
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 12 October 2021
Sec. Geriatric Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.744012
This article is part of the Research TopicAdvances on Pathogenesis and Treatment of Lower Urinary Tract Symptoms and Pelvic Floor Dysfunction DiseasesView all 22 articles
Background: Tadalafil has been approved for the treatment of benign prostatic hyperplasia (BPH) for nearly 10 years. However, there are insufficient evidence-based studies of the efficacy and safety of tadalafil in treating lower urinary tract symptoms of BPH (LUTS/BPH).
Objective: To evaluate the therapeutic effect and clinical safety of tadalafil monotherapy (5 mg once daily for 12 weeks) for LUTS/BPH.
Methods: A total of 13 studies (15 randomized clinical trials [RCTs]) were extracted from the following databases: PubMed, Cochrane Central Register of Controlled Trials, Embase, and Web of Science for the period up to July 2021. The quality of the included RCTs was evaluated independently by two authors, who, respectively, extracted data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses principles. Conflicts were settled by a discussion with two-third of senior authors. All data analyses were conducted by the Review Manager, version 5.4.
Results: Regarding efficacy, 12-week trials indicated that 5 mg once daily tadalafil showed a significantly lower and, consequently, better total International Prostate Symptom Score (IPSS) than the placebo did (mean difference [MD]: −1.97, 95% CI: −2.24 to −1.70; P < 0.00001). In addition, significant differences were found between the tadalafil regimen and the placebo in the IPSS voiding subscore (MD: −1.30, 95% CI: −1.48 to −1.11; P < 0.00001), the IPSS storage subscore (MD: −0.70, 95% CI: −0.82 to −0.58; P < 0.00001), the IPSS quality of life (MD: −0.29, 95% CI: −0.35 to −0.22; P < 0.00001), and BPH impact index (MD: −0.58, 95% CI: −0.76 to −0.40; P < 0.00001). The safety analysis did not show a significant difference in serious adverse events between the two groups (risk ratio: 1.27, 95% CI: 0.80–2.01; P = 0.31), although the adverse events occurred at a higher incidence in the tadalafil group than in the placebo.
Conclusions: This study demonstrates that once daily 5 mg tadalafil is a potentially effective and safe treatment choice with excellent tolerability for patients with LUTS/BPH.
Systematic Review Registration: Identifier (CRD42021228840).
Benign prostatic hyperplasia (BPH), which is caused by the proliferation of epithelial and stromal cells in the transition zone of the prostate, is characteristic of nonmalignant hyperplasia of prostatic tissue and is highly prevalent in older men (1). Lower urinary tract symptoms (LUTS) associated with BPH include storage or irritative (mainly including urinary frequency, urgency, and nocturia), voiding or obstructive (mainly including urinary hesitancy, straining, retention, and a decreased force of urination), and postmicturition symptoms, which can significantly and negatively affect the quality of life (QoL) of the elderly (2, 3). More than 50% of men > 50 years and over 80% of men > 80 years old experience LUTS/BPH (4).
In addition to surgical intervention, alpha-blockers (ABs), 5-alpha reductase inhibitors (5ARIs), and phytotherapies (monotherapy or cotherapy) have been prescribed for the treatment of LUTS related to BPH for decades (5, 6). Although these drug treatments are effective, they can cause troublesome adverse reactions, such as hypotension, dizziness, and sexual dysfunction (7, 8). Tadalafil, one of the phosphodiesterase type 5 inhibitors (PDE5Is) used to improve the symptoms of erectile dysfunction (ED), has been increasingly investigated and gained approval for the treatment of BPH in many countries since 2011 (9).
This is mainly because PDE5Is are believed to likely act mainly by increasing the concentration of nitric oxide (NO), which improves the activity of intracellular cyclic guanosine monophosphate (cGMP) (10). This system is collectively called the NO-cGMP signaling pathway, and its activation could lead to the relaxation of smooth muscle in the detrusor, bladder neck, and prostate (10). In addition, PDE5Is not only ameliorate intraprostatic inflammation-associated BPH but also improve tissue oxygenation and blood supply and thereby playing a vital part in alleviating LUTS (9, 11).
Recent meta-analyses comparing the effect of tamsulosin as a monotherapy and a cotherapy with tadalafil in improving LUTS/BPH, have concluded that cotherapy might be more suitable for patients than monotherapy (12, 13). However, analyses of tadalafil 5 mg monotherapy administered once daily for the treatment of LUTS/BPH are rare. Despite the use of tadalafil monotherapy, LUTS/BPH has been reported to improve significantly following treatment with this strategy (14). Therefore, in this study, we conducted the latest and high-quality synthesis of current worldwide evidence to evaluate the therapeutic effect and clinical safety of tadalafil 5 mg once daily monotherapy for 12 weeks in men with LUTS/BPH.
The study registration application has been submitted to the International Prospective Register of Systematic Reviews (CRD42021228840) and the report was prepared using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
A comprehensive and systematic search of PubMed, Cochrane Central Register of Controlled Trials, Embase, and Web of Science databases was performed for the period up to July 2021 by using the following key terms: [(“Prostatic Hyperplasia”[Mesh]) OR (Benign Prostatic Hyperplasia) OR (Hyperplasia, Prostatic) OR (Prostatic Hypertrophy) OR (Adenoma, Prostatic) OR (Adenomas, Prostatic) OR (Prostatic Adenomas) OR (Prostatic Adenoma) OR (Prostatic Hyperplasia, Benign) OR (Prostatic Hypertrophy, Benign) OR (Benign Prostatic Hypertrophy) OR (Hypertrophy, Benign Prostatic) OR (“lower urinary tract symptoms”[Mesh]) OR (lower urinary tract symptom)] AND [(“Tadalafil”[Mesh]) OR (Pyrazino (1′,2′:1,6) pyrido (3,4-b)indole-1,4 -dione,6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-) OR (IC-351) OR (IC 351) OR (Cialis)]. Reference lists of relevant articles were also manually searched and the “related articles” function was used to identify additional articles in PubMed. The subsequent document filtration and data extraction were performed independently by the two authors (JW Cui, DH Cao), and conflicts on suitable articles were settled by discussion and consultation with two-third senior authors (Q Wei, J Wang).
Trials included in our study met the following criteria according to the population, intervention, comparator, outcome, and study design (PICOS) approach: (P) male patients were diagnosed as LUTS/BPH; (I) taking medication: 12-week monotherapy with once daily 5 mg tadalafil; (C) treatment with an oral isodose placebo in the same way; (O) evaluating the following outcomes: changes in the total International Prostate Symptom Score (IPSS), IPSS storage (or irritative) subscore, IPSS voiding (or obstructive) subscore, IPSS quality of life (IPSS QoL), adverse events (AEs), and serious adverse events (SAEs); and (S) randomized controlled trials (RCTs). In addition, we excluded reviews, case reports, meeting abstracts, comments, letters to the editor, and animal research.
The outcome indicators were the QoL affected by LUTS/BPH scored using the IPSS, IPSS storage subscore, IPSS voiding subscore, BPH impact index (BII), IPSS QoL, the maximum flow rate (Qmax), and the postvoid residual (PVR). Furthermore, safety-related findings assessed based on AEs and SAEs were additional indicators. Efficacy was evaluated by analyzing index changes from baseline to 12 weeks, whereas safety was assessed by comparing AE-related data. The analyses were mainly conducted using the Review Manager, version 5.4 (Cochrane Collaboration, Oxford, the UK). Specifically, heterogeneity was evaluated with Cochrane's Q-statistic test and the I2 tests. The random-effects model was used when heterogeneity was accepted at a PQ < 0.10 or I2 > 50%, otherwise, the fixed-effects model was used. The risk of bias was independently assessed by two authors using the Jadad scale (≥3 scores represented high-quality studies), funnel plot, and the Cochrane Collaboration's tool for assessing the risk of bias, and conflicts were resolved by reaching a consensus through discussions. Sensitivity analysis was performed in the outcome indicators, respectively, by sequentially excluding each study. To find out more information about the ideal candidate for tadalafil monotherapy, several linear regressions were performed. Continuous variables reported as a mean and SD in the original articles were converted from standard error into SD for this study: SD = standard error *(sample size)1/2. A 95% CI was chosen whether mean difference (MD) was used to express the continuous data or risk ratio (RR) was used for dichotomous data.
A total of 1,425 relevant articles were extracted from the databases through systematical and manual search strategies. Eventually, 13 articles (15–27) involving 9,525 participants were identified, of which 15 trials that met the eligibility criteria were included in this study. While the follow-up period was different in each study, we pooled data of the change from baseline to 12 weeks from the studies.
Figure 1 illustrates the literature search process used and Table 1 shows the mean baseline characteristics. The symmetrical funnel plot roughly revealed no selection biases or no small sample studies with poor methodological quality (Figure 2A). The risk of biases was presented further in the risk of bias graph summary and risk of bias graph, which illustrated that the probability of each bias in every study is quite small (Figure 2B). Apart from Qmax, the rest of the indicators have reached the insubstantial heterogeneity standard (PQ > 0.10 or I2 < 50% in forest plots) in Cochrane Reviewers' Handbook. In addition, no significant differences from the original analysis were found after sensitivity analysis.
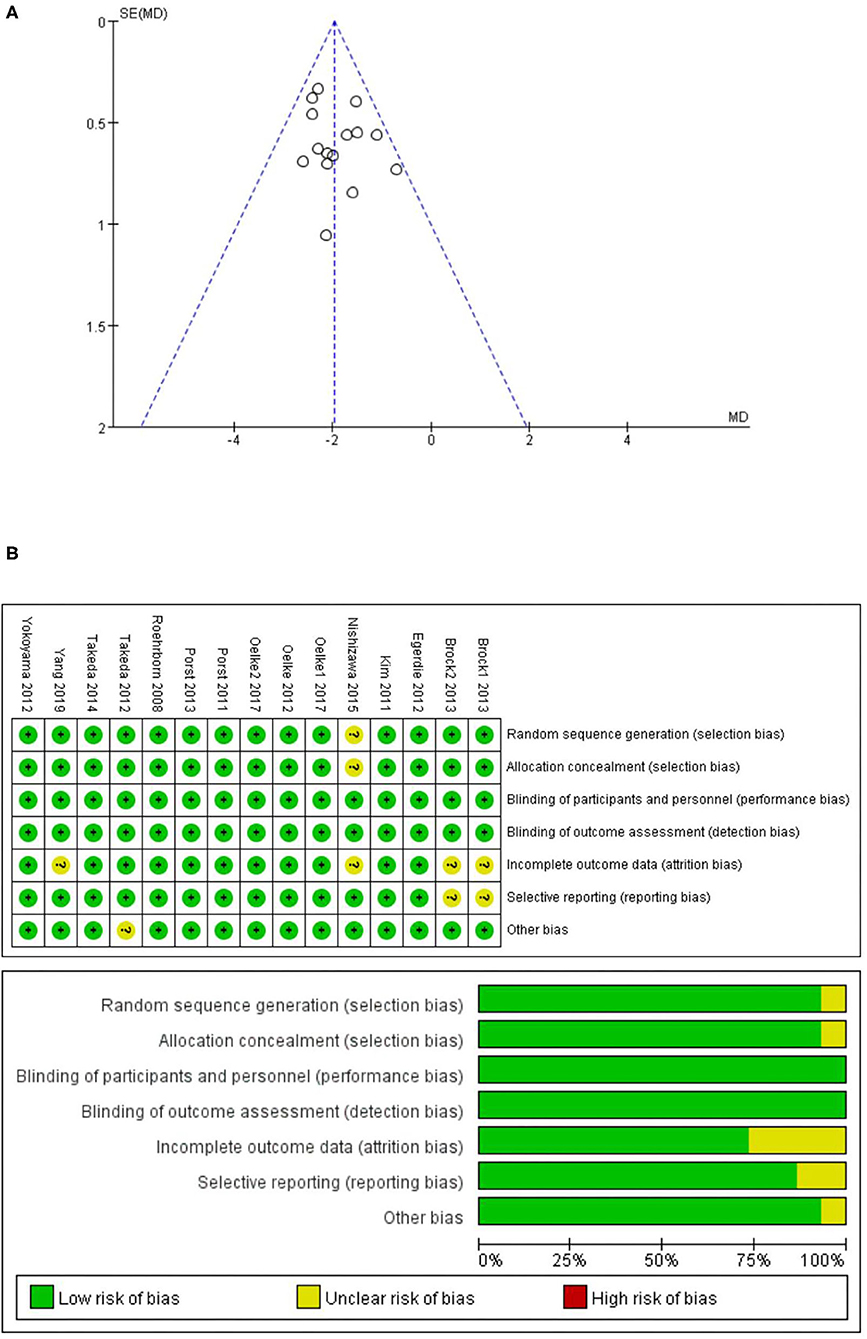
Figure 2. (A) Funnel plot of the studies represented in our meta-analysis; (B) risk of bias graph summary and risk of bias graph.
The total IPSS, IPSS voiding subscore, and IPSS storage subscore were reported in all the trails we selected. The data pooled from 15 studies (15–27) including 9,525 participants (4,937 and 4,588 in the tadalafil and placebo groups, respectively), and provided the necessary information for this study. The subsequent analysis result revealed a significantly larger total IPSS change from the baseline to 12 weeks in the tadalafil group (MD: −1.97 95% CI: −2.24 to −1.70, P < 0.00001, Figure 3A) than in the placebo group.
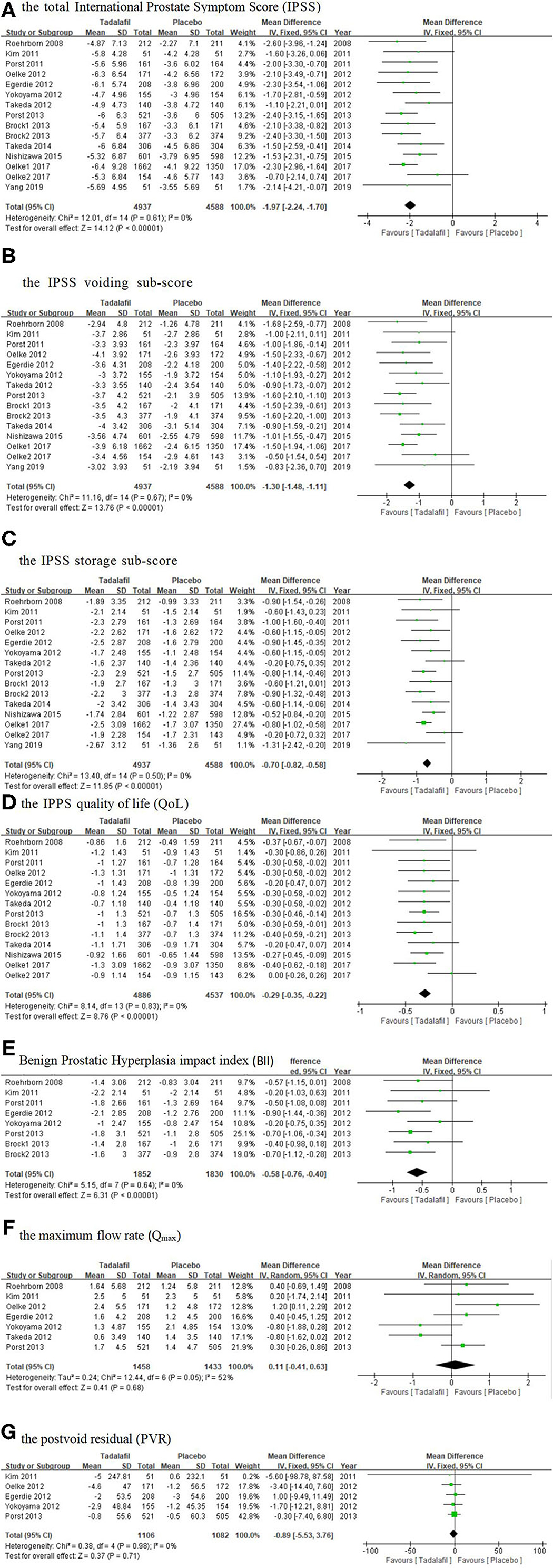
Figure 3. Forest plot of (A) the total IPSS; (B) the IPSS voiding subscore; (C) the IPSS storage subscore; (D) the IPSS QoL; (E) BII; (F) Qmax; and (G) PVR. IPSS, International Prostate Symptom Score; PVR, postvoid residual; QoL, quality of life.
A total of 15 studies (15–27) included in the analysis recorded the change in the IPSS voiding and storage subscores from baseline to 12 weeks. The analysis of the combined data showed that tadalafil significantly reduced the IPSS voiding and storage subscores more than the placebo did, and the MD of the change in the two subscores was −1.30 (95% CI: −1.48 to −1.11, P < 0.00001, Figure 3B) and −0.70 (95% CI: −0.82 to −0.58, P < 0.00001, Figure 3C), respectively.
A total of 14 studies (15–26) including 9,423 participants (4,886 and 4,537 in the tadalafil and placebo groups, respectively) investigated the IPSS QoL improvement data. The fixed-effects estimate of the MD was −0.29 (95% CI: −0.35 to −0.22, P < 0.00001, Figure 3D).
Eight studies (15–18, 21–23) including 3,682 participants (1,852 and 1,830 in the tadalafil and placebo groups, respectively) compared the effect of the treatments on the BII. Overall, the pooled MD was −0.58 (95% CI: −0.76 to −0.40, P < 0.00001, Figure 3E) in the fixed-effect model, which indicated the BII was significantly lower in patients administered tadalafil therapy than in those who received the placebo.
These results suggested that tadalafil oral monotherapy (5 mg once daily for 12 weeks) significantly improved LUTS/BPH in the treated patients. Interestingly, the better improvement in IPSS seemed to be in connection with the younger age, the high BMI, and the high baseline IPSS (Figure 4).
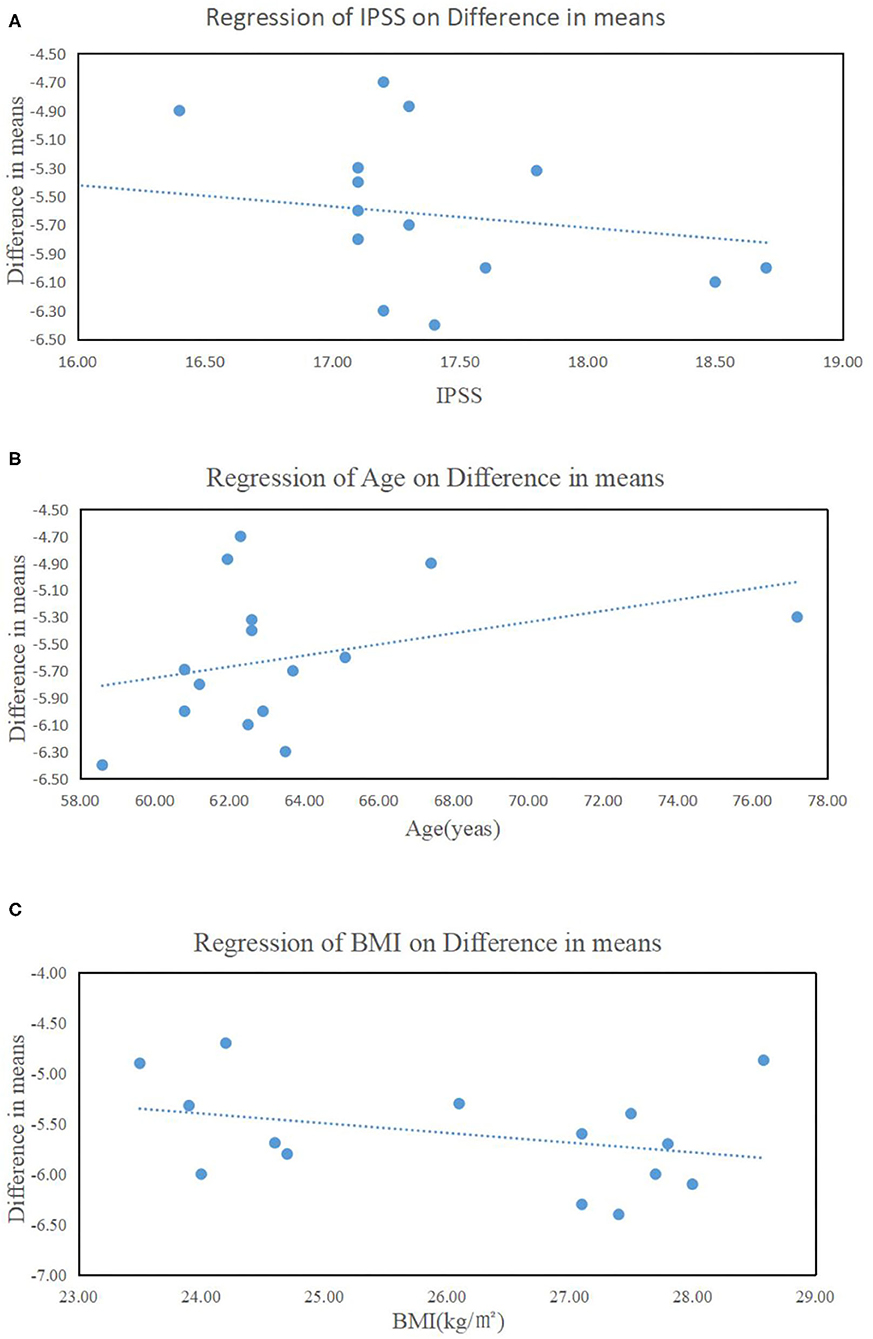
Figure 4. Influence of (A) the baseline of the total International Prostate Symptom Score (IPSS), (B) age, and (C) body mass index on IPSS improvement.
The mean change in the Qmax was calculated from pooled data from seven studies (15, 16, 18–21, 23) including 2,891 participants (1,458 and 1,433 in the tadalafil and placebo groups, respectively). The results showed an insignificant difference between tadalafil and the placebo, with an MD of 0.11 (95% CI: −0.41 to 0.63, P = 0.68, Figure 3F).
Five studies (16, 18, 19, 21, 23) including 2,188 participants (1,106 and 1,082 in the tadalafil and placebo groups, respectively), provided information about the change in the PVR from baseline to 12 weeks. The results indicated that the decrease in PVR was similar between these two groups, with an MD of −0.89 (95% CI: −5.53 to 3.76, P = 0.71, Figure 3G).
In our study, the most common AEs were headache (3.59%), nasopharyngitis (3.21%), dyspepsia (2.92%), and back pain (2.29%). The results of meta-analyses showed that a higher incidence of headache, dyspepsia, and back pain was associated with tadalafil monotherapy (Table 2).
The AEs and SAEs that occurred in the tadalafil and placebo groups were compared in this review. All the studies included (15–27) in the analysis reported the incidence of AEs, which showed a significant difference between the tadalafil and placebo groups with a RR of 1.27 (95% CI: 1.19–1.36, P < 0.00001, Figure 5A). However, the comparison of incidences of SAE did not show any significant difference between the two groups (RR: 1.27, 95% CI: 0.80–2.01, P = 0.31, Figure 5B) in the 13 studies including 8,436 participants (4,393 and 4,043 in the tadalafil and placebo groups, respectively).
This systematic review and meta-analysis have demonstrated comprehensively the therapeutic effect and clinical safety of 12-week monotherapy with once daily 5 mg tadalafil for LUTS/BPH. In our study, the total IPSS improvement was clinically meaningful after a tadalafil monotherapy, which significantly improved the IPSS voiding and storage subscores, IPSS QoL, and BII, whereas no statistically significant difference was found in terms of Qmax and PVR. In addition, the result shows that tadalafil and placebo were similar in the matter of the incidence of SAEs, although the difference in AEs was statistically significant.
Medicinal management has become the standard of therapy in patients diagnosed with moderate to severe LUTS/BPH, whereas surgery is performed in those who fail to respond to medication. Tamsulosin is regarded as an effective treatment for LUTS/BPH because it selectively blocks α1 receptors to induce smooth muscle relaxation in the bladder, neck, and prostate (28). Furthermore, tamsulosin 0.4 mg administered once daily is used in Western countries and is the safest AB in combination with PDE5Is (12). As a recommended medicine, tamsulosin could improve the symptom score by 4–6 points in the European Association of Urology guidelines. Likewise, the total IPSS was reduced by an average of 4.7–6.4 points with tadalafil monotherapy in the studies we included. Coincidentally, the study by Oelke (19), comparing with tamsulosin, monotherapy with tadalafil seemed to be a similar therapeutic effect in improving moderate to severe LUTS/BPH. All these make it necessary to test whether tadalafil is effective in LUTS/BPH with more sufficient evidence.
Tadalafil, the only PDE5I used for the treatment of BPH, was initially prescribed for the treatment of ED in 2003 but later proved to be effective in LUTS/BPH. Consequently, this agent was approved by the US Food and Drug Administration (FDA) in 2011 for the treatment of symptoms of BPH especially when accompanied by ED (29). A dose-finding study was conducted with a sample size of 1,058 patients experiencing LUTS/BPH who were randomly assigned to receive tadalafil (2.5, 5, 10, or 20 mg) or a placebo once daily. The results showed that tadalafil administered at all the tested doses significantly improved the symptoms more than the placebo did, and tadalafil 5 mg daily showed a more positive risk-benefit profile (15). In fact, an oral dose of 5 mg per day is the standard for tadalafil (12). Dong et al. (30) performed a meta-analysis of studies reporting the use of monotherapy for LUTS in 2012, whereas Wang et al. (31) conducted a similar study in 2016. However, the tadalafil dose used in the included trials in the article by Dong et al. (30) was not unified in the analysis of the comprehensive effect of four different doses, and the number of studies included was small in the latter passage (30, 31). Furthermore, three related literature reports (25–27) (four RCTs) have been published over the last 5 years. Therefore, the latest and largest pooled analysis and systematic review are urgently required to evaluate the therapeutic effect and clinical safety of tadalafil with a unified dose (5 mg once daily) and a uniform endpoint (12 weeks) in patients with LUTS/BPH.
The reduced indexes, including the total IPSS, IPSS voiding and storage subscores, IPSS QoL, and BII, showed the relief of symptoms of BPH and the improvement of QoL. As we all know, IPSS QoL indicates the view of the patient if always accompanied by current LUTS/BPH in a future life, among which are six options: from 0 (pleasurable) to 3 (acceptable), to 6 points (terrible), in turn. Therefore, although the score of QoL was only reduced by about one point, it meant a qualitative leap in the satisfaction of patients with the improvement of urination symptoms and reflected the need of the patient for treatment. Storage symptoms, especially the relief in nocturia, made a greater contribution to the improvement in QoL (32). These statistically significant results illustrated a great therapeutic effect of tadalafil in improving LUTS, but the specific mechanisms of action remain unclear. The PDE5 enzyme is expressed in the bladder, urethra, corpora cavernosa, prostate, ureter, and kidney (33). Matsukawa et al. (34) conducted a urodynamic-based study that demonstrated that tadalafil significantly improved bladder outlet obstruction and bladder storage function. In addition, in vitro studies showed that a PDE5I relaxed the smooth muscle of the bladder, neck, and prostate, and decreased detrusor muscle overactivity (35). The relaxant effect of PDE5Is on the bladder neck and urethra mediated by the NO-cGMP signaling pathway might result in symptom relief in patients with LUTS/BPH. Other related mechanisms include decreased activation of the RhoA/Rho kinase pathway, improvement of oxygenation, regulation of proliferation and transdifferentiation, modulation of the afferent nerves of the bladder and the prostate along with autonomic nervous system overactivity, and anti-inflammatory effects (7, 36). These mechanisms identified by our results might mediate the effective alleviation of the symptoms of BPH by this agent.
The corpus cavernosum, bladder, and prostate have similar pathophysiological pathways, and, therefore, LUTS/BPH and ED are often comorbid in the older population and have a higher incidence in older men (37). More importantly, LUTS is considered a risk factor for ED (38). Although many PDE5Is have similar mechanisms of action, tadalafil is the only PDE5I approved for the treatment of BPH because of its safety, tolerability, and longer duration of action (7). Nevertheless, a study of more than 1,000 men conducted by Broderick et al. (39) reported that tadalafil exhibited a similar efficacy in improving LUTS/BPH in men with or without comorbid ED. Moreover, Dong et al. (30) performed a subgroup analysis and reported that tadalafil showed a more significant improvement in BPH symptoms in men without ED than in those with ED. The effect of tadalafil on LUTS does not appear to mainly involve its mechanism of action on ED. However, the inhibitory effects of tamsulosin on neurogenic contractions have been reported to be enhanced by tadalafil in the human bladder, neck, and prostate. Those findings along with the study showing that cotreatment with ABs and tadalafil produces an additional relaxation effect on the corpus cavernosum in vitro (40, 41), suggests there might exist synergistic effects among tadalafil and tamsulosin accounting for the better effect in patients who are suffering from LUTS/BPH and ED. Thus, when an ABs or tadalafil alone does not show sufficient efficacy, cotherapy with both agents may be considered.
No statistically significant difference was found in Qmax and PVR in this study, which is consistent with the finding by Liu et al. (42). However, trials studying cotreatment with ABs and PDE5Is demonstrated that it increased the urinary flow rate (43, 44). The relaxant effect of PDE5Is on the bladder neck and prostate, which should have increased the urinary flow, might be counteracted by the concomitant relaxation of the detrusor muscle. Nevertheless, cotherapy may exert a synergistically positive effect on the detrusor muscle, thereby improving the urinary flow rate (12). More studies are required to elucidate the mechanism.
Although the difference in AEs was statistically significant, the side effects were mild and sustainable, such as headache, dyspepsia, and back pain. Furthermore, the difference in the incidence of SAEs was not significant. Interestingly, one patient reported an SAE in the tadalafil group whereas six participants reported in the placebo group of the study by Roehrborn (15) reported SAEs, suggesting that some AEs might be caused by psychological effects. Moreover, Wada et al. (45) demonstrated that the main reason for withdrawal of tadalafil was insufficient efficacy, followed by AEs and improvement of symptoms (45). In addition, calculated finally to be 2.5% on average, the incidence of discontinuation was low. Therefore, tadalafil 5 mg daily had no obvious adverse effects and is highly effective in improving LUTS/BPH.
Previous trials have reported that a higher baseline IPSS score correlates with a better therapeutic effect of ABs (46), which is consistent with our present observation (Figure 4A). Moreover, the improvement in IPSS appeared to be related to age and obesity (Figures 4B,C). These results suggested that younger, more obese men with more severe urinary symptoms may be the ideal candidates for tadalafil monotherapy. This could be explained by the changes in metabolism and hormonal levels that accompany aging and obesity. Testosterone levels decrease with increasing age and the hormonal dependency of PDE5 expression has been found in the animal models (47). Additional research is needed to further verify these results and explore the underlying mechanisms of action.
There are some limitations to our study that should be recognized. We were restricted to studies comparing tadalafil with placebo, rather than other pharmacological treatments recommended in guidelines to manage LUTS. Nonetheless, we focus on whether tadalafil is effective and safe in treating LUTS/BPH, rather than assessing which is better after comparing tadalafil and other drugs. In addition, relatively few experiments reported Qmax and PVR, and those that did were conducted before 2013, which may have caused the lack of statistical significance observed. More large-scale, well-designed, and long-term prospective clinical studies using comprehensive indicators should be conducted to further confirm the effectiveness and safety of tadalafil monotherapy in improving LUTS/BPH vs. active control.
Despite the several limitations to this study, we have reported the latest and most comprehensive evidence-based medical proof to demonstrate that 5 mg once daily tadalafil was effective and safe for the treatment of LUTS/BPH. All the studies in our analysis were RCTs and the follow-up deadline of 3 months was long enough, and the heterogeneity of our forest plots was also calculated to be low, except for the Qmax (I2 = 52%). These findings indicate the reliability of the curative effect of tadalafil. As the population ages, more and more aging men are being troubled with LUTS/BPH together with ED. As an effective treatment for ED, tadalafil, shown in our study, has also a therapeutic effect in relieving LUTS/BPH. So, tadalafil is a better choice for men suffering from both LUTS/BPH and ED. What is more, due to its safety and tolerable AEs, tadalafil may provide another option for those who are intolerant to the side effects of ABs or 5ARIs. This study could provide a more meaningful reference for clinicians and researchers, and provide more concrete evidence for future guidelines for the treatment of BPH.
Treatment with 5 mg tadalafil once daily effectively improved total IPSS, IPSS voiding subscore, IPSS storage subscore, IPSS QoL, and BII. Moreover, monotherapy with tadalafil appeared to be well tolerated and safe because no significant difference was detected in SAEs between the tadalafil- and placebo-treated patients. Finally, 5 mg tadalafil administered once daily could be a therapeutic option for patients with LUTS/BPH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Conception and design, and administrative support by JW and QW. Provision of study materials or patients were administered by JC and DC. Collection and assembly of data were administered by JC, DC, YB, WW, and YX. Data analysis and interpretation were administered by JC, DC, and YB. Manuscript writing and final approval of manuscript were done by all the authors.
This work was funded by the National Natural Science Foundation of China (Grant no. 82000721 and no. 81770756); the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant no. 2019HXBH089); and the Health Commission of Sichuan Province (Grant no. 20PJ036).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (www.editage.cn) for the English language.
1. Madersbacher S, Sampson N, Culig, Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: A mini-review. Gerontology. (2019) 65:458–64. doi: 10.1159/000496289
2. Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. (2015) 67:1099–109. doi: 10.1016/j.eururo.2014.12.038
3. MacDonald R, Brasure M, Dahm P, Olson CM, Nelson VA, Fink HA, et al. Efficacy of newer medications for lower urinary tract symptoms attributed to benign prostatic hyperplasia: A systematic review. Aging Male. (2019) 22:1–11. doi: 10.1080/13685538.2018.1434503
4. Lokeshwar SD, Harper BT, Webb E, Jordan A, Dykes TA, Neal DE Jr., et al. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl Androl Urol. (2019) 8:529–39. doi: 10.21037/tau.2019.10.01
5. Morton A, Williams M, Perera M, Teloken PE, Donato P, Ranasinghe S, et al. Management of benign prostatic hyperplasia in the 21st century: Temporal trends in Australian population-based data. BJU Int. (2020) 126:18–26. doi: 10.1111/bju.15098
6. Yamanishi T, Kaga K, Sakata K, Yokoyama T, Kageyama S, Fuse M, et al. A randomized controlled study of the efficacy of tadalafil monotherapy versus combination of tadalafil and mirabegron for the treatment of persistent overactive bladder symptoms in men presenting with lower urinary tract symptoms (contact study). Neurourol Urodyn. (2020) 39:804–12. doi: 10.1002/nau.24285
7. Monica FZ, De Nucci. G. Tadalafil for the treatment of benign prostatic hyperplasia. Expert Opin Pharmacother. (2019) 20:929–37. doi: 10.1080/14656566.2019.1589452
8. Ückert S, Kedia GT, Tsikas D, Simon A, Bannowsky A, Kuczyk M.A. Emerging drugs to target lower urinary tract symptomatology (LUTS)/benign prostatic hyperplasia (BPH): Focus on the prostate. World J Urol. (2020) 38:1423–35. doi: 10.1007/s00345-019-02933-1
9. Sebastianelli A, Spatafora P, Morselli S, Vignozzi L, Serni S, McVary KT, et al. Tadalafil alone or in combination with tamsulosin for the management for LUTS/BPH and ED. Current Urology Reports. (2020) 21:56. doi: 10.1007/s11934-020-01009-7
10. Rezk MR, Tantawy MA, Wadie M, Weshahy S.A. Smart spectrophotometric assessment of tamsulosin hydrochloride and tadalafil in their new pharmaceutical formulation for treatment of benign prostatic hyperplasia and erectile dysfunction. Spectrochimica Acta. Part A. Molecular & Biomolecular Spectroscopy. (2020) 227:117547. doi: 10.1016/j.saa.2019.117547
11. Alhakamy NA, Fahmy UA, Ahmed O.A.A. Attenuation of benign prostatic hyperplasia by optimized tadalafil loaded pumpkin seed oil-based self nanoemulsion: In vitro and in vivo evaluation. Pharmaceutics. (2019) 11:640. doi: 10.3390/pharmaceutics11120640
12. Nagasubramanian S, John NT, Antonisamy B, Mukha RP, Jeyachandra Berry CS, Kumar S, et al. Tamsulosin and placebo vs tamsulosin and tadalafil in male lower urinary tract symptoms: A double-blinded, randomised controlled trial. BJU Int. (2020) 125:718–24. doi: 10.1111/bju.15027
13. Zhou Z, Zheng X, Wu J, Gao Z, Xu Z, Cui Y. Meta-analysis of efficacy and safety of tadalafil plus tamsulosin compared with tadalafil alone in treating men with benign prostatic hyperplasia and erectile dysfunction. Am J Mens Health. (2019) 13:1557988319882597. doi: 10.1177/1557988319882597
14. Zhang Z, Li H, Zhang X, Dai Y, Park HJ, Jiann BP, et al. Efficacy and safety of tadalafil 5 mg once-daily in Asian men with both lower urinary tract symptoms associated with benign prostatic hyperplasia and erectile dysfunction: A phase 3, randomized, double-blind, parallel, placebo- and tamsulosin-controlled study. Int J Urol. (2019) 26:192–200. doi: 10.1111/iju.13828
15. Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A dose finding study. J Urol. (2008) 180:1228–34. doi: 10.1016/j.juro.2008.06.079
16. Kim SC, Park JK, Kim SW, Lee SW, Ahn TY, Kim JJ, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: Results from a placebo-controlled pilot study using tamsulosin as an active control. Low Urin Tract Symptoms. (2011) 3:86–93. doi: 10.1111/j.1757-5672.2011.00088.x
17. Porst H, Kim ED, Casabé AR, Mirone V, Secrest RJ, Xu L, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. (2011) 60:1105–13. doi: 10.1016/j.eururo.2011.08.005
18. Egerdie RB, Auerbach S, Roehrborn CG, Costa P, Garza MS, Esler AL, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: Results of a randomized, placebo-controlled, double-blind study. J Sex Med. (2012) 9:271–81. doi: 10.1111/j.1743-6109.2011.02504.x
19. Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. (2012) 61:917–25. doi: 10.1016/j.eururo.2012.01.013
20. Takeda M, Nishizawa O, Imaoka T, Morisaki Y, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms in Japanese men with benign prostatic hyperplasia: Results from a 12-week placebo-controlled dose-finding study with a 42-week open-label extension. Low Urin Tract Symptoms. (2012) 4:110–19. doi: 10.1111/j.1757-5672.2012.00144.x
21. Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, Morisaki Y, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. (2012) 20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x
22. Brock G, Broderick G, Roehrborn CG, Xu L, Wong D, Viktrup L. Tadalafil once daily in the treatment of lower urinary tract symptoms(LUTS) suggestive of benign prostatic hyperplasia(BPH) in men without erectile dysfunction. BJU Int. (2013) 112:990–7. doi: 10.1111/bju.12251
23. Porst H, Roehrborn CG, Secrest RJ, Esler A, Viktrup L. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: Analyses of pooled data from four randomized, placebo-controlled tadalafil clinical studies. J Sex Med. (2013) 10:2044–52. doi: 10.1111/jsm.12212
24. Takeda M, Yokoyama O, Lee SW, Murakami M, Morisaki Y, Viktrup L. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int J Urol. (2014) 21:670–5. doi: 10.1111/iju.12410
25. Nishizawa O, Yoshida M, Takeda M, Yokoyama O, Morisaki Y, Murakami M, et al. Tadalafil 5 mg once daily for the treatment of Asian men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: Analyses of data pooled from three randomized, double-blind, placebo-controlled studies. Int J Urol. (2015) 22:378–84. doi: 10.1111/iju.12699
26. Oelke M, Wagg A, Takita Y, Büttner H, Viktrup L. Efficacy and safety of tadalafil 5 mg once daily in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia in men aged ≥75 years: Integrated analyses of pooled data from multinational, randomized, placebo-controlled clinical studies. BJU Int. (2017) 119:793–803. doi: 10.1111/bju.13744
27. Yang DY, Jeong HC, Ko K, Lee SH, Lee SK, Shin TY, et al. Effect of tadalafil 5 mg on post-micturition dribble in men with lower urinary tract symptoms: A multicentre, double-blind, randomized, placebo-controlled trial. BJU Int. (2019) 124:862–69. doi: 10.1111/bju.14849
28. Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Treatment of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. (2018) 21:272–80. doi: 10.1080/13685538.2018.1432586
29. Singh I, Tk A, Gupta S. Efficacy and safety of tadalafil vs tamsulosin in lower urinary tract symptoms (luts) as a result of benign prostate hyperplasia (BPH)-open label randomised controlled study. Int J Clin Pract. (2020) 74:e13530. doi: 10.1111/ijcp.13530
30. Dong Y, Hao L, Shi Z, Wang G, Zhang Z, Han C. Efficacy and safety of tadalafil monotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A meta-analysis. Urol Int. (2013) 91:10–8. doi: 10.1159/000351405
31. Wang Y, Bao Y, Liu J, Duan L, Cui Y. Tadalafil 5 mg once daily improves lower urinary tract symptoms and erectile dysfunction: A systematic review and meta-analysis. Low Urin Tract Symptoms. (2016) 10:84–92. doi: 10.1111/luts.12144
32. Choi WS, Son H. The change of ipss 7 (nocturia) score has the maximum influence on the change of qol score in patients with lower urinary tract symptoms. World J Urol. (2019) 37:719–25. doi: 10.1007/s00345-018-2410-8
33. Rossanese M, Crestani A, Inferrera A, Giannarini G, Bartoletti R, Tubaro A, et al. Medical treatment for benign prostatic hyperplasia: Where do we stand? Urologia Journal. (2019) 86:115–21. doi: 10.1177/0391560319859785
34. Matsukawa Y, Majima T, Matsuo K, Funahashi Y, Kato M, Yamamoto T, et al. Effects of tadalafil on storage and voiding function in patients with male lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A urodynamic-based study. Int J Urol. (2018) 25:246–50. doi: 10.1111/iju.13489
35. Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, Gacci M, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. (2011) 8:2746–60. doi: 10.1111/j.1743-6109.2011.02416.x
36. Gacci M, Andersson KE, Chapple C, Maggi M, Mirone V, Oelke M, et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. (2016) 70:124–33. doi: 10.1016/j.eururo.2015.12.048
37. Kim SW, Park NC, Lee SW, Yang DY, Park JK, Moon DG, et al. Efficacy and safety of a fixed-dose combination therapy of tamsulosin and tadalafil for patients with lower urinary tract symptoms and erectile dysfunction: Results of a randomized, double-blinded, active-controlled trial. J Sex Med. (2017) 14:1018–27. doi: 10.1016/j.jsxm.2017.06.006
38. Kallidonis P, Adamou C, Kotsiris D, Ntasiotis P, Verze P, Athanasopoulos A. Combination therapy with alpha-blocker and phosphodiesterase-5 inhibitor for improving lower urinary tract symptoms and erectile dysfunction in comparison with monotherapy: A systematic review and meta-analysis. Eur Urol Focus. (2020) 6:537–58. doi: 10.1016/j.euf.2019.05.007
39. Broderick GA, Brock GB, Roehrborn CG, Watts SD, Elion-Mboussa A, Viktrup L. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunction. Urology. (2010) 75:1452–58. doi: 10.1016/j.urology.2009.09.093
40. Journal of Sexual Medicine. Conference: International Symposium on Prostate, A.a.M.s.S.H. J, Cuevas P, Fernandez A, La Fuente JM, Allona A, Moncada I, et al. Tadalafil enhances the inhibitory effects of tamsulosin on neurogenic contractions of human prostate and bladder neck. J Sex Med. (2012) 9:2293–306. doi: 10.1111/j.1743-6109.2012.02821.x
41. Oger S, Behr-Roussel D, Gorny D, Lebret T, Denoux Y, Alexandre L, et al. Combination of alfuzosin and tadalafil exerts an additive relaxant effect on human detrusor and prostatic tissues in vitro. Eur Urol. (2010) 57:699–707. doi: 10.1016/j.eururo.2009.04.039
42. Liu L, Zheng S, Han P, Wei Q. Phosphodiesterase-5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review and meta-analysis. Urology. (2011) 77:123–9. doi: 10.1016/j.urology.2010.07.508
43. Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, Kaplan SA, et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. (2012) 61:994–1003.
44. Yan H, Zong H, Cui Y, Li N, Zhang Y. The efficacy of PDE5 inhibitors alone or in combination with alpha-blockers for the treatment of erectile dysfunction and lower urinary tract symptoms due to benign prostatic hyperplasia: A systematic review and meta-analysis. J Sex Med. (2014) 11:1539–45. doi: 10.1111/jsm.12499
45. Wada N, Kikuchi D, Tateoka J, Abe N, Watanabe M, Tamaki G, et al. Persistence rate with tadalafil for treatment of male lower urinary tract symptoms. Urol Int. (2020) 104:373–77. doi: 10.1159/000507230
46. Roehrborn CG, Siami P, Barkin J, Damiao R, Becher E, Minana B, et al. The influence of baseline parameters on changes in international prostate symptom score with dutasteride, tamsulosin, and combination therapy among men with symptomatic benign prostatic hyperplasia and an enlarged prostate: 2-year data from the combat study. Eur Urol. (2009) 55:461–71. doi: 10.1016/j.eururo.2008.10.037
Keywords: benign prostatic hyperplasia (BPH), lower urinary tract symptoms (LUTS), tadalafil, efficacy, safety
Citation: Cui J, Cao D, Bai Y, Wang J, Yin S, Wei W, Xiao Y, Wang J and Wei Q (2021) Efficacy and Safety of 12-week Monotherapy With Once Daily 5 mg Tadalafil for Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia: Evidence-based Analysis. Front. Med. 8:744012. doi: 10.3389/fmed.2021.744012
Received: 19 July 2021; Accepted: 06 September 2021;
Published: 12 October 2021.
Edited by:
Xiulin Zhang, Second Hospital of Shandong University, ChinaReviewed by:
Hui Ding, Lanzhou University Second Hospital, ChinaCopyright © 2021 Cui, Cao, Bai, Wang, Yin, Wei, Xiao, Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wei, d2VpcWlhbmc5MzNAMTI2LmNvbQ==; Jia Wang, d2FuZ2ppYXdjaEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.