–7. doi: 10.1136/bmj.38975.390370.7C
42. Waikar SS, Bonventre JV. Can we rely on blood urea nitrogen as a biomarker to determine when to initiate dialysis? Clin J Am Soc Nephrol. (2006) 1:903–4. doi: 10.2215/CJN.02560706
43. Musch W, Verfaillie L, Decaux G. Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. (2006) 1:909–4. doi: 10.2215/CJN.00320106
Keywords: critical care, AKI (acute kidney injury), serum osmolality, independent risk factor, outcome
Citation: Yang J, Cheng Y, Wang R and Wang B (2021) Association Between Serum Osmolality and Acute Kidney Injury in Critically Ill Patients: A Retrospective Cohort Study. Front. Med. 8:745803. doi: 10.3389/fmed.2021.745803
Received: 22 July 2021; Accepted: 21 September 2021;
Published: 15 October 2021.
Edited by:
José António Lopes, Centro Hospitalar Lisboa Norte (CHLN), PortugalReviewed by:
Cale Kassel, University of Nebraska Medical Center, United StatesCopyright © 2021 Yang, Cheng, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wang, d2NoaWN1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.