- Division of Chest Medicine, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan
Introduction: The coronavirus disease 2019 (COVID-19) lockdown strategies were associated with a significant decrease in the common respiratory viral diseases and decreased the need for hospitalization among children in the COVID-19 outbreak. However, the trend of non-COVID-19 pneumonia in adult people remains uncertain. Our aim is to assess the impact of the COVID-19 pandemic on the incidence of the non-COVID-19 pneumonia in adult people and understand whether the substantial decrease in pneumonia cases is the same as the decline in the incidence of respiratory viral disease activity.
Methods: We conducted a retrospective analysis of adult patients presenting with pneumonia from January 2019 to December 2020. Details on all the demographics of the patient of pneumonia, hospital course details, prior admission history within 3 months, respiratory culture, and antibiotics sensitivity test were also obtained.
Results: The number of adult patients with community-acquired pneumonia in 2020 was lower than that in 2019, which decreased by 74 patients in 2020. The decreasing number of patients with community-acquired pneumonia between 2019 and 2020 was from −13.9% in January to March 2020 to −39.7% in October to December 2020. The decreasing number of patients with community-acquired pneumonia between 2019 and 2020 was from −14.8% in the youngest cohort to −28.7% in those aged ≥85 years. The number of reduced patients with community-acquired pneumonia is greater in late seasons and older age, respectively. The number of adult patients with hospital-acquired pneumonia in 2020 was lower than that in 2019, which decreased by 23 patients in 2020. The decreasing number of patients with hospital-acquired pneumonia between 2019 and 2020 was from −20.0% in January to March 2020 to −52.4% in October to December 2020. The decreasing number of patients with hospital-acquired pneumonia between 2019 and 2020 was from 0% in the youngest cohort to −45.6% in those aged ≥ 85 years. The number of reduced patients with hospital-acquired pneumonia is greater in late seasons and older age, respectively.
Conclusion: Interventions applied to control the COVID-19 pandemic were effective not only in substantial changes in the seasonal influenza activity, but also in decreasing adult pneumonia cases.
Introduction
The coronavirus disease 2019 (COVID-19) outbreak first occurred in December 2019 in Wuhan, Hubei Province, China. Within 3 months, the COVID-19 had quickly spread worldwide. The incidence of COVID-19 then substantially increased and it was declared a pandemic by the WHO on March 11, 2020 (1). The governments of various countries worldwide have widely promoted several measures to prevent the COVID-19 outbreak such as education on hand hygiene and cough etiquette, staying at home if respiratory symptoms persist, using a mask in public places, social distancing, travel restrictions, and closure of schools. The government of Taiwan undertook several rapid measures since January 2020 to prevent the COVID-19 outbreak such as border control, case identification, quarantine of suspicious cases, and travel restriction. As of March 14, 2020, people returning directly to Taiwan from other countries had to stay under quarantine at home for 14 days. Taiwanese citizens were advised to avoid all the non-essential travels. All the healthcare workers were prohibited from going abroad. The Taiwan Centers for Disease Control announced social distancing measures to encourage the general public to maintain social etiquette and to cancel or postpone conferences and social gatherings. The rules specified separate social distancing standards for restaurants, school campuses, offices, mass transport, supermarkets, and special institutions such as long-term care facilities and prisons. The use of face masks during all the instances of close contact was widely recommended throughout the country (2). The following strategies were implemented to prevent the COVID-19 outbreak in our hospital: (1) Only three entrance doors to the hospital buildings were opened including the entrance door of the emergency room (ER), (2) Only one car entrance was allowed during daytime, (3) Body temperature of individuals entering the premises was checked by using an IR thermal camera, (4) We checked the National Health Insurance identification card data of all the visitors who entered our hospital when the confirmed COVID-19 cases were increasing in the community, (5) All the people had to wear surgical masks and were sprayed with 75% alcohol for hand hygiene when they entered the hospital, (6) All the febrile hospital visitors were transferred to the ER for further evaluation and management, (7) All the persons with respiratory symptoms had a travel history of epidemic countries or closely contact with patients with the confirmed COVID-19 diagnosis and were transferred to the ER for further evaluation and management, and (8) The number and time of inpatient visitor caregivers and visitors were restricted. Moreover, the implementation period of government-sponsored influenza vaccination was from October 2019 in Taiwan. The total number of outpatient and ER visits for influenza-like illness decreased since February 2020, which continued to decrease each week. Seasonal influenza activity continued to reduce to its lowest point in April 2020 (Figure 1) (https://www.cdc.gov.tw/En/File/Get/a-VtY5lEvxa8VSLx9KN-7A). The COVID-19 lockdown strategies were associated with a significant decrease in infectious diseases disseminated through the airborne or fecal–oral transmissions (3). In Korea, efforts to stimulate the national response not only decreased the spread of the COVID-19, but also substantially decreased seasonal influenza activity (4). The incidence of hospitalization of young children owing to respiratory syncytial virus and acute respiratory infections dramatically decreased after implementing social distancing in the Alaskan population (5). Social distancing and other lockdown strategies effectively impeded the spread of common respiratory viral diseases and decreased the need for hospitalization among children in Finland (6). Winter surveillance data from Australia and Japan also demonstrated a decrease in seasonal influenza activity compared with previous seasons (7, 8). Thus, a decline in the incidence of respiratory viral diseases was reported following the COVID-19 outbreak. However, the trend of the non-COVID-19 pneumonia in adults remains uncertain. Therefore, this study aimed to assess the impact of the COVID-19 pandemic on the incidence of pneumonia in adult people and understand whether the substantial decrease in pneumonia cases correlated with the decline in the incidence of respiratory viral disease activity.
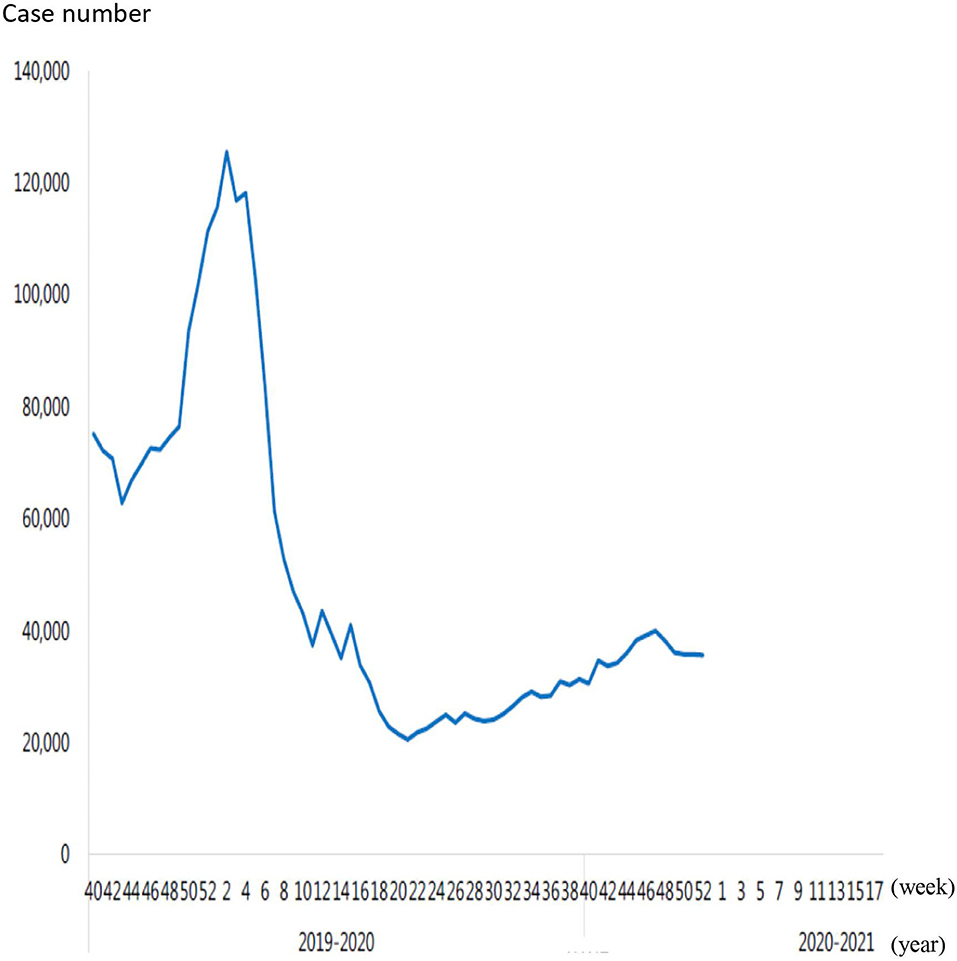
Figure 1. The total number of outpatient and emergency visits for influenza-like illness in 2019, 2020 and 2021.
Methods
We conducted a retrospective analysis of all the patients (age > 18 years) presenting with pulmonary infection who were admitted to the Dalin Tzu Chi Hospital between January 2019 and December 2020. Patients were diagnosed with pulmonary infection if their clinical symptoms and signs indicated infection and their chest radiology displayed a new acute pulmonary infiltration. Community-acquired pneumonia (CAP) was defined as a pulmonary parenchymal infection in the patients who acquire the condition in the community. Hospital-acquired pneumonia (HAP) was defined as a pulmonary parenchymal infection in the patients who acquire the condition at least 48 h after the hospital admission or within 14 days of hospital discharge (9). All the patients with HAP in this study were diagnosed with pulmonary parenchymal infection within 14 days of hospital discharge.
Data were obtained from the clinical information system of hospital, microbiology laboratory report system, and medical chart review. Details on all the CAP and HAP (excluding pulmonary tuberculosis and pulmonary fungal infection), demographics of the patient, hospital course details, prior admission history within 3 months, respiratory culture, and antibiotics sensitivity test were also obtained.
Respiratory secretions were collected for culture. Bacterial cultures of respiratory secretions and examination for antibiotic susceptibility were performed by using the VITEK 2 system with the ASTGN87 cards (bioMérieux, Marcy-l'Étoile, France, UK) based on the Clinical and Laboratory Standards Institute interpretive criteria M100-S25. Respiratory secretions were cultured on chocolate agar, blood agar, eosin methylene blue agar, and colistin–nalidixic acid agar plates immediately after collection. Gram staining and microscopy were performed. Identification cards were inoculated with microorganism suspensions by using an integrated vacuum apparatus. A test tube containing the microorganism suspension was placed into a cassette. One identification card for each Gram-negative card, Gram-positive card, and VITEK 2 AST-N322 (for susceptibility testing against specified antimicrobial agents) was placed in the neighboring slot along with the transfer tube and corresponding suspension tube. Calculations were performed by using raw data and compared with thresholds to determine reactions for each test.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median (range), whereas categorical variables were expressed as frequencies and percentages. The trend in change of pneumonia cases was analyzed by linear-by-linear association by using the chi-squared test. All the statistical analyses were conducted by using the Statistical Package for the Social Sciences (SPSS) for Windows (version 17.0, SPSS Incorporation, Chicago, Illinois, USA). p < 0.05 was considered as statistically significant.
Results
A total of 704 patients with pulmonary infection were admitted to the Dalin Tzu Chi Hospital during the study period including 574 patients with CAP, 121 patients with HAP, 7 patients with pulmonary tuberculosis, and 2 patients with pulmonary fungal infection.
Community-Acquired Pneumonia
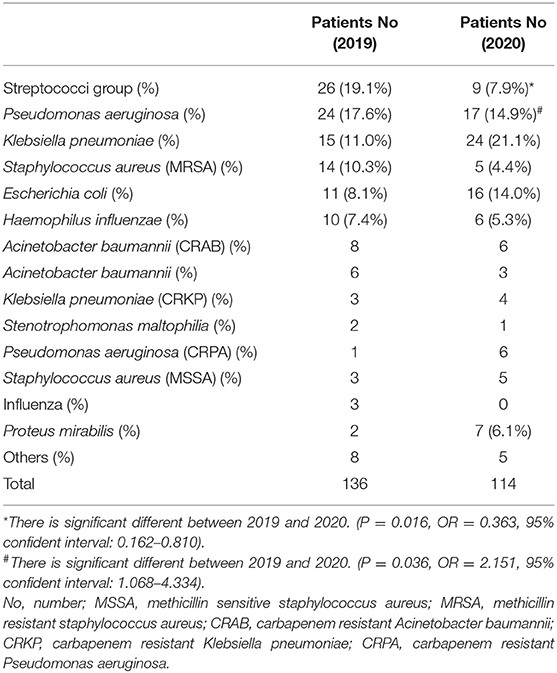
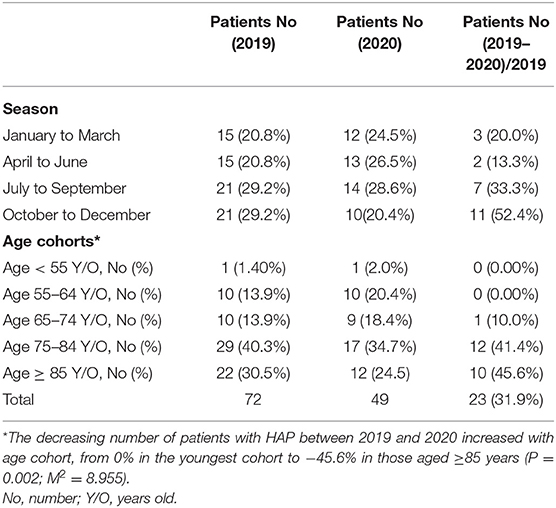
The number of adult patients with CAP who were admitted to our hospital between January and December 2020 was compared with those from the same period in 2019. A total of 324 patients were diagnosed with CAP in 2019 (including 67 nursing home residents) and a total of 250 patients were diagnosed with CAP in 2020 (including 67 nursing home residents). No significant difference was observed in the number of nursing home residents with CAP between 2019 and 2020 (p = 0.091). We further divided these patients with CAP into four groups based on seasons (January to March, April to June, July to September, and October to December) as shown in Table 1. It showed that the number of patients with CAP in 2020 was lower than that the number of patients with CAP in 2019, which decreased by 74 patients (−22.9%) in 2020. The decreasing number of patients with CAP between 2019 and 2020 increased with season from −13.9% in January to March 2020 to −39.7% in October to December 2020 (p = 0.001; M2 = 11.899). This study explored the influence of increasing age on the number of patients with CAP by comparing the following five age cohorts: <55, 55–64, 65–74, 75–84, and ≥85 years in 2 years as shown in Table 1. The decreasing number of patients with CAP between 2019 and 2020 increased with age cohorts from −14.8% in the youngest cohort to −28.7% in those aged ≥85 years (p = 0.042; M2 = 4.256). In 2019, 136 patients with CAP were detected with pneumonia pathogens with the Streptococci group (19.1%) being the most common followed by Pseudomonas aeruginosa (P. aeruginosa) (17.6%) and Klebsiella pneumoniae (K. pneumoniae) (11.0%), whereas in 2020, 114 patients with CAP were detected with pneumonia pathogens with K. pneumoniae (21.1%) being the most common followed by P. aeruginosa (14.9%) and Escherichia coli (14.0%) (Table 2).
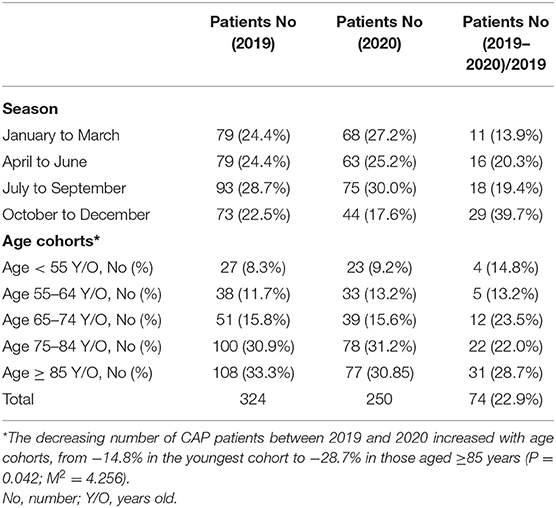
Table 1. Community-acquired pneumonia patients requiring hospitalization, by season and age cohorts.
Hospital-Acquired Pneumonia
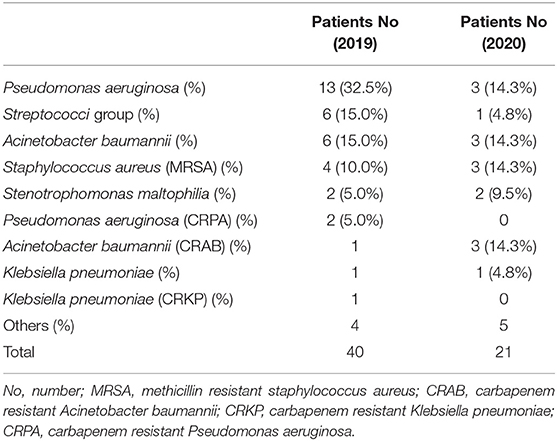
The number of adult patients with HAP who were admitted to our hospital from January to December 2020 was compared with those from the same period in 2019. A total of 72 patients were diagnosed with HAP in 2019 (including 23 nursing home residents) and a total of 49 patients were diagnosed with HAP in 2020 (including 19 nursing home residents). No significant difference in the number of nursing home residents with HAP was observed between 2019 and 2020 (p = 0.445). These patients were further divided into four groups based on seasons (January to March, April to June, July to September, and October to December) as shown in Table 3. It showed that the number of patients with HAP in 2020 was lower than that the number of patients with HAP in 2019, which decreased by 23 patients (−31.9%) in 2020. The decreasing number of patients with HAP between 2019 and 2020 increased with season from −20.0% in January to March 2020 to −52.4% in October to December 2020 (p = 0.016; M2 = 5.865). This study explored the influence of increasing age on the number of patients with HAP by comparing the following five age cohorts: <55, 55–64, 65–74, 75–84, and ≥85 years during the study period as shown in Table 3. The decreasing number of patients with HAP between 2019 and 2020 increased with age from 0% in the youngest cohort to −45.6% in the oldest cohort (p = 0.002; M2 = 8.955). In 2019, 40 patients with HAP were detected with pneumonia pathogens with P. aeruginosa (32.5%) being the most common followed by Streptococci group (15.0%) and Acinetobacter baumannii (A. baumannii) (15.0%), whereas in 2020, 21 patients with HAP were detected with pneumonia pathogens with P. aeruginosa (14.3%), A. baumannii (14.3%), carbapenem-resistant A. baumannii (CRAB) (14.3%), and methicillin-resistant Staphylococcus aureus (MRSA) (14.3%) (Table 4).
Discussion
The reduction in the incidence of viral diseases may be attributed to the COVID-19 preventive measures. However, no study has investigated the impact of preventive infection control measures in reducing the incidence of bacterial pneumonia. To the best of our knowledge, viral infection can trigger the development of bacterial pneumonia. Weinberger et al. reported a significant increase in pneumonia-related hospitalizations during the 2009–2010 H1N1 influenza pandemic.
Conversely, this study showed that the number of patients with CAP reduced by an average of 22.9% and that the number of patients with HAP reduced by an average of 31.9% in 1 year. The longer the number of days, the more the number of patients will decrease. To compare October to December season, the number of patients with CAP reduced by 39.7% in 2020 and the number of patients with HAP reduced by 52.4% in 2020. With respect to the age of the patients, the older the age, the greater the reduction in the number of patients in both the CAP and HAP groups. The strategy to prevent the COVID-19 outbreak has also been extremely effective in promoting the protection of the public from pneumonia, especially the elderly people. With regard to nursing home residents, the number of pneumonia did not significantly decrease in both the CAP and HAP groups during the COVID-19 outbreak period. Several modifiable factors were related to nursing home resident pneumonia such as sedative medications, tube feeding, depression, reduced level of consciousness, several chronic diseases, malnutrition, mobility limitation, poor oral hygiene, and dysphagia (10–17). Therefore, strategies to prevent the COVID-19 outbreak did not provide protection to nursing home residents against pneumonia.
Identification of the pneumonia pathogen is challenging. In the most pneumonia studies, between 35 and 70% of participants had no pathogen identified (18). The literatures of Taiwan showed that the percentage of unknown infectious pathogen of three pneumonia series was 25, 28, and 50%, respectively (19–21). The unknown infectious pathogen was 44.4% in 2019 and 45.2% in 2020 in our series, which was similar as literature reports.
With respect to CAP pathogens, the incidence of patients infected with the Streptococci group in this study showed a decreasing trend in 2020, which could be related to the decreased influenza activity in Taiwan. McCullers et al. showed that influenza infections increase the risk of developing secondary bacterial disease, particularly with streptococcal pneumonia (22). Daniel et al. reported a significant increase in hospitalizations owing to pneumococcal infections that corresponded to the timing of highest pandemic influenza activity (23). Besides, the number of K. pneumonia infections in this study increased in 2020. Lauderdale et al. showed that S. pneumoniae is the most common pathogen of adult patients with CAP requiring hospitalization and gram-negative bacilli, particularly K. pneumoniae, also play an important role in the infections of hospitalized patients with CAP in Taiwan (24). A prospective observational study of adult patients with CAP in 14 hospitals of eight Asian countries (including Taiwan) in 2008 displayed that S. pneumoniae was the most common isolate followed by K. pneumoniae and Haemophilus influenzae (25). A significant decrease in hospitalizations owing to pneumococcal infections in this study corresponded to the lowest influenza activity during the COVID-19 pandemic; however, a significant increase in hospitalizations owing to K. pneumoniae infections was observed in Taiwan. No difference was observed in the pathogens that caused HAP between 2019 and 2020, which may be owing to the extremely small number of HAP in this study.
More than 29 viruses have been linked to pneumonia. Among viral pneumonia, influenza virus remains the most common causes of viral pneumonia in adult. The ways for respiratory virus detection included isolation of the virus by viral culture, antigen detection in respiratory secretions, and reverse transcriptase-PCR in respiratory secretions (26). Influenza PCR was performed for intensive care unit patient who was suspected influenza pneumonia in our hospital. Therefore, there were only three influenza virus pneumonia cases in our series. According to the study by Peto et al., the viral pneumonia accounted for 9.8% of hospitalized CAP in Asian studies and 10.9% of hospitalized CAP in European studies. There were many studies on CAP in the literature and none of these series included viral pneumonia (27–30).
Only one rapid communication discussed the issue of the COVID-19 pandemic and the incidence of the non-COVID-19 pneumonia in the literature. Yamamoto et al. reported that the number of patients with CAP began to decrease in February 2020. The number was significantly lower than those during the same period in the last 3 years. The author thought that these COVID-19 outbreak measures might have indirectly contributed to a decrease in the number of cases through the prevention of common viral infections that could be a trigger of CAP (31). The findings of this study are the same as those of Yamamoto et al. showing that the COVID-19 outbreak prevention measures have indirectly contributed to the decrease in the number of pneumonia cases. Influenza and other respiratory viral infections are the most common type of acute respiratory infection. Viral infections predispose the patients to secondary bacterial infections, especially post-influenza secondary bacterial infection. A study by Klein et al. showed the proportion of bacterial pneumonia in patients with influenza that was found to range between 11 and 35% (32). A decline in the incidence of respiratory viral diseases had been reported following the COVID-19 outbreak in the literature. The COVID-19 infection control measures may prevent general viral infections and subsequently bacterial pneumonia.
There are the two COVID-19 screening and treatment center in Chiayi County, Taiwan. Our hospital is one of the two COVID-19 screening and treatment center. We setup the 40 COVID-19 isolation general beds and the 10 COVID-19 intensive care bed during the COVID-19 pandemic. Our hospital provides outpatient and inpatient medical services for general patients and COVID-19 inpatient medical services for the patients with COVID-19. We infer the decrease in the number of pneumonia patients by a reflection of changes in the disease incidence.
Limitations
The major limitation of this study is that it is a retrospective 2-year observational study at a single hospital and, therefore, can provide only minimal clinical experience. The results may, therefore, not be generalizable owing to different populations of the patient in other hospitals and countries.
Conclusion
The number of adult patients with CAP in 2020 was lower than that in the same period in 2019 with the number of adult patients with CAP markedly decreasing (−39.7%) in October to December period of 2020. The number of adult patients with HAP in 2020 was lower than that in the same period in 2019 with the number of adult patients with HAP markedly decreasing (−52.4%) in October to December period of 2020. Therefore, interventions applied to control the COVID-19 pandemic were effective not only in influencing substantial changes in the seasonal influenza activity, but also in decreasing pneumonia cases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study conformed to the Declaration of Helsinki 1975, revised Hong Kong 1989. The study was approved by the Buddhist Dalin Tzu Chi General Hospital Research Ethics Committee (Approved IRB No. B11002015).
Author Contributions
The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (accessed April 23, 2020).
2. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. (2020) 323:1341–2. doi: 10.1001/jama.2020.3151
3. Angoulvant F, Ouldali N, Yang DD, Filser M, Gajdos V, Rybak A, et al. COVID-19 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections, a time series analysis. Clin Infect Dis. (2021) 72:319–22. doi: 10.1093/cid/ciaa710
4. Lee H, Lee H, Song KH, Kim ES, Park JS, Jung J, et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin Infect Dis. (2020) 73:e132–40. doi: 10.1093/cid/ciaa672
5. Nolen LD, Seeman S, Bruden D, Klejka J, Desnoyers C, Tiesinga J, et al. Impact of social distancing and travel restrictions on non-COVID-19 respiratory hospital admissions in young children in rural Alaska. Clin Infect Dis. (2020) 72:2196–8. doi: 10.1093/cid/ciaa1328
6. Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in finland during early 2020. Pediatr Infect Dis J. (2020) 39:e423–7. doi: 10.1097/INF.0000000000002845
7. Australian Influenza Surveillance Report No. 11, 24 August to 6 September 2020. Australian Government Department of Health (2020). Available online at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/ozflusurveil-no11-20.htm (accessed September 11, 2020).
8. Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARSCoV- 2 outbreak in Japan. JAMA. (2020) 323:1969–71. doi: 10.1001/jama.2020.6173
9. Chou CC, Shen CF, Chen SJ, Chen HM, Wang YC, Chang WS, et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. (2019) 52:172–99. doi: 10.1016/j.jmii.2018.11.004
10. Quagliarello V, Ginter S, Han L, Van Ness P, Allore H, Tinetti M. Modifiable risk factors for nursing home-acquired pneumonia. Clin Infect Dis. (2005) 40:1–6. doi: 10.1086/426023
11. Alvarez S, Shell C, Woolley T, Berk S, Smith J. Nosocomial infections in long term care facilities. J Gerontol. (1988) 43:M9–17. doi: 10.1093/geronj/43.1.M9
12. Harkness G, Bentley D, Roghmann K. Risk factors for nosocomial pneumonia in the elderly. Am J Med. (1990) 89:457–63. doi: 10.1016/0002-9343(90)90376-O
13. Magaziner J, Tenney J, DeForge B, Hebel R, Muncie H, Warren J. Prevalence and characteristics of nursing home acquired infections in the aged. J Am Geriatr Soc. (1991) 39:1071–8. doi: 10.1111/j.1532-5415.1991.tb02871.x
14. Langmore S, Terpenning M, Schork A, Chen Y, Murray JT, Lopatin D, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. (1998) 13:69–81. doi: 10.1007/PL00009559
15. Vergis EN, Brennan C, Wagener M, Muder RR. Pneumonia in long term care: a prospective case-control study of risk factors and impact on survival. Arch Intern Med. (2001) 161:2378–81. doi: 10.1001/archinte.161.19.2378
16. Yoshino A, Ebihara T, Ebihara S, Fuji H, Sasaki H. Daily oral care and risk factors for pneumonia among elderly nursing home patients. JAMA. (2001) 286:2235–6. doi: 10.1001/jama.286.18.2235
17. Hollaar VRY, van der Putten GJ, van der Maarel-Wierink CD, Bronkhorst EM, de Swart BJM, de Baat C, et al. Nursing home acquired pneumonia, dysphagia and associated diseases in nursing home residents: a retrospective, cross-sectional study. Geriatr Nurs. (2017) 38:437–41. doi: 10.1016/j.gerinurse.2017.02.007
18. Peto L, Nadjm B, Horby P, Ngan TT, van Doorn R, Van Kinh N, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. (2014) 108:326–37. doi: 10.1093/trstmh/tru058
19. Lee YT, Chen SC, Chan KC, Wu TC, Tsao SM, Chan CH. Impact of infectious etiology on the outcome of Taiwanese patients hospitalized with community acquired pneumonia. J Infect Dev Ctries. (2013) 7:116–24. doi: 10.3855/jidc.2834
20. Yen MY, Hu BS, Chen YS, Lee SS, Lin YS, Wann SR, et al. A prospective etiologic study of community-acquired pneumonia in Taiwan. J Formos Med Assoc. (2005) 104:724–30.
21. Wu CL, Ku SC, Yang KY, Fang WF, Tu CY, Chen CW, et al. Antimicrobial drug-resistant microbes associated with hospitalized community-acquired and healthcare-associated pneumonia: a multi-center study in Taiwan. J Formos Med Assoc. (2013) 112:31–40. doi: 10.1016/j.jfma.2011.09.028
22. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. (2006) 19:571–82. doi: 10.1128/CMR.00058-05
23. Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. (2012) 205:458–65. doi: 10.1093/infdis/jir749
24. Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiologicalcharacteristics in Taiwan, 2001–2008. BMC Infect Dis. (2010) 10:307. doi: 10.1186/1471-2334-10-307
25. Song JH, Oh WS, Kang CI, Chung DR, Peck KR, Ko KS, et al. Epidemiology and clinical outcomes of community-acquired pneumoniain adult patients in Asian countries: a prospective study by the Asiannetwork for surveillance of resistant pathogens. Int J Antimicrob Agents. (2008) 31:107–14. doi: 10.1016/j.ijantimicag.2007.09.014
26. Dandachi D, Rodriguez-Barradas MC. Viral pneumonia: etiologies and treatment. J Investig Med. (2018) 66:957–65. doi: 10.1136/jim-2018-000712
27. Loh LC, Khoo SK, Quah SY, Visvalingam V, Radhakrishnan A, Vijayasingham P, et al. Adult community-acquired pneumonia in Malaysia: prediction of mortality from severity assessment on admission. Respirology. (2004) 9:379–86. doi: 10.1111/j.1440-1843.2004.00604.x
28. Ngeow YF, Suwanjutha S, Chantarojanasriri T, Wang F, Saniel M, Alejandria M, et al. An Asian study onthe prevalence of atypical respiratory pathogens in community-acquiredpneumonia. Int J Infect Dis. (2005) 9:144–53. doi: 10.1016/j.ijid.2004.06.006
29. Reechaipichitkul W, Lulitanond V, Tantiwong P, Saelee R, Pisprasert V. Etiologies and treatment outcomes in patients hospitalized with community-acquired pneumonia (CAP) at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. (2005) 36:156–61.
30. Horie M, Ugajin M, Suzuki M, Noguchi S, Tanaka W, Yoshihara H, et al. Diagnostic and prognostic value ofprocalcitonin in community-acquired pneumonia. Am J Med Sci. (2012) 343:30–5. doi: 10.1097/MAJ.0b013e31821d33ef
31. Yamamoto T, Komiya K, Fujita N, Okabe E, Hiramatsu K, Kadota JI. COVID-19 pandemic and the incidence of community-acquired pneumonia in elderly people. Respir Investig. (2020) 58:435–6. doi: 10.1016/j.resinv.2020.09.001
Keywords: COVID-19, community acquired pneumonia, hospital acquired pneumonia, nursing home acquired pneumonia, pandemic
Citation: Huang C (2021) The COVID-19 Pandemic and the Incidence of the Non-COVID-19 Pneumonia in Adults. Front. Med. 8:737999. doi: 10.3389/fmed.2021.737999
Received: 08 July 2021; Accepted: 12 October 2021;
Published: 11 November 2021.
Edited by:
Laurent Pierre Nicod, University of Lausanne, SwitzerlandReviewed by:
Mats W. Johansson, Morgridge Institute for Research, United StatesSanda Predescu, Rush University Medical Center, United States
Copyright © 2021 Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chienhsiu Huang, aGdzc3BvcnRAeWFob28uY29tLnR3; ZG01NTA2NzFAdHp1Y2hpLmNvbS50dw==; orcid.org/0000-0003-1239-9584
 Chienhsiu Huang
Chienhsiu Huang