- 1Department of Kidney Transplantation, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Department of Neurosurgery, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Objectives: We aimed to analyze the effect of cold ischemia time (CIT) on post-transplant graft function through mixed-effect model analysis to reduce the bias caused by paired mate kidneys.
Methods: We reviewed all kidney transplantation records from 2015 to 2019 at our center. After applying the exclusion criteria, 561 cases were included for analysis. All donor characteristics, preservation and matching information, and recipient characteristics were collected. Transplant outcomes included delayed graft function (DGF) and estimated glomerular filtration rate (eGFR). Generalized linear mixed models were applied for analysis. We also explored potential effect modifiers, namely, donor death category, expanded criteria donors, and donor death causes.
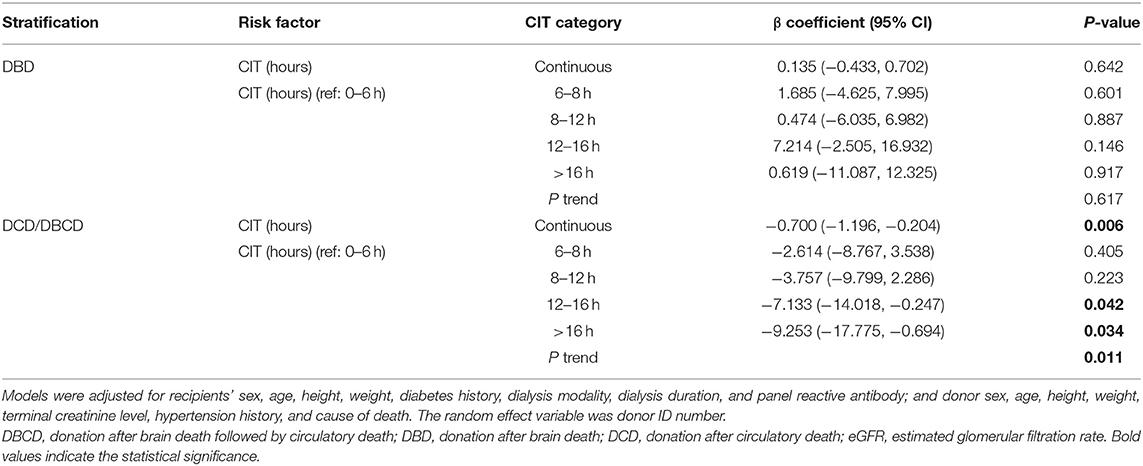
Results: Among the 561 cases, 79 DGF recipients developed DGF, and 15 recipients who died after surgery were excluded from the eGFR estimation. The median stable eGFR of the 546 recipients was 60.39 (47.63, 76.97) ml/min/1.73 m2. After adjusting for confounding covariates, CIT had a negative impact on DGF incidence [odds ratio = 1.149 (1.006, 1.313), P = 0.041]. In the evaluation of the impact on eGFR, the regression showed that CIT had no significant correlation with eGFR [β = −0.287 (−0.625, 0.051), P = 0.096]. When exploring potential effect modifiers, only the death category showed a significant interaction with CIT in the effect on eGFR (Pinteraction = 0.027). In the donation after brain death (DBD) group, CIT had no significant effect on eGFR [β = 0.135 (−0.433, 0.702), P = 0.642]. In the donation after circulatory death/donation after brain death followed by circulatory death (DCD/DBCD) group, CIT had a significantly negative effect on eGFR [β= −0.700 (−1.196, −0.204), P = 0.006]. Compared to a CIT of 0–6 h, a CIT of 6–8 or 8–12 h did not decrease the post-transplant eGFR. CIT over 12 h (12–16 h or over 16 h) significantly decreased eGFR. With the increase in CIT, the regenerated eGFR worsened (Ptrend = 0.011).
Conclusion: Considering the effect of paired mate kidneys, the risk of DGF increased with prolonged CIT. The donor death category was an effect modifier between CIT and eGFR. Prolonged CIT did not reduce the eGFR level in recipients from DBDs but significantly decreased the eGFR in recipients from DCDs/DBCDs. This result indicates the potential biological interaction between CIT and donor death category.
Introduction
Kidney transplantation is an effective alternative to dialysis treatment for end-stage renal disease patients and has advantages over it. Currently, expanded criteria donors are gradually being accepted to expand the donor pool. Cold ischemia time (CIT) is one of the numerous factors affecting transplant outcomes. It is generally considered that acute kidney injury induced by ischemia is the cause of delayed graft function (DGF) (1, 2). Ischemia time naturally affects the degree of acute kidney injury, thus possibly affecting transplant outcomes. However, the effect of CIT on transplant outcomes is controversial in the literature. Foroutan et al. performed a systematic review of risk factors for 1-year graft survival and observed no or little effect of CIT on graft survival (3). The unit analyzed in their study was each increased hour of CIT. Large cohorts from the United Kingdom and the Scientific Registry of Transplant Recipients (SRTR) in the United States have shown that CIT did not impact graft survival (4–7). In other studies, prolonged CIT significantly reduced graft survival (8–13). The inconsistency of these results may result from how different populations are affected by local public policies, the sample size, the analytic methods and models, or other causes of bias.
Regarding the analytic methods, previous studies have neglected the pre-conditions of case independence in their multivariate regression adjustments. There exists a similitude effect of transplant outcomes in both recipients of mate kidneys. In other words, the graft recovery in both recipients of mate kidneys is not independent because they share mate kidneys from the same donor. There are several methods to address cluster data, namely, robust cluster standard error estimation, mixed effect models, generalized estimating equations, and multistate analysis (14). In this article, we aimed to analyze the effect of CIT on post-transplant graft function through mixed-effect model analysis to reduce the bias caused by paired mate kidneys.
Methods
Study Design and Patients
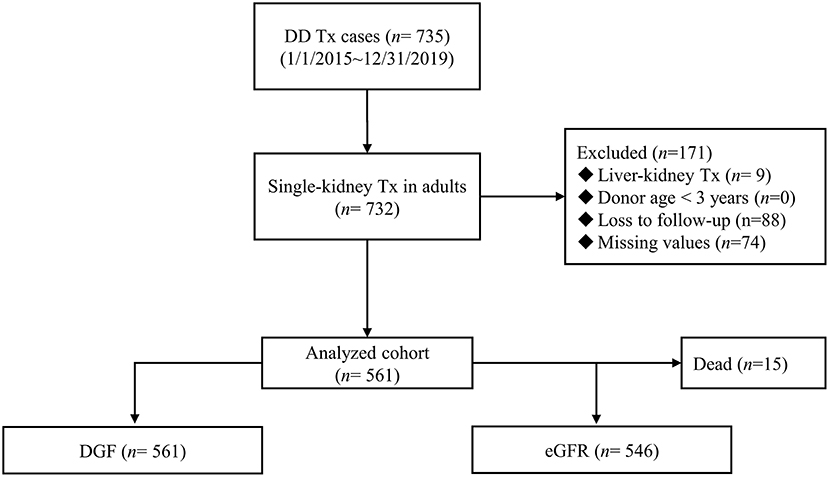
The China Organ Transplant Response System (COTRS) is the sole legitimate official registry designated by the National Health Commission of China for solid organ donation, matching, and allocation. It includes a potential donor registry system maintained by the donation coordinators in each organ procurement organization (OPO), an allocation system maintained by the OPO (merged), and a waiting list maintained by each transplant registry center. We accessed and retrieved cases in our transplant center from January 1, 2015 to December 31, 2019. The inclusion criteria were single-kidney transplantation, donor age ≥ 3 years, and recipient age ≥ 18 years. Any recipient who was lost to follow-up was excluded. The detailed screen diagram is shown in Figure 1. All donors were deceased citizen donors, as this is the only legal avenue for non-relative solid organ transplantation procurement in the Chinese mainland as of January 1, 2015. All donors and procured organs were numbered, matched, and allocated at COTRS (https://www.cot.org.cn/). This study was approved by the research ethics committee at the Third Affiliated Hospital of Sun Yat-sen University [IRB Approval: (2020)02-243-01]. Donor profile access was permitted after ethical review. This research complied with the Declaration of Helsinki. The clinical and research activities reported here are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Data Sources and Variables
The primary outcomes of this research comprised DGF and stable graft function [estimated glomerular filtration rate (eGFR)] after transplantation. DGF was defined as the need for dialysis during the 1st week after transplantation (15). Graft recovery function (i.e., stable eGFR) was manually evaluated at each follow-up visit. It was estimated by the range of creatinine values within the 1st year. We used the median creatinine value as the index of stable function and calculated the eGFR by the Chronic Kidney Disease Epidemiology Collaboration formula (16). All data were from COTRS (including its waitlist and allocation system), medical records, tests, and examinations from the donation hospitals and transplant hospitals. These data were legally protected and inspected for their authenticity. The authors were authorized to retrieve the relevant data from these platforms.
Common variables of donors included age, sex, blood type, height, weight, terminal serum creatinine, and cause of death. Expanded criteria donors were defined using Rao's definition (17). The donor death category was divided into three classifications: donation after brain death (DBD), donation after circulatory death (DCD), and donation after brain death followed by circulatory death (DBCD) (18). Because the sample size of DBCD was small, we merged DCD and DBCD into the single group DCD/DBCD. CIT was estimated as the interval from donor death to reperfusion of the anastomotic kidney, ignoring the short duration (several minutes) between death and initial cold perfusion in the donor. All donated kidneys were placed in static cold storage and transported. Common variables of recipients included recipient age, sex, height, weight, diabetes, dialysis mortality, dialysis vintage, and peak panel reactive antibody.
Statistical Analysis
Categorical variables are described as percentages and were compared between groups by the Fisher's exact-test or the chi-squared-test. Continuous variables are expressed as the mean and standard deviation (for normal distributions) or median and interquartile range (for skewed distributions) and were compared by the Wilcoxon test. To demonstrate the impact of CIT on transplant outcomes, considering the intercorrelation within mate kidneys from the same donor, we used mixed-effect models for analysis. The random variable was donor ID code. In addition, we used the P-values of the interaction and the effect size in each subgroup to explore the effect-modifying factors. Potential factors included donor death category, expanded criteria donors, and donor death causes. A mixed logit model was used for binary outcomes, and mixed linear regression was applied for continuous outcomes. The standard error of each coefficient was calculated via robust estimation. All statistical analyses were performed using Stata 16.1 IC (StataCorp, College Station, TX, USA) and R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P-value < 0.05 was considered statistically significant.
Results
Baseline Demographics
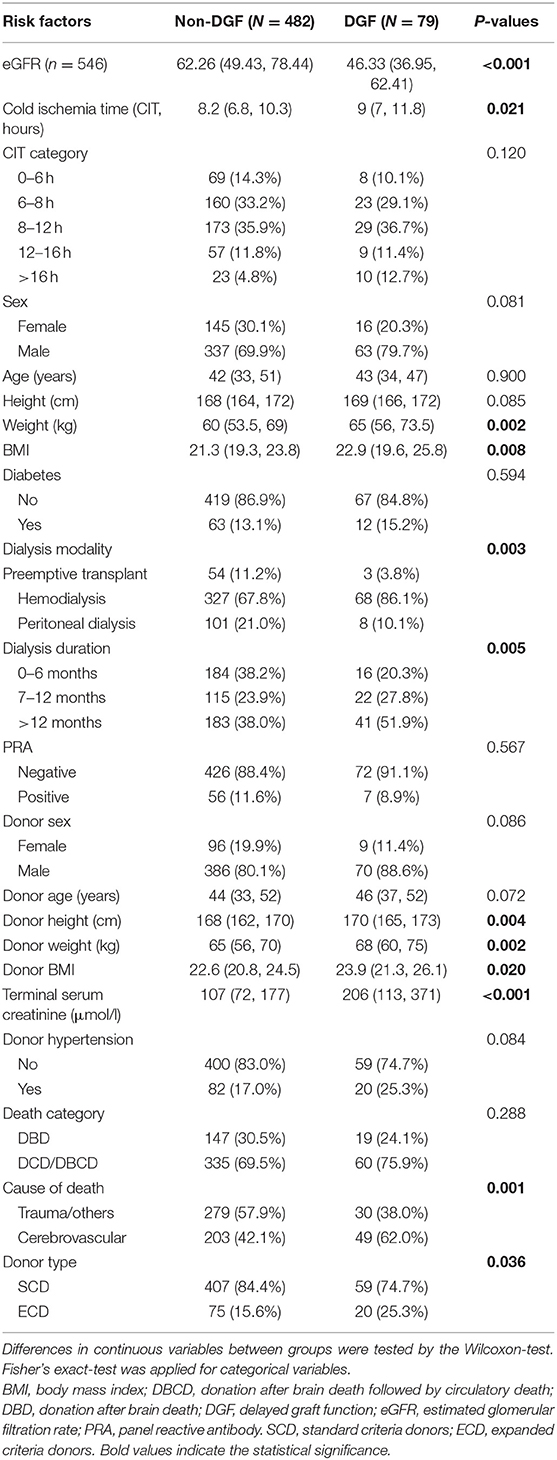
From 2015 to 2019, 735 recipients had deceased-donor kidney transplantation records. After excluding child recipients (n = 3), liver-kidney transplantations (n = 9), recipients lost to follow-up (n = 88), and donors with key values not reported in COTRS (missing values due to no access to other OPOs, n = 74). A total of 561 kidney transplant cases were remaining for analysis. Among them, 15 recipients died in the post-operative period, which was attributed to transplant failure (Figure 1). The baseline characteristics of the analyzed cohort are listed in Table 1. There were 79 DGF recipients and 15 recipients who died after surgery. The median stable eGFR of the 546 recipients was 60.39 (47.63, 76.97) ml/min/1.73 m2. The stable post-transplant eGFR was much lower in DGF recipients (P < 0.001) than in non-DGF recipients. In addition, DGF recipients had higher body weight, longer dialysis duration, more hemodialysis modalities, longer CIT, higher donor body height and weight, higher terminal serum creatinine level, higher cerebrovascular death rate, and higher expanded criteria donors (ECD) rate.
Cold Ischemia Time and Graft Function Recovery Outcomes
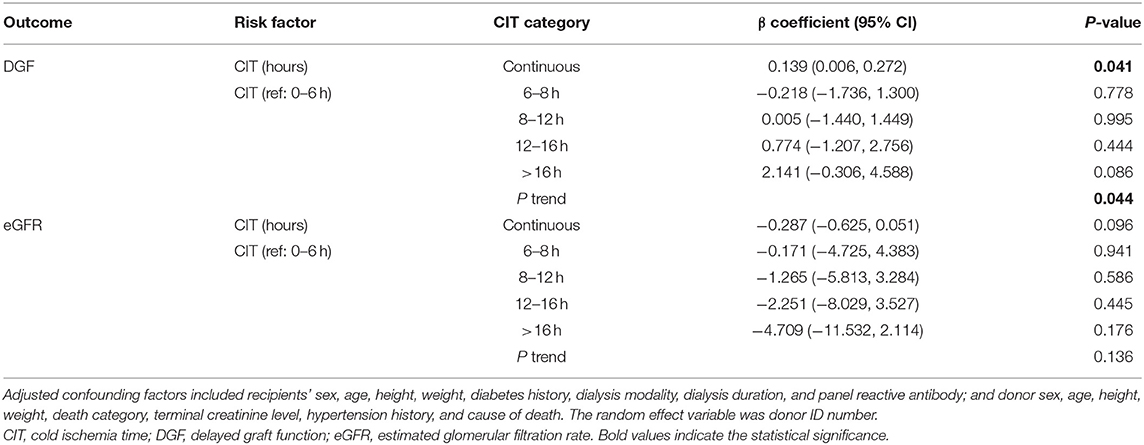
The mean CIT was 9.4 ± 4.1 h. Recipients with DGF had longer CIT (P = 0.021). After adjusting for confounding covariates, CIT had a negative impact on DGF incidence [odds ratio = 1.149 (1.006, 1.313), P = 0.041]. In the entire analyzed cohort of 546 cases, simple correlation analysis showed that CIT had no significant correlation with post-transplant eGFR (P = 0.410). Multivariate adjustment regression showed that CIT had no significant correlation with eGFR (Table 2). In addition, we divided CIT into several categories and performed multivariate regression analysis. The regression results are listed in Table 2. No significant effect was observed in any subgroup for either DGF or eGFR outcomes. The effect size trend of CIT on DGF was statistically significant (P = 0.044). As CIT increased, the effect size of DGF risk increased, although no significance was observed in any group compared to the CIT of 0–6 h.
Potential Effect-Modifying Factors
We explored the correlation of CIT with DGF and eGFR in the entire cohort and each subgroup of potential effect-modifying factors. Figure 2 shows that in both donor type and death category stratifications, CIT was not significantly different between the DGF and non-DGF groups. In the cause of death subgroups (Figure 2D), recipients with DGF had longer CITs when the donor had cerebrovascular death. However, no differences were observed in the recipients from donors who had trauma/other causes of death.
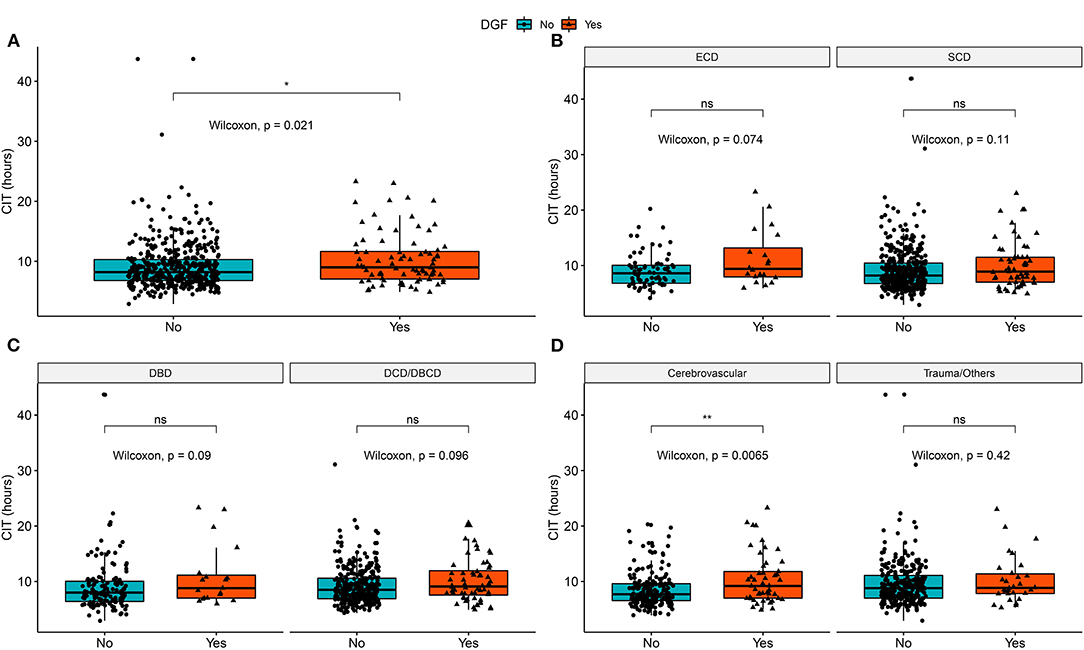
Figure 2. Distribution of cold ischemia hours in DGF/non-DGF groups in different subgroups and the entire cohort. (A) Cold ischemia time in the DGF and non-DGF groups (P = 0.021). (B) The difference in cold ischemia time between the DGF groups in SCD (P = 0.114) and ECD (P = 0.074). (C) The difference in cold ischemia time between DGF groups in DBD donors (P = 0.089) and DCD donors (P = 0.095). (D) The difference in cold ischemia time between DGF groups in donors who had cerebrovascular death (P = 0.007) and trauma/other cause of death (P = 0.423). DBD, donation after brain death; DCD, donation after circulatory death; DBCD, donation after brain death followed by circulatory death; SCD, standard criteria donors; ECD, expanded criteria donors; DGF, delayed graft function. *p < 0.05, **p < 0.01; ns, no significance.
When exploring potential effect-modifying factors between CIT and eGFR, as shown in Figure 3, there was no correlation between CIT and eGFR in the global cohort, in each donor type or each cause of death strata. In the death category stratification, CIT was negatively correlated with eGFR in the DCD/DBCD group (P = 0.012), but there was no significant correlation in the DBD group (P = 0.149).
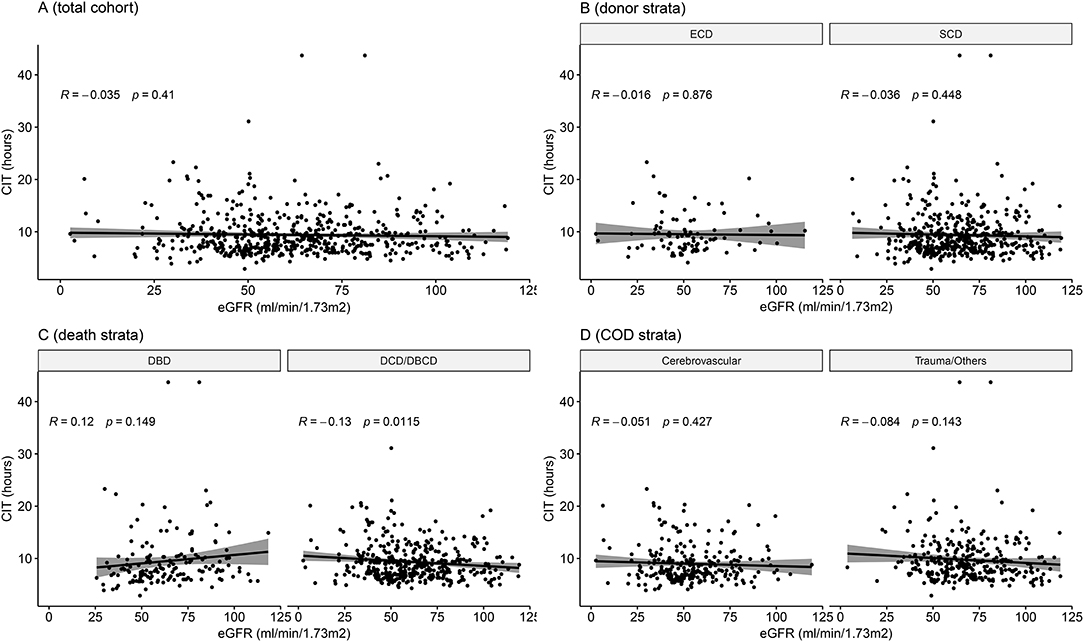
Figure 3. Correlation between cold ischemia time and eGFR in the entire cohort and in subgroups. (A) Entire cohort of 546 cases (P = 0.410). (B) Stratified by donor type (P = 0.876 in ECDs and P = 0.448 in SCDs). (C) Stratified by death category (P = 0.149 in DBDs and P = 0.012 in DCD/DBCDs). (D) Stratified by cause of death (P = 0.427 in the cardiovascular disease group and P = 0.143 in the trauma/other group). DBCD, donation after brain death followed by circulatory death; DBD, donation after brain death; DCD, donation after circulatory death; DBCD, donation after brain death followed by circulatory death; SCD, standard criteria donors; ECD, expanded criteria donors; eGFR, estimated glomerular filtration rate.
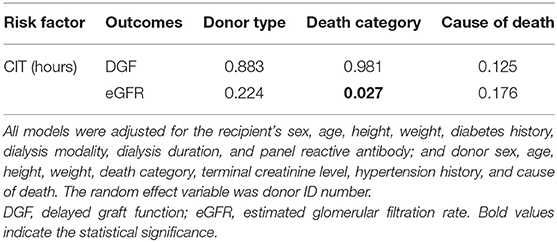
To quantitatively explore the existence of potential effect-modifying factors, we incorporated interaction terms in the regression model to test the interactions of CIT with donor type, death category, and cause of death. An interaction term with a P-value < 0.05 was considered to indicate an effect modification. Each regression result is listed in Table 3. Neither donor type nor the cause of death interacted with CIT. Only the death category showed a significant interaction with CIT on the eGFR outcome (Pinteraction= 0.027).
We further explored the effect size of CIT on eGFR in different death category strata (shown in Table 4). In the DBD group, CIT had no significant effect on eGFR (P = 0.642). In the DCD/DBCD group, CIT had a significantly negative effect on eGFR [β = −0.700 (−1.196, −0.204), P = 0.006]. Compared to a CIT of 0–6 h, a CIT of 6–8 or 8–12 h did not significantly increase the risk of eGFR recovery. CIT over 12 h (12–16 h or over 16 h) significantly decreased eGFR. With increasing CIT, the regenerated eGFR worsened. The trend test of effect size was significant (P = 0.011).
Discussion
In this study, we considered the effect of mate kidneys and found that prolongation of CIT increased DGF risk. In general, CIT did not correlate with the regenerated eGFR. However, we found that donor death category was an effect-modifying factor between CIT and eGFR. In DBD recipients, prolonged CIT did not reduce the eGFR level. In DCD/DBCD recipients, each additional hour of CIT significantly decreased eGFR by an average of 0.7 ml/min/1.73 m2.
It is common sense that CIT increases the risk of DGF, and our results bore this out. Our results did not reveal any interaction between CIT and donor type, death category, or cause of death when there was DGF. The eGFR decrease along with the CIT increase was modified by death category. In a previous publication, Wong et al. (19) proposed a significant interaction between donor death category and total ischemia time in the effect on death-censored graft loss (DCGL). There was a significantly increased risk of DCGL along with increased ischemia time in recipients of DCD kidneys [hazard ratio (HR), 1.08; 95% CI, 1.01–1.17; P = 0.03] but not in recipients of DBD kidneys (HR, 0.99; 95% CI, 0.98–1.01; P = 0.83). They also found that donor age was an effect modifier between ischemia time and eGFR at 12 months. The eGFR at 12 months decreased significantly with each ischemia hour in recipients of older donors but not in recipients of young donors (age <55 years). Our results agree with this conclusion when we treated donor age as a bivariate category with a cutoff of 55 years [β= −1.190 (−2.070, −0.310) ml/min/1.73 m2, P = 0.008 in recipients of donor age over 55 years and β = −0.204 (−0.570, 0.162) ml/min/1.73 m2 in recipients of donor age <55 years, P = 0.274; Pinteraction = 0.014]. However, when we treated donor age as the original continuous variable, we did not find a significant interaction between CIT and donor age (Pinteraction = 0.436). In addition, they did not explore whether the death category was an effect modifier. In another study, Summers et al. (20) also showed greater graft loss risk from cold ischemia prolongation in kidneys from DCD donors than in kidneys from DBD donors, especially when the CIT was over 24 h (Pinteraction = 0.004); their study did not explore the effect modification by death category on eGFR recovery. Based on the previous consensus that eGFR recovery is a determining factor highly correlated with graft survival (21–25), the factors affecting graft survival very likely have the same impact trends on eGFR recovery. On this point, our results coincide with these other publications.
In a regression model, the presence of effect modification does not always imply biological interaction. However, a product term in a linear statistical model for a causal dependency can only arise from the presence of biological interaction in the dependent-action sense (26). Our study outcome was eGFR as a continuous variable, and the analytic model was a linear statistical model. Thus, we speculate that there was a potential biological interaction between the death category and CIT in the effect on eGFR recovery. The underlying mechanisms by which CIT influences transplant outcomes remain unclear. There exists a certain time-dependent molecular detriment mechanism since the effect of different CIT durations can be significantly different. Le Pape et al. (27) demonstrated that the unfolded protein response plays a critical role in elaborating the relationship between CIT and transplant outcomes. During the first 1–8 h, the eIF2a-ATF4 pathway is inhibited, while ATF6 is activated at 12–24 h and is associated with cell death. The IRE1a-XBP1 pathway is activated in reperfusion only if CIT surpasses 8 h, and IRE1a RNase activation is not detected before reperfusion but rather is only observable when preceded by at least 12 h of ischemia. These results coincide with our timeline trend outcomes in the subgroup analysis. Our results showed that in DCD recipients, CIT over 12 h (12–16 and >16) significantly decreased the eGFR compared to 0–6 h, while 6–12 h (6–8 and 8–12 h) did not. Our study showed that CIT was more detrimental to kidneys from DCD than to kidneys from DBD. How does the CIT interact with DCD, and does DCD or DBD trigger exacerbation or alleviation of subsequent detriment? The mechanism is still unclear. Saat et al. (28) compared kidney inflammatory, cytoprotective, and injury gene expression profiles from DCD and DBD in rat models. The results showed massive upregulation of proinflammatory genes, namely, IL-1β, IL-6, TNF-α, MCP-1, P-selectin, and E-selectin, in DBD kidneys compared with DCD kidneys immediately after kidney retrieval. During 18 h of cold ischemia, the expression levels of these genes did not significantly change. Interestingly, the expression of the cytoprotective gene heme oxygenase-1 (HO-1), whose upregulation is an adaptive response to oxidative stress, was significantly higher in DBD kidneys than in DCD kidneys during cold storage. A previous study demonstrated that HO-1 induction in brain-dead donors could improve allograft survival (29). In addition, the major difference between DCD and DBD kidneys is that DCD kidneys suffer worse warm ischemia injury before cold preservation. Warm ischemia time periods as short as a few minutes can lead to major metabolic changes. Baniene et al. (30) proved that warm ischemia leads to renal mitochondrial injury, which increases progressively with the increased duration of ischemia. As Saeb-Parsy et al. (31) summarized, mitochondria play central roles in ischemia-reperfusion injury and generate damaging reactive oxygen species. Mitochondrial injury promotes the release of damage-associated molecular patterns, which enhance inflammatory damage to the tissue. Thus, mitochondrial dysfunction caused by warm ischemia injury may induce susceptibility to cold ischemia and reperfusion injury. Mitochondrial transplantation significantly improves graft function and decreases graft tissue injury in a murine heart transplantation model (32). This evidence may explain why CIT had a more drastic impact on graft recovery in recipients from DCD.
Cold ischemia damage plays a critically important role in ischemia-reperfusion injury during kidney transplantation. Currently, there are some advances in reducing the detrimental effect of CIT on donors' kidneys. Hypothermic machine perfusion has been proven superior to static cold storage in terms of reducing DGF risk in deceased donor kidney transplantation (33). Modifying perfusion solution by adding agents against ATP depletion, Ca2+ overload, cell apoptosis, mitochondrial dysfunction, oxidative stress, inflammation, etc., is a practical direction to take (34). Gregorini et al. applied mesenchymal stromal cells (MSCs) and found that the addition of MSC/MSC-derived extracellular vesicles to Belzer solution during hypothermic machine perfusion protected the kidney from ischemic injury by preserving the enzymatic machinery essential for cell viability and protecting the kidney from reperfusion damage (35). To reduce the injury caused by hypoxia, oxygenated perfusion seems to be a reasonable alternative (36). However, current clinical trial results of oxygenated hypothermic machine perfusion are discouraging (36, 37). Normothermic machine perfusion (NMP) can mimic normal physiological perfusion of kidneys by oxygen carriers and can enable the reduction or avoidance of cold ischemia. Clinical trials assessing the effects of NMP on early and longer-term graft outcomes are needed (38). In addition, absorption of proinflammatory cytokines during cold perfusion breaks the cytokine cascade and amplification; absorption during perfusion reduces the inflammatory response and improves renal blood flow and graft viability in animal models (39, 40). It provides another means for reducing ischemia-reperfusion injury. By increasing the understanding of the potential mechanism of ischemia-reperfusion injury during kidney transplantation, therapeutic strategies based on different pathway targets are emerging. Our study revealed that DCD kidneys are more susceptible to cold ischemia damage than DBD kidneys. Although the underlying mechanism is unclear, understanding such a mechanism would provide new targets to reduce ischemia damage and improve DCD graft outcomes.
There are some limitations to this study. First, our sample was derived from our single transplantation center and had quite a few missing values or losses to follow-up. Second, the sample size was relatively small compared to the cohorts from transplant registry data. Finally, when we analyzed post-transplant eGFR, we excluded 15 recipients who died during the perioperative period after transplantation. These recipients would have had a lower eGFR if they had survived. This may have resulted in bias.
In conclusion, by considering the paired effect of mate kidneys, we showed that the risk of DGF increased with prolonged CIT. The donor death category was an effect modifier between CIT and eGFR. A longer CIT did not reduce the eGFR level in DBD recipients but significantly decreased the eGFR by an average of 0.7 ml/min/1.73 m2 with each prolonged CIT hour in DCD/DBCD recipients. This result indicates the potential biological interaction between CIT and donor death category.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee at the Third Affiliated Hospital of Sun Yat-sen University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
FQ and NN conceived and designed this study. YL, ZD, XH, ZT, JZ, XW, WD, and BM collected the data. YL and XH analyzed the data. YL and ZD wrote the manuscript. BM, NN, and FQ supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81970652), the Guangdong Basic and Applied Basic Research Foundation (2019A1515011219), the Bioengineering Research Center Training Project of the Third Affiliated Hospital of Sun Yat-sen University (SW201904), the Third Affiliated Hospital of Sun Yat-sen University Clinical Research Program (YHJH201906), and the Science and Technology Planning Project of Guangzhou (201803010016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.743085/full#supplementary-material
References
1. Mannon RB. Delayed graft function: the AKI of kidney transplantation. Nephron. (2018) 140:94–8. doi: 10.1159/000491558
2. Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. (2011) 11:2279–96. doi: 10.1111/j.1600-6143.2011.03754.x
3. Foroutan F, Friesen EL, Clark KE, Motaghi S, Zyla R, Lee Y, et al. Risk factors for 1-year graft loss after kidney transplantation: systematic review and meta-analysis. Clin J Am Soc Nephrol. (2019) 14:1642–50. doi: 10.2215/CJN.05560519
4. Xia Y, Friedmann P, Cortes CM, Lubetzky ML, Kayler LK. Influence of cold ischemia time in combination with donor acute kidney injury on kidney transplantation outcomes. J Am Coll Surg. (2015) 221:532–8. doi: 10.1016/j.jamcollsurg.2015.05.003
5. Kayler L, Yu X, Cortes C, Lubetzky M, Friedmann P. Impact of cold ischemia time in kidney transplants from donation after circulatory death donors. Transplant Direct. (2017) 3:e177. doi: 10.1097/TXD.0000000000000680
6. Sampaio MS, Chopra B, Tang A, Sureshkumar KK. Impact of cold ischemia time on the outcomes of kidneys with Kidney Donor Profile Index >/=85%: mate kidney analysis - a retrospective study. Transpl Int. (2018) 31:729–38. doi: 10.1111/tri.13121
7. Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. (2011) 11:2647–56. doi: 10.1111/j.1600-6143.2011.03741.x
8. Perez Valdivia MA, Gentil MA, Toro M, Cabello M, Rodriguez-Benot A, Mazuecos A, et al. Impact of cold ischemia time on initial graft function and survival rates in renal transplants from deceased donors performed in Andalusia. Transplant Proc. (2011) 43:2174–6. doi: 10.1016/j.transproceed.2011.06.047
9. Debout A, Foucher Y, Trebern-Launay K, Legendre C, Kreis H, Mourad G, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. (2015) 87:343–9. doi: 10.1038/ki.2014.304
10. Krishnan AR, Wong G, Chapman JR, Coates PT, Russ GR, Pleass H, et al. Prolonged ischemic time, delayed graft function, and graft and patient outcomes in live donor kidney transplant recipients. Am J Transplant. (2016) 16:2714–23. doi: 10.1111/ajt.13817
11. Hansson J, Mjornstedt L, Lindner P. The risk of graft loss 5 years after kidney transplantation is increased if cold ischemia time exceeds 14 hours. Clin Transplant. (2018) 32:e13377. doi: 10.1111/ctr.13377
12. Postalcioglu M, Kaze AD, Byun BC, Siedlecki A, Tullius SG, Milford EL, et al. Association of cold ischemia time with acute renal transplant rejection. Transplantation. (2018) 102:1188–94. doi: 10.1097/TP.0000000000002106
13. Peters-Sengers H, Houtzager JHE, Idu MM, Heemskerk MBA, van Heurn ELW, Homan van der Heide JJ, et al. Impact of cold ischemia time on outcomes of deceased donor kidney transplantation: an analysis of a national registry. Transplant Direct. (2019) 5:e448. doi: 10.1097/TXD.0000000000000888
14. Galbraith S, Daniel JA, Vissel B. A study of clustered data and approaches to its analysis. J Neurosci. (2010) 30:10601–8. doi: 10.1523/JNEUROSCI.0362-10.2010
15. Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. (2013) 96:885–9. doi: 10.1097/TP.0b013e3182a19348
16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
17. Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD–fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. (2009) 4:1827–31. doi: 10.2215/CJN.02270409
18. Na N, Li K, Huang Z, Miao B, Hu C, Li H, et al. Posttransplant outcomes of kidneys donated after brain death followed by circulatory death: a cohort study of 128 chinese patients. Transplant Direct. (2017) 3:e189. doi: 10.1097/TXD.0000000000000704
19. Wong G, Teixeira-Pinto A, Chapman JR, Craig JC, Pleass H, McDonald S, et al. The impact of total ischemic time, donor age and the pathway of donor death on graft outcomes after deceased donor kidney transplantation. Transplantation. (2017) 101:1152–8. doi: 10.1097/TP.0000000000001351
20. Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. (2013) 381:727–34. doi: 10.1016/S0140-6736(12)61685-7
21. First MR. Renal function as a predictor of long-term graft survival in renal transplant patients. Nephrol Dial Transplant. (2003) 18(Suppl. 1):i3–6. doi: 10.1093/ndt/gfg1027
22. Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Patient outcomes in renal transplantation I. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. (2011) 57:466–75. doi: 10.1053/j.ajkd.2010.10.054
23. Schnitzler MA, Lentine KL, Axelrod D, Gheorghian A, You M, Kalsekar A, et al. Use of 12-month renal function and baseline clinical factors to predict long-term graft survival: application to BENEFIT and BENEFIT-EXT trials. Transplantation. (2012) 93:172–81. doi: 10.1097/TP.0b013e31823ec02a
24. Baek CH, Kim H, Yang WS, Han DJ, Park SK. A postoperative 1-Year eGFR of more than 45 ml/min may be the cutoff level for a favorable long-term prognosis in renal transplant patients. Ann Transplant. (2016) 21:439–47. doi: 10.12659/AOT.897938
25. Mottola C, Girerd N, Duarte K, Aarnink A, Giral M, Dantal J, et al. Prognostic value for long-term graft survival of estimated glomerular filtration rate and proteinuria quantified at 3 months after kidney transplantation. Clin Kidney J. (2020) 13:791–802. doi: 10.1093/ckj/sfaa044
26. Greenland S. Effect Modification and Interaction. Wiley StatsRef: Statistics Reference Online. (2015). Availble online at: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118445112.stat03728.pub2 (accessed July 1, 2021).
27. Le Pape S, Pasini-Chabot O, Couturier P, Delpech PO, Volmer R, Quellard N, et al. Decoding cold ischaemia time impact on kidney graft: the kinetics of the unfolded protein response pathways. Artif Cells Nanomed Biotechnol. (2018) 46:S873–85. doi: 10.1080/21691401.2018.1518908
28. Saat TC, Susa D, Roest HP, Kok NF, van den Engel S, Ijzermans JN, et al. A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation. (2014) 98:15–21. doi: 10.1097/TP.0000000000000136
29. Kotsch K, Francuski M, Pascher A, Klemz R, Seifert M, Mittler J, et al. Improved long-term graft survival after HO-1 induction in brain-dead donors. Am J Transplant. (2006) 6:477–86. doi: 10.1111/j.1600-6143.2005.01208.x
30. Baniene R, Trumbeckas D, Kincius M, Pauziene N, Raudone L, Jievaltas M, et al. Short ischemia induces rat kidney mitochondria dysfunction. J Bioenerg Biomembr. (2016) 48:77–85. doi: 10.1007/s10863-016-9643-2
31. Saeb-Parsy K, Martin JL, Summers DM, Watson CJE, Krieg T, Murphy MP. Mitochondria as therapeutic targets in transplantation. Trends Mol Med. (2021) 27:185–98. doi: 10.1016/j.molmed.2020.08.001
32. Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, et al. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant. (2019) 38:92–9. doi: 10.1016/j.healun.2018.09.025
33. Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. (2019) 3:CD011671. doi: 10.1002/14651858.CD011671.pub2
34. Chen Y, Shi J, Xia TC, Xu R, He X, Xia Y. Preservation solutions for kidney transplantation: history, advances and mechanisms. Cell Transplant. (2019) 28:1472–89. doi: 10.1177/0963689719872699
35. Gregorini M, Corradetti V, Pattonieri EF, Rocca C, Milanesi S, Peloso A, et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J Cell Mol Med. (2017) 21:3381–93. doi: 10.1111/jcmm.13249
36. Darius T, Nath J, Mourad M. Simply adding oxygen during hypothermic machine perfusion to combat the negative effects of ischemia-reperfusion injury: fundamentals and current evidence for kidneys. Biomedicines. (2021) 9:993. doi: 10.3390/biomedicines9080993
37. Husen P, Boffa C, Jochmans I, Krikke C, Davies L, Mazilescu L, et al. Oxygenated end-hypothermic machine perfusion in expanded criteria donor kidney transplant: a randomized clinical trial. JAMA Surg. (2021) 156:517–25. doi: 10.1001/jamasurg.2021.0949
38. Elliott TR, Nicholson ML, Hosgood SA. Normothermic kidney perfusion: an overview of protocols and strategies. Am J Transplant. (2021) 21:1382–90. doi: 10.1111/ajt.16307
39. Hosgood SA, Moore T, Kleverlaan T, Adams T, Nicholson ML. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J Transl Med. (2017) 15:216. doi: 10.1186/s12967-017-1314-5
Keywords: kidney transplantation, cold ischemia time, delayed graft function, DCD, DBD, effect modification
Citation: Luo Y, Dong Z, Hu X, Tang Z, Zhang J, Deng W, Wei X, Miao B, Qin F and Na N (2021) Donor Death Category Is an Effect Modifier Between Cold Ischemia Time and Post-transplant Graft Function in Deceased-Donor Kidney Transplant Recipients. Front. Med. 8:743085. doi: 10.3389/fmed.2021.743085
Received: 17 July 2021; Accepted: 25 October 2021;
Published: 23 November 2021.
Edited by:
Pasquale Esposito, University of Genoa, ItalyReviewed by:
Marilena Gregorini, Fondazione Ospedale San Matteo (IRCCS), ItalyAndrea Angeletti, Giannina Gaslini Institute (IRCCS), Italy
Copyright © 2021 Luo, Dong, Hu, Tang, Zhang, Deng, Wei, Miao, Qin and Na. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Qin, cWluZmVuZzJAbWFpbC5zeXN1LmVkdS5jbg==; Ning Na, bmFuaW5nQG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
 You Luo1†
You Luo1† Zhanwen Dong
Zhanwen Dong Xiao Hu
Xiao Hu Zuofu Tang
Zuofu Tang Jinhua Zhang
Jinhua Zhang Weiming Deng
Weiming Deng Xiangling Wei
Xiangling Wei Bin Miao
Bin Miao Feng Qin
Feng Qin Ning Na
Ning Na