
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 November 2021
Sec. Family Medicine and Primary Care
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.742581
This article is part of the Research Topic Rising Stars: Family Medicine and Primary Care 2021 View all 9 articles
 Xiangling Deng1,2
Xiangling Deng1,2 Min Yang1,2
Min Yang1,2 Shunan Wang1,2
Shunan Wang1,2 Qiong Wang1,2
Qiong Wang1,2 Bo Pang1,2
Bo Pang1,2 Kundi Wang3*
Kundi Wang3* Zhixin Zhang2*
Zhixin Zhang2* Wenquan Niu4*
Wenquan Niu4*This study was prepared to identify and characterize potential factors associated with childhood asthma and wheeze in Chinese preschool-aged children. A comprehensive questionnaire was designed for children aged 3–6 years and their parents or guardians in Beijing and Tangshan from September to December 2020. The least absolute shrinkage and selection operator (LASSO) model was used to identify factors in a significant association with childhood asthma and wheeze, respectively. The LASSO model was internally validated using bootstrap resampling with 100 replications. A total of 9,529 questionnaires were certified as eligible for inclusion after stringent quality control. The prevalence of doctor-diagnosed childhood asthma and parent-reported wheeze was 2.8 and 6.2%, respectively. Factors simultaneously associated with childhood asthma and wheeze were children with a history of allergic rhinitis, hay fever, eczema, initial age of using antibiotics, body mass index category, and family history of asthma. Specifically, children's vitamin D supplement duration was significantly associated with childhood asthma, whereas the association with childhood wheeze was significant for intake frequency of night meals for children and their screen time. Modeling of significant factors in nomograms had decent prediction accuracies, with C-index reaching 0.728 and 0.707 for asthma and wheeze, respectively. In addition, internal validation was good, with bootstrap C-statistic of being 0.736 for asthma and 0.708 for wheeze. Taken together, our findings indicated that the development of asthma and wheeze among preschool-aged children was probably determined by the joint contribution of multiple factors including inherited, nutritional, unhealthy lifestyles, and history of allergic disease. Further validation in other groups is necessary.
Asthma is a heterogeneous disease characterized by chronic airway inflammation, and it is defined by the history of respiratory symptoms, such as wheeze, chest tightness, shortness of breath, and cough that are varying over time and in intensity, together with variable expiratory airflow limitation (1). Asthma has become the most common non-communicable disease among children (2), and it imposes a heavy burden on health and healthcare systems (3). According to the reports of the International Study of Asthma and Allergies in Childhood (ISAAC), the prevalence of asthma symptoms has exhibited an increasing tendency globally in both children (from 11.1 to 11.6%) and adolescents (13.2–13.7%). Preschool-aged children have a relatively high asthma-related morbidity, with about 48% of preschool-aged children with asthma experiencing an asthma attack in the preceding year (4). Meanwhile, a wide variation existed in the symptom prevalence of childhood asthma worldwide (5). Compared with some developed countries, such as Austria (11.3%), the United States (8.7%), the United Kingdom (15.4%), and Canada (13%) (6, 7), the prevalence of childhood asthma in China was much lower. The 3rd Nationwide Survey of Childhood Asthma in Urban Areas of China recorded that the prevalence of asthma in children under 14 years had been increased to 3% in 2020 from 2% in 2000.
It is worth noting that childhood asthma is preventable. Asthma usually starts before school age, and approximately one-third to half of the children with moderate to severe asthma may persist into adulthood (8). As evidenced, childhood asthma may predispose people to chronic obstructive pulmonary disease (9). Much worse, a proportion of children with asthma had severe symptoms and frequent exacerbations even with the availability of effective drugs (10). It is hence necessary to gain a better understanding of factors responsible for the development of childhood asthma and to inform alternative public health strategies that can effectively reduce or control the prevalence of asthma worldwide.
To yield more information, we designed a large-scale cross-sectional survey in Beijing and Tangshan, aiming to identify and characterize potential factors associated with childhood asthma and wheeze in Chinese preschool-aged children.
During the period between September and December in 2020, we undertook a cross-sectional survey of preschool children from Beijing and Tangshan, according to the principles of the Declaration of Helsinki. The survey was reviewed and approved by the Ethics Committee of China–Japan Friendship Hospital and all parents or guardians of study children read and signed informed consent forms prior to participation.
In this survey, study subjects were restricted to preschool-aged children (3–6 years old) who attended junior to senior classes in kindergartens. We utilized a stratified cluster random sampling strategy in 16 districts from Beijing and one city (Tangshan) in Hebei. In total, 30 kindergartens were eligible for data collection.
A comprehensive questionnaire was self-designed for both children and their parents or guardians, aiming to collect potential factors responsible for the development of childhood asthma and wheeze. Items from the questionnaire were selected a priori based upon published literature and clinical experience.
From children, items of interest included age, sex, region, body mass index (BMI), birth weight, ABO blood type, delivery mode, breastfeeding duration, daily sleep duration, secondhand smoke exposure, vitamin D supplement duration, probiotics supplement, exposure of average daily screen time, intake frequency of night meals, history of allergic rhinitis, hay fever, eczema, and initial age of using antibiotics. Body weight (to the nearest 0.1 kg) and height (to the nearest 0.1 cm) were measured by trained health physicians.
From parents or guardians, data on age, sex, weight, height, education, family income, maternal pre-pregnancy weight, gestational weight gain (GWG), maternal pregnancy smoking exposure, gestational hypertension, gestational diabetes mellitus, and family history of asthma were self-reported and recorded simultaneously.
Kindergarten teachers from 30 designated kindergartens were responsible for explaining and circulating the questionnaires to the parents or guardians of all children. Children with a history of the disease, including chronic kidney disease, hypothyroidism, congenital heart disease, chronic respiratory diseases, and inherited metabolic diseases, were also eliminated. Information on chronic medical histories of study children was obtained from their latest health records.
For children, asthma was defined according to the records of doctors, and wheezing symptoms were collected based on parental reports, including the onset of wheeze during the past 12 months. BMI was calculated and divided into obesity, overweight, and non-overweight according to the China criteria (2009) (11). Both break time during the day and sleep time at night was summed to calculate sleep duration of children, that is, sleep time on working days × 5 and corresponding time on weekends × 2 divided by 7. Average daily screen time was calculated as the same as the daily sleep duration and grouped into four groups, viz. <0.5, 0.5–1, 1–1.5, and >1.5 h. Intake frequency of night meals was defined as weekly eating food within 2 h before bedtime, which was classified as often (≥3 times), occasional (one to two times), once in a while, and none. Vitamin D supplement duration was classified into four categories, viz. ≤3, 3–6, 6–12, and >12 months. The initial age of using antibiotics was divided into seven groups, that is, <28 days, 28 days−1 month, 1–6 months, 6 months−1 year, 1–3 years, 3–6 years, and without antibiotics in the first 6 years of life.
For parents, family income (RMB per year) was classified into ≤100,000, 100,000–300,000, 300,000–600,000, 600,000–900,000, and >1,000,000. Education was defined as a high school degree or below, college degree, master's degree, and doctor degree or above. Adequate GWG was defined as weight gain of 12.5–18 kg in underweight mothers, 11.5–16 kg in normal-weight mothers, 7–11.5 kg in overweight mothers, and 5–9 kg in obese mothers. Inadequate GWG was grouped as less than the lower limits of adequate levels, and excessive GWG meant greater than the upper limits of adequate levels. All reference criteria were based on the recommendations of the Institute of Medicine (2009) (12). Secondhand smoke exposure and maternal smoking were defined as smoking exposure during maternal pregnancy.
Statistical analyses were completed using the STATA software (version 14.0, Stata Corp., College Station, TX, USA) and the R programming environment (version 4.0.2, https://www.r-project.org/). The power to detect statistical significance was estimated by the PS-Power Simple Size software (version 3.1.2, Copyright 1997–2009 by William D. Dupont and Walton D. Plummer.).
As data under study were collected from preschool children from 30 kindergartens, intraclass correlation coefficient (ICC) was calculated to quantify the degree to which observations within a cluster differ from those between clusters (13). The ICC statistic ranges from 0 to 1, with a lower value indicating a lower likelihood of between-kindergarten variation.
Continuous data are expressed as median (interquartile range), and categorical data are summarized as count (percentage). Two-group comparison was done by using the Wilcoxon rank-sum test or χ2 test, when appropriate. Two-sided p < 5% was defined to be statistically significant.
To account for missing data, we used multiple imputations, based on five replications and a chained equation approach method in the R MI procedure (14). All children were divided into asthmatic/wheezing or non-asthmatic groups.
To minimize the effects of potential confounding factors including age, sex, region, blood type, delivery mode, and family income, a 1:3 propensity score matching (PSM) method was performed to compare the relationship of various factors and these two groups by using the psmatch2 routine in the STATA software (Stata Corp., College Station, TX, USA).
The least absolute shrinkage and selection operator (LASSO) model (15) was used to quantify the contribution of all possible factors in this survey. LASSO is effective in declining covariance between multiple factors, reducing the possibility of over-fitting and removing unnecessary covariates. The “glmnet” package (version 2.0-16, https://glmnet.stanford.edu) was used to fit the logistic LASSO regression, and 10-fold cross-validation was used to select the penalty term, λ. Selected factors of statistical significance formed the elements of the prediction model.
The R “rms” package was used to create a prediction nomogram model based on selected factors of significance for the early identification of childhood asthma or wheeze. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to evaluate the discrimination ability of nomogram models. The predictive accuracy of nomogram models was determined using both calibration plots and C-index.
Models for predicting childhood asthma and wheeze were internally validated using bootstrap resampling with 100 replications (16, 17). For each step of resampling, models were refitted, and model discrimination and calibration were assessed on bootstrapped data and validated on original data. The difference in performance between the two datasets was calculated and averaged over the 100 replications to calculate optimism-adjusted C-statistics.
In total, our questionnaires were sent to the parents or guardians of 10,441 children, and the response rate was 98% (n = 10,230) within the scheduled time. Finally, 9,529 questionnaires were certified as eligible for inclusion after strict quality control. In addition, the ICC statistics for all study factors were lower than 0.05, indicating a lower likelihood of clustering across kindergartens.
The baseline characteristics of 9,529 children in the present study are shown in Table 1. The prevalence of childhood asthma and wheeze was 2.8% and 6.2%, respectively. After PSM, for asthma, the number of children with asthma and without was 265 and 752, separately. For wheeze, the number of children with asthma and without was 594 and 1,611, severally.
As shown in Table 2, significant differences between children with and without asthma were observed for probiotics supplement, allergic rhinitis, history of hay fever, eczema, initial age of using antibiotics, and family history of asthma (p ≤ 0.05). Univariate analyses suggested statistically significant associations of sex, BMI category, intake frequency of night meals, probiotics supplement, screen time, allergic rhinitis, history of hay fever, eczema, initial age of using antibiotics, and family history of asthma with childhood wheeze.
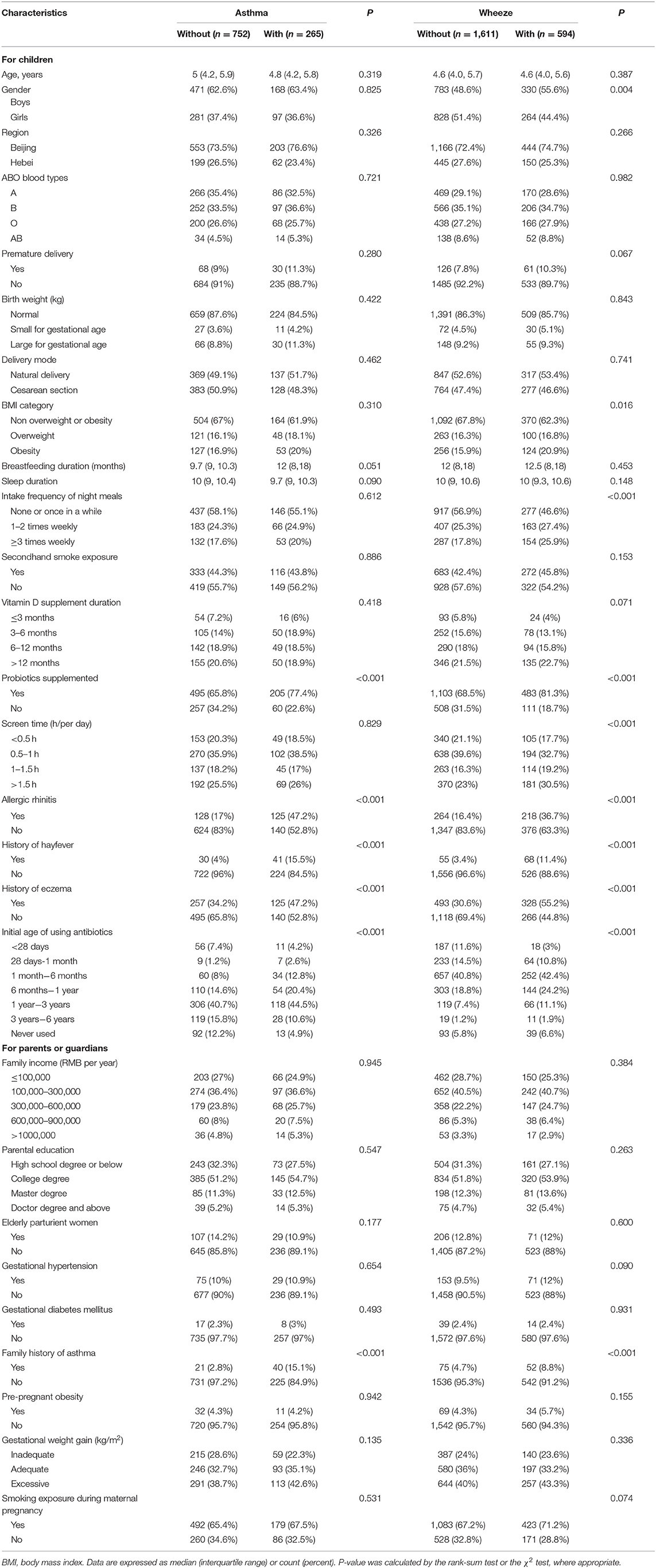
Table 2. Baseline characteristics of study participants after propensity score matching in this study.
Table 3 shows the estimated coefficients for candidate factors with childhood asthma and wheeze in the logistic LASSO regression analysis. For doctor-diagnosed childhood asthma, the λ-values ranged from 0.000328 to 0.1344, and the opted λ-value was 0.02475 in this study (Figure 1). Finally, the LASSO regression analysis revealed that seven factors contributed to the development of childhood asthma, including children with a history of allergic rhinitis (β = 1.35), hay fever (β = 0.88), eczema (β = 0.34), initial age of using antibiotics (β = 0.10), vitamin D supplement duration (β = −0.19), BMI category (β = 0.24), and family history of asthma (β = 1.81).
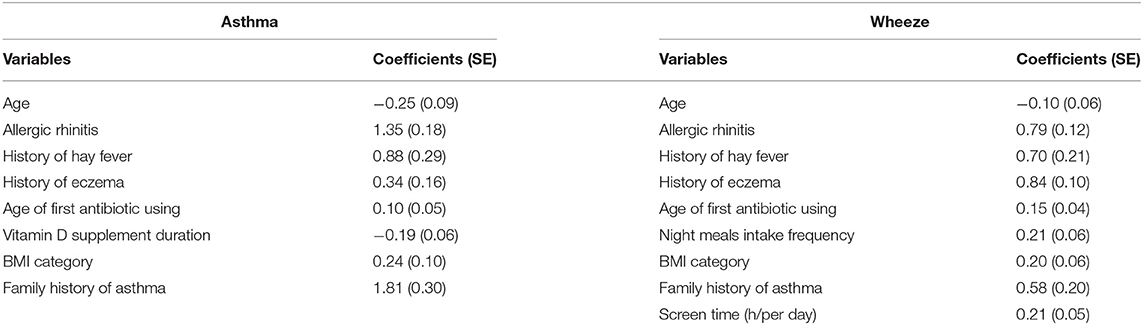
Table 3. The estimated coefficients for logistic least absolute shrinkage and selection operator (LASSO) regression between candidate risk factors with doctor-diagnostic childhood asthma and parent-reported wheeze.
For parent-reported wheeze, the λ-values ranged from 0.1002 to 0.000356, and the opted λ-value was 0.02689 (Figure 2). Eight factors were selected to be significantly associated with childhood wheeze, including children with a history of allergic rhinitis (β = 0.79), hay fever (β = 0.70), eczema (β = 0.84), initial age of using antibiotics (β = 0.15), intake frequency of night meals (β = 0.21), BMI category (β = 0.20), family history of asthma (β = 0.58), and screen time (h/day) (β = 0.21). The plots for LASSO regression coefficients over different values of the penalty parameter could be seen in Supplementary Figure 1.
For each selected factor associated with childhood asthma and wheeze, the power to detect significance was consistently over 90%, indicating the robustness of our findings.
As the relative contribution of each individual factor to the development of childhood asthma or wheeze might be small, it is of importance to consider the joint contribution of selected factors of significance. To achieve this goal, we constructed risk prediction nomogram models for childhood asthma and wheeze, respectively, by incorporating factors of significance selected by the logistic LASSO regression analyses (Figure 3). Furthermore, ROC analyses were performed to evaluate the discrimination ability of the nomogram model for childhood asthma, with decent results (AUC = 0.746, 95% CI: 0.7109–0.7803). Similarly, the nomogram model for the prediction of childhood wheeze (Figure 4) had higher prediction efficacy (AUC = 0.715, 95% CI: 0.6913–0.7388). The predictive accuracy of the nomograms was determined using calibration plots (Supplementary Figure 2A for asthma and Supplementary Figure 2B for wheeze), and the C-index were 0.728 and 0.707, respectively.
To better understand the utility of the nomogram model, an example was given here. Taking the risk prediction nomogram model for childhood asthma as an example: assuming a child aged 6 years old (14 points), with a history of allergic rhinitis (75 points), without a history of hay fever and eczema, using antibiotics within 1 month of birth (28 points), with vitamin D supplement duration over 1 year (0 points), with a family history of asthma (100 points), and being overweight (15 points), the probability of asthma for this child was estimated to be 75%.
The two constructed nomogram models were internally validated using bootstrap resampling with 100 replications. For asthma, the C-statistic was 0.728 in the derivation cohort, with good internal validation (bootstrap C-statistic of 0.736) and excellent calibration of predicted and observed risk. For wheeze, the C-statistic was 0.707 in the derivation cohort, and the bootstrap C-statistic was 0.708.
In the present study, via a large-scale cross-sectional survey, data from 9,529 Chinese preschool-aged children were collected to identify and characterize potential factors associated with childhood asthma and wheeze in China. By means of the logistic LASSO regression, which minimizes multicollinearity between variables, we separately identified seven and eight factors in a significant association with childhood asthma and wheeze. Importantly, the incorporation of these factors into nomogram models can robustly predict the risk of childhood asthma and wheeze, with decent accuracies. To the best of our knowledge, this is to date the first comprehensive research in China that has combined genetic, prenatal, perinatal, and postnatal factors to examine the possible association with asthma and wheeze among preschool-aged children.
Asthma is a consequence of complex gene environment interactions. It is widely recognized that family history plays a key role. As reported by a meta-analysis of 33 studies, maternal asthma increased offspring asthma risk to approximately three-fold greater than those free of maternal asthma (18). Consistently, in the present study, family history of asthma played a crucial part in predicting childhood asthma and wheeze. Meanwhile, the impact of the external environment on childhood asthma and wheeze is equally important. External factors, including maternal weight gain and obesity during pregnancy (19), childhood overweight and obesity (20), preterm delivery (21), breastfeeding duration (22), environmental tobacco smoke (23), and use of antibiotics (24), have been well-acknowledged to be associated with the risk of childhood asthma in agreement with the findings of the present survey. The implication of obesity in the development of childhood asthma is biologically plausible. There is evidence that obesity-related reduction in lung volume, changes in the hormone, dyslipidemia, and inflammatory mediators were identified as possible mechanisms behind the relationship between overweight and childhood asthma (25, 26). Meanwhile, we found premature exposure to antibiotics contributed to the susceptibility of childhood asthma. Early-life host–microbiome interactions resulted in the proper development of the immune system. Antibiotics affected microbial composition markedly, even transient perturbations during critical developmental periods may compromise both immune tolerance and inflammatory responses (27).
Several important findings specializing in the present study merit adequate discussion. First, we found the children with a history of allergic rhinitis, hay fever, and eczema were significantly associated with childhood asthma and wheeze. Recently, many studies have explored that the coexistence of eczema, rhinitis, and asthma in the same child was more common than expected, as 44% of the observed comorbidity at age 4 years and 50% at age 8 years (28), and several studies have shown that allergic rhinitis was a risk factor for subsequent wheezing onset (29), and uncontrolled asthma (30). In the CAPS cohort, children with atopic eczema were more likely than were those without atopic eczema to have a history of food allergies, allergic rhinitis, and current wheeze (31). Sensitization to allergens (atopy) is a key component of atopic diseases such as asthma and allergic rhinitis. However, some studies found that both IgE-mediated and non-IgE-mediated mechanisms were probably involved in their intimate connection (28).
Second, we found intake frequency of night meals among preschool-aged children was another noteworthy factor contributing to the development of childhood wheeze. In this survey, we found nearly 8.9% of children had night meals every day, and ~9.9% of children had night meals >3 times a week. It is well-known that eating too much food before bedtime can easily lead to esophageal reflux, especially for children with a more flaccid cardia and a less powerful esophageal sphincter. Gastrooesophageal reflux disease has been found in 25–80% of adults and children with asthma. Mechanisms include increased acid reflux during exacerbations with hyperinflation, microaspiration triggering neurogenic inflammation, and β2 agonists reducing lower esophageal sphincter pressure (32). More importantly, frequent late-night snacks make children overweight and obese and further increase the risk of developing wheeze and asthma. The same is suitable for long electronic screen time. Longer screen time is associated with a variety of health harms for children, with evidence strongest for adiposity, unhealthy diet (33). Altogether, reducing the frequency of late-night snacks and electronic screen time may be important lifestyle changes of interest to effectively reduce wheezing and asthma in children.
Several limitations should be acknowledged for the present study. First, this survey is cross-sectional in nature, which precludes further comments on the cause–effect relationship between selected factors of significance and childhood asthma or wheeze. Second, recall bias cannot be entirely excluded, as the majority of data were obtained via questionnaires. Third, diagnosis biases could not be avoided because the wheezing symptoms caused by a cold or other respiratory illness cannot be excluded.
Taken together, our findings indicated that the development of asthma and wheeze among preschool-aged children was probably determined by the joint contribution of multiple factors including inherited, nutritional, unhealthy lifestyles, and history of allergic disease. More attention to poor feeding practices and lifestyles of preschool-aged children and active prevention of allergic diseases is needed for the prevention of childhood asthma. Further validation of our findings in other independent groups is necessary.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Institutional Review Boards of the China-Japan Friendship Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
KW, ZZ, and WN contributed to the conceptualization of the study. XD, MY, SW, BP, and QW involved in the data collection. MY, XD, and SW participated in the investigation. XD helped in the data detection. WN and XD performed the statistical analysis and involved in writing. All authors read and approved the final manuscript prior to submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.742581/full#supplementary-material
1. National Heart and Institute. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. Bethesda, MD: National Institutes of Health (2002).
2. Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. (2012) 3:1–58.
3. Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. (2014) 18:1269–78. doi: 10.5588/ijtld.14.0170
4. Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Rep. (2008) 19:45–50.
5. Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. (2007) 62:758–66. doi: 10.1136/thx.2006.070169
6. Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. (2004) 59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x
7. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. (2009) 64:476–83. doi: 10.1136/thx.2008.106609
8. Van Aalderen WM. Childhood asthma: diagnosis and treatment. Scientifica. (2012) 2012:674204. doi: 10.6064/2012/674204
9. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. (2018) 391:350–400. doi: 10.1016/S0140-6736(17)30879-6
10. Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. (2016) 138:1608–18.e1612. doi: 10.1016/j.jaci.2016.09.028
11. Li H, Ji CY, Zong XN, Zhang YQ. [Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi. (2009) 47:493–8.
12. National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington: National Research Council (2010).
13. Donner A. A review of inference procedures for the intraclass correlation coefficient in the one-way random effects model. Int Stat Rev. (1986) 54:67–82. doi: 10.2307/1403259
14. Su YS, Gelman AE, Hill J, Yajima M. Multiple imputation with diagnostics (mi) in R: opening windows into the black box. J Stat Softw. (2011) 45:1–31. doi: 10.18637/jss.v045.i02
15. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B. (1996) 58:267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x
16. Kohavi R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. Montreal, QC: IJCAI (1995). p. 1137–45.
17. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans M, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. (2001) 54:774–81. doi: 10.1016/S0895-4356(01)00341-9
18. Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE. (2010) 5:e10134. doi: 10.1371/journal.pone.0010134
19. Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. (2014) 134:e535–46. doi: 10.1542/peds.2014-0439
20. Deng X, Ma J, Yuan Y, Zhang Z, Niu W. Association between overweight or obesity and the risk for childhood asthma and wheeze: an updated meta-analysis on 18 articles and 73 252 children. Pediatr Obes. (2019) 14:e12532. doi: 10.1111/ijpo.12532
21. Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. (2006) 118:823–30. doi: 10.1016/j.jaci.2006.06.043
22. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. (2015) 104:38–53. doi: 10.1111/apa.13132
23. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. (2012) 129:735–44. doi: 10.1542/peds.2011-2196
24. Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. (2011) 127:1125–38. doi: 10.1542/peds.2010-2092
25. Sood A, Shore SA. Adiponectin, leptin, and resistin in asthma: basic mechanisms through population studies. J Allergy. (2013) 2013:785835. doi: 10.1155/2013/785835
26. Nasiri Kalmarzi R, Ataee P, Mansori M, Moradi G, Ahmadi S, Kaviani Z, et al. Serum levels of adiponectin and leptin in asthmatic patients and its relation with asthma severity, lung function and BMI. Allergol Immunopathol. (2017) 45:258–64. doi: 10.1016/j.aller.2016.09.004
27. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378
28. Pinart M, Benet M, Annesi-Maesano I, Von Berg A, Berdel D, Carlsen KC, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. (2014) 2:131–40. doi: 10.1016/S2213-2600(13)70277-7
29. Rochat MK, Illi S, Ege MJ, Lau S, Keil T, Wahn U, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. (2010) 126:1170–5.e1172. doi: 10.1016/j.jaci.2010.09.008
30. Dixon AE, Kaminsky DA, Holbrook JT, Wise RA, Shade DM, Irvin CG. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest. (2006) 130:429–35. doi: 10.1378/chest.130.2.429
31. Kusel MM, Holt PG, De Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. (2005) 116:1067–72. doi: 10.1016/j.jaci.2005.06.038
32. Parsons JP, Mastronarde JG. Gastroesophageal reflux disease and asthma. Curr Opin Pulm Med. (2010) 16:60–3. doi: 10.1097/MCP.0b013e328332ca2f
Keywords: childhood asthma, wheeze, preschool-aged children, risk factors, LASSO regression, nomogram
Citation: Deng X, Yang M, Wang S, Wang Q, Pang B, Wang K, Zhang Z and Niu W (2021) Factors Associated With Childhood Asthma and Wheeze in Chinese Preschool-Aged Children. Front. Med. 8:742581. doi: 10.3389/fmed.2021.742581
Received: 16 July 2021; Accepted: 30 September 2021;
Published: 04 November 2021.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
Jian Gu, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2021 Deng, Yang, Wang, Wang, Pang, Wang, Zhang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kundi Wang, a3VuZGk1MjNAMTYzLmNvbQ==; Zhixin Zhang, emhhbmd6aGl4aW4wMzJAMTYzLmNvbQ==; Wenquan Niu, bml1d2VucXVhbl9zaGNuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.