- Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY, United States
SARS-CoV2 infection results in a range of symptoms from mild pneumonia to cardiac arrhythmias, hyperactivation of the immune response, systemic organ failure and death. However, the mechanism of action has been hard to establish. Analysis of symptoms associated with COVID-19, the activity of repurposed drugs associated with lower death rates or antiviral activity in vitro and a small number of studies describing interventions, point to the importance of electrolyte, and particularly potassium, homeostasis at both the cellular, and systemic level. Elevated urinary loss of potassium is associated with disease severity, and the response to electrolyte replenishment correlates with progression toward recovery. These findings suggest possible diagnostic opportunities and therapeutic interventions. They provide insights into comorbidities and mechanisms associated with infection by SARS-CoV2 and other RNA viruses that target the ACE2 receptor, and/or activate cytokine-mediated immune responses in a potassium-dependent manner.
Introduction
SARS-CoV2 infects cells via interaction with the ACE2 receptor which is found primarily on the surface of the heart, liver, kidney, and lungs (1). ACE2 is a negative regulator of the renin-angiotensin system (RAS) that acts in conjunction with ion transporters and the insulin receptor to protect against hypertension, diabetes, cardiovascular disease, and organ damage (2). It does this by regulating electrolyte balance and blood pressure, cell volume, intercellular signaling, filtering of urine in the kidney, membrane potential, and the firing rate of electrically active cells (3, 4). Binding of ACE2 by the SARS-CoV2 virus and the processes of viral entry and replication, enhance degradation of the receptor, which decreases inhibition of the classical RAS system. The net result is increased reabsorption of sodium and water, and raised blood pressure (5). Hypokalemia/low intracellular potassium can also lead to cellular hyperpolarity, increased resting potential, and depolarization in cardiac and lung cells that can trigger ventricular arrhythmia and respiratory dysfunction (6). In parallel, expression of the viral viroporin, Orf3a protein actively promotes potassium efflux, and stimulates activation of the innate immune response. It does so by triggering the cell-intrinsic Nod-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome (7–9), which promotes cytokine release. Inflammasome responses play fundamental roles in clearing viruses and promoting tissue repair (10), however, hyperactivation of this immune response, gives rise to the devastating “cytokine storm” that is associated with severe infection, and a major cause of death (11).
This mini-perspective discusses the effects of electrolyte and potassium imbalance in SARS-CoV2 infection, describes how a number of comorbidities of COVID-19 affect ion homeostasis and, identifies some drugs effective against SARS-CoV2 in vivo that have also been shown to affect pH or K+ balance. Collectively, these findings highlight the importance of maintaining, and promoting electrolyte homeostasis. They also provide a framework for beginning to understand the broad, and seemingly unrelated, range of symptoms associated with COVID-19 and possibly other RNA viruses, that target the ACE2 receptor and/or those that activate the NRPL3 inflammasome in a potassium-dependent manner.
Potassium Imbalance is Common Among Patients With Severe SARS-CoV2 Infection
Potassium homeostasis is maintained at a systemic level, in the balance between dietary intake (~100 mmol/day) and excretion (95% via the kidney; 5% via the colon) and via internal balance of K+ between intracellular and extracellular fluid compartments (4). Hypokalemia, typically defined as <3.5 mmol/L in plasma, shares many of the features of SARS-CoV2 infection, including muscle weakness, palpitations, cardiac dysrhythmias, and poor diabetic control (4, 12).
In the course of SARS-CoV2 infection, hypokalemia is primarily caused by elevated aldosterone, which promotes excretion of potassium in urine (13). One study involving 1,415 patients, found electrolyte imbalance and hypokalemia were associated with disease severity (Weighted Mean Difference:0.12 mmol/L [95% CI: −0.18 to −0.07 mmol/ L], I21/433%) (14). Another found that hypokalemia around the time of admission was associated with a requirement for invasive mechanical ventilation (15), while a smaller study observed that although only 54% of the patients (n = 175) had low potassium levels, of the severely ill patients 85% had hypokalemia (13). A case-controlled study of three emergency rooms in France found that hypokalemia and hyponatremia (sodium <135 mmol/L) were independently associated with COVID-19 infection, but that low sodium, and not potassium levels were associated with ICU admission (16). Disease severity is also related to the degree of response to potassium replacement as mildly ill COVID-19 patients with hypokalemia in the Chen study achieved normokalemia within 5–8 days of potassium replacement (3 g potassium chloride or 40 mEq/day), whereas, it took 10–14 days to achieve homeostasis potassium in severely ill patients (13). Severe hypokalemia may be harder to correct as it is associated with alkalosis (29% had a ≥ pH 7.45) (13). This is due to hydrogen-potassium exchange between the intra and extracellular fluid (4). Patients with COVID-19 are also susceptible to pro-arrhythmic effects (17).
A Number of Comorbidities for COVID-19 Affect Ion Homeostasis
Patients with severe symptoms of COVID-19 are more likely to have kidney or cardiovascular disease, hypertension, diabetes mellitus (DM) or other comorbidities than those with milder symptoms (18–22). The association between COVID-19 and a number of these comorbidities is bidirectional (23, 24): patients with diabetes are more likely to develop severe symptoms or die of COVID-19 (12, 22) and acute diabetes or acid-ketosis can develop as a result of SARS-CoV2 infection (25–28). High levels of insulin are found in the olfactory bulb in the brain. Insulin modulates the voltage-dependent potassium channel, Kv1.3, and suppresses the Kv1.3-contributed current in cultured olfactory bulb neurons (OBNs) of rodents (29, 30),while deletion of the Kv1.3 channel results in “super smeller” mice (31). There is little data on the effect of decreased insulin production on the Kv1.3 channel, however it may contribute to the anosmia experienced by some COVID-19 patients (32).
A Number of Repurposed Drugs Effective Against SARS-CoV2 Affect Potassium Balance
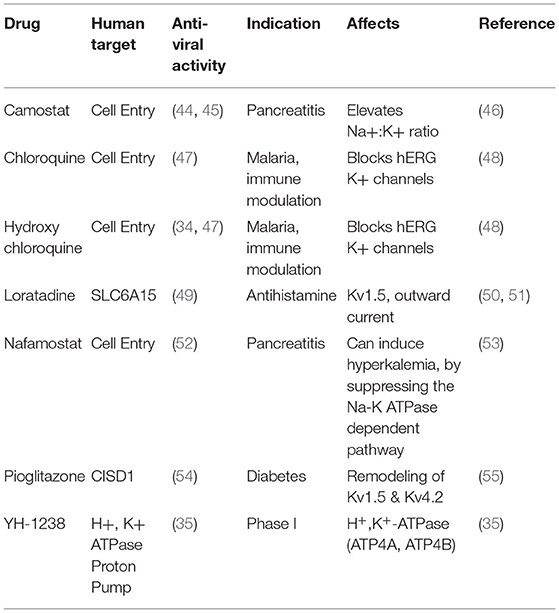
It has been hard to obtain insights into the mechanism by which SARS-CoV2 acts, based on the diversity of symptoms identified in infected individuals. Likewise, FDA approved drugs that act in vitro to reduce viral replication and plaque formation, increase cell viability, or are associated with lower death rates in patients target a range of host factors. These drugs are used for a wide range of purposes from treatment of malaria to pancreatitis and diabetes (33–36) (Table 1). However, some patterns are emerging: 17 of 66 FDA approved drugs with anti-viral activity were found to target the Sigma-1 receptor (σ1-R) and sigma-2 receptor (σ2-R) (SIGMAR1/SIGMAR2) (34). Sigma receptors are ubiquitously expressed in mammalian tissues and are involved in cellular signaling in a number of conditions including retinal and neurodegenerative disorders (37, 38). A number of σ1-R and σ2-R receptor agonists have been found to inhibit Kv2.1 potassium channel activity in a receptor-independent manner (39), suggesting that they act to modulate potassium currents directly. Another 7 of the 69 drugs inhibit protein synthesis (34). Although the mechanism is not known, protein synthesis, and potassium abundance are inversely correlated in systems as diverse as yeast, algae, and mouse fibroblasts (40–43), such that inhibition of protein synthesis would be expected to result in greater intracellular potassium abundance. A further 17 drugs have been shown to affect osmotic or ion homeostasis. Agonists of potassium channels, angiotensin II, and protein synthesis were also found to be enriched among drugs with anti-SARS-CoV2 activity in an independent study (35).
Some of these repurposed drugs many act to reduce disease severity via their effects on the immune system. Sex hormones, such as progesterone, promote immune tolerance, and anti-inflammatory responses and that may account for lower COVID-related disease severity and mortality in women and during pregnancy (56, 57). Clinical studies of drug efficacy also point to the key role of the renin-angiotensin system and electrolyte balance in influencing patient outcomes. A retrospective study of COVID-19 patients taking famotidine, an antiacid, found that hospitalized patients taking the drug were more than twice as likely to survive (33). Famotidine was also identified in a computational screen of drugs likely to have anti-SARS-CoV2 activity (36). Another drug, Nafamostat, acts on potassium balance by reducing urinary excretion of potassium via the Na+/K+ ATPase-dependent pathway (58, 59). These data support the idea that restoring potassium balance promotes a better host response against viral infection. Conversely some of these drugs pose a risk as they promote hyperkalemia (48, 60). This is a complication found in a number of patients who die of COVID-19 (37% of those who died (n = 113) compared with 14% (n = 161) of those who recovered (61).
Potassium dysregulation is also likely to form part of the mechanism that promotes viral pathogenicity. A study that ectopically expressed the SARS-CoV2 envelope (E) protein in HEK 293 and NIH3T3 cells found that it formed a pH-dependent ion channel permeable to potassium and sodium ions (62). Only a small proportion of the E protein ends up in the viral envelope and most is localized to the endoplasmic reticulum-Golgi complex where it multimerizes to form a virioporin, that promotes an increase in intra-golgi pH (62, 63). The E protein channel is critical for infectivity and for the pathogenicity of SARS-CoV2, as it is for other coronaviruses, and thus presents a good target for therapeutic intervention (63, 64).
Discussion
Taken together, these observations drawn from comorbidities, clinical features of disease and the possible targets of drugs that are effective against viral infection show that symptoms associated with low intracellular potassium are similar to those that result from SARS-CoV2 infection, and that potassium efflux can promote hyperactivation of the innate immune response. Although we do not yet understand how SARS-CoV2 acts in detail, potassium balance is likely to be important for both the propagation and pathogenicity of the virus, via effects on both the virus, and on homeostatic mechanisms in the host.
It is likely that this line of enquiry will have relevance for understanding the consequences of viral infection more broadly. Ion disturbance, mediated by virioporins, is central to the mechanism of action of a range of viruses from influenza, and rhinovirus to COVID-19 and HIV (8), and a number of RNA viruses modulate activity of the NLRP3 inflammasome in a potassium-dependent manner (65, 66). In bats, dampening of the inflammasome and proinflammatory responses confers tolerance to a range of RNA viruses, suggesting that modulating the inflammasome may prove a useful therapeutic target for reducing disease severity in humans too (10).
Similarities between SARS-CoV2 and other coronaviruses offer further mechanistic insight and opportunities for drug repurposing. SARS-CoV1 also enters the cell via the ACE2 receptor and can cause acute lung failure, cardiac arrhythmia, gastrointestinal disorders, hyperkalemia and diabetes (4, 5, 67, 68). Nafamostat, which induces hyperkalemia, inhibits the activity of SARS-CoV1, 2 and MERS-CoV (52, 53, 60, 69). Approximately 50 FDA-approved drugs are known to have activity against all 3 viruses (70). These results present a strong argument for gaining a fundamental understanding of how electrolyte balance functions in both the healthy host and in response to viral infection. This knowledge is expected to identify strategies for diagnosis and therapeutic intervention in patients suffering from a number of virally induced diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
2. Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. (2020) 92:726–730. doi: 10.1002/jmv.25785
3. Santos RA, Ferreira AJ, Simoes AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. (2008) 93:519–27. doi: 10.1113/expphysiol.2008.042002
4. Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. (2011) 7:75–84. doi: 10.1038/nrneph.2010.175
5. Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med. (2006) 84:814–20. doi: 10.1007/s00109-006-0094-9
6. Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS, Banach M. The meaning of hypokalemia in heart failure. Int J Cardiol. (2012) 158:12–7. doi: 10.1016/j.ijcard.2011.06.121
7. Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. (2019) 10:50. doi: 10.3389/fmicb.2019.00050
8. Farag NS, Breitinger U, Breitinger HG, El Azizi MA. Viroporins and inflammasomes: a key to understand virus-induced inflammation. Int J Biochemistr Cell Biol. (2020) 122:105738. doi: 10.1016/j.biocel.2020.105738
9. Xu H, Chitre SA, Akinyemi IA, Loeb JC, Lednicky JA, McIntosh MT, Bhaduri-McIntosh S. SARS-CoV-2 viroporin triggers the NLRP3 inflammatory pathway, BioRxiv (2020). doi: 10.1101/2020.10.27.357731
10. Nagaraja S, Jain D, Kesavardhana S. Inflammasome regulation in driving COVID-19 severity in humans and immune tolerance in bats. J Leukoc Biol. (2021) 21:93. doi: 10.1002/JLB.4COVHR0221-093RR
11. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. (2021) 93:250–256. doi: 10.1002/jmv.26232
12. Docherty A, Harrison E, Green C, Hardwick H, Pius R, Norman L, et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. (2020) 369:m1985.
13. Chen D, Li X, Song Q, Hu C, Su F, Dai J, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. (2020) 3:e2011122. doi: 10.1001/jamanetworkopen.2020.11122
14. Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. (2020) 57:262–265. doi: 10.1177/0004563220922255
15. Moreno-Perez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JMJ, et al. CA.r. group, Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect. (2021) 82:378–383. doi: 10.1016/j.jinf.2021.01.004
16. De Carvalho H, Richard MC, Chouihed T, Goffinet NQ, Freund Y, Kratz A, et al. Electrolyte imbalance in COVID-19 patients admitted to the emergency department: a case-control study. Intern Emerg Med. (2021) 6:32. doi: 10.1007/s11739-021-02632-z
17. Wu CI, Postema PG, Arbelo E, Behr ER, Bezzina CR, Napolitano C, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. (2020) 17:1456–62. doi: 10.1016/j.hrthm.2020.03.024
18. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int (2020) 97:829-838. doi: 10.1016/j.kint.2020.03.005
19. Dworakowska D, Grossman AB. Renin-angiotensin system inhibitors in management of hypertension during the COVID-19 pandemic. J Physiol Pharmacol (2020) 71:20. doi: 10.26402/jpp.2020.2.01
20. Lim JH, Jung HY, Choi JY, Park SH, Kim CD, Kim YL, et al. Hypertension and Electrolyte Disorders in Patients with COVID-19. Electrolyte Blood Press. (2020) 18:23–30. doi: 10.5049/EBP.2020.18.2.23
21. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3
22. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metabolism. (2020) 31:1068–1077. doi: 10.1016/j.cmet.2020.04.021
23. Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. (2020) 14:513-517. doi: 10.1016/j.dsx.2020.04.049
24. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. (2020) 17:543–558. doi: 10.1038/s41569-020-0413-9
25. Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diab. Endocrinol. (2020) 8:546–550. doi: 10.1016/S2213-8587(20)30152-2
26. Chee YJ S., Ng JH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabet Res Clinic Pract. (2020) 164:108166. doi: 10.1016/j.diabres.2020.108166
27. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID−19 infection may cause ketosis and ketoacidosis. Diabet Obesity Metabol. (2020) 22:1935–41. doi: 10.1111/dom.14057
28. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-onset diabetes in Covid-19. N Engl J Med. (2020) 383:789–90. doi: 10.1056/NEJMc2018688
29. Fadool DA, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol. (2000) 83:2332–48. doi: 10.1152/jn.2000.83.4.2332
30. Das P, Parsons AD, Scarborough J, Hoffman J, Wilson J, Thompson RN, et al. Electrophysiological and behavioral phenotype of insulin receptor defective mice. Physiol Behav. (2005) 86:287–96. doi: 10.1016/j.physbeh.2005.08.024
31. Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, et al. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, biophysics. Neuron. (2004) 41:389–404. doi: 10.1016/S0896-6273(03)00844-4
32. Lechien JR, Chiesa-Estomba DR, Horoi MSD, Le Bon D, Rodriguez A, Dequanter D, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 277:2251–2261. doi: 10.1007/s00405-020-05965-1
33. Freedberg D, Conigliaro J, Wang T, Tracey K, Callahan M, Abrams J, et al. Famotidine use is associated with improved clinical outcomes in hospitalized covid-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. (2020) 159:1129–31. doi: 10.1053/j.gastro.2020.05.053
34. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. (2020) 583:459–68. doi: 10.1038/s41586-020-2286-9
35. Riva L, Yuan S, Yin X L., Martin-Sancho, Matsunaga N, Pache LS, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. (2020) 586:113-119. doi: 10.1038/s41586-020-2577-1
36. Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. (2020) 10:766–88. doi: 10.1016/j.apsb.2020.02.008
37. Wang J, Saul A, Roon P, Smith SB. Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc Natl Acad Sci U S A. (2016) 113:E3764–72. doi: 10.1073/pnas.1521749113
38. Maurice T, Goguadze N. Sigma-1 (sigma1) receptor in memory and neurodegenerative diseases. Handbook Experiment Pharmacol. (2017) 244:81–108. doi: 10.1007/164_2017_15
39. Liu X, Fu Y, Yang H, Mavlyutov T, Li J, McCurdy CR, et al. Potential independent action of sigma receptor ligands through inhibition of the Kv2.1 channel. Oncotarget. (2017) 8:59345–59358. doi: 10.18632/oncotarget.19581
40. Mahmoud S, Planes MD, Cabedo M, Trujillo C, Rienzo A, Caballero-Molada M, Sarma SC, Montesinos C, et al. TOR complex 1 regulates the yeast plasma membrane proton pump and pH and potassium homeostasis. FEBS Lett. (2017) 591:1993–2002. doi: 10.1002/1873-3468.12673
41. Primo C, Ferri-Blazquez, Leowith R, Yenush L. Reciprocal regulation of target of rapamycin complex 1 and potassium accumulation. J Biol Chem. (2017) 292:563–574. doi: 10.1074/jbc.M116.746982
42. O'Neill JS, Hoyle NP, Robertson JB, Edgar R, Frezza C, Day JH, et al. Eukaryotic cell biology is temporally coordinated to support the energetic demands of protein homeostasis. Nat Commun. (2020) 14:955521. doi: 10.1101/2020.05.14.095521
43. Stangherlin A, Wong D, Barbiero S, Watson J, Zeng A, Seinkmane E, et al. Compensatory ion transport buffers daily protein rhythms to regulate osmotic balance and cellular physiology. BioRxiv preprint. (2020) 28:118398. doi: 10.1101/2020.05.28.118398
44. Sun G, Sui Y, Zhou Y, Ya J, Yuan C, Jiang L, Huang M. Structural Basis of Covalent Inhibitory Mechanism of TMPRSS2-Related Serine Proteases by Camostat. J Virol. (2021) 95:e0086121. doi: 10.1128/JVI.00861-21
45. Hoffmann MH, Smith JC, Kruger N, Arora P, Sorensen LK, Sogaard OS, et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. (2021) 65:103255. doi: 10.1016/j.ebiom.2021.103255
46. Kitamura K, Tomita K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin Exp Nephrol. (2012) 16:44–8. doi: 10.1007/s10157-011-0506-1
47. Rakedzon S, Neuberger A, Domb AJ, Petersiel N, Schwartz E. From hydroxychloroquine to ivermectin: what are the anti-viral properties of anti-parasitic drugs to combat SARS-CoV-2? J Travel Med. (2021) 28:5. doi: 10.1093/jtm/taab005
48. Szendrey M, Guo J, Li W, Yang T, Zhang S. COVID-19 drugs chloroquine and hydroxychloroquine, but not azithromycin and remdesivir, block hERG potassium channels. J Pharmacol Experiment Therapeut. (2021) 377:265–72. doi: 10.1124/jpet.120.000484
49. Hou Y, Ge S, Li X, Wang C, He H, He L. Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis. Chem Biol Interact. (2021) 338:109420. doi: 10.1016/j.cbi.2021.109420
50. Lacerda AE, Roy ML, Lewis EW, Rampe D. Interactions of the nonsedating antihistamine loratadine with a Kv1.5-type potassium channel cloned from human heart. Mol Pharmacol. (1997) 52:314–22. doi: 10.1124/mol.52.2.314
51. Crumb WJ. Rate-dependent blockade of a potassium current in human atrium by the antihistamine loratadine. Br J Pharmacol. (1999) 126:575–80. doi: 10.1038/sj.bjp.0702273
52. Hoffmann M, Schroeder SH, Kleine-Weber H, Muller MA, Drosten C, Pohlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemotherap. (2020) 64:20. doi: 10.1128/AAC.00754-20
53. Ookawara S, Tabei K, Sakurai T, Sakairi Y, Furuya H, Asano Y. Additional mechanisms of nafamostat mesilate-associated hyperkalaemia. Eur J Clin Pharmacol. (1996) 51:149–51. doi: 10.1007/s002280050176
54. Imamura K, Sakurai Y, Enami T, Shibukawa R, Nishi Y, Ohta A, Shu T, et al. iPSC screening for drug repurposing identifies anti-RNA virus agents modulating host cell susceptibility. FEBS Open Bio. (2021) 11:1452–1464. doi: 10.1002/2211-5463.13153
55. Gu J, Hu W, Liu X. Pioglitazone improves potassium channel remodeling induced by angiotensin II in atrial myocytes. Med Sci Monit Basic Res. (2014) 20:153–60. doi: 10.12659/MSMBR.892450
56. Slowik A, Lammerding L, Zendedel A, Habib P, Beyer C. Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. J Steroid Biochem Mol Biol. (2018) 183:18–26. doi: 10.1016/j.jsbmb.2018.05.003
57. Pinna G Sex and COVID-19: a protective role for reproductive steroids. Trends Endocrinol Metab. (2021) 32:3–6. doi: 10.1016/j.tem.2020.11.004
58. Muto S, Imai M, Asano Y. Mechanisms of the hyperkalaemia caused by nafamostat mesilate: effects of its two metabolites on Na+ and K+ transport properties in the rabbit cortical collecting duct. Br J Pharmacol. (1994) 111:173–8. doi: 10.1111/j.1476-5381.1994.tb14040.x
59. Muto S, Sebata K, Watanabe H, Shoji F, Yamamoto Y, Ohashi M, Yamada T, et al. Effect of oral glucose administration on serum potassium concentration in hemodialysis patients. Am J Kidney Dis. (2005) 46:697–705. doi: 10.1053/j.ajkd.2005.06.013
60. Okajima M, Takahashi Y, Kaji T, Ogawa N, Mouri H. Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reports. World J Clin Cases. (2020) 8:5320–5325. doi: 10.12998/wjcc.v8.i21.5320
61. Chen T, Wu D, Chen H, Yan W, Yang D, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
62. Cabrera-Garcia D Bekdash R Abbott GW Yazawa M Harrison NL he envelope protein of SARS-CoV2 increases intra-Golgi pH and forms a cation channel that is regulated by pH. J Physiol. (2021) 599:2851–68. doi: 10.1113/JP281037
63. Trobec T. The role of the SARS-CoV-2 envelope protein as a pH-dependent cation channel. J Physiol. (2021) 599:3435–3436. doi: 10.1113/JP281785
64. Singh Tomar PP Arkin IT. SARS-CoV-2 E protein is a potential ion channel that can be inhibited by Gliclazide and Memantine. Biochem Biophys Res Commun. (2020) 530:10–14. doi: 10.1016/j.bbrc.2020.05.206
65. da Costa LS, Outlioua A, Anginot A, Akarid K, Arnoult D. RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux. Cell Death Dis. (2019) 10:346. doi: 10.1038/s41419-019-1579-0
66. Choudhury S, Ma X, Abdullah SW, Zheng H. Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses. J Inflamm Res. (2021) 14:1145–1163. doi: 10.2147/JIR.S295706
67. Tsang OT, Chau TN, Choi KW, Tso EY, Lim W, Chiu MC, et al. Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg Infect Dis. (2003) 9:1381–7. doi: 10.3201/eid0911.030400
68. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. (2010) 47:193–9. doi: 10.1007/s00592-009-0109-4
69. Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, et al. Identification of nafamostat as a potent inhibitor of Middle East Respiratory Syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemotherap. (2016) 60:6532–39. doi: 10.1128/AAC.01043-16
Keywords: electrolyte, renin-angiotensin system, drug repurposing, SARS-CoV-2 infection, potassium
Citation: Causton HC (2021) SARS-CoV2 Infection and the Importance of Potassium Balance. Front. Med. 8:744697. doi: 10.3389/fmed.2021.744697
Received: 20 July 2021; Accepted: 30 September 2021;
Published: 27 October 2021.
Edited by:
Ihsan Ullah, Khyber Medical University, PakistanReviewed by:
Yan Li, Oregon Health and Science University, United StatesIgnacija Vlašić, Rudjer Boskovic Institute, Croatia
Copyright © 2021 Causton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen C. Causton, aGMyNDE1QGN1bWMuY29sdW1iaWEuZWR1
 Helen C. Causton
Helen C. Causton