- 1Department of Microbiology and Immunology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran
- 2Cellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 3Department of Laboratory Sciences, School of Paramedical Sciences, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 4Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran
Introduction: Novel coronavirus (COVID-19) and tuberculosis (TB) are the newest and one of the oldest global threats, respectively. In the COVID-19 era, due to the health system's focus on the COVID-19 epidemic, the national TB control program received less attention, leading to a worsening of the global TB epidemic. In this study, we will review the characteristics of TB patients coinfected with COVID-19.
Material and Methods: Using Scopus, PubMed/Medline, Embase, and Web of Science databases, a systematic search was performed. Case reports and case series on TB/COVID-19 coinfection published from January 1, 2019 to February 24, 2021 were collected. There were no limitations regarding publication language.
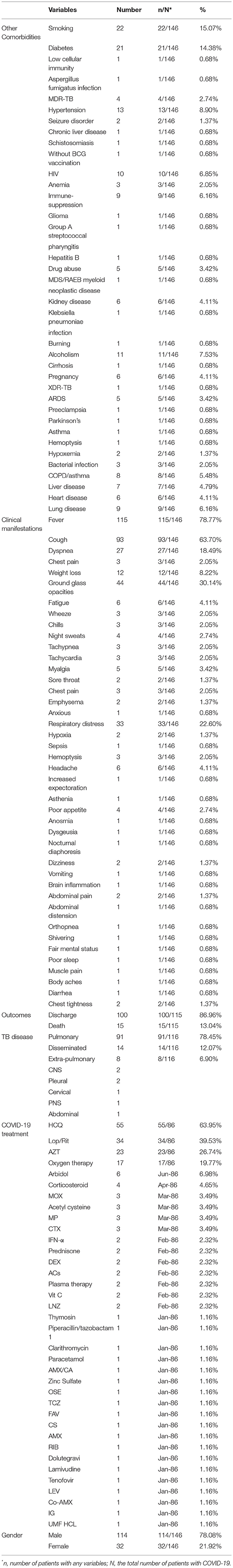
Results: Eleven case series and 20 case reports were identified from 18 countries, with the majority them being from India (N = 6) and China (N = 4). Overall, 146 patients (114 men and 32 women) coinfected with TB and COVID-19 enrolled. Smoking (15.1%), diabetes (14.4%), and hypertension (8.9%) were the most frequent comorbidities among these patients. The COVID-19 patients with TB mainly suffered fever (78.8%), cough (63.7%), and respiratory distress (22.6%). Hydroxychloroquine (64.0%) and lopinavir/ritonavir (39.5%) were the most common treatments for them. The mortality rate was 13.0% and the rate of discharged patients was 87.0%.
Conclusion: Global prevalence of COVID-19-related deaths is 6.6%. Our results showed that 13.0% of patients with TB/COVID-19 died. Thus, this study indicated that coinfection of TB and COVID-19 can increase the mortality. The respiratory symptoms of TB and COVID-19 are very similar, and this causes them to be misdiagnosed. In addition, TB is sometimes diagnosed later than COVID-19 and the severity of the disease worsens, especially in patients with underlying conditions. Therefore, patients with TB should be screened regularly in the COVID-19 era to prevent the spread of the TB/COVID-19 coinfection.
Introduction
The most recent problem in the world today is the spread of the 2019 novel coronavirus (2019-nCoV). The virus originated in China and is now affecting many countries around the world (1). COVID-19 is the name of the respiratory syndrome caused by 2019-nCoV (2). Various symptoms, including fever, breathlessness, and lung lesions are seen in infected persons (3). Interhuman transmission occurs in hospitals and medical staff and also among family members (4). Mycobacterium tuberculosis (M. tuberculosis) is the causative agent of tuberculosis (TB) (5, 6). Airborne transmission is the most common method in which M. tuberculosis spreads between healthy and TB-active infected persons (7). The prevalence of TB in 2019 is approximately 10 million people and decreases by only 1–2% every year (8). Contact with people coinfected with TB and HIV is one of the main risk factors to increase the prevalence of TB (9). Social factors such as poverty, living environment, population, and economic status affect the COVID-19 and TB incidence. Therefore, improving social life can be effective in controlling TB and COVID-19 (10). Bacterial coinfection is a common problem in COVID-19 patients (11). During the outbreak of COVID-19, many cases of TB/COVID-19 coinfection were reported (12–16). Some studies reported death in coinfected patients with TB and COVID-19 (16–18). A previous study has shown an association between the high incidence of COVID-19 in TB patients (19). Both COVID-19 and TB have respiratory symptoms (20). The presence of respiratory diseases in patients causes dysfunction of the respiratory system and lungs, making TB a risk factor for COVID-19 (19). Prioritizing financial and human resources to combat the COVID-19 epidemic and increasing the risk of SARS-CoV-2 in people with TB raises concerns about TB control. These may reverse the achievements of TB control and increase the prevalence of TB in the future (10, 21). In this systematic review, we reviewed the case reports and case series presenting the TB/COVID-19 coinfection to evaluate the various aspects such as symptoms, diagnosis, treatment, and mortality rate.
Materials and Methods
This systematic review was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (22).
Information Source and Search Strategies
We performed a systematic review using Web of Science, Embase, Scopus, and PubMed/Medline. Search criteria included case series and case reports articles published from January 1, 2019 to February 24, 2021. There were no limitations regarding the publication language, but for eligible non-English studies, we used Google Translate. The search terms for our review included Mycobacterium tuberculosis, tuberculosis, COVID-19, severe acute respiratory syndrome coronavirus 2, novel coronavirus, SARS-CoV-2, nCoV disease, SARS2, 2019-nCoV disease, coronavirus disease-19, coronavirus disease 2019, novel coronavirus 2019, Wuhan coronavirus, Wuhan seafood market pneumonia virus, and Wuhan pneumonia outbreak.
Study Selection
Studies included in this review met the following criteria: retrospective, descriptive, and prospective case series and case reports of COVID-19 in hospital settings. The studies presenting diagnostic methods, laboratory and clinical features, and treatment and its outcomes were also included. Reviews, publications without peer review processes, and papers describing experimental approaches were excluded. All potentially related articles followed two steps for eligibility. In the first step, two independent reviewers screened the titles and abstracts of the articles and eliminated duplicate papers using the EndNote X7 program (Thomson Reuters, New York, NY, USA). In the second step of evaluation, the same reviewers retrieved and reviewed the full text of those abstracts that met the inclusion criteria. Technical uncertainties and disagreements were resolved between the authors.
Data Extraction
The extracted data entailed first author's name, study country, year of publication, type of the study, number of co-infected patients, median age, gender, TB diagnostic method, TB treatment, TB disease, SARS-CoV-2 diagnosis method, clinical manifestations, comorbidities, and outcomes. Three authors independently extracted the data from the selected articles. The reviewed data was jointly reconciled, and disagreements resolved between authors.
Quality Assessment
The critical appraisal checklist for case reports and case series provided by the Joanna Briggs Institute (JBI) was used for the qualitative evaluation of the studies (23).
Results
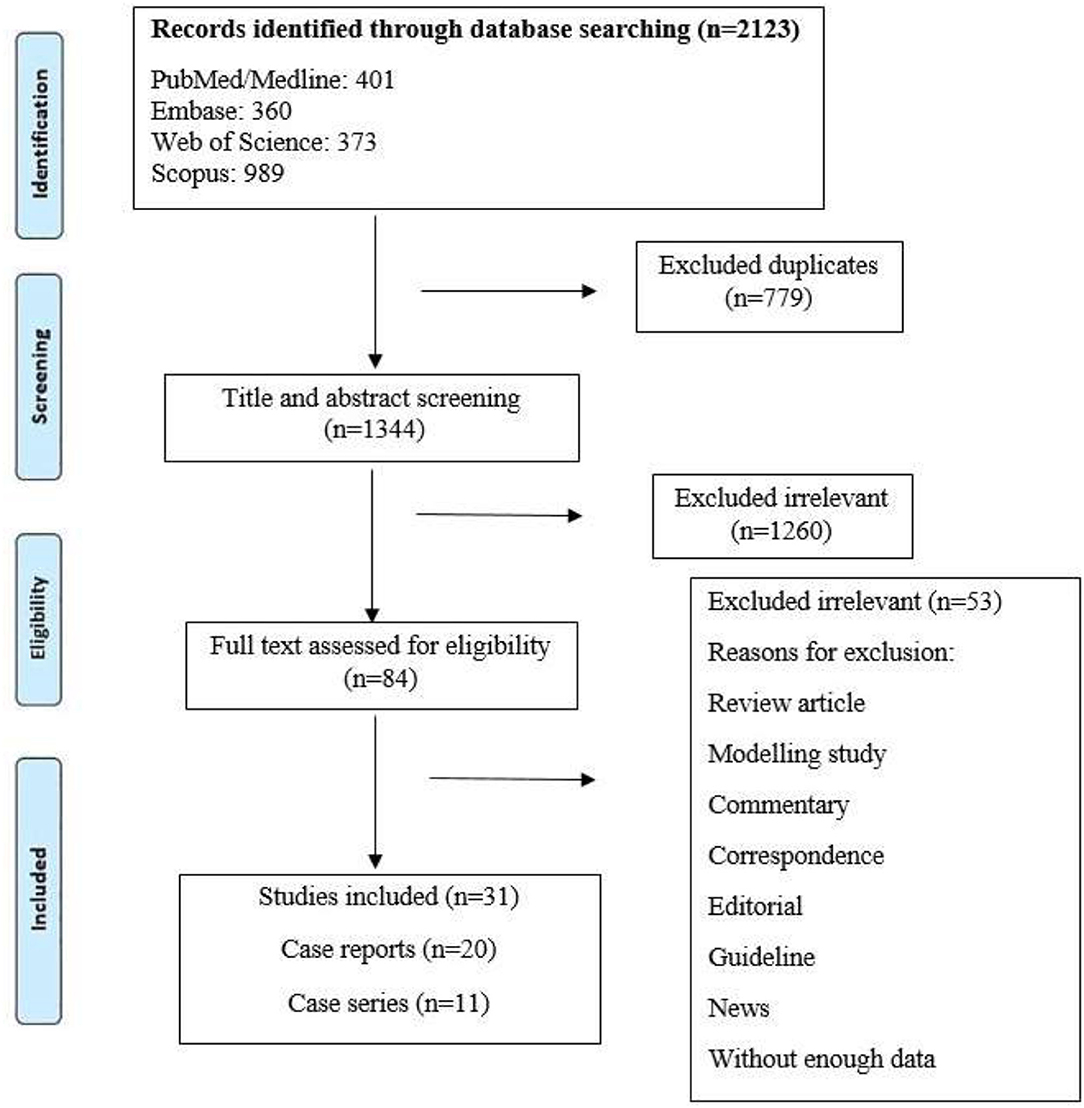
Our systematic search resulted in 2,123 potentially relevant articles, of which 1,260 were excluded after title and abstract evaluation (Figure 1). By applying the inclusion/exclusion criteria to the full-text documents, we found that 31 articles were eligible for inclusion in our systematic review. A total of 146 TB/COVID-19 coinfected patients were confirmed with different methods.
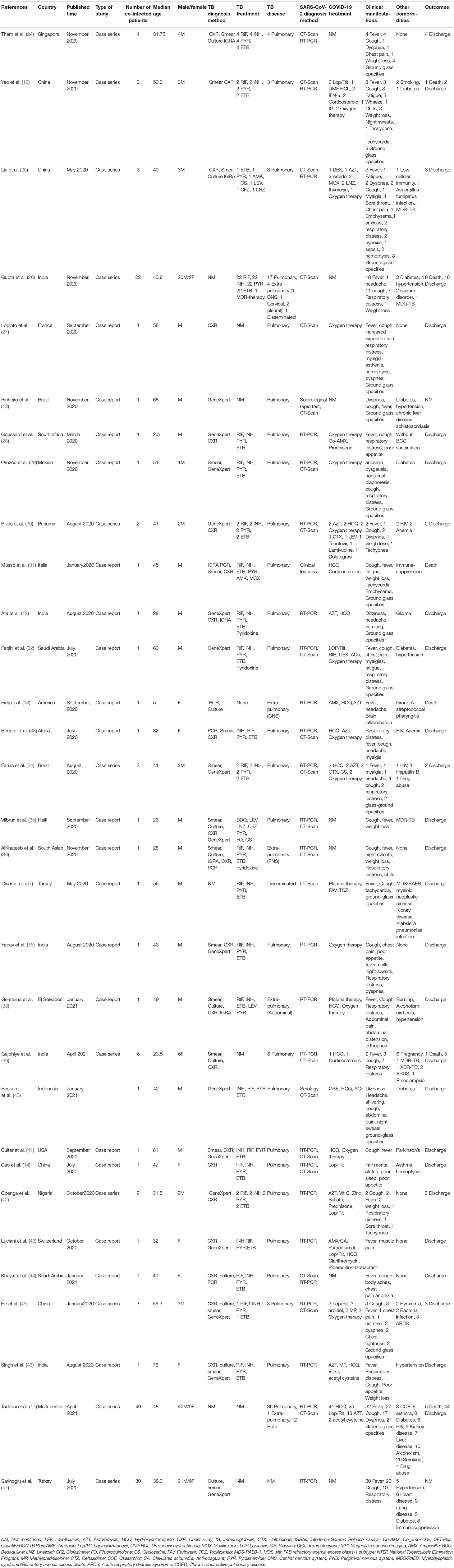
In the search, we detected 11 case series and 20 case reports from 18 countries, and the majority of studies were from India (N = 6) and China (N = 4). Characteristics of the eligible studies are listed in Table 1. We included case reports in our review because they reported new laboratory findings, new CT findings and clinical features, atypical manifestations, treatment outcomes, and some of these reports were the first in a specific country.
Regarding TB, the main diagnostic methods in eligible studies were chest X-ray (CXR), smear, and GeneXpert. CT scan served as a diagnostic tool in 21 (67.74%) COVID-19 articles. In addition to CT scan, RT-PCR for COVID-19 was present in 25 (80.64%) papers as inclusion criteria.
A summary of the case report and case series findings is shown in Table 2. Of the total patients, 114 (78.082%) and 32 (21.917%) were men and women, respectively. The most frequent comorbidities in the included studies were smoking (n = 22; 15.068%), diabetes (n = 21; 14.383%), hypertension (n = 13; 8.904%), and alcoholism (n = 11; 7.534%). CT images commonly showed ground-glass opacification (GGO) patterns (n = 44; 30.136%). The most common treatment drugs for COVID-19 were hydroxychloroquine (n = 55; 63.953%), lopinavir/ritonavir (n = 34; 39.534%), and azithromycin (n = 23; 26.744%).
Extrapulmonary TB (EPTB) and disseminated form were reported in eight and 14 (12.068 vs. 6.896%) TB cases. Concerning viral and bacterial infectious diseases, 10 (6.849%), one (0.684%), and five (3.424%) patients had HIV, HBV, and bacterial infection, respectively. In addition, MDR and XDR M. tuberculosis strains were identified in four and one patient, respectively. The most frequent clinical complications were fever (78.767%), cough (63.698%), GGO (30.136%), dyspnea (18.493%), and respiratory distress (22.602%).
The present systematic review reported that the mortality rate in patients with TB/COVID-19 coinfection was 13.04% and the rate of discharged patients was 86.96%.
Discussion
The novel coronavirus has declared as a public health emergency of international concern on January 31, 2020. The virus has had irreversible effects on the health and economy of the world and has placed limitations on society (48). TB is the most important cause of death through a single infectious agent (49, 50). Both M. tuberculosis and SARS-CoV-2 are transmitted through the respiratory tract and often affect the lungs. Thus, there are concerns about the effect of COVID-19 on the clinical course of TB and its outcome (49). In this study, we described the different dimensions of concurrent COVID-19 and TB infection.
The most common clinical manifestations in TB patients include coughing up mucus or blood, chronic cough (>2 months), chest pain, loss of appetite, weight loss, and chills (21). In contrast, the most clinical manifestations of COVID-19 include fever, cough, dyspnea, muscle ache, confusion, and headache, respectively (51). The findings of the present study introduce different clinical manifestations for coinfection with TB and COVID-19. The highest incidence of clinical manifestations belonged to fever, cough, ground-glass opacities, respiratory distress, dyspnea, and weight loss with 78.767, 63.698, 30.136, 22.602, 18.493, and 8.219%, respectively. COVID-19 has rapid clinical manifestation, but TB is time-consuming, and the onset of symptoms takes longer. This feature can help differentiate between the two diseases.
Our study has demonstrated that the most common comorbidities were smoking, diabetes, hypertension, alcohol consumption, and HIV. Stochino et al. have shown that old age, diabetes, and respiratory diseases are the main factors increasing the mortality in patients with TB/COVID-19 coinfection (49). In addition, it has proven that respiratory illnesses can cause lung dysfunction and reduce resistance to the virus. Thus, TB patients are more likely to develop severe COVID-19 (19).
Tuberculosis and COVID-19 have similarities in symptoms and chest radiographs that can lead to misdiagnosis (31). COVID-19 aggravates or obscures latent TB, leading to increased TB mortality (18). Motta et al. did not consider TB a leading cause of death and reported higher mortality rates in the elderly with coinfection than the others (52). In dead patients, COVID-19 infection was during the primary phases of the epidemic, indicating the importance of careful implementation of infection-control interventions for inpatients and hospital staff (52). Although the signs and symptoms of the two diseases are similar, the treatment and clinical course are different (31). Anti-TB therapies are not effective against COVID-19 because COVID-19 occurs during the use of these therapies (53). According to this study, most treatment of COVID-19 includes hydroxychloroquine (63.95%), lopinavir/ritonavir (39.53%), azithromycin (26.74%), and oxygen therapy (19.7%). Stochino et al. have observed no drug interactions between anti-TB drugs and hydroxychloroquine (49). On March 28, 2020, hydroxychloroquine received an emergency use permit for use in patients with COVID-19. However, clinical trials have shown that the drug has no particular advantage in treating COVID-19, so the FDA revoked the drug's emergency license (54).
Mortality rate was reported among patients coinfected with TB and COVID-19 (18, 31). A systematic review conducted by Nasiri et al. in 2020 reported that the pooled mortality rate from COVID-19 was 6.6% (55). The present review includes case reports and case series around the world. The mortality rate in patients with concurrent infection of TB and COVID-19 in this study is 13.04%, and the rate of discharging from the hospital is 86.96%. The difference in mortality rates in the two studies mentioned above indicates that the coinfection of TB and COVID-19 increases mortality.
Various viruses such as influenza, measles, and HIV cause the malfunction of macrophages that inhibit the growth of M. tuberculosis (56, 57). TypeI IFN signaling pathway plays a key role in increasing the growth of M. tuberculosis among patients coinfected with respiratory viruses and M. tuberculosis (58). Intracellular growth and response of macrophages to IFN-γ increases with increasing typeI interferon (59). Interferon-gamma is highly present in the early stages of COVID-19 (60). Therefore, COVID-19 is a risk factor for TB (31). COVID-19 weakens the immune system in various ways: (1) Destroying lymphocytes (2) destroying B cells (3) damage to T-cell (4) damage to NK cell (61). Lymphocyte depletion is one of the common causes of coinfection (62). The immune system, especially CD4 T cell-mediated immunity, is exposed to TB. In addition, both M. tuberculosis and SARS-CoV-2 can interfere with the immune system. It leads to immunodeficiency and lymphopenia with a decrease in CD4+ T cells. (31). Lymphopenia is a good indicator of the severity of the disease. Defected immune systems worsen in patients with COVID-19. On the other hand, TB causes respiratory failure in patients with COVID-19 due to interference with the cellular immune system and lymphopenia (31).
In 2020, Nigeria reported a decreasing number of diagnoses and possible cases of TB compared with 2019, which could be due to the prevalence of COVID-19 and restrictions on the assessment of diagnosis and uninterrupted treatment of TB (53). Lai et al. in Taiwan declared that the prevalence of TB decreased in the first 20 weeks of 2020 (63). Failure to diagnose TB promptly not only delays the treatment of patients but also infects others. On the other hand, late treatment also increases the severity of the disease and consequently increases the mortality rate. Since the global health system today focuses on COVID-19, the TB control program has received less attention. This has led to delays in the “End TB Strategy” (21). With proper care, the effect of COVID-19 on active TB can be controlled clinically (49).
SARS-CoV-2/bacteria coinfections can cause problems in the diagnosis, treatment, and prognosis of COVID-19 and increase the symptoms of the disease and mortality rate (11). According to the results of a systematic review by Gao et al., the prevalence of TB among COVID-19 patients was 0.37 to 4.47% (19). To prevent coinfection of TB and COVID-19 during the epidemic, physicians should be aware of the following: (1) Pneumonia associated with COVID-19 (2) Methods of distinguishing TB and COVID-19 from each other (3) The differences between TB and COVID-19 (31).
The COVID-19 epidemic has led to a decline in immunization services and increase in deaths from vaccine-preventable diseases (64). Among the vaccines proposed against COVID-19, the BCG vaccine has found a special place to prevent COVID-19 in the scientific community. In addition, the BCG has nonspecific effects on the immune system that lead to protective immunity from other infections. The BCG vaccine may reduce viremia after exposure to SARS-CoV-2 and decrease the severity of COVID-19 (65). People with active and latent TB should avoid COVID-19. Therefore, appropriate vaccination should be provided for them (21).
There are several limitations to this review. First, only case report/case series studies were included in this review, and the observational studies were excluded because of their absence or insufficient data. Second, case reports and case series are more likely to be biased than other studies. Third, case studies and case series are descriptive and describe the patient's signs and symptoms. The prevalence and percentage of coinfection in them have not been studied. For this reason, it was not possible to perform metaanalysis calculations in this review. Therefore, the prevalence of TB among COVID-19 patients and vice versa has not been calculated. Fourth, one of the problems with the SARS-CoV-2 virus is the existence of new variants (66). In recent months, the appearance of a new variant of this virus called the SARS-CoV-2 Delta variant (B.1.617.2) has spread in different parts of the world. This variant was first found in India and, its main feature is its high ability to transfer and escape from the immune system (67). One of the challenges ahead in curbing the spread of M. tuberculosis during the COVID-19 epidemic is the new variants of the virus. However, the extent to which new variants may play a role in the prevalence of coinfection has not yet been determined and requires further and more extensive studies.
Conclusions
There have been many reported cases of viral, fungal, and bacterial infections associated with COVID-19. In this study, we studied the association between TB and COVID-19. We discussed clinical characteristics, diagnosis, treatment, and mortality rate of TB/COVID-19 coinfectetd patients. Patients with TB are more likely to get COVID-19 infection. Testing TB patients for COVID-19 and vice versa is a controversial topic in the scientific community. COVID-19 and TB, although slightly different in how they are transmitted, have similar clinical features and manifestations such as fever, shortness of breath, and cough. However, these symptoms are also slightly different from each other. A noteworthy point in distinguishing these two diseases from each other is the time of onset of clinical symptoms, which is rapid in patients with COVID-19 but time-consuming in people with TB. Since the symptoms of both the diseases are similar, it is difficult to diagnose them, and sometimes the diagnosis of TB occurs later, which causes the progression and severity of the disease. Both diseases have similar risk factors, such as old age, diabetes, immunodeficiency, HIV, and COPD. Several reasons show that TB can facilitate COVID-19 establishment. One of these factors is related to the damage caused by TB in the lungs that predispose a person. The second reason is the immune system of a patient with TB that can increase the susceptibility to COVID-19. Finally, a regular program is recommended to detect TB during the outbreak of COVID-19 and follow it up continuously to prevent the occurrence of these two diseases simultaneously.
Author Contributions
MH and SK designed the study and revised the manuscript. MH, MK, AN, NM, PM, FT, and SG performed the search, acquisition of data, and wrote the first draft of the article. MH and NM analyzed and interpreted the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med (Lausanne). (2020) 7:231. doi: 10.3389/fmed.2020.00231
3. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. (2020) 63:364–74. doi: 10.1007/s11427-020-1643-8
4. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
5. Gagneux S. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. (2018) 16:202–13. doi: 10.1038/nrmicro.2018.8
6. Heidary M, Bostanabad SZ, Amini SM, Jafari A, Nobar MG, Ghodousi A, et al. The anti-mycobacterial activity of Ag, ZnO, and Ag-ZnO nanoparticles against MDR-and XDR-Mycobacterium tuberculosis. Infect Drug Resist. (2019) 12:3425. doi: 10.2147/IDR.S221408
7. Kanabalan RD, Lee LJ, Lee TY, Chong PP, Hassan L, Ismail R, et al. Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiol Res. (2021) 246:126674. doi: 10.1016/j.micres.2020.126674
8. Cords O, Martinez L, Warren JL, O'Marr JM, Walter KS, Cohen T, et al. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health. (2021) 6:e300–e8. doi: 10.1016/S2468-2667(21)00025-6
9. Heidary M, Nasiri MJ. Why has HIV/AIDS prevalence increased in Iran? Clin Infect Dis. (2016) 63:846. doi: 10.1093/cid/ciw361
10. Duarte R, Aguiar A, Pinto M, Furtado I, Tiberi S, Lonnroth K, et al. Different disease, same challenges: social determinants of tuberculosis and COVID-19. Pulmonology. (2021) 27:338–44. doi: 10.1016/j.pulmoe.2021.02.002
11. Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. (2020) 104:7777–85. doi: 10.1007/s00253-020-10814-6
12. Ata F, Yousaf Q, Parambil JV, Parengal J, Mohamedali MG, Yousaf Z, et al. 28-Year-Old Man from India with SARS-Cov-2 and Pulmonary Tuberculosis Co-Infection with Central Nervous System Involvement. Am J Case Rep. (2020) 21:e926034–1. doi: 10.12659/AJCR.926034
13. Pinheiro DO, Pessoa MSL, Lima CFC, Holanda JLB. Tuberculosis and coronavirus disease 2019 coinfection. Rev Soc Brasileira Med Tropical. (2020) 53. doi: 10.1590/0037-8682-0671-2020
14. Cao B, Wei M, Du Y, Xiao K, Li Q, Lu W, et al. Coronavirus disease 2019 with comorbid pulmonary tuberculosis: a case report. Iranian Red Cresc Med J. (2020) 22. doi: 10.32592/ircmj.2020.22.10.196
15. Yadav S, Rawal G. The case of pulmonary tuberculosis with COVID-19 in an Indian male-a first of its type case ever reported from South Asia. Pan African Med J. (2020) 36. doi: 10.11604/pamj.2020.36.374.24260
16. Yao Z, Chen J, Wang Q, Liu W, Zhang Q, Nan J, et al. Three patients with COVID-19 and pulmonary tuberculosis, Wuhan, China, January–February 2020. Emerg Infect Dis. (2020) 26:2754. doi: 10.3201/eid2611.201536
17. Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar J-W, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. (2020) 56. doi: 10.1183/13993003.02328-2020
18. Freij BJ, Gebara BM, Tariq R, Wang A-M, Gibson J, El-Wiher N, et al. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. (2020) 20:1–7. doi: 10.1186/s12887-020-02308-1
19. Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol. (2021) 93:194–6. doi: 10.1002/jmv.26311
20. Echeverria G, Espinoza W, de Waard JH. How TB and COVID-19 compare: an opportunity to integrate both control programmes. Int J Tuberc Lung Dis. (2020) 24:971–4. doi: 10.5588/ijtld.20.0417
21. Visca D, Ong CWM, Tiberi S, Centis R, D'Ambrosio L, Chen B, et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology. (2021) 27:151–65. doi: 10.1016/j.pulmoe.2020.12.012
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
23. Institute J. The joanna briggs institute critical appraisal tools for use in JBI systematic reviews checklist for analytical cross sectional studies. Joanna Briggs Institute North Adelaide, Australia. (2017).
24. Tham SM, Lim WY, Lee CK, Loh J, Premkumar A, Yan B, et al. Four patients with COVID-19 and tuberculosis, Singapore, April–may 2020. Emerg Infect Dis. (2020) 26:2763–5. doi: 10.3201/eid2611.202752
25. Liu C, Yu Y, Fleming J, Wang T, Shen S, Wang Y, et al. Severe COVID-19 cases with a history of active or latent tuberculosis. Int J Tuberc Lung Dis. (2020) 24:747–9. doi: 10.5588/ijtld.20.0163
26. Gupta N, Ish P, Gupta A, Malhotra N, Caminero JA, Singla R, et al. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Eur Respir J. (2020) 56:2003408. doi: 10.1183/13993003.03408-2020
27. Lopinto J, Teulier M, Milon A, Voiriot G, Fartoukh M. Severe hemoptysis in post-tuberculosis bronchiectasis precipitated by SARS-CoV-2 infection. BMC Pulm Med. (2020) 20:1–3. doi: 10.1186/s12890-020-01285-6
28. Goussard P, Solomons RS, Andronikou S, Mfingwana L, Verhagen LM, Rabie H. COVID-19 in a child with tuberculous airway compression. Pediatr Pulmonol. (2020) 55:2201–03. doi: 10.1002/ppul.24927
29. Orozco JAM, Tinajero ÁS, Vargas EB, Cueva AID, Escobar HR, Alcocer EV, et al. COVID-19 and tuberculosis coinfection in a 51-year-old taxi driver in Mexico city. Am J Case Rep. (2020) 21:e927628–1. doi: 10.12659/AJCR.927628
30. Rivas N, Espinoza M, Loban A, Luque O, Jurado J, Henry-Hurtado N, et al. Case Report: COVID-19 Recovery from Triple Infection with Mycobacterium tuberculosis, HIV, and SARS-CoV-2. Am J Trop Med Hyg. (2020) 103:1597–9. doi: 10.4269/ajtmh.20-0756
31. Musso M, Di Gennaro F, Gualano G, Mosti S, Cerva C, Fard SN, et al. Concurrent cavitary pulmonary tuberculosisand COVID-19 pneumonia with in vitro immune cell anergy: a case report. (2020) 49:1061–4. doi: 10.21203/rs.3.rs-54297/v1
32. Faqihi F, Alharthy A, Noor A, Balshi A, Balhamar A, Karakitsos D. COVID-19 in a patient with active tuberculosis: A rare case-report. Respir Med Case Rep. (2020) 31:101146. doi: 10.1016/j.rmcr.2020.101146
33. Bouaré F, Laghmari M, Etouche FN, Arjdal B, Saidi I, Hajhouji F, et al. Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin. Pan African Med J. (2020) 35:110. doi: 10.11604/pamj.supp.2020.35.2.24952
34. Farias LABG, Moreira ALG, Corrêa EA, de Oliveira Lima CAL, Lopes IMP, de Holanda PEL, et al. Case report: Coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: Report of two cases. Am J Trop Med Hyg. (2020) 103:1593–6. doi: 10.4269/ajtmh.20-0737
35. Vilbrun SC, Mathurin L, Pape JW, Fitzgerald D, Walsh KF. Case Report: Multidrug-Resistant Tuberculosis and COVID-19 Coinfection in Port-au-Prince, Haiti. Am J Trop Med Hyg. (2020) 103:1986–8. doi: 10.4269/ajtmh.20-0851
36. AlKhateeb MH, Aziz A, Eltahir M, Elzouki A. Bilateral foot-drop secondary to axonal neuropathy in a tuberculosis patient with co-infection of COVID-19: a case report. Cureus. (2020) 12:e11734. doi: 10.7759/cureus.11734
37. Çinar OE, Sayinalp B, Karakulak EA, Karataş AA, Velet M, Inkaya AÇ, et al. Convalescent (immune) plasma treatment in a myelodysplastic covid-19 patient with disseminated tuberculosis. Transfusion Apheresis Sci. (2020) 59:102821. doi: 10.1016/j.transci.2020.102821
38. Gerstein S, Khatri A, Roth N, Wallach F. Coronavirus disease and extra-pulmonary tuberculosis co-infection–A case report and review of literature. J Clin Tubercul Mycobacterial Dis. (2019) 2021:100213. doi: 10.1016/j.jctube.2021.100213
39. Gajbhiye RK, Mahajan NN, Kamath N, Bahirat S, Patokar G, Bhurke AV, et al. Clinical presentations, pregnancy complications, and maternal outcomes in pregnant women with COVID-19 and tuberculosis: a retrospective cohort study. Int J Gynecol Obstet. (2021) 153:176–9. doi: 10.1002/ijgo.13588
40. Baskara MA, Makrufardi F, Dinisari A. COVID-19 and active primary tuberculosis in a low-resource setting: a case report. Ann Med Surg. (2021) 62:80–3. doi: 10.1016/j.amsu.2020.12.052
41. Cutler T, Scales D, Levine W, Schluger N, O'Donnell MA. Novel viral epidemic collides with an ancient scourge: COVID-19 associated with tuberculosis. Am J Respir Crit Care Med. (2020) 202:748–9. doi: 10.1164/rccm.202003-0828IM
42. Gbenga TA Oloyede T Ibrahim OR Sanda A Suleiman BM. Pulmonary tuberculosis in coronavirus disease-19 patients: a report of two cases from Nigeria. Open Access Macedonian. J Med Sci. (2020) 8:272–5. doi: 10.3889/oamjms.2020.5298
43. Luciani M, Bentivegna E, Spuntarelli V, Lamberti PA, Guerritore L, Chiappino D, et al. Coinfection of tuberculosis pneumonia and COVID-19 in a patient vaccinated with Bacille Calmette-Guerin (BCG): case report. SN Comprehensive Clin Med. (2020) 2:2419–22. doi: 10.1007/s42399-020-00601-9
44. Khayat M, Fan H, Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: a case report. Respir Med Case Rep. (2021) 32:101344. doi: 10.1016/j.rmcr.2021.101344
45. He G, Wu J, Shi J, Gamber M, Jiang X, Sun W, et al. COVID-19 in Tuberculosis patients: a report of three cases. (2020) 92:1802–6. doi: 10.1002/jmv.25943
46. Singh A, Gupta A, Das K. Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis coinfection: double trouble. Indian J Med Special. (2020) 11:164. doi: 10.4103/INJMS.INJMS_72_20
47. Sarinoglu RC, Sili U, Eryuksel E, Yildizeli SO, Cimsit C, Yagci AK. Tuberculosis and COVID-19: an overlapping situation during pandemic. J Infect Develop Country. (2020) 14:721–5. doi: 10.3855/jidc.13152
48. Kenny G, Mallon PW. COVID19- clinical presentation and therapeutic considerations. Biochem Biophys Res Commun. (2021) 538:125–31. doi: 10.1016/j.bbrc.2020.11.021
49. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. (2020) 56:2001708. doi: 10.1183/13993003.01708-2020
50. Khoshnood S, Goudarzi M, Taki E, Darbandi A, Kouhsari E, Heidary M, et al. Bedaquiline: current status and future perspectives. J Global Antimicrobial Resistance. (2021) 25:48–59. doi: 10.1016/j.jgar.2021.02.017
51. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
52. Motta I, Centis R, D'Ambrosio L, Garcia-Garcia JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. (2020) 26:233–40. doi: 10.1016/j.pulmoe.2020.05.002
53. Adewole OO. Impact of COVID-19 on TB care: experiences of a treatment centre in Nigeria. Int J Tuberc Lung Dis. (2020) 24:981–2. doi: 10.5588/ijtld.20.0418
54. Saghir SA, AlGabri NA, Alagawany MM, Attia YA, Alyileili SR, Elnesr SS, et al. Chloroquine and hydroxychloroquine for the prevention and treatment of COVID-19: a fiction, hope or hype? An updated review. Therapeutics Clin Risk Manage. (2021) 17:371. doi: 10.2147/TCRM.S301817
55. Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med (Lausanne). (2020) 7:459. doi: 10.3389/fmed.2020.00459
56. Whittaker E, López-Varela E, Broderick C, Seddon JA. Examining the complex relationship between tuberculosis and other infectious diseases in children. Frontiers in pediatrics. (2019) 7:233. doi: 10.3389/fped.2019.00233
57. Pathak S, Wentzel-Larsen T, Åsjö B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect Immun. (2010) 78:4022–32. doi: 10.1128/IAI.00106-10
58. Redford PS, Mayer-Barber KD, McNab FW, Stavropoulos E, Wack A, Sher A, et al. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J Infect Dis. (2014) 209:270–4. doi: 10.1093/infdis/jit424
59. Scriba T, Coussens A, Fletcher H. Human immunology of tuberculosis. Microbiol Spectr. (2017) 5:5–1. doi: 10.1128/microbiolspec.TBTB2-0016-2016
60. Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Ossoski RGC, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. (2020) 289:198171. doi: 10.1016/j.virusres.2020.198171
61. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis. (2020) 96:148–50. doi: 10.1016/j.ijid.2020.04.060
62. Luo Y, Xie Y, Zhang W, Lin Q, Tang G, Wu S, et al. Combination of lymphocyte number and function in evaluating host immunity. Aging (Albany NY). (2019) 11:12685. doi: 10.18632/aging.102595
63. Lai CC Yu WL. The COVID-19 pandemic and tuberculosis in Taiwan. J Infect. (2020) 81:e159–e61. doi: 10.1016/j.jinf.2020.06.014
64. Jain VK, Iyengar KP, Samy DA, Vaishya R. Tuberculosis in the era of COVID-19 in India. Diabetes Metab Syndr. (2020) 14:1439–43. doi: 10.1016/j.dsx.2020.07.034
65. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. (2020) 395:1545–6. doi: 10.1016/S0140-6736(20)31025-4
66. Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. New England J Med. (2021) 384:1866–8. doi: 10.1056/NEJMc2100362
Keywords: tuberculosis, COVID-19, review, coinfection, TB
Citation: Koupaei M, Naimi A, Moafi N, Mohammadi P, Tabatabaei FS, Ghazizadeh S, Heidary M and Khoshnood S (2021) Clinical Characteristics, Diagnosis, Treatment, and Mortality Rate of TB/COVID-19 Coinfectetd Patients: A Systematic Review. Front. Med. 8:740593. doi: 10.3389/fmed.2021.740593
Received: 13 July 2021; Accepted: 20 October 2021;
Published: 01 December 2021.
Edited by:
Malaisamy Muniyandi, National Institute of Research in Tuberculosis (ICMR), IndiaReviewed by:
Charles J. Vukotich Jr., University of Pittsburgh, United StatesRoland Ruscher, James Cook University, Australia
Copyright © 2021 Koupaei, Naimi, Moafi, Mohammadi, Tabatabaei, Ghazizadeh, Heidary and Khoshnood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsen Heidary, bW9oc2VuaGVpZGFyeTQwQGdtYWlsLmNvbQ==; Saeed Khoshnood, c2FlZWQua2hvc2hub29kMjJAZ21haWwuY29t
†These authors have contributed equally to this work and share second authorship
‡These authors have contributed equally to this work and share third authorship
 Maryam Koupaei1
Maryam Koupaei1 Mohsen Heidary
Mohsen Heidary